Abstract
Objective
The BARI 2D trial compared insulin provision (IP) versus insulin sensitization (IS) for the primary outcome of total mortality in participants with T2DM and cardiovascular disease (CVD). In this analysis we examine baseline characteristics that are associated with successful long-term glycemic control.
Research Design and Methods
In a 2×2 factorial design, 2,368 participants were randomized to either IP or IS therapy, and to either prompt revascularization with medical therapy or medical therapy alone. Successful long-term glycemic control (success) was defined by simultaneously meeting 1) a mean HbA1c level of <7.0% after each participant's third year of follow-up period, and 2) adherence with medications only from the assigned glycemic treatment arm during >80% of the BARI 2D follow-up. The association between baseline variables and success was determined using unadjusted and adjusted logistic regression models.
Results
1,917 participants (962 IP and 955 IS participants) had sufficiently long follow-up and data for this analysis. Among these IP and IS participants, 235 and 335 participants met both criteria of success, respectively (p <0.001). Those not on insulin at entry had higher odds of success (OR 2.25; CI 1.79-2.82) when treated with IS versus IP medications, irrespective of baseline HbA1c levels. Younger age, shorter duration of T2DM, and lower HbA1c at baseline were also each independently associated with higher success when treated with IS versus IP medications.
Conclusion
Patients similar to those in the BARI 2D trial may have a higher chance of achieving success with IS versus IP medications if they are younger, have shorter duration of T2DM, have lower HbA1c levels, have moderate or strenuous physically activity, and are not on insulin. In contrast, increasing age, longer duration of T2DM, higher HbA1c, and insulin therapy are associated with increased chance of success if treated with IP medications.
Index words: Cardiovascular Disease, Plasma Insulin levels, Predictors of Glycemic Control
Introduction
Glycemic control is an important goal in the management of type 2 diabetes (T2DM) (1). Maintaining an HbA1c <7.0%, provided that it can be safely achieved, is deemed important in prevention of complications of diabetes (1-3). However, close to half of the patients with T2DM continue to have HbA1c levels of >7.0% (4). Glycemic control varies among geographic locations and racial/ethnic groups, and is influenced by obesity, dyslipidemia, duration of diabetes, basal HbA1c, and type of medication used (5-7). Family history of T2DM is associated with “poor” glycemic control (8), although this association was not found in Mexicans and Mexican Americans (9). Other factors associated with “poor” glycemic control include access to health care, socioeconomic status, body mass index, depression, knowledge of diabetes, co-morbid conditions, and perceived health status (10-12).
The five-year, multinational multicenter randomized controlled Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial with a 2-by-2 factorial design tested effect of (i) insulin-providing (IP) versus insulin-sensitizing (IS) therapy prompt, and (ii) revascularization plus intensive medical therapy versus initial intensive medical therapy alone with the option of revascularization if necessary to relieve symptoms, for a primary outcome of total mortality in participants with T2DM (13-16). Insulin formulations, sulfonylureas, or both were employed in the IP arm, while metformin, a thiazolidinedione (mostly rosiglitazone), or both were used in the IS arm of the study. On average, participants were 62.4 years old with type 2 diabetes of 10.4 years duration, and had advanced stable documented coronary artery disease.
At trial's end, we noted that a sizable fraction of participants in both IS and IP arms of the study had successful glycemic control (defined as an average HbA1c of <7.0% after three years of follow-up while remaining predominantly on their group-assigned glycemic therapy). We found this observation to be intriguing, since as clinicians we seek better predictors of which patient would respond well for prolonged periods to IS or IP therapy.
The purpose of this analysis is to determine whether any baseline patient characteristics are associated with successful long-term glycemic control (success as defined above) during the BARI 2D follow-up. We chose to include both HbA1c and sustained therapy in the definition of “success” since these criteria are deemed important in the management of patients with T2DM. In addition, we specifically chose to examine baseline factors since this setting mimics the first encounter that a health-care provider may have with a patient with established diabetes and cardiovascular disease (CVD).
Methods
The general characteristics and the primary outcome of the BARI 2D cohort, as well as the design of the trial, have been published (13-16). In general, older participants (average age 62.4 years) with established T2DM and documented coronary artery disease (CAD) were enrolled
HbA1c values used in this analysis were those obtained by standardized methods in the laboratories at each clinic. Fasting insulin assays were performed as described (17).
Definition of Successful Long-Term Glycemic Control (Success)
In this analysis, success was defined by simultaneously meeting two criteria: namely, i) a mean HbA1c level of <7.0% starting at each participant's third year anniversary during follow-up and ending with their last available measurement, and ii) adherence (ascertained by medical history) defined as using medications only from the assigned glycemic treatment arm during >80% of their BARI 2D follow-up period starting six months after entry into the trial and ending at the exit visit.
Statistical Analysis
The calculation of the mean HbA1c was based on an estimate of the “area-under-the-curve” (AUC), where the curve consists of a sequence of straight lines connecting consecutive HbA1c values. For each patient, the HbA1c mean was obtained by dividing the area under these lines by the appropriate length of time for that patient. The latter is measured from the HbA1c value taken nearest to the 3-year anniversary to the last HbA1c measured while in the BARI 2D trial. Note that in selecting the HbA1c value nearest to the 3-year anniversary, HbA1c values obtained after 2.5 years into the study were considered.
Continuous variables are summarized as means ± SE, and categorical variables are summarized as percentages. When comparing groups of participants, t-tests were used to assess the statistical significance of differences between means, and chi-square tests were used to assess the statistical significance of differences between percentages.
