Abstract
Horses that have undergone infection caused by Streptococcus equi subspecies equi (strangles) were found to have significantly increased serum antibody titers against three previously characterized proteins, FNZ (cell surface-bound fibronectin binding protein), SFS (secreted fibronectin binding protein), and EAG (α2-macroglobulin, albumin, and immunoglobulin G [IgG] binding protein) from S. equi. To assess the protective efficacy of vaccination with these three proteins, a mouse model of equine strangles was utilized. Parts of the three recombinant proteins were used to immunize mice, either subcutaneously or intranasally, prior to nasal challenge with S. equi subsp. equi. The adjuvant used was EtxB, a recombinant form of the B subunit of Escherichia coli heat-labile enterotoxin. It was shown that nasal colonization of S. equi subsp. equi and weight loss due to infection were significantly reduced after vaccination compared with a mock-vaccinated control group. This effect was more pronounced after intranasal vaccination than after subcutaneous vaccination; nearly complete eradication of nasal colonization was obtained after intranasal vaccination (P < 0.001). When the same antigens were administered both intranasally and subcutaneously to healthy horses, significant mucosal IgA and serum IgG antibody responses against FNZ and EAG were obtained. The antibody response was enhanced when EtxB was used as an adjuvant. No adverse effects of the antigens or EtxB were observed. Thus, FNZ and EAG in conjunction with EtxB are promising candidates for an efficacious and safe vaccine against strangles.
Streptococcus equi subsp. equi is the causative agent of strangles, one of the most widespread and costly horse diseases worldwide (4, 5). The disease is highly contagious, and common symptoms are fever, lymphadenitis, and abscesses mainly in the head and neck regions. Other more generalized symptoms such as endocarditis, rheumatic fever, and glomerulonephritis as well as fatal outcomes also occur (1). The disease is characterized by a long convalescence period, and diseased horses are isolated for at least 4 weeks in order to prevent further spread of the disease (1). Affected animals may harbor the bacteria for several months in, among other places, the guttoral pouches and, thus, shed and act as reservoirs of S. equi subsp. equi (17, 25). Although S. equi subsp. equi is quite sensitive to penicillin and other antibiotics, antibiotic treatment is for various reasons mostly ineffective (4). In order to combat the disease and mitigate serious clinical complications, research has mainly been aimed at developing efficient vaccines. Vaccines against strangles such as bacterins or adjuvanted extracts have been used since the 1980s. Bacterins or adjuvanted extracts do not, however, confer efficient protection against infection with S. equi subsp. equi (8, 23). During recovery and convalescence, horses develop a protective immune response, indicating that efficient vaccines might be developed (3). The antibody response is strong predominantly against the antiphagocytic cell wall-associated M-like protein SeM. Convalescent horses have antibodies of immunoglobulin G (IgG) and IgA types against SeM both in serum and in nasal secretions, and because of the strong antibody response, SeM has attracted great interest as a vaccine component (16, 21). The vaccination of horses with SeM, with different adjuvants and by different routes, however, gave no significant protection against infection with S. equi subsp. equi (20, 21).
Many different pathogenic bacteria have evolved extracellular proteins that bind host proteins. These proteins are considered as potential virulence factors and are candidate components for vaccine development. Many bacterial species have been reported to express extracellular proteins that bind the serum proteins fibronectin (Fn) or IgG, indicating that that these interactions are of particular importance for virulence. Fn-binding is considered to be of importance for bacterial adherence and internalization into mammalian cells (15), whereas the binding of IgG can affect antibody-mediated complement activation and phagocytosis (9, 19). Two different Fn-binding proteins have been identified in S. equi subsp. equi, FNE and SFS. FNE was first identified in Streptococcus equi subsp. zooepidemicus and named FNZ (11, 13); later a homologous gene was found in S. equi subsp. equi. However, there is one major difference between fne and fnz, in that the fne gene contains a base pair deletion, which results in a truncated Fn-binding protein of ∼30 kDa. The sfs-gene encodes a 40-kDa extracellular protein, and the gene is found in all tested strains of S. equi subsp. equi and in 41 of 48 S. equi subsp. zooepidemicus strains (10). Furthermore, an IgG, serum albumin, and α2-macroglobulin-binding surface protein called ZAG first isolated from S. equi subsp. zooepidemicus has attracted interest as a virulence factor (6, 7). The zag gene has previously been detected in both subspecies by Southern blot analysis (12). The zag gene in S. equi subsp. equi has in this study been named eag, encoding the protein EAG.
The aim of the present study was to evaluate the extracellular antigens FNZ, EAG, and SFS as components in a vaccine against strangles by using a mouse strangles model. The antibody responses to these antigens were also studied in convalescent horses, in horses without a history of strangles, and in experimentally immunized horses.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. equi subsp. equi strain 1866 was obtained from NordVacc Läkemedel, Stockholm. The Escherichia coli strain ER2566 and plasmid vector pTYB4 were obtained from New England Biolabs (Beverly, Mass.) Streptococcal strains were grown on horse blood agar (BG) plates or in Todd-Hewitt broth (THB; Oxoid, Basingstoke, Hampshire, United Kingdom) supplemented with 0.5% yeast extract. E. coli was cultured in Luria-Bertani broth supplemented with ampicillin (100 μg/ml) or on plates coated with Luria-Bertani broth with ampicillin and agar [15g/liter]).
Adjuvant preparation.
Recombinant E. coli heat-labile enterotoxin B subunit (EtxB) was expressed in Vibrio sp. strain 60 (pMMB68) and purified from the culture supernatant as reported previously by Richards et al. (18). Briefly, EtxB was recovered from the culture medium by diafiltration and then subjected to ammonium sulfate precipitation (30% saturation), followed by hydrophobic interaction and anion-exchange chromatography using phenyl-Superose HR5/5 (Amersham Biosciences) and Resource Q (Amersham), columns, respectively. The single peak eluting from the Resource Q column was desalted by using a NAP-10 column equilibrated in phosphate buffered saline (PBS; 0.15 M NaCl, sodium phosphate [pH 7.2]). Purified preparations of EtxB were depleted of residual lipopolysaccharide by using Detoxi-Gel columns (Pierce, Rockford, Ill.), followed by dialysis against PBS. The purified protein was stored at −80°C prior to use.
Construction of clones and preparation of antigens.
