Abstract
Ligand-stimulated receptor tyrosine kinases (RTKs) are phosphorylated/ubiquitinated, endocytosed and transported to the lysosomes via endosomes/multivesicular bodies, resulting in the attenuation of signal transmission. If this physiological mechanism of RTK signal downregulation is perturbed, signal transduction persists and may contribute to cellular transformation. This article presents several such examples. In some cases, endocytosis is impaired, and the activated RTK remains on the plasma membrane. In other cases, the activated RTK is endocytosed into endosomes/multivesicular bodies, but not subsequently sorted to the lysosomes for degradation. The latter cases indicate that even endocytosed RTKs can transmit signals. Transport of RTKs is accomplished via the formation and movement of membrane vesicles. Blockage or delay of endocytosis/trafficking can be caused by genetic alterations in the RTK itself or by mutations in CBL, Arf GAPs, or other components involved in internalization and vesicle transport. A survey of the literature indicates that, in some cases, even RTKs synthesized de novo can initiate signaling at the endoplasmic reticulum/Golgi before reaching the plasma membrane. The spectrum of molecules targeted by the signal is likely to be different between cell surface- and endoplasmic reticulum/Golgi-localized RTKs.
Keywords: endocytosis, endosomes, lysosomes, membrane vesicles, oncogenesis, receptor tyrosine kinase, signal transduction, ubiquitination
Introduction
Growth factor receptors on the cell surface comprise three parts: an extracellular domain for ligand binding, a transmembrane domain, and an intracellular domain harboring a tyrosine kinase domain. Binding of an extracellular ligand triggers intracellular autophosphorylation of the receptor tyrosine kinase (RTK) followed by adaptor association, recruitment and phosphorylation of associated kinases, and the activation of downstream signaling molecules.1 The eventual outcome of this signal transmission is either a positive or a negative effect on the growth of the cell. It must be noted that ligand-induced activation of RTKs occurs only transiently. Phosphorylated RTKs are then ubiquitinated, triggering internalization into endocytic endosomes via clathrin-dependent or -independent pathways. The early endosomes (EE) are the site of cargo sorting, at which point it is determined whether RTKs will be recycled back to the plasma membrane via recycling endosomes (RE), or transported to the lysosomes via late endosomes (LE)/multivesicular bodies (MVB). In lysosomes, RTKs are enzymatically degraded, which downregulates the signal. Under physiological conditions, growth-stimulating signals are transient due to the above-mentioned mechanisms of signal downregulation, which are necessary to prevent unlimited cell growth. By contrast, if cells should acquire the capacity to escape these downregulating mechanisms, persistent transduction of growth-promoting signals could become a causative mechanism of oncogenesis.2-4
Growth-promoting signals are believed to be elicited mainly from activated RTKs located on the cell surface; however, when RTKs are endocytosed into the cytoplasm, they may not be automatically downregulated. Thus, the first question to be addressed is whether endocytosed activated RTKs continue to participate in signal transduction. Similarly, new RTK polypeptides are synthesized at the endoplasmic reticulum (ER) and then transported to and glycosylated in the Golgi apparatus. Newly synthesized RTK molecules on their way to the plasma membrane have been believed to be inactive because of the unavailability of ligand in the cytoplasmic membrane-bound organelles. A second question is whether RTKs synthesized de novo are capable of initiating signaling, particularly when the RTK is activated by mutation. Accumulated evidence indicates that the answers to these two questions are indeed yes. In this mini-review, we describe several examples of these phenomena and discuss the significance of altered trafficking of mutated RTKs in human oncology.