A logistic regression model was used to study the association between baseline variables and success during follow-up. The variables presented in the patient description in Table 1 were allowed to enter the model sequentially if their association with the outcome was significant (p<0.05) either among participants in the IP arm or among participants in the IS arm. This selection process was started with the following baseline variables reflecting patient status at study entry forced to be in the model: diabetes therapy category (one oral IP agent, one oral IS agent, two or more oral agents, insulin alone, and insulin plus oral agents), HbA1c, patient age, and number of years with diabetes. In a second logistic regression model, the association between baseline insulin use and success was assessed in both the IP and IS arms after controlling for baseline HbA1c, age, duration of diabetes, and also for the same baseline variables selected to build the first logistic regression model. A third set of logistic regression models was used to examine the association between the strategy for glycemic control (IS vs. IP) and the outcome of actually achieving success. Because of the critical role that insulin use at baseline would be expected to have on the relative effectiveness of IS versus IP strategies, all models were developed separately, once among participants not on insulin at baseline and then again among participants on insulin at baseline. For each group, the association between randomized glycemic control strategy and success was estimated (with odds ratios) overall, and also within subgroups defined by baseline HbA1c (<6.0%, 6.0-6.5%, 6.6-7.0%, and >7.0%), age (<50, 50-60, 61-70, >70 years), duration of diabetes (<5, 5-10, 11-20, >20 years), level of physical activity (sedentary or mild vs. moderate or strenuous), and by fasting plasma insulin concentration (≤5 vs. >5 μIU/ml).
Table 1.
Diabetes-related variables at baseline and during follow-up, and successful glycemic control (unadjusted for baseline variables).
| N (1917) |
Mean baseline HbA1c (%) (7.75) |
Glycemic control during follow-up | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| IP Arm | IS Arm | |||||
|
| ||||||
| N (962) |
% with successful control (24.4) | N (955) |
% with successful control (35.1) |
|||
|
| ||||||
| Baseline DM therapy groups | ||||||
| None | 164 | 6.9 | 78 | 24.4 | 86 | 45.3 |
| One oral agent | 577 | 7.3 | 284 | 29.2 | 293 | 57.3 |
| α-glucosidase inhibitor | 1 | 5.2 | 0 | -- | 1 | 0.0 |
| Sulfonylurea | 316 | 7.5 | 154 | 33.8 | 162 | 48.1 |
| Meglitinide | 3 | 7.6 | 3 | 33.3 | 0 | -- |
| Biguanide | 225 | 7.0 | 112 | 24.1 | 113 | 69.0 |
| Thiazolidinedione | 32 | 6.9 | 15 | 20.0 | 17 | 70.6 |
| Two or more oral agents | 652 | 7.8 | 327 | 22.9 | 325 | 31.1 |
| Sulfonylurea + biguanide | 412 | 8.0 | 209 | 23.0 | 203 | 36.0 |
| Sulfonylurea + biguanide + TZD | 133 | 7.6 | 72 | 19.4 | 61 | 14.8 |
| Other combinations | 107 | 7.6 | 46 | 28.3 | 61 | 31.1 |
| Insulin alone | 205 | 8.5 | 117 | 23.9 | 88 | 11.2 |
| Insulin plus oral agent(s) | 319 | 8.3 | 156 | 19.2 | 163 | 11.0 |
| Ins. + biguanide | 106 | 8.4 | 52 | 23.1 | 54 | 14.8 |
| Ins. + sulfonylurea + biguanide | 60 | 8.3 | 25 | 20.0 | 35 | 8.6 |
| Ins. + thiazolidinedione | 42 | 8.2 | 22 | 27.3 | 20 | 10.0 |
| Ins. + sulfonylurea | 39 | 8.7 | 25 | 8.0 | 14 | 14.3 |
| Ins. + biguanide + thiazolidinedione | 39 | 8.2 | 19 | 10.5 | 20 | 5.0 |
| Other combinations | 33 | 7.9 | 13 | 23.1 | 20 | 10.0 |
|
| ||||||
| Age at baseline, years | ||||||
| <50 | 167 | 8.4 | 81 | 11.1 | 86 | 31.4 |
| 50-55 | 318 | 8.2 | 158 | 17.1 | 160 | 28.1 |
| 56-60 | 380 | 7.9 | 191 | 19.9 | 189 | 36.5 |
| 61-65 | 411 | 7.7 | 217 | 22.1 | 194 | 33.0 |
| 66-70 | 312 | 7.4 | 164 | 31.1 | 148 | 40.5 |
| 71+ | 329 | 7.2 | 151 | 41.1 | 178 | 39.3 |
|
| ||||||
| Duration of diabetes at baseline, years | ||||||
| 0-5 | 759 | 7.4 | 370 | 23.2 | 389 | 49.1 |
| 6-10 | 419 | 7.8 | 198 | 23.7 | 221 | 33.9 |
| 11-15 | 311 | 8.2 | 162 | 25.3 | 149 | 22.8 |
| 16-20 | 221 | 8.1 | 111 | 25.2 | 110 | 21.8 |
| 21+ | 207 | 8.1 | 121 | 27.3 | 86 | 12.8 |
|
| ||||||
| HbA1c at baseline | ||||||
| <6.0% | 202 | -- | 105 | 37.1 | 97 | 60.8 |
| 6.0%-6.5% | 295 | -- | 139 | 30.9 | 156 | 50.6 |
| 6.6%-7.0% | 281 | -- | 147 | 31.2 | 134 | 45.5 |
| 7.1%-7.5% | 254 | -- | 121 | 26.4 | 133 | 34.6 |
| 7.6%-8.0% | 221 | -- | 122 | 21.3 | 99 | 28.3 |
| 8.1%-8.5% | 153 | -- | 80 | 17.5 | 73 | 21.9 |
| 8.6%-9.0% | 130 | -- | 68 | 19.1 | 62 | 25.8 |
| 9.1% + | 381 | -- | 180 | 11.7 | 201 | 14.9 |
Results
The patient-selection process employed for this analysis is shown in Figure 1. From the total of 2,368 participants randomized in the BARI 2D trial, a full set of baseline variables was available in 2,314 participants, among whom 1,917 participants had at least three years of active follow-up, and at least one HbA1c value obtained at or after 3 years. The present analysis includes 962 participants in the IP arm and 955 participants in the IS arm of the study.
Figure 1.