The SFS protein (GenBank accession number AF136451) from S. equi subsp. equi has previously been described by Lindmark and Guss (10). A clone expressing the C-terminal fragment of this protein was produced as follows. The 3′ end of the sfs gene was amplified by PCR by using chromosomal DNA from S. equi subsp. equi strain 1866 as a template and the synthetic oligonucleotides OSFS25 (5′-GGTCCCATGGCAACTCCGAATTTAGAAGGA) and OSFS23 (5′-CAGACTCGAGGTCGGGATTGTAAGAATAG). After amplification the PCR product was digested with NcoI and XhoI and ligated into plasmid vector pTYB4, which previously had been digested with the same enzymes. After electrotransformation of the ligation mix into E. coli strain ER2566, clones harboring the correct insert were identified by sequence analysis. The clone pSFSC1, encoding the truncated SFS protein (SFSC1), was chosen for the preparation of the antigen according to a manufacturing procedure provided by New England Biolabs.
Protein FNZ from S. equi subsp. zooepidemicus (EMBL accession number X99995) has previously been described by Lindmark et al. (11-13). A similar protein, called FNE (GenBank accession number AF360373), is also expressed by strains of S. equi subsp. equi (13). The construction of the clone pT2fnzN encoding the N-terminal part of FNZ, called FNZN, has previously been described by Lindmark et al. (13).
In the study by Lindmark et al. (12) a gene similar to zag (the GenBank accession number for the zag gene is U25852) from S. equi subsp. zooepidemicus was also described in S. equi subsp. equi. The gene present in S. equi subsp. equi is hereafter called eag. A part of this gene was cloned and expressed essentially as described above by using the primers OZAG43B (5′-TTT TCT CGA GCT ACG GTA GAG CTG ATA AAA TCT C-3′) and OZAG15 (5′-TCA GCC ATG GCT CTA GAT GCT ACA ACG GTG TT-3′). The PCR-amplified DNA fragment corresponds to amino acids 34 to 262 in protein ZAG. Correct transformants were identified by colony screening by using horseradish peroxidase (HRP)-labeled human serum albumin. The insert of the clone pEAG4B chosen for further work was sequenced to verify the correct insertion into the vector and the presence of a stop codon between EAG and the intein affinity tag. The stop codon between the albumin binding domain and the intein tag was introduced since a previous intein fusion construct was not cleaved in the presence of dithiothreitol. To purify the truncated EAG protein (EAG4B), human serum albumin was immobilized on a HiTrap N-hydroxysuccinimide-activated column (Amersham) according to the manufacturer's instructions. The column was washed with 20 mM Na-phosphate, pH 7.0 (binding buffer), before the lysed cell sample was loaded onto the column. The column was washed with binding buffer, and the bound EAG4B protein was eluted in 0.1 M glycine-HCl, pH 3.0. After elution, the pH of the sample was neutralized with 2 M Tris buffer, pH 8, and dialyzed against PBS.
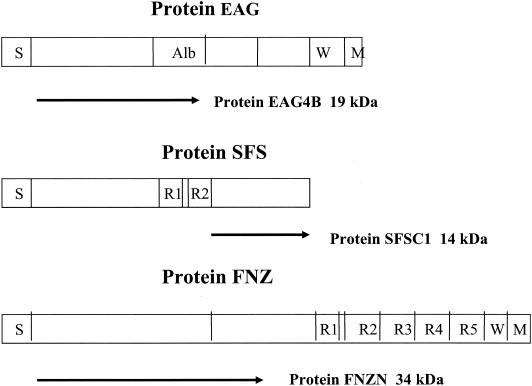
For simplicity, the recombinant proteins SFSC1, FNZN, and EAG4B will in this study be called SFS, FNZ, and EAG, respectively (Fig. 1).
FIG. 1.
Schematic presentation of the three S. equi proteins EAG, SFS, and FNZ. Abbreviations: S, the signal sequence; W, the cell wall-associated domain; M, the membrane-spanning region. For protein EAG the albumin-binding region Alb is indicated. For proteins FNZ and SFS different repetitive regions are indicated by R. Arrows indicate the part of the respective protein that was used for antigen preparation. The deduced molecular mass for proteins EAG4B, SFSC1, and FNZN are indicated.
Immunization of mice.
Seven mice were immunized on four occasions at 1-week intervals with a combination of FNZ, SFS, and EAG; four were immunized with EtxB adjuvant, and three were immunized without adjuvant. Mice were immunized either subcutaneously (s.c.) or intranasally (i.n.). For each antigen (FNZ, SFS, and EAG), 12 μg of antigen was mixed with an equal amount of EtxB so that all components achieved a final concentration of 300 μg/ml. In the case of i.n. immunization, the total volume was kept to less than 40 μl and applied into the nostrils of mice anaesthetized with Hypnorm (Janssen Pharmaceutica, Beerse, Belgium). For both s.c. and i.n. immunizations, four doses were given with 1-week intervals. Challenge i.n. with S. equi subsp. equi (106 CFU in a 10-μl volume) (see below) was given 1 week after the last booster dose. Control mice were given only EtxB in the same volume. Animals were divided into groups of four in each cage in all experiments.
Immunization of horses.
Horses with no previous history of strangles and with low antibody titers against FNZ, SFS, and EAG were selected. An experimental design with four groups (A to D) of horses with three horses in each group was used. The horses were randomly allocated into four groups and were immunized as follows: group A received EAG, FNZ, and SFS with EtxB as adjuvant, given both i.n. and s.c.; group B received EAG, FNZ, and SFS with EtxB as adjuvant, given only i.n.; group C received EAG, FNZ, and SFS but without EtxB as adjuvant, given both i.n. and s.c; and group D received only EtxB and no antigens, given both i.n. and s.c.
Each dose consisted of 35 μg of each antigen and 100 μg of EtxB in a volume of 2 ml for i.n. and/or s.c. immunization. Immunizations were given on days 0, 14, 28, and 42. The immunizations of the horses were performed at the Centre for Preventative Medicine, Animal Health Trust, Newmarket, United Kingdom. Blood samples for the determination of serum IgG antibody titers and nasal washings for the determination of mucosal IgA were collected immediately prior to the first immunization and on day 56 after the first immunization. The experimental horses were inspected daily during the whole study period for possible signs of adverse side effects. The rectal temperature of each horse was checked once daily.
Determination of antibody titers from horses by enzyme-linked immunosorbent assay (ELISA).