Implication of altered trafficking of mutated RTKs on cancer
Epidermal growth factor receptor (EGFR)
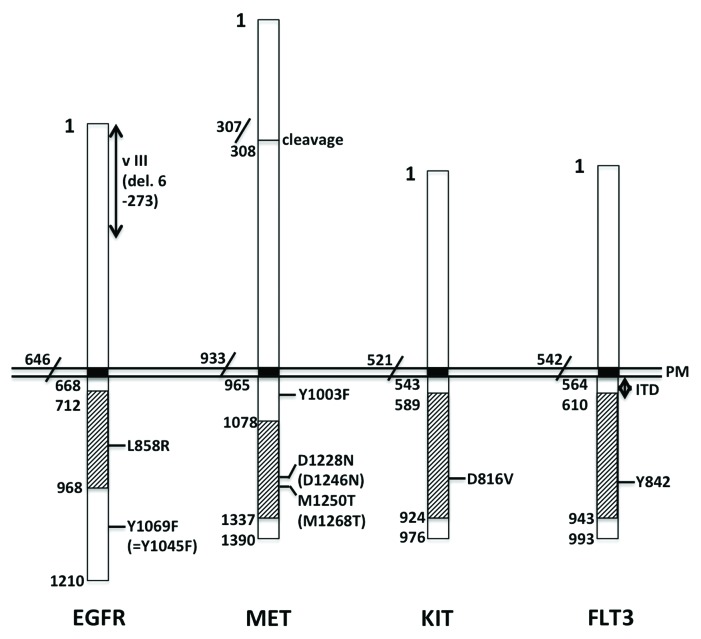
The L858R point mutation resides in the tyrosine kinase domain of EGFR and is frequently associated with non-small cell lung cancer (Fig. 1).5-7 This mutation results in impaired endocytosis of EGFR.8 Although L858R-EGFR is internalized after EGF stimulation, its internalization occurs more slowly than that of wild-type EGFR, so it remains intact on the cell surface for a longer time. Since the tyrosine phosphorylation of mutant EGFR is increased and prolonged, downstream signal transduction is also more strongly activated, as reflected by the increased phosphorylation of ERK, AKT and STAT3. The L858R mutation causes defective coupling to CBL, an E3 ubiquitin-conjugating enzyme. The poorly ubiquitinated mutant EGFR is therefore endocytosed less efficiently. Note that Y1045 of mutant EGFR, the CBL-docking site, is well phosphorylated, but that phosphorylation of CBL is poor. The authors suggest that the weak association between L858R-EGFR and CBL is probably related to the formation of a heterodimer between L858R-EGFR and HER2, another member of the EGFR family.8 This heterodimerization appears to occur even in the absence of ligand stimulation.
Figure 1. Cancer/leukemia-associated mutations found in RTKs. The numbers in the figure correspond to those of the amino acid sequences registered in the NCBI Reference Sequence. The loci IDs of human EGFR, MET, KIT and FLT3 polypeptides are NP_005219.2, NP_000236, NP_000213 and NP_004110, respectively. Note that Y1045 of EGFR described in the text corresponds to Y1069 in the figure, and that D1246 and M1268 of MET described in the text are numbered in NP_001120972 and correspond to D1228 and M1250 in the figure, respectively. Black boxes represent transmembrane domains, whereas slashed boxes represent tyrosine kinase domains. PM, plasma membrane; v III, variant type 3; del, deletion; ITD, internal tandem duplication.
The type III variant of EGFR, EGFRvIII, is a mutant (deletion of amino acid residues 6–273) found in various types of cancers, including those of the brain, lung, prostate and ovary (Fig. 1).9-12 This deletion in the extracellular domain renders the receptor incapable of binding EGF. Instead, the tyrosine residues in EGFRvIII are phosphorylated independently of ligand engagement, but at a much lower level compared with EGF-stimulated wild-type EGFR.13 Y1045, whose phosphorylation is necessary for EGFR to recruit CBL, is also barely phosphorylated in EGFRvIII. However, weakly phosphorylated EGFRvIII can still associate with Grb2, and hence indirectly associate with CBL. Ubiquitination of EGFRvIII by this putative EGFRvIII-Grb2-CBL complex is limited. Consequently, constitutive endocytosis of EGFRvIII remains minimal. Furthermore, a small amount of cytoplasm-incorporated EGFRvIII reaching the EE/RE is not brought to lysosomes, but is instead recycled back to the plasma membrane. Overall, constitutively activated EGFRvIII is not downregulated but instead continues to transmit cell growth signals.13
Mmesenchymal epithelial transition factor (MET)