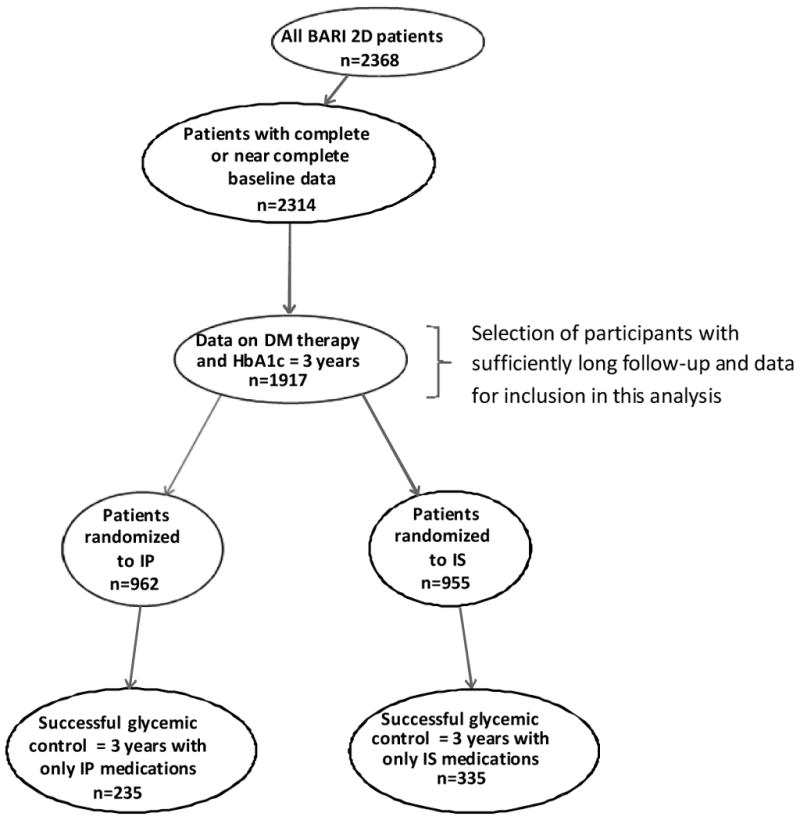
Flow-chart of participants included in the analysis.
Baseline characteristics of participants in the IP and IS arms included in this analysis are summarized in Table A in the Appendix. Very few differences were found between the two groups and compared to the entire BARI 2D cohort (14, 15). The average patient was 62 years old (29% female) with a 10.3 year history of diabetes, had a BMI of 32 kg/m2 and an HbA1c of 7.7%. At entry, 27% were being treated with insulin, and all had advanced coronary heart disease, 65% had ≥ 2 diseased regions, and the mean myocardial jeopardy index was 44% (15). Baseline serum creatinine averaged 1.0 mg/dL (88.4 μmol/L (creatinine >2.0 mg/dL [176.8 μmol/L] was an exclusion criterion), and 22.5% had microalbuminuria and 8.7% had macroalbuminuria.
Among the 962 participants in the IP arm, 706 (73.4%) satisfied the medication-adherence criterion, 358 (37.2%) met the HbA1c criterion, and 235 (24.4%) met both criteria for success (Table B in the Appendix and Figure 1). Among the 955 participants in the IS arm, 417 (43.7%) met the medication-adherence criterion, 522 (54.7%) met the HbA1c criterion, and 335 (35.1%) met both criteria for success. The difference in the percentage of participants achieving success (24.4% in the IP versus 35.1% in the IS arm) was highly significant (p <0.0001), while their baseline HbA1c was not significantly different (7.18% and 7.06% in the IP and IS groups, respectively; p=0.30). The mean in-trial HbA1c of the 962 IP and 955 IS participants was 7.57 ± 0.04% and 7.12 ± 0.04%, respectively (p<0.001), and the mean in-trial HbA1c of the 706 adherent IP and 417 adherent IS participants was 7.62 ± 0.05% and 6.53 ± 0.03%, respectively (p<0.001).
Table 1 describes the relationship between several important diabetes-related variables at baseline and successful glycemic control during follow-up (unadjusted for baseline variables). Considering HbA1c levels, participants taking no glycemia-lowering medications on average had lower baseline HbA1c values than participants taking one or more oral agents (p<0.001), with the highest values being among those treated with insulin alone or in combination with other agents. Baseline HbA1c values were highest among younger participants (p<0.001 for trend), and lower among those with shorter duration of the disease (p<0.001 for trend). Considering successful outcomes, those who entered the trial taking no or only one oral glycemia-lowering agent had higher success if randomized to the IS arm (p<0.001). In contrast, those who entered using insulin alone or in combination with oral agents had more success if randomized into the IP arm (p=0.001). Participants up to age 70 and those with 10 or fewer years of T2DM had relatively more success if randomized to the IS arm (p<0.001 for both). Finally, those with HbA1c values of ≤7.5% had higher success if randomized to the IS arm (p<0.001). Not surprisingly, the success rate in both groups decreased with increasing HbA1c at entry (p<0.001 for trend for both the IP and IS arms).
Table 2 summarizes the independent association between baseline variables and successful glycemic control (adjusted odds-ratios). Among participants in the IP arm, those taking one oral IP agent at entry were more likely to achieve success compared to participants not taking any glycemia-lowering medication (OR=2.21; p=0.02). Among IS participants, those taking one oral IS agent at entry had the highest likelihood of success compared to participants taking no medications (OR=3.79, p<0.001). In IS participants, those who were on insulin at entry were less likely to achieve success, regardless of whether their therapy included oral agents (OR=0.37, p=0.01) or not (OR=0.38, p=0.04). In both the IP and the IS arms, higher HbA1c at entry was associated with lesser odds of success (OR≈0.7 for every 1% increase in HbA1c). In the IP arm, the association between increasing age and a higher likelihood of success was statistically significant (OR 1.5) but not significantly different from the OR (1.2) in the IS arm (p=0.081). In the IS arm, longer duration of diabetes was associated with a smaller likelihood of success (OR=0.80 for every additional 5 years with the disease, p=0.001), whereas among IP participants this association was not evident (OR=1.02, p=.69) with the difference in OR between the two arms being significant (p=0.005).