Serum samples were taken from horses without any previous or present sign of strangles (n = 16), as well as from horses with clinical signs of strangles and positive cultivation of S. equi subsp. equi (n = 10). Microtiter wells (Costar) were coated overnight with 100 μl of FNZ, SFS, or EAG at a concentration of 10 μg per ml overnight in PBS. The plates were then blocked with 2% (wt/vol) bovine serum albumin for 1 h at 37°C. After the plates were washed, horse serum samples were added to the wells at a 20-fold dilution in PBS with 0.05% Tween, followed by twofold serial dilutions. The plates were washed after incubation for 1 h at 37°C. After washing, the detection of antibody binding was performed with antibodies, diluted 1,000 times, against horse IgG raised in rabbit and conjugated with HRP (Sigma Chemical Co). The development of colorimetric reaction was done with o-phenylenediamine dihydrochloride tablets (Dako, Glostrup, Denmark). Absorbance was determined spectrophotometrically at 492 nm.
Levels of antigen-specific IgA in nasal wash samples from immunized horses were determined by an indirect ELISA. Plates (Immulon 4; Dynatech Laboratories) were coated with 50 μl of the antigens (at a concentration of 4 μg/ml) at 4°C for 16 to 18 h and blocked with 5% Marvel skim milk in PBS-0.05% Tween at 37°C for 1 h. Samples (50 μl) containing IgA were added and incubated at 37°C for 1 h. Mouse monoclonal antibody K129-3E7 (volume, 50 μl; concentration, 2 μg/ml) to equine IgA was used to detect bound IgA in the samples by incubation at 37°C for 1 h, followed by a goat anti-mouse Ig HRP conjugate (2 μg/ml;Dako) (incubation at 37°C for 1 h). HRP was detected by incubation with 100 μl of TMB microwell peroxidase substrate (3,3′,5,5′-tetramethylbenzidine; Kirkegaard & Perry Laboratories) at room temperature for 10 min. The reaction was stopped by the addition of 100 μl of 0.18 M H2SO4, and absorbance was read at 450 nm. Background values (plates without antigens) were subtracted from the result, and all samples were analyzed in triplicate. The ELISA data of antigen-specific IgA are expressed relative to the total IgA in order to correct for nasal secretion dilution during the nasal wash procedure. Total IgA in nasal wash samples was determined by a capture ELISA that used two different monoclonal antibodies specific to equine IgA. One antibody (K129-2G5) was used to coat wells at a concentration of 10 μg/ml (100 μl/well). IgA samples (100 μl) were added and incubated at 37°C for 1 h. After washing, the second biotinylated monoclonal antibody (K129-3E7) was used to detect IgA. K129-3E7 was biotinylated by using a biotinylation kit (Amersham) according to the manufacturer's instructions. The biotinylated monoclonal antibody was detected by using a streptavidin-HRP conjugate at a concentration of 0.25 μg/ml (Amersham). Monoclonal antibody and streptavidin-HRP concentrations were optimized such that none were limiting in the assay. Samples were analyzed in triplicate. Monoclonal antibodies against horse IgA have been described (14, 21).
Analysis of IgA in immunized horses was performed at the Centre for Preventive Medicine, Animal Health Trust, Newmarket, United Kingdom.
Serum samples were also taken from the immunized horses and analyzed for IgG as described above for convalescent and noninfected horses.
Determination of antibody titers in mouse by ELISA.
Serum samples were taken from mice (NMRI) that had undergone experimental S. equi subsp. equi infection for 2 weeks. Control samples were taken before infection. The samples were analyzed by ELISA as described above for horses; detection of mice Igs was done by using HRP-conjugated antibodies from rabbit (Dako) to detect all classes of Igs. The bronchoalveolar lavage (BAL) procedure was performed as described previously (22). IgA in BAL fluid was determined in a similar way but by using specific anti-mouse IgA antibodies from goat conjugated with HRP (Sigma).
Experimental infection of mice with S. equi subsp. equi.
The model described by Chanter et al. was followed (2). Strain 1866 was first passaged through an animal by inoculating ca. 106 CFU into each nostril of an anaesthetized mouse (NMRI), although the necessity to do this has not been assessed. Bacteria were recovered after 6 days from the submandibular glands and grown on BG plates at 37°C in 10% CO2. A single colony was grown in THB overnight at 37°C. The culture was kept at −80°C in vials, and a new vial was used for each experiment. To infect mice, bacteria were grown on BG plates at 37°C in 10% CO2 overnight, followed by inoculation into THB supplemented with 1% yeast extract and grown without shaking over night. The culture was then diluted 10 times into THB with 1% yeast extract and 10% horse serum (Sigma) and grown for 4 h at 37°C. The culture was centrifuged and resuspended in THB. To determine an optimal inoculation dose, 20 mice were divided into four groups and given i.n. 5 × 107, 1.3 × 107, 3 ×106, or 8 × 105 CFU of strain 1866 in a 10-μl volume. After 5 days, the mice were killed, and the presence of bacteria was determined in submandibular glands, trachea, kidneys, blood, and liver. Daily during the infection, and without killing the mice, growth was also determined quantitatively in the nostrils by gently touching the nostrils to BG plates. A dose containing 106 CFU in a 10-μl volume was used for infections of mice. Bacterial growth was scored on a four-point scale from 0 to 3, where 0 means no growth or <8 colonies, 1 means 9 to ca. 100 colonies, 2 means more than ca. 100 colonies, and 3 means confluent growth. The weight was determined every day and animals losing >15% of their body weight were killed; this point is here defined as the clinical end point. However, in one experiment (see Fig. 5A and B) some animals lost more than 15% of their body weight but were included.
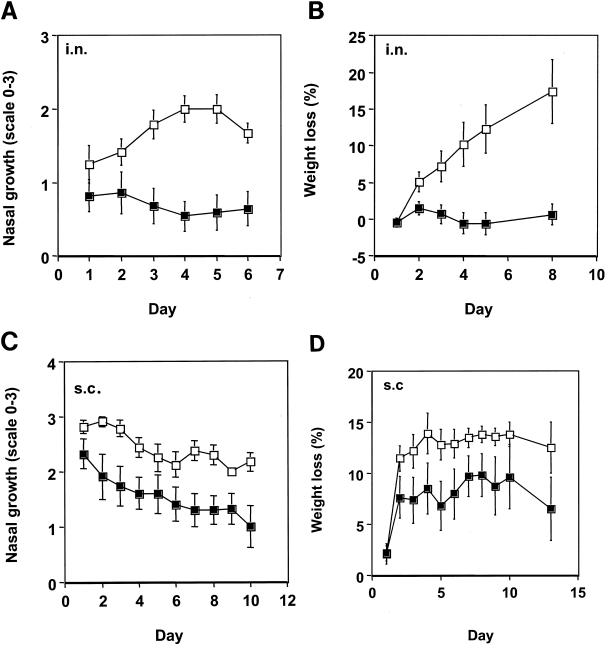
FIG. 5.