MET is the receptor for hepatocyte growth factor (HGF) and is expressed in various types of epithelial cells. Y1003 is located in the juxta-transmembrane domain of MET, and the Y1003F-MET, an experimentally introduced mutation, is capable of transforming cells in vitro and in vivo (Fig. 1).14 Ligand-induced phosphorylation of Y1003 is necessary to recruit CBL, since phosphorylated Y1003 serves as a CBL-docking site. Therefore, Y1003F-MET is only poorly ubiquitinated, even after HGF stimulation, although tyrosine phosphorylation in the cytoplasmic tail is unaffected by the mutation. Thus, phosphorylated but not ubiquitinated Y1003F-MET is endocytosed from the cell surface, transported to EE, and sorted to LE/MVB as efficiently as wild-type MET. This means that ubiquitination of MET is dispensable for its endocytosis and transport to MVB. However, tyrosine phosphorylation of Hrs, an essential component of MVB, does not occur in cells with Y1003F-MET, thus preventing the mutated MET from being transported to the lysosomes. This results in a substantial delay in the degradation of Y1003F-MET, which remains intact in its phosphorylated form. This causes persistent activation of downstream signaling molecules, including H-RAS, MEK and ERK. An important implication of the above findings is that both cell surface-activated MET and endocytosed cytoplasmic MET are capable of transducing signals to the downstream molecules.14
The two mutations in MET D1246N and M1268T were originally identified in papillary renal carcinomas and are located in the kinase domain (Fig. 1).15 The tyrosine residues of mutated MET are constitutively phosphorylated, even in the absence of HGF.16 Interestingly, roughly half of the mutated MET molecules are located on the cell surface, while the remaining molecules are detected in the cytoplasm. This cytoplasmic, mutated MET resides in the EE and RE, as indicated by its colocalization with EEA1 and Rab11, respectively. When internalization and recycling are assessed individually, both are substantially increased in cells with mutated MET, indicating that active shuttling occurs between the plasma membrane and the EE/RE.
Mutated MET is constitutively active and can elicit signals to downstream molecules. RAC1 is activated, inducing remodeling of the actin cytoskeleton (namely, disappearance of stress fibers), and cell migration is stimulated. All of these events appear to confer a transformed phenotype to cells expressing mutated MET. Importantly, blocking the endocytosis of mutated MET abolishes RAC1 activation, reducing cell migration and cell transformation. Thus, in the case of constitutively active MET in the absence of HGF ligation, it is likely that cytoplasmic MET localized to endosomes is responsible for the induction of cellular transformation.16
Tyrosine-protein kinase (KIT)
D816V is a mutation in KIT found in certain subtypes of human acute myeloid leukemia (AML).17,18 As shown in Figure 1, D816 is located in the kinase domain of KIT, and D816V-KIT represents a ligand-independent, constitutively active form of the protein.19 When overexpressed in human cells, human D816V-KIT is mainly distributed on the Golgi apparatus and, to a lesser extent, the plasma membrane, and is capable of transforming cells. Strangely, when expressed in murine cells, the same protein is synthesized de novo and retained on the ER, but fails to reach either the Golgi apparatus or the plasma membrane, and is not able to transform cells. However, the expression of a truncated form of human D816V-KIT, which is comprised primarily of the intracytoplasmic domain (but not the extracellular domain or transmembrane domain), is still capable of transforming murine cells. This truncated protein appears to be distributed throughout the cytoplasmic membrane-bound organelles, including the Golgi apparatus, but is not present on the cell surface. Thus, for D816V-KIT to transform cells, ER retention is not sufficient, Golgi localization is sufficient, and plasma membrane localization is dispensable.19 In murine cells, the intracytoplasmic domain of D816V-KIT can transduce signals to downstream molecules, including ERK, AKT and STAT3, whereas the ER-retained intact form of D816V-KIT can activate ERK, but not AKT or STAT3.