Table 2. General baseline predictors of successful long-term glycemic control in participants in the IP and IS arms Adjusted odds ratios (OR) from logistic regression models.
| IP Arm | IS Arm | OR in IP arm compared to OR in IS arm p |
||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N=962 n=235 successful control |
N=9541 n=335 successful control |
|||||||
|
| ||||||||
| OR | 95% C.I. | p | OR | 95% C.I. | p | |||
| Compared to participants not on any diabetes drug1, | n=164 | |||||||
| On one oral IP agent | n=319 | 2.208 | 1.12-4.35 | 0.022 | 1.518 | 0.84-2.75 | 0.17 | 0.42 |
| On one oral IS agent | n=257 | 1.202 | 0.59-2.44 | 0.61 | 3.794 | 2.01-7.15 | <.001 | 0.018 |
| On two or more oral agents | n=652 | 1.368 | 0.71-2.63 | 0.35 | 0.993 | 0.56-1.75 | 0.98 | 0.47 |
| On insulin: | ||||||||
| Insulin alone | n=205 | 1.593 | 0.70-3.64 | 0.27 | 0.378 | 0.15-0.95 | 0.039 | 0.023 |
| Insulin plus oral agent(s) | n=319 | 1.149 | 0.53-2.49 | 0.73 | 0.368 | 0.17-0.79 | 0.010 | 0.040 |
|
| ||||||||
| HbA1c (increment of 1%) | 0.697 | 0.61-0.79 | <.001 | 0.673 | 0.60-0.76 | <.001 | 0.69 | |
|
| ||||||||
| Age (increment of 10 years) | 1.530 | 1.23-1.90 | <.001 | 1.179 | 0.97-1.44 | 0.10 | 0.081 | |
|
| ||||||||
| Duration of DM (increment of 5 years) | 1.023 | 0.92-1.14 | 0.69 | 0.804 | 0.71-0.92 | 0.001 | 0.005 | |
|
| ||||||||
| Other clinical and demographic variables: | ||||||||
|
| ||||||||
| Canada vs. US | 0.997 | 0.63-1.57 | 0.99 | 1.060 | 0.68-1.64 | 0.80 | 0.85 | |
| Mexico vs. US | 1.486 | 0.57-3.89 | 0.42 | 2.736 | 1.18-6.35 | 0.019 | 0.35 | |
| Brazil vs. US | 2.868 | 1.71-4.80 | <.001 | 3.462 | 2.01-5.98 | <.001 | 0.62 | |
| Vienna, Prague vs. US | 0.822 | 0.29-2.37 | 0.72 | 0.437 | 0.16-1.20 | 0.11 | 0.40 | |
|
| ||||||||
| High school education or higher | 0.694 | 0.48-1.00 | 0.0496 | 0.773 | 0.53-1.12 | 0.17 | 0.68 | |
|
| ||||||||
| Moderate or strenuous level of physical activity | 0.733 | 0.52-1.04 | 0.08 | 1.807 | 1.28-2.55 | <.001 | <.001 | |
|
| ||||||||
| Current smoker | 0.754 | 0.42-1.36 | 0.35 | 0.573 | 0.34-0.98 | 0.041 | 0.50 | |
|
| ||||||||
| Non-compressible artery (i.e., ABI cannot be measured) | 1.991 | 1.00-3.96 | 0.0496 | 1.039 | 0.46-2.36 | 0.93 | 0.23 | |
|
| ||||||||
| High creatinine: >1.5 mg/dl males, >1.4 mg/dl females (x88.4 for μmol/L) | 1.417 | 0.74-2.73 | 0.30 | 0.398 | 0.18-0.88 | 0.024 | 0.016 | |
|
| ||||||||
| Diastolic blood pressure (10 mmHg increment) | 0.814 | 0.70-0.95 | 0.010 | 0.881 | 0.75-1.03 | 0.11 | 0.48 | |
|
| ||||||||
| Prior PCI | 0.719 | 0.47-1.10 | 0.13 | 0.640 | 0.42-0.98 | 0.042 | 0.70 | |
|
| ||||||||
| Insulin > 5 μIU/ml vs. =< 5 μIU/ml (x6.945 for pmol/L) | 1.135 | 0.73-1.76 | 0.57 | 1.575 | 1.05-2.37 | 0.030 | 0.29 | |
One person was excluded who was taking acarbose and no other anti-glycemic medication, a therapy that does not fit into any of the groups analyzed in this table.
The association between baseline variables and success is quantified with odds ratios (OR), with values larger or smaller than 1.0 indicating respectively a higher or lower likelihood of having successful glycemic control. These odds ratios, and their associated confidence intervals and p-values were estimated separately for participants randomized to IP and IS arms; the p value of potential difference between the two arms is given in the last column.
Examination of other clinical and demographic variables showed that compared to participants from the US, Brazilian participants in both IP and IS arms exhibited higher success (Table 2). Because of this intriguing observation, additional information about the baseline characteristics of the cohort from Brazil versus the United States is supplied in Appendix Table C. In general, the cohort from Brazil had lower duration of disease, higher percentage with less than high school education, lower BMI and waist circumference, higher BP, lower health distress score, and higher myocardial jeopardy. High school or higher levels of education was associated with lower odds of success in both study arms. Participants with moderate or strenuous physical activity (compared to those without) had higher odds of success if randomized to the IS arm (OR=1.81; p<0.001). The association between high creatinine at entry (>1.5 and >1.4 mg/dL in males and females [132.6 and 123.8 μmol/L], respectively) and the likelihood of success among IS participants was significantly less than 1.0 (OR=0.40; p=0.024), whereas this association was reversed in IP participants (OR=1.42, p=0.30) and significantly different than the IS arm (p=0.016). A history of prior PCI was associated with less success in both arms. Finally, among participants in the IS arm, the association between plasma insulin levels higher than 5 μIU/mL (34.8 pmol/L) and success was positive and statistically significant (OR=1.58; p=0.030).
Because of the clinical importance of BMI as a predictor of long-term glycemic control, we considered its effect on the outcome. Analyzed as a continuous variable, and after controlling for the variables shown in Table 2, BMI was found not to be significantly associated with the odds of successful long-term control in the IP arm (p=0.27) or in the IS arm (p=0.49). As a result, BMI did not enter the model summarized in Table 2. We also examined BMI as a categorical variable using ≤25, 25.1-30, 30.1-35, and >35 as cut-points. The association between BMI and successful glycemic control was again not statistically significant: p=0.12 in the IP arm, and p=0.08 in the IS arm.