Protection against infection by S. equi subsp. equi. Mice (n = 12) were immunized with FNZ, SFS, and EAG with EtxB (filled symbols), and control groups (n = 12) were given only EtxB (open symbols). Mean values with standard errors are shown. Panels A and B show results after i.n. immunization; panels C and D show results after s.c. immunization. Panels A and C show growth of S. equi subsp. equi in noses. The nasal growth of S. equi subsp. equi was determined by placing the noses against BG plates, and the bacteria were streaked out to single colonies. The number of bacteria was given a value from 0 to 3, where 0 means no growth or <8 colonies, 1 means 9 to ca. 100 colonies, 2 means more than ca. 100 colonies, and 3 means confluent growth. Panels B and D show the daily percentage weight loss. Significance values are given in the text.
Statistical methods.
The serum dilutions required to achieve an absorbance value of 1.0 were calculated by an ELISA of sera from horses with or without strangles. The log10 values of these dilutions were compared by using a nonpaired Student's t test. For the ELISA of sera from mice taken before and after experimental infection, a cutoff absorbance value of 0.5 was accepted, due to lower absorbance values compared with the horses, and the log10 values of the dilutions were calculated. In this case, a paired t test was used for comparison. For comparison of weight changes and nasal growth in mice, a nonpaired t test was used. Fisher′s exact test was used for comparisons of the proportions of mice that lost more than 15% of their weight or died.
RESULTS
Antibody responses against FNZ, SFS, and EAG in horses with strangles.
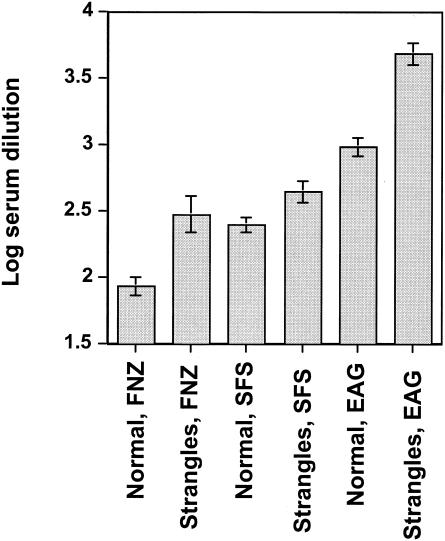
Sera were collected from 10 horses with a recent history of strangles and from 16 healthy horses without strangles. These sera were tested by an ELISA for IgG recognizing FNZ, SFS, and EAG. For each sample, the dilution was determined which was required to give an absorbance value of 1.0, which is on the linear part of the titration curves (data not shown). The log10 values of these dilutions are shown in Fig. 2. It is clear that the sera from horses with strangles had elevated antibody levels to all three antigens compared to horses without strangles. The differences were significant, with P values for the two groups for FNZ, SFS, and EAG of <0.001, 0.02, and <0.0001, respectively. These findings indicate that all three antigens are expressed during natural infection with strangles and that they are able to elicit a serum antibody response.
FIG. 2.
Antibody titers in sera from horses. The log10 dilution of sera required to give an absorbance value at a cutoff of 1.0 was calculated for each individual serum sample. Mean values with standard errors are shown. P values for comparing normal (n = 16) versus strangles (n = 10) sera against FNZ, SFS, and EAG are <0.001, 0.02, and <0.0001, respectively.
Evaluation of experimental infection in mice with S. equi subsp. equi.
Preliminary studies of different doses of bacteria revealed that 106 CFU per 10-μl volume, administered i.n., was sufficient to infect 80 to 100% of mice. Clinical symptoms, bacterial growth in submandibular glands, trachea, kidneys, and blood were followed and used as criteria for infection. It was also noted that loss of weight and growth of bacteria in the nostrils correlated perfectly (P < 10−4) with the other signs of infection. Consequently, in the following experiments, only the two latter parameters were followed as correlates of infection.
A time study was performed by infecting 20 mice that were followed for 23 days. Eight animals that lost more than ca. 15% of their body weight were killed. In some animals, bacterial growth in the nostrils peaked between days 4 to 8. After 8 days, bacterial growth in the nostrils declined. In other animals, a high level of bacterial growth was evident from day 1. Weight loss was also highest on days 6 to 8 (mean value, 12%). Some mice slowly regained weight after that time in an apparent spontaneous recovery. Three randomly chosen animals were analyzed for bacterial growth in lung, trachea, heart, and brain. The median CFU values of S. equi subsp. equi per gram of tissue were 8.9 × 106, 3 × 105, 4.6 × 103, and 2.4 × 105, respectively.
Antibody response in mice infected with S. equi subsp. equi.
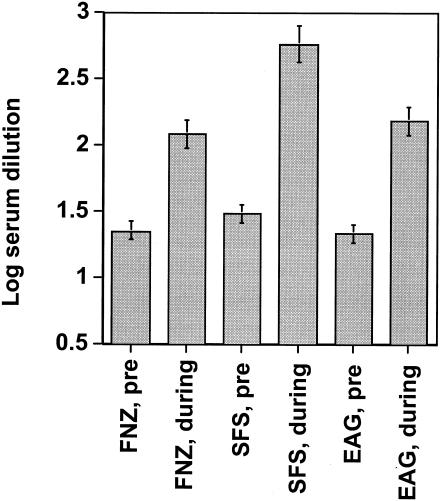
Serum samples were taken before and 13 days after infection of 15 mice that had been experimentally infected with S. equi subsp. equi. The serum antibody responses to FNZ, SFS, and EAG are shown in Fig. 3, where the log10 dilution values required to give an absorbance value of 0.5 are shown. Infection with S. equi subsp. equi led to highly significant (P ≤ 0.0001) rises in antibody titers to all three antigens. These findings indicate that FNZ, SFS, and EAG are all expressed during experimental S. equi subsp. equi infection of mice.
FIG. 3.
Antibody titers in sera from mice. The log10 dilution of sera required to give an absorbance value at a cutoff of 0.5 was calculated for each individual serum sample. Mean values with standard errors are shown. Samples taken before challenge, which at the lowest dilution (20 times) gave an absorbance value of <0.5, were assigned a value of 1.30. P values for comparing sera taken before (pre) challenge and during infection against all three antigens are <10−4.