Fms-like tyrosine kinase 3 (FLT3)
An internal tandem duplication (ITD) mutation in FLT3 occurs in the juxta-transmembrane domain of the protein. This mutation is also associated with AML (Fig. 1).20,21 FLT3-ITD is a constitutively active form and is therefore autophosphorylated at Y591 (and Y842) independently of ligand binding.22 With regard to its subcellular localization, FLT3-ITD is detected not only on the cell surface but also on the ER. To examine the different signaling activities elicited by cell surface FLT3-ITD and ER-located FLT3-ITD, various techniques have been employed. These strategies include the use of chemical compounds, such as tunicamycin and brefeldin A, as well as the introduction of FLT3-ITD molecules that contain modifications, such as the deletion of an extracellular domain or tagging of the myristoylation motif. FLT3-ITD located on the cell surface activates the RAS-MAPK (phosphorylation of ERK) and PI3K (phosphorylation of AKT) pathways, whereas FLT3-ITD located in the ER activates STAT5. This differential signaling elicited by differentially localized FLT3-ITD molecules appears to be applicable to both overexpressed and endogenous proteins. Furthermore, it is similar to the signaling activity observed for KIT-ITD, another mutation associated with AML.22
Implication of CBL mutations on RTK signaling and cancer
CBL encodes an E3 ubiquitin ligase and contains a phosphotyrosine-binding domain and a Ring finger domain.23 Interaction with activated RTKs is mediated by the phosphotyrosine-binding domain, whereas ubiquitination of RTKs occurs via the Ring finger domain. Ubiquitination of RTKs is generally believed to trigger endocytosis and subsequent degradation, the main mechanism of downregulation of RTK signaling.
R420Q is a mutation of CBL found in an AML patient.24 R420 lies in a site of contact between CBL and the E2 ubiquitin-conjugation enzyme UbcH7. Although R420Q-CBL can associate with FLT3, the mutated CBL only weakly ubiquitinates FLT3. Thus, the ligand-induced internalization of RTKs, including FLT3 and EGFR, is substantially delayed in R420Q-CBL-expressing cells, resulting in increased RTK signaling as evidenced by sustained phosphorylation of ERK. Eventually, the R420Q mutation of CBL induces cellular transformation.
Chronic myelomonocytic leukemia (CMML) presents a unique pattern of gene alteration involving CBL.25 In this condition, an allele from one parent is lost, whereas another allele from the other parent is duplicated, thus giving rise to an apparently normal diploidy (called acquired uniparental disomy). Loss of one allele is in accordance with the concept of CBL as a tumor suppressor, since CBL is involved in downregulating ligand-induced RTK signaling. By contrast, the duplicated allele is found to harbor various missense mutations in the Ring finger domain that are positioned at the E2-binding interface. Note that these mutations are often duplicated due to uniparental disomy. This results in some apparently paradoxical observations. On one hand, bone marrow cells from CBL(−/−) mice exhibit increased colony-forming activity, indicating that CBL functions as a tumor suppressor gene. On the other hand, cells transduced by missense-mutated CBL also exhibit a transformed phenotype, indicating that CBL acts as a proto-oncogene. Because of the locations of the mutations in CBL, its enzymatic activity as an E3 ubiquitin ligase is reduced. In addition, and more importantly, CBL molecules containing mutations such as Q367P and Y371S tend to attenuate the activity of wild-type CBL when both are coexpressed. As a result, ubiquitination of RTKs, including KIT, FLT3, JAK2 and EGFR, is inhibited, allowing prolongation of RTK signaling. In this sense, the mutated CBL appears to exert a gain-of-function effect on wild-type CBL. It must be noted here that, in the CMML samples, one allele of CBL is lost, whereas the other allele is duplicated. Therefore, it is possible that mutated CBL negatively regulates other members of the CBL family, such as CBL-B.25
Involvement of the Arf GAPs AGFG1 and SMAP1 in transferrin receptor/RTK trafficking and cancer
Arf genes form a family that encodes small GTPases belonging to the RAS superfamily. GTPase-activating proteins (GAPs) mediate the conversion of the GTP-bound, active form of a GTPase to the GDP-bound, inactive form. There are 31 human genes that encode Arf GAPs, most of which are implicated in the process of vesicle formation from membranes.26 Arf GAPs exert terminator (similar to other GAPs) as well as effector (uniquely to Arf GAP) functions in vesicle budding.27
AGFG1
AGFG1 (also known as Hrb) is a member of the Arf GAP family that is involved in endocytosis of the transferrin receptor via clathrin-coated vesicles.28 Activating mutations of Notch1 are frequently observed, particularly in pediatric T cell, acute lymphoblastic lymphoma (T-ALL).29 Evidence suggesting the involvement of AGFG1 in T-ALL comes from bone marrow transplantation experiments in mice and in vitro experiments using T-ALL cell lines.30 Notch signaling directly activates transcription of AGFG1, which is necessary for the efficient incorporation of transferrin into the cytoplasm. The resulting increase in cytoplasmic iron levels promotes the post-transcriptional upregulation of p21 (CIP1/WAF1), a CDK inhibitor that serves a pro-survival function in T cells. Thus, one effect of activated Notch 1 is AGFG1 upregulation, resulting in an increase in proliferation/survival of hematopoietic cells and/or T cell precursors.