We also assessed the association between insulin therapy at baseline and success (Appendix, Table D). Among 1917 participants who had at least three years of active follow-up, 524 were being treated with insulin of any type at baseline. Among participants randomized to the IS arm, insulin use at entry was less likely to result in success (OR=0.28, p<0.001), and this association was significantly more pronounced (p<0.001) than that observed among IP participants (OR=0.86, p=0.48). Higher HbA1c values were associated with lower odds of success in both arms. The effect of increasing age and duration of T2DM showed a similar direction to that summarized in Table 2.
Table 3 compares achieving success between participants in the IS and IP arms, using both percentages and odds ratios of success with IS over IP (IS/IP) from logistic regression models, and the findings stratified by insulin use at baseline are shown in Figures 2A and 2B. The results show the independent association between baseline variables and success of IS/IP strategy (adjusted odds-ratios). Overall, among 1,393 participants who were not on insulin at entry, the odds of success was 2.25 times higher if they were randomized to the IS versus the IP arm. In marked contrast, among 524 participants who were being treated with insulin at baseline, the odds of success in the IS arm was 0.45 compared to those randomized to the IP arm (p<0.001). For those who entered the study on no insulin, there was higher odds of success in the IS arm irrespective of the HbA1c at entry (Figure 2A). In addition, lower HbA1c, lower age, shorter duration of T2DM, and moderate or strenuous physical activity were each independently associated with increasing odds of success if treated with IS versus IP medications (Table 3). The effect of HbA1c, age and duration of diabetes on decreasing OR of success of IS/IP therapy was evident irrespective of entering on or off insulin, with the effect being more prominent in those with no insulin therapy at baseline (Figures 2A and 2B). Finally, among participants not on insulin at baseline with fasting serum insulin of >5.0 μIU/mL (34.8 pmol/L), the superiority of the IS arm was more pronounced (OR=2.41) than among participants with lower insulin levels (OR=1.61) (Figure 2A).
Table 3. Comparison of successful long-term glycemic control between participants in the IS versus the IP arm.
Simple percentages and odds ratios (OR) of IS/IP success rates from logistic regression models
| Successful Long-Term Glycemic Control (Success) During Follow-up | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| All participants | ||||||
|
| ||||||
| IP Arm | IS Arm | OR: IS / IP | 95% C.I. | |||
|
|
|
|||||
| N | % Success | N | % Success | |||
|
| ||||||
| 962 | 24.4 | 955 | 35.1 | 1.67 | 1.37, 2.04 | |
|
| ||||||
| Insulin therapy at baseline | ||||||
| No Insulin (N=1393) | 689 | 25.7 | 704 | 43.8 | 2.25 | 1.79, 2.82 |
|
| ||||||
| Insulin (N=524) | 273 | 21.2 | 251 | 10.8 | 0.45 | 0.27, 0.73 |
|
| ||||||
| Test for heterogeneity1 of ORs | p<0.0001 | |||||
|
| ||||||
| HbA1c at baseline | ||||||
| <6.0% | 105 | 37.1 | 97 | 60.8 | 2.63 | 1.49, 4.64 |
|
| ||||||
| 6.0-6.5% | 139 | 30.9 | 156 | 50.6 | 2.29 | 1.42, 3.69 |
|
| ||||||
| 6.6-7.0% | 147 | 32.0 | 134 | 45.5 | 1.78 | 1.09, 2.89 |
|
| ||||||
| >7.0% | 571 | 18.6 | 568 | 23.9 | 1.38 | 1.04, 1.84 |
|
| ||||||
| Test for heterogeneity1 of ORs | p=0.12 | |||||
|
| ||||||
| Age at baseline | ||||||
| <50 yrs | 81 | 11.1 | 86 | 31.4 | 3.66 | 1.60, 8.38 |
|
| ||||||
| 50-60 yrs | 349 | 18.6 | 349 | 32.7 | 2.12 | 1.49, 3.01 |
|
| ||||||
| 61-70 yrs | 381 | 26.0 | 342 | 36.3 | 1.62 | 1.18, 2.23 |
|
| ||||||
| >70 yrs | 151 | 41.1 | 178 | 39.3 | 0.93 | 0.60, 1.45 |
|
| ||||||
| Test for heterogeneity1 of ORs | p=0.008 | |||||
|
| ||||||
| Duration of DM | ||||||
| < 5 yrs | 370 | 23.2 | 389 | 49.1 | 3.19 | 2.33, 4.36 |
|
| ||||||
| 5-10 yrs | 198 | 23.7 | 221 | 33.9 | 1.65 | 1.07, 2.54 |
|
| ||||||
| 11-20 yrs | 273 | 25.3 | 259 | 22.4 | 0.85 | 0.57, 1.27 |
|
| ||||||
| > 20 yrs | 121 | 27.3 | 86 | 12.8 | 0.39 | 0.19, 0.83 |
|
| ||||||
| Test for heterogeneity1 of ORs | p<0.0001 | |||||
|
| ||||||
| Level of physical activity | ||||||
| Sedentary or mild | 591 | 26.9 | 605 | 33.2 | 1.35 | 1.06, 1.73 |
|
| ||||||
| Moderate or strenuous | 371 | 20.5 | 350 | 38.3 | 2.41 | 1.73, 3.36 |
|
| ||||||
| Test for heterogeneity1 of ORs | p=0.006 | |||||
|
| ||||||
| Basal insulin | ||||||
| ≤ 5 μIU/ml** | 166 | 24.1 | 188 | 31.9 | 1.48 | 0.92, 2.36 |
|
| ||||||
| > 5 μIU/ml** | 737 | 24.4 | 710 | 35.5 | 1.70 | 1.36, 2.14 |
|
| ||||||
| Test for heterogeneity1 of ORs | p=0.59 | |||||
Interaction between randomization arm (IS or IP) and each row variable.
multiply by 6.945 to obtain pmol/L
Figure 2a. Comparison (IS vs. IP) of successful long–term glycemic control.