Immunization of mice with FNZ, SFS, and EAG.
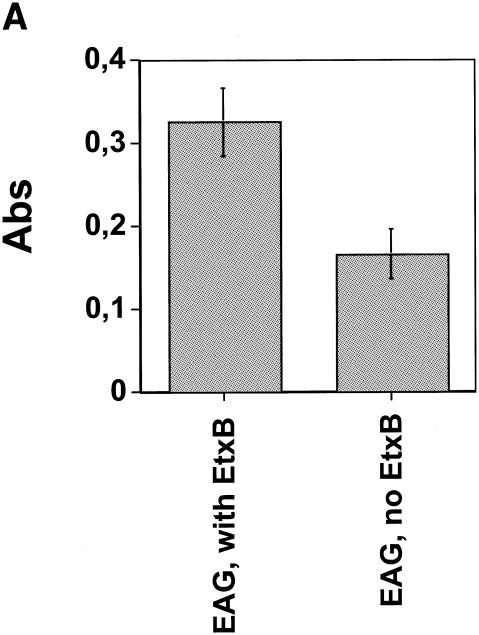
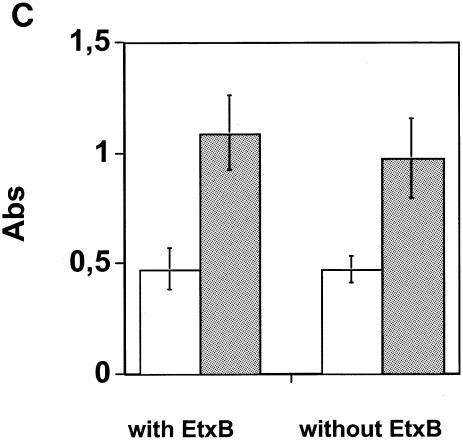
Seventeen mice were immunized i.n. on four occasions at 1-week intervals with a combination of FNZ, SFS, and EAG, 11 with EtxB adjuvant and 6 without adjuvant. An analysis of the BAL fluid taken after the last immunization revealed significant IgA levels to EAG in the animals immunized with the three antigens and the EtxB adjuvant (Fig. 4A). By contrast, mice immunized with the three S. equi antigens in the absence of EtxB gave significantly lower IgA responses (P = 0.02) (Fig. 4A). IgA levels against the other antigens were more variable and not significantly different with or without EtxB (data not shown). The total serum Ig and IgA responses to the three antigens following i.n. immunization are shown in Fig. 4B and C. Although serum Ig responses to the three S. equi antigens tended to be slightly higher in animals immunized with the EtxB adjuvant, these differences were not statistically significant (Fig. 4B). Likewise, there was no apparent difference in serum IgA levels in mice given S. equi antigens with or without the adjuvant.
FIG. 4.
Antibodies in immunized mice. Seventeen mice were immunized i.n. with FNZ, SFS, and EAG, 11 of the mice with EtxB and 6 without EtxB. Panel A shows the mean values and standard error of IgA levels against EAG in BAL fluid (P = 0.02). Panel B shows the mean values and standard error of serum dilutions giving an absorbance of 1.0 for the three antigens by an ELISA detecting all classes of antibodies (n = 4 for animals where EtxB was included; n = 3 for animals without EtxB). Panel C shows IgA levels in sera (same animals as described in the legend to 4B) at 40 dilutions. White bars, preimmunization; gray bars, after immunization.
Protection against S. equi subsp. equi infection in vaccinated mice.
When mice (n = 12) were immunized i.n., as above, with FNZ, SFS, and EAG together with EtxB and then challenged with S. equi subsp. equi 1 week after the last booster immunization, a highly significant difference in the course of the infection was observed compared with a control group (n = 12) that received only EtxB. Figures 5A and B show data reflecting bacterial nasal growth and weight loss, respectively. S. equi subsp. equi failed to colonize or grow to any significant levels in animals that had been immunized with S. equi antigens and EtxB, and there was virtually no weight loss in the animals over the 8 days following experimental infection. This result contrasted with the control group that showed an increase in bacterial growth in the nose, which peaked on day 4, and a striking loss of weight. The difference in bacterial growth and weight loss between the immunized and control groups was highly significant, with P values of <0.001 and <0.005, respectively, from day 4 and onwards. We therefore conclude that i.n. immunization of mice with FNZ, SFS, EAG, and EtxB affords significant protection against nasal challenge with S. equi subsp. equi.
In addition to i.n. immunization, an alternative route of s.c. immunization was also evaluated. Mice (n = 12) were immunized s.c. with FNZ, SFS, EAG, and EtxB and then infected with S. equi subsp. equi 1 week later, as above. The control group (n = 12) was given only EtxB. Figure 5C shows that bacterial nasal growth was significantly different in vaccinated mice compared with control mice. For example on days 2, 3, and 7, the P values were 0.03, 0.02, and 0.01, respectively. Figure 5D shows that the weight loss in mice was higher in the nonvaccinated group, although this was not statistically significant. However, the number of animals that did not survive or lost more than 15% of their weight (which was the clinical end point in this experiment) was significantly higher in the control group (P < 0.05 by Fisher′s exact test). These findings demonstrate that s.c. immunization with S. equi antigens together with EtxB affords protection against experimental challenge with S. equi. A difference between the control groups represented by the data shown in Fig. 5B and D can be seen; a faster progression of the infection as shown in Fig. 5D is due to the natural variability between challenge experiments. Therefore, one cannot unambiguously say that i.n. immunization is better than s.c. immunization after comparing the data shown in Fig. 5A and C to B and D.
Since strangles is a highly contagious disease, we also analyzed the rate of spread of the infection between animals. Each of two noninfected mice was introduced into one cage (each) with three infected mice. Importantly, no signs of infection in these newcomers were observed (data not shown), implying that the extent of infection in the mice used in the vaccination studies can be regarded as independent cases, with infection not being the result of a few animals contaminating the others.
To explore whether the combination vaccine consisting of all three S. equi antigens afforded better protection than immunization with a single antigen (EAG) and whether inclusion of the EtxB adjuvant enhanced the protective efficacy of the vaccine, an additional vaccination experiment was carried out. Animals were immunized i.n. with FNZ, SFS, and EAG either with EtxB (group A; n = 8) or without EtxB (group B; n = 10) or with EAG together with EtxB (group C; n = 10) or with EtxB alone (group D control; n = 9). One week after the final booster immunization, animals were challenged by nasal inoculation with S. equi subsp. equi as above. Table 1 shows the weight loss and nasal growth of bacteria in each of groups A, B, and C on day 14 after challenge. The weight loss of group D is not included since only two out of nine mice were left. Nasal growth of bacteria in group D, as shown in Table 1, is calculated at the time of clinical end point, i.e., 15% weight loss or death.
TABLE 1.
Weight change and nasal growth of bacteria in mice treated with different vaccines
| Group (n) | Treatment | % Weight change (mean + SE)a | Nasal growth of bacteria (mean + SE)b | No. of mice with <15% weight loss on day 7 |
|---|---|---|---|---|
| A (8) | FNZ, SFS, and EAG plus EtxB | 0.7 ± 1.9 | 0.83 ± 0.17 | 2 |
| B (10) | FNZ, SFS, and EAG | −6.8 ± 6.8 | 1.5 ± 0.23 | 3 |
| C (10) | EAG plus EtxB | −8.11 ± 5.24 | 1.44 ± 0.26 | 4 |
| D (9) | ExtB | 1.78 ± 0.22c | 7 |
Weight change is based on day 14 as a percentage of initial weight.
Growth was measured on a scale from 0 to 3 (see Materials and Methods) on day 14.
Nasal growth at death or at the point of 15% weight loss.
By day 7 after challenge, seven mice in the control group (D) had lost >15% of their body weight and were sacrificed. By contrast, in the three immunized groups (A plus B plus C) only 9 of the 28 animals had died (P = 0.02 by Fisher's exact test). Bacterial nasal growth also differed between control group D and vaccinated group A (P = 0.004), thus confirming the protective effect of the immunizations.
At day 14 after challenge, the mean body weight of the vaccinated mice in group A had increased compared to their initial weight, and the numbers of CFU of S. equi subsp. equi recovered from the nostrils were very low (Table 1). An analysis of whether the inclusion of EtxB altered the level of protection in groups A and B revealed that protection on day 14, measured as bacterial nasal growth, was more prominent when EtxB was included in the vaccination (P < 0.05). This finding is in line with the observation (Fig. 4A) that including EtxB results in a better mucosal IgA response against EAG.
The vaccination of mice with EAG as a single antigen and EtxB (group C) was less effective at affording protection than immunization with FNZ, SFS, EAG, and EtxB (group A) (Table 1), although not statistically significant (P = 0.08 for nasal growth). This result implies that the inclusion of several different antigens from S. equi in a strangles vaccine is likely to be beneficial.
Immune responses of horses.
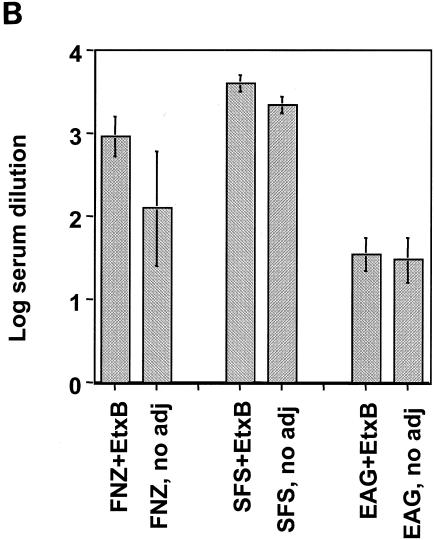
The promising findings on the induction of mucosal IgA antibodies to FNZ, SFS, and EAG, as well as the protective efficacy to challenge following immunization of mice with the three S. equi antigens and EtxB prompted us to test whether a similar regime of vaccination might enhance antigen-specific immune responses in horses. Twelve horses were used in the study and divided into four groups A to D (n = 3 for each group) as follows: group A received both i.n. and s.c. immunizations with FNZ, SFS, and EAG with EtxB; group B received the same immunizations as group A but only i.n.; group C received both i.n. and s.c. immunizations with FNZ, SFS, and EAG but without EtxB; and group D was immunized both i.n. and s.c. with EtxB alone. Serum samples were taken before each immunization on days 0 and 56, and nasal washings were taken on day 0 and day 56. As can be seen from the results shown in Fig. 6, only horses immunized both i.n. and s.c. with the three S. equi antigens and EtxB (group A) exhibited high mucosal antigen-specific IgA responses against FNZ and EAG. For horses vaccinated i.n. only, whether immunized with or without EtxB (groups B and C), the levels of mucosal IgA responses to FNZ and EAG were modest and not significantly different from those of the control group. It was also found that the IgA responses to SFS were very low in all of the animals, irrespective of the route of administration or inclusion of EtxB (data not shown).
FIG. 6.
IgA antibodies in nasal washings of immunized horses. Mean absorbance values (n = 3) in ELISAs are shown for groups A to D. The horses were divided into four groups: group A received the antigens plus EtxB, given both s.c. and i.n.; group B received the same immunizations as group A but given only i.n.; group C received the same treatment as group A but without EtxB; and group D (control group) was given only EtxB both i.n. and s.c. Open bars, samples from day 0; and filled bars, samples from day 56.
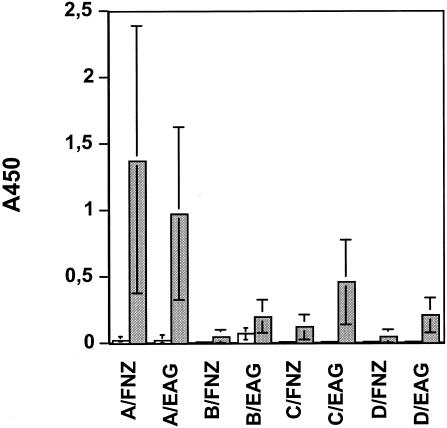
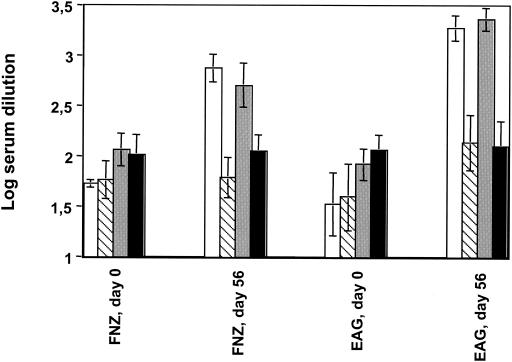
An analysis of the serum IgG levels revealed that the antigen-specific responses to FNZ and EAG were most pronounced in groups A and C (Fig. 7). This result was expected for group A, since the horses were immunized both i.n. and s.c., but the high titers in group C indicate that i.n. vaccination alone is sufficient to trigger seroconversion in horses. As with IgA, it was also found that the serum IgG responses to SFS were very low in all of the animals, irrespective of the route of administration or inclusion of EtxB (data not shown).