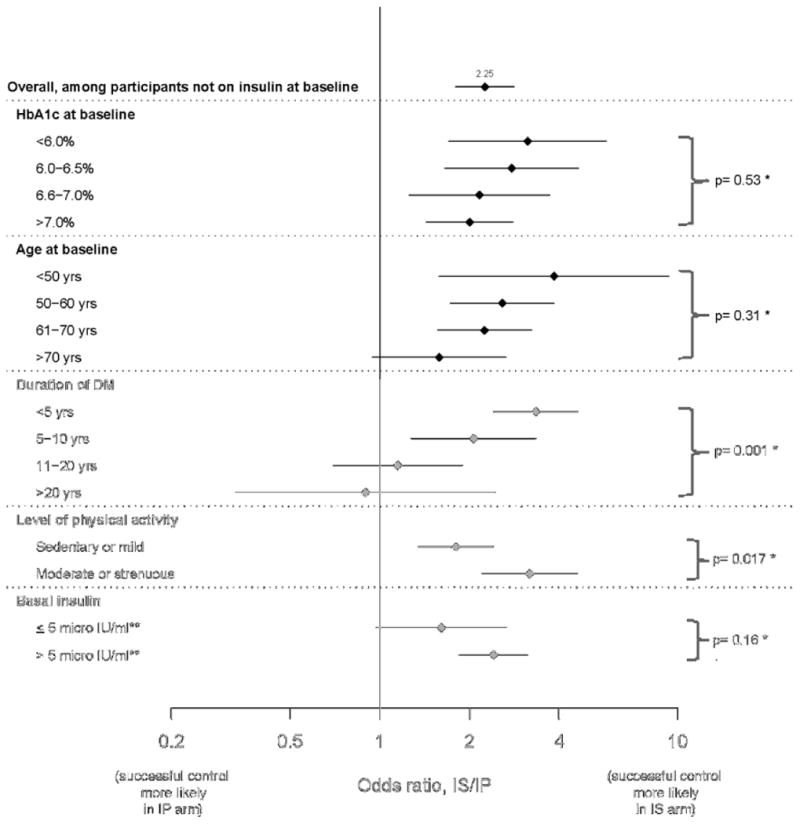
Odds ratio (IS/IP) estimates for participants not on insulin at baseline
*testing whether difference in odds ratio estimates is statistically significant
**multiply by 6.945 to obtain pmol/L
Figure 2b. Comparison (IS vs. IP) of successful long–term glycemic control.
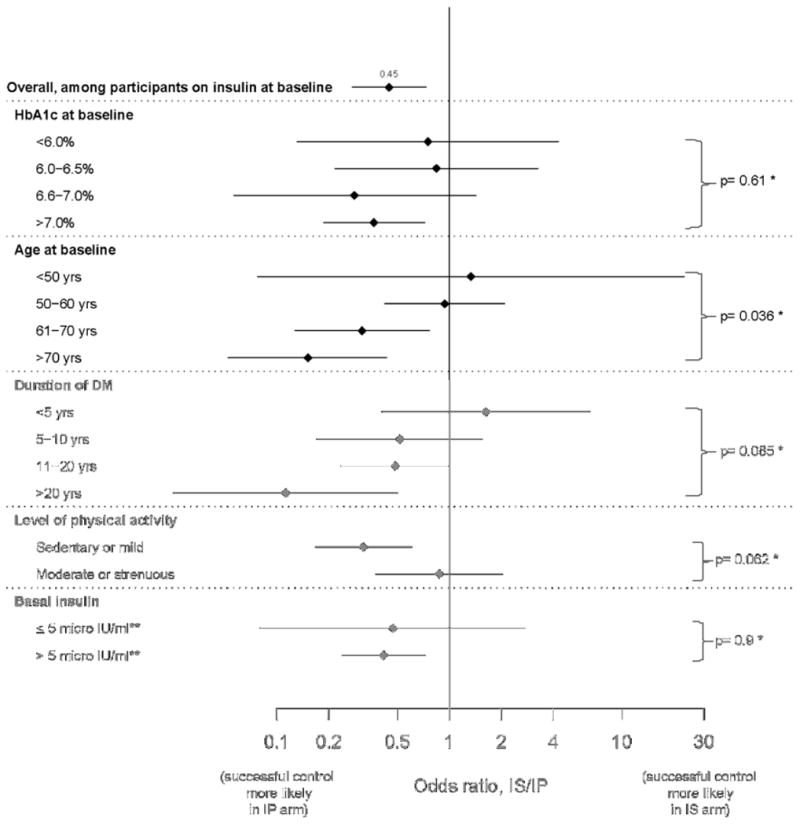
Odds ratio (IS/IP) estimates for participants not on insulin at baseline
*testing whether difference in odds ratio estimates is statistically significant
**multiply by 6.945 to obtain pmol/L
Discussion
This analysis was performed with the aim of identifying clinically relevant factors that could help in selecting treatment strategies that are apt to be positively associated with success in patients similar to those participating in the BARI 2D trial. Because most of the participants in either arm of the trial did not achieve success during follow-up (as defined herein), no specific “road-map” for selection of best medical management strategy based on baseline parameters can be offered. Nevertheless, the results of this analysis do offer some clinically useful insights on certain patient-specific attributes that may lead to success in selected subgroups of patients.
Overall, participants randomized to IS who were treated with IS drugs more than 80% of the time were more likely to maintain HbA1c < 7.0% (35%) than participants randomized to IP who were treated with IP medications more than 80% of the time (24%). This is in keeping with results of a randomized study comparing monotherapy with metformin, rosiglitazone, and glyburide which found that the effect of the sulfonylurea in controlling glycemia was least durable (18). The mean in-trial HbA1c of the above IS group was 6.53 ± 0.04% compared to the above IP group at 7.62 ± 0.03%. This difference when extrapolated to large groups of similar patients with T2DM and CVD would be expected to decrease the number of microvascular complication events significantly (19-21). Participants randomized to the IS arm who entered the trial while being treated with insulin were much less likely to achieve success with IS medications alone compared to those who entered the trial not on insulin (OR 0.28, p<0.001; Table C in Appendix). However in those not on insulin at baseline, IS was more effective than IP (OR 2.25) whereas the IS/IP OR was 0.45 in those on insulin at baseline (Figures 2A and B). The superiority of the IS arm became significantly less pronounced with increasing duration of diabetes. These finding are not unexpected, since IS medications appear to work best on a background of circulating insulin (22). Moreover, patients with longer duration of diabetes often have less beta-cell function, are apt to be on insulin therapy (23), and may be less responsive to IS medications when used alone. In keeping with this premise, we found that participants with longer duration of diabetes were less likely to achieve success in the IS arm. In addition, among participants who were not on insulin at baseline, those with insulin levels >5.0 μIU/mL (34.8 pmol/L) in the IS arm had a higher chance of success than those in the IP arm.