FIG. 7.
IgG antibodies in sera of immunized horses. The log dilution of sera required to give an absorbance value at a cutoff of 1.0 was calculated for each individual serum sample. Mean values (n = 3) with standard errors are shown. Samples taken before (day 0) and after (day 56) immunizations are shown. The horses, the same as described in the legend of Fig. 6, were divided into four groups: group A (white bars) received the antigens plus EtxB, given both s.c. and i.n.; group B (striped bars) received the same immunizations as group A but given only i.n.; group C (gray bars) received the same treatment as group A but without EtxB; and group D (black bars; control group) was given only EtxB both i.n. and s.c..
DISCUSSION
Horses that have undergone infection with S. equi subsp. equi (strangles) do not acquire strangles soon afterwards. This fact suggests that strangles triggers an immune response that affords protection. The nature of such protection is not, however, clear but is generally thought to involve the induction of neutralizing antibodies.
Apparently, the cell-bound antigen SeM, in spite of high serum and mucosal antibody titers following various administration routes, does not afford protection against challenge with S. equi subsp. equi (16, 20). Vaccination with live attenuated strains of S. equi subsp. equi may induce protection against infection with S. equi subsp. equi. Since 1998, Pinnacle, a live mutant attenuated nonencapsulated S. equi subsp. equi strain administered i.n., has been widely used in North America as a strangles vaccine, however, not without complications. It causes abscesses and other adverse effects probably due to revertant mutations (24). Recently, a nonencapsulated deletion mutant of the Pinnacle strain lacking two of the genes encoding hyaluronate synthase was constructed. However, this strain has not yet been reported as a strangles vaccine (24). Another attenuated deletion mutant of S. equi subsp. equi was recently used as a vaccine in challenge experiments (5). This strain was reported to protect horses against challenge with a heterologous S. equi subsp. equi strain. However, various adverse effects in the form of local reactions were observed. Thus, live attenuated strains, to date, seem to be inadequate for use as a vaccine. It would, therefore, be of particular interest to study further the antibody response after vaccination with recombinant subunit vaccines, since such vaccines may elicit a protective effect and minimal adverse effects.
Horses with a recent history of S. equi subsp. equi infection had significantly increased levels of antibodies against the three antigens used here. Little is known about the exact role of FNZ, SFS, and EAG during infection, but it can be assumed that they influence the pathogenicity of S. equi. The rise in antibody titers against the three antigens after both natural infection in horses and induced infection in mice demonstrates that the genes encoding these proteins are expressed during infection. Thus, antibodies against these proteins may neutralize their function.
The individual variation in antibody levels between the horses was large. Some horses without a known history of strangles had antibody titers near the level of convalescent horses. This result may be due to cross-reactivity between antigens from S. equi subsp. equi and S. equi subsp. zooepidemicus, the latter of which commonly colonizes horses. It is also possible that some of the investigated horses have had a previous undiagnosed subclinical infection with S. equi subsp. equi.
The infection model mimicking strangles in mouse that was used in this study has been described earlier (2). We have here confirmed these findings and observations. In horses, systemic infection is observed in about 5 to 10% of cases of strangles, whereas in the mouse systemic infection seems to be much more common. The mucosal route of infection and the ability of the bacteria to disseminate from the upper respiratory tract in mice resemble the events during a clinical case of equine strangles. We therefore conclude that this murine model of strangles is the best model at hand for testing vaccine components. As with most experimental infection models in animals, the individual response to bacterial challenge varies. Likewise, the average severity of infection was found to vary between experiments. Systemic infection correlated very well with weight loss, which was an easy method for evaluation of infection and also of recovery. A systemic infection leading to a weight loss exceeding ca. 15% was found eventually to be fatal, and such animals were killed for ethical reasons.
Because of the antibody response observed against FNZ, SFS, and EAG after infection, we decided to investigate the protective effect of all antigens in combination in vaccination trials. A significant protection against S. equi subsp. equi infection was found upon vaccination. An s.c. immunization route led to less bacterial nasal colonization and fewer animals loosing more than 15% of their weight. After nasal vaccination, the protective effect appeared more pronounced although comparison between experiments may be inexact.
An IgA response was found after nasal vaccination, particularly against SFS, which seemed to be the most immunogenic of the three antigens in mice. A nasal immunization giving a better IgA response is presumably important to prevent the early establishment of infection in the nasal mucosa. The inclusion of EtxB resulted in better protection (Table 1) and also higher mucosal IgA levels (Fig. 4A). The dissemination of bacteria from this site via the bloodstream to peripheral sites is prevented by circulating IgG. Therefore, it is likely that both IgA and IgG are required for effective protection.
It has not been the main focus in these studies to pinpoint which is the best of the three antigens. However, the results imply a synergistic effect, since better protection was obtained with all three antigens than with EAG alone.
When horses were vaccinated, a poor response against SFS was obtained, contrary to the results with mice (Fig. 4B and C). From the data obtained here, it seems as if i.n. immunization is more important for a good response in mice than in horses. The combination of s.c. and i.n. immunization in the horse gave far better responses than i.n. immunization alone. It was also seen that the addition of EtxB increased the IgA antibody titer in the nasal mucosa (Fig. 6). The difference in immunological behavior between the mouse and the horse strongly implies that the route of immunization and choice of adjuvant are best assessed in the target animal. The mouse model, however, serves well for the selection of target antigens for a vaccine.
In conclusion, we found that vaccination with FNZ, SFS, and EAG together gives an antibody response that is highly protective in the mouse. When administered both i.n. and s.c. in horses, significant mucosal IgA and serum IgG antibody responses against FNZ and EAG were obtained. The antibody titers tended to be higher when EtxB was used as an adjuvant. These antigens had no adverse effects on horses and neither had the adjuvant EtxB. Thus, these antigens are promising candidates for an effective and safe vaccine against strangles.
Acknowledgments
Financial support was obtained from The Swedish Agency for Innovation Systems (project number 2001-00846), FORMAS (project number 22.1/2001-0911), and NordVacc Läkemedel AB.
Editor: J. T. Barbieri
REFERENCES
- 1.Chanter, N. 2002. Bacterial infections including mycoplasmas. In P. Lekeux (ed.), Equine respiratory diseases. International Veterinary Information Services, Ithaca, N.Y.