Among participants in the IS arm, those already on monotherapy with one IS medication at baseline were more likely to achieve success when compared to those on no medication at entry (OR = 3.79; p<0.001). Similarly, among participants in the IP group, those on one non-insulin IP medication at entry (usually a sulfonylurea) were more likely to achieve success when compared to those on no medication at entry (OR = 2.21; p=0.022). Within the IP group, older age and those with advanced systemic atherosclerosis (manifested by “non-compressible” arteries) were less likely to achieve success. The reasons for this observation, especially those with advanced atherosclerosis, are not entirely clear. In studies of mechanisms underlying the increasing prevalence of hyperglycemia with increasing age among non-diabetic and pre-diabetic people, insulin resistance corrected for BMI remains relatively constant while beta-cell function diminishes with age with the rate being faster in those with prediabetes (24-27). In addition, increasing age is associated with higher percentage of fat (even with constant body mass) which can be associated with increased insulin resistance (25-27). Hence, it may be surmised that older patients with less beta-cell function are apt to be more successful at glycemic control when treated with IP medications. In addition, IS agents such as metformin, may be less efficacious in older people, as was demonstrated in the Diabetes Prevention Trial (28). While the explanation for the observation that older participants had more success in the IP arm remains elusive, the finding that participants with less duration of disease had higher success in the IS arm is consistent with the natural history of the disease, namely decreasing beta-cell function with increasing duration of T2DM.
Certain baseline characteristics were associated with success (or lack thereof) irrespective of the glycemic treatment strategy. Higher HbA1c was associated with lowered odds of success in both arms of the study. This finding is in keeping with observations showing that higher HbA1c values are often associated with longer duration of disease, and ever-increasing failure of beta-cell function associated with the progressive nature of T2DM (29). Having a high school degree (or higher) was associated with lowered chance of success in both the IP and IS arms of the study; this apparently paradoxical finding has been reported in other studies (19, 20) and may reflect a presumed lower adherence to protocols. A striking finding was that participants from Brazil (and to a certain extent, those from Mexico) had remarkably higher odds of success (compared to those from the United States) in both the IP and IS arms. Potential explanations for this finding, while uncertain, could include higher adherence to the assigned medical regimen, or differences in the phenotype and physiological derangements underlying type 2 diabetes among different ethnic groups (30-32).
A smaller percentage of participants in the IP group versus the total IS group achieved success; this may reflect the difficulties associated with use of insulin, fear of hypoglycemia, and aversion to weight gain that may limit maximally effective use of insulin-providing medications. Among IS participants, those who were on insulin at entry were much less likely to achieve success than those not on insulin (OR=0.284 with p<0.001; Figures 2A and B). This finding suggests that these participants most probably had advanced disease requiring concomitant insulin-providing medications. In contrast, IS participants who had higher plasma insulin levels at entry had higher odds of success when compared to participants with lower plasma insulin levels. With better beta-cell function, reduction of insulin resistance may more effectively alleviate hyperglycemia.
Parameters that independently contributed to success in participants treated with IS versus IP medications deserve commenting. The major distinction for successful glycemic control is use of insulin at baseline. Those not on insulin at entry had a significantly higher probability of success when treated with IS compared to IP medications. This finding probably reflects the common practice of initiating insulin therapy late in the course of the disease when beta-cell function has deteriorated significantly and patients' glycemia can no longer be adequately controlled with oral medications alone. It also suggests that the clinician might measure fasting plasma insulin as a guide in choosing appropriate initial pharmacotherapy. Increasing age, and increasing duration of T2DM are both associated with decreasing odds of success when treated with IS versus IP medications, irrespective of being treated or not treated with insulin at entry.
The strengths of this analysis include the randomized, multicenter and multinational design of the BARI 2D trial. The similarity between participants in the IS and IP arms of the study at baseline strengthens the assertion that predictors of successful glycemic control are not explained by patient disparities. Weaknesses include the post-hoc nature of the analysis, the fact that the findings cannot be generalized to the general patient population with T2DM without established coronary artery disease, and our inability to compare our findings to similar patients randomly assigned to treatment with a combination of IS and IP medications.
In conclusion, and as summarized in Figure 3, patients with T2DM similar to those enrolled in the BARI 2D trial may have higher chance of achieving successful long-term glycemic control with IS medications if at presentation they are not being treated with insulin, are younger, have lower HbA1c levels, are physically active and have shorter duration (<10 years) of T2DM. In contrast, patients may have a higher chance of achieving long-term successful glycemic control on IP medications if they are already being treated with insulin, are older, have higher HbA1c levels, are mostly sedentary, and have had T2DM for longer durations; continuation of insulin and/or IP medications in such patients is associated with an increased likelihood of successful glycemic control.
Figure 3. Baseline Characteristics Associated with Higher Rates of Success for Glycemic Control.

A more detailed description of some of the characteristics is given in the text. At entry, the average age was 62 years, duration of diabetes was 10 years, 27% were being treated with insulin, and HbA1c averaged 7.7%; status of physical activity was classified as moderate or strenuous versus sedentary or mild.
Supplementary Material
Acknowledgments
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) is funded by the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases (U01 HL061744, U01 HL061746, U01 HL061748, U01 HL063804).
BARI 2D receives significant supplemental funding from GlaxoSmithKline, and additional funding from Lantheus Medical Imaging, Inc. (formerly Bristol-Myers Squibb Medical Imaging, Inc.), Astellas Pharma US, Inc., Merck & Co., Inc., Abbott Laboratories, Inc. and Pfizer, Inc. Medications and supplies were donated by Abbott Laboratories Ltd., MediSense Products, Bayer Diagnostics, Becton, Dickinson and Company, J. R. Carlson Labs, Centocor, Inc., Eli Lilly and Company, LipoScience, Inc., Merck Sante, Novartis Pharmaceuticals Corporation, and Novo Nordisk, Inc.