- 2.Chanter, N., K. C. Smith, and J. A. Mumford. 1995. Equine strangles modelled in mice. Vet. Microbiol. 43:209-218. [DOI] [PubMed] [Google Scholar]
- 3.Hamlen, H. J., J. F. Timoney, and R. J. Bell. 1994. Epidemiologic and immunologic characteristics of Streptococcus equi infection in foals. JAVMA 204:768-775. [PubMed] [Google Scholar]
- 4.Harrington, D. J., I. C. Sutcliffe, and N. Chanter. 2002. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 4:501-510. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs, A. A., D. Goovaerts, P. J. Nuijten, R. P. Theelen, O. M. Hartford, and T. J. Foster. 2000. Investigations towards an efficacious and safe strangles vaccine: submucosal vaccination with a live attenuated Streptococcus equi. Vet. Rec. 147:563-567. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsson, K., H. Jonsson, H. Lindmark, B. Guss, M. Lindberg, and L. Frykberg. 1997. Shot-gun phage display mapping of two streptococcal cell-surface proteins. Microbiol. Res. 152:121-128. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson, H., H. Lindmark, and B. Guss. 1995. A protein G-related cell surface protein in Streptococcus zooepidemicus. Infect. Immun. 63:2968-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorm, L. R. 1990. Strangles in horse studs: incidence, risk factors and effect of vaccination. Aust. Vet. J. 67:436-439. [DOI] [PubMed] [Google Scholar]
- 9.Kihlberg, B.-M., M. Collin, A. Olsén, and L. Björck. 1999. Protein H, an antiphagocytic surface protein in Streptococcus pyogenes. Infect. Immun. 67:1708-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindmark, H., and B. Guss. 1999. SFS, a novel fibronectin-binding protein from Streptococcus equi, inhibits the binding between fibronectin and collagen. Infect. Immun. 67:2383-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindmark, H., K. Jacobsson, L. Frykberg, and B. Guss. 1996. Fibronectin-binding protein of Streptococcus equi subsp. zooepidemicus. Infect. Immun. 64:3993-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindmark, H., H. Jonsson, E. Olsson Engvall, and B. Guss. 1999. Pulsed-field gel electrophoresis and distribution of the genes zag and fnz in isolates of Streptococcus equi. Res. Vet. Sci. 66:93-99. [DOI] [PubMed] [Google Scholar]
- 13.Lindmark, H., M. Nilsson, and B. Guss. 2001. Comparison of the fibronectin-binding protein FNE from Streptococcus equi subspecies equi with FNZ from S. equi subspecies zooepidemicus reveals a major and conserved difference. Infect. Immun. 69:3159-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lunn, D. P., M. A. Holmes, D. F. Antczak, N. Agerwal, J. Baker, S. Bwendali-Ahcene, M. Blanchard-Chanelle, K. M. Byrne, K. Cannizz, W. Davis, M. J. Hamilton, D. Hannan, T. Kondo, J. H. Kydd, M. C. Monier, P. F. Moore, P. F. O′Neil, B. R. Schram, A. S. Sheoran, J. L. Stotte, T. Sugiura, and K. E. Vagnonia. 1998. Report of the Second Equine Leucocyte Antigen Workshop, Squaw Valley, California, July 1995. Vet. Immunol. Immunopath. 62:101-143. [DOI] [PubMed] [Google Scholar]
- 15.Molinari, G., M. Rhode, C. A. Guzman, and G. S. Chhatwal. 2000. Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell Microbiol. 2:145-154. [DOI] [PubMed] [Google Scholar]
- 16.Nally, J. E., S. Artiushin, A. S. Sheoran, P. J. Burns, B. Simon, R. M. Gilley, J. Gibson, S. Sullivan, and J. F. Timoney. 2001. Induction of mucosal and systemic antibody specific for SeMF3 of Streptococcus equi by intranasal vaccination using a sucrose acetate isobutyrate based delivery system. Vaccine 19:492-497. [DOI] [PubMed] [Google Scholar]
- 17.Newton, J. R., N. Dunn, M. N. de Brauwere, and N. Chanter. 1997. Naturally occurring persistent and asymptomatic infection of the guttoral pouches of horses with Streptococcus equi. Vet. Rec. 140:84-90. [DOI] [PubMed] [Google Scholar]
- 18.Richards, C. M., A. T. Aman, T. R. Hirst, T. J. Hill, and N. A. Williams. 2001. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J. Virol. 75:1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schalen, C., L. Truedsson, K. K. Christensen, and P. Christensen. 1985. Blocking of antibody complement-dependent effector functions by streptococcal IgG Fc-receptor and staphylococcal protein A. Acta Pathol. Microbiol. Immunol. Scand. 93:395-400. [DOI] [PubMed] [Google Scholar]
- 20.Sheoran, A. S., S. Artiushin, and J. F. Timoney. 2002. Nasal mucosal immunogenicity for the horse of a SeM peptide of Streptococcus equi genetically coupled to cholera toxin. Vaccine 20:1653-1659. [DOI] [PubMed] [Google Scholar]
- 21.Sheoran, A. S., B. T. Sponseller, M. A. Holmes, and J. F. Timoney. 1997. Serum and mucosal antibody isotype responses to M-like protein (SeM) of Streptococcus equi in convalescent and vaccinated horses. Vet. Immunol. Immunopathol. 59:239-251. [DOI] [PubMed] [Google Scholar]
- 22.Takao, S.-I., K. Kiyotani, T. Sakaguchi, Y. Fujii, M. Seno, and T. Yoshida. 1997. Protection of mice from respiratory Sendai virus infections by recombinant vaccinia virus. J. Virol. 71:832-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timoney, J. F., and D. Eggers. 1985. Serum bactericidal responses to Streptococcus equi of horses following infection or vaccination. Equine Vet. J. 17:306-310. [DOI] [PubMed] [Google Scholar]
- 24.Walker, J. A., and J. F. Timoney. 2002. Construction of a stable non-mucoid deletion mutant of the Streptococcus equi Pinnacle vaccine strain. Vet. Microbiol. 89:311-321. [DOI] [PubMed] [Google Scholar]
- 25.Wood, J. L., N. Dunn, N. Chanter, and M. N. de Brauwere. 1993. Persistent infection with Streptococcus equi and the epidemiology of strangle. Vet. Rec. 133:375. [DOI] [PubMed] [Google Scholar]