As an NIH funded trial, we are required to abide by the NIH PubMed Central Policy that we retain the right to provide a copy of the final manuscript to the NIH upon acceptance for publication by your journal, for public archiving in PubMed Central as soon as possible, but no later than 12 months after publication.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institute of Diabetes And Digestive And Kidney Diseases, or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Diabetes Association. Standards of medical care in diabetes – 2011. Diabetes Care. 2011;34(Suppl. 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 3.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EAM, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials. A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 4.Shaya FT, Yan X, Lin PJ, Simoni-Wastila L, Bron M, Baran R, Donner TW. US Trends in glycemic control, treatment, and comorbidity burden in patients with diabetes. J Clin Hypertens (Greenwich) 2010;12:826–832. doi: 10.1111/j.1751-7176.2010.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egede LE, Gebregziabher M, Hunt KJ, Axon RN, Echols C, Gilbert GE, Mauldin PD. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011;34:938–943. doi: 10.2337/dc10-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suh DC, Choi IS, Plauschinat C, Kwon J, Baron M. Impact of comorbid conditions and race/ethnicity on glycemic control among the US population with type 2 diabetes, 1988–1994 to 1999–2004. J Diabetes Complications. 2010;24:382–391. doi: 10.1016/j.jdiacomp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama S, Sakura H, Kanno H, Iwamoto Y. Factors associated with glycemic control after an inpatient program. Metabolism. 2009;58:843–7. doi: 10.1016/j.metabol.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Kuo CK, Lin LY, Yu YH, Chang CH, Kuo HK. A family history of diabetes mellitus is associated with poor glycemic control and increased metabolic risks among people with diabetes: data from the National Health and Nutrition Examination Survey 1999-2004. Int Med. 2010;49:549–55. doi: 10.2169/internalmedicine.49.2880. [DOI] [PubMed] [Google Scholar]
- 9.Ross SE, Franks SF, Hall J, Young R, Cardarelli R. Levels of acculturation and effect on glycemic control in Mexicans and Mexican Americans with type 2 diabetes. Postgrad Med. 2011;123:66–72. doi: 10.3810/pgm.2011.01.2246. [DOI] [PubMed] [Google Scholar]
- 10.Ben Abdelaziz A, Soltane I, Gaha K, Thabet H, Tlili H, Ghannem H. Facteurs determinants du controle glycemique des patients diabetiques de type 2 suivis en premiere ligne. Rev Epidemiol Sante Publique. 2006;54:443–52. doi: 10.1016/s0398-7620(06)76742-6. [DOI] [PubMed] [Google Scholar]
- 11.Dirmaier J, Watzke B, Koch U, Schulz H, Lehnert H, Pieper L, Wittchen HU. Diabetes in primary care: prospective associations between depression, nonadherence and glycemic control. Psychother Psychosom. 2010;79:172–8. doi: 10.1159/000296135. [DOI] [PubMed] [Google Scholar]
- 12.Bains SS, Egede LE. Associations between health literacy, diabetes knowledge, self-care behaviors, and glycemic control in a low income population with type 2 diabetes. Diabetes Technol Ther. 2011;13(3):335–41. doi: 10.1089/dia.2010.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks MM, Frye RL, Genuth S, Detre KM, Nesto R, Sobel BE, Kelsey SF, Orchard TJ. Hypotheses, design, and methods for the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol. 2006;97:9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group. Baseline characteristics of patients with diabetes and coronary artery disease enrolled in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am Heart J. 2008;156:528–36. doi: 10.1016/j.ahj.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Bypass Angioplasty Revascularization Investigation 2 Diabetes Investigators. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magee MF, Isley WL for the BARI 2D Trial Investigators. Rationale, Design, and Methods for Glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Am J Cardiol. 2006;97(suppl 12A):20G–30G. doi: 10.1016/j.amjcard.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Sobel BE, Hardison RM, Genuth S, Brooks MM, McBane RD, Schneider DJ, et al. For the BARI 2D Investigators. Profibrinolytic, antithrombotic, and antiinflammatory effects of an insulin-sensitizing strategy in patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Circulation. 2011;124:695–703. doi: 10.1161/CIRCULATIONAHA.110.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn S, Haffner S, Heise M, Herman W, Holman R, Jones N, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. The ACCORD Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. ACCORD Study Group. Effect of intensive treatment of hyperglycemia on microvascular complications of type 2 diabetes: an analysis of the ACCORD randomized trial. Lancet. 2010;376:419–30. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chehade JM, Mooradian AD. A rational approach to drug therapy of type 2 diabetes mellitus. Drugs. 2000;60:95–113. doi: 10.2165/00003495-200060010-00006. [DOI] [PubMed] [Google Scholar]
- 23.Leahy JL, Hirsch IB, Peterson KA, Scneider D. Targeting β-cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:4206–4216. doi: 10.1210/jc.2010-0668. [DOI] [PubMed] [Google Scholar]
- 24.Chiu KC, Lee NP, Cohan P, Chuang LM. β-Cell function declines with age in glucose tolerant Caucasians. Clin Endocrinol (Oxf) 2000;53:569–575. doi: 10.1046/j.1365-2265.2000.01132.x. [DOI] [PubMed] [Google Scholar]
- 25.Yates AP, Laing I. Age-related increase in hemoglobin A1c and fasting plasma glucose is accompanied by a decrease in β-cell function without change in insulin sensitivity: evidence from a cross-sectional study of hospital personnel. Diabet Med. 2002;19:254–258. doi: 10.1046/j.1464-5491.2002.00644.x. [DOI] [PubMed] [Google Scholar]
- 26.Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52:1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 27.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–E12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. Diabet Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 31.Haffner SM, D'Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with nonHispanic whites The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 32.Chandler-Laney PC, Phadke RP, Granger WM, Fernández JR, Muñoz JA, Man CD, Cobelli C, Ovalle F. Gower BA Age-related changes in insulin sensitivity and β-cell function among European-American and African-American women. Obesity (Silver Spring) 2011;19:528–35. doi: 10.1038/oby.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