SMAP1
SMAP1 is another member of the Arf GAP family and is involved in clathrin-dependent endocytosis of the transferrin receptor and E-cadherin.31,32 The coding region of SMAP1 harbors a unique 10-adenine repeat downstream of the Arf GAP domain. In colorectal cancers displaying microsatellite instability, short deletions or insertions frequently occur in this 10-A repeat in a monoallelic or biallelic manner, giving rise to a premature termination codon.33 As a result, SMAP1 protein levels are reduced or abolished in colorectal tumors. This decline in SMAP1 expression likely contributes to enhanced cell proliferation by shortening the G2/M phase of the cell cycle.
Using gene-targeted mice, we demonstrated that SMAP1 deficiency can become a predisposing factor for the occurrence of a hematological disorder called myelodysplastic syndrome (MDS).34 SMAP1-deficient mice develop MDS only after they reach 1 y of age, and MDS-bearing mice subsequently suffer from AML. Since these features mimic closely those of human MDS, SMAP1(−/−) mice provide a useful model. As for membrane trafficking, hematopoietic progenitors of SMAP1(−/−) mice endocytose transferrin more efficiently than wild-type cells. By contrast, the extent of endocytosis of KIT is not different between wild-type and SMAP1(−/−) cells. Therefore, KIT in SMAP1(−/−) cells is phosphorylated, ubiquitinated and properly transported to the EE. However, in SMAP1(−/−) cells, KIT that has reached LE/MVB tends to remain there for a longer time. As a result, transport of KIT to the lysosomes and subsequent degradation are substantially impaired. The retained KIT is associated with GRB2, and is thus capable of continuing to signal to downstream molecules, as exemplified by increased phosphorylation of ERK. Increased incorporation of transferrin and the persistence of endocytosed KIT likely stimulate the proliferation of hematopoietic progenitors. As transport machinery, SMAP1 appears to function at the site of vesicle budding from LE/MVB to the lysosomes. However, the effector function of SMAP1 appears to be specific to KIT; other RTKs, such as EGFR, are efficiently transported to the lysosomes regardless of SMAP1 status.34
HIP1 and CTTN in RTK trafficking and cancer
The Huntingtin interacting protein 1 (HIP1) is involved in clathrin-mediated endocytosis.35-39 HIP1 associates and colocalizes with clathrin and the adaptor protein AP-2. In human prostate and breast cancers, HIP1 is overexpressed.40 The effects of HIP1 overexpression have been investigated in multiple cell lines.41 In these cells, expression of EGFR (and transferrin receptor) is substantially upregulated, and its auto- and ligand-induced phosphorylation is increased. Accordingly, intracellular uptake and accumulation of EGF (and transferrin) is increased, resulting in the activation of a downstream signaling cascade, including RAS/MEK/ERK and PI3K signaling. This increased signaling activity likely serves as the basis for cellular transformation.
Cortactin (CTTN) is a multidomain protein that connects membrane endocytosis and the actin cytoskeleton.42 CTTN binds both filamentous actin and dynamin2, and regulates the fission of budding vesicles. In human tumors of the breast, and head and neck regions, CTTN is amplified and overexpressed,43,44 and the effects of CTTN overexpression have been investigated in multiple cell lines.45 In these cells, EGF-induced phosphorylation and internalization of EGFR is not altered. However, phosphorylation of CBL and its association with EGFR is impaired, resulting in poor ubiquitination of EGFR. Thus, upon EGF stimulation in CTTN-overexpressing cells, downregulation of EGFR is delayed and impaired. Persistent activation of downstream signals, such as ERK, appears to be responsible for the increased growth of CTTN-overexpressing cells.
Caveats and perspectives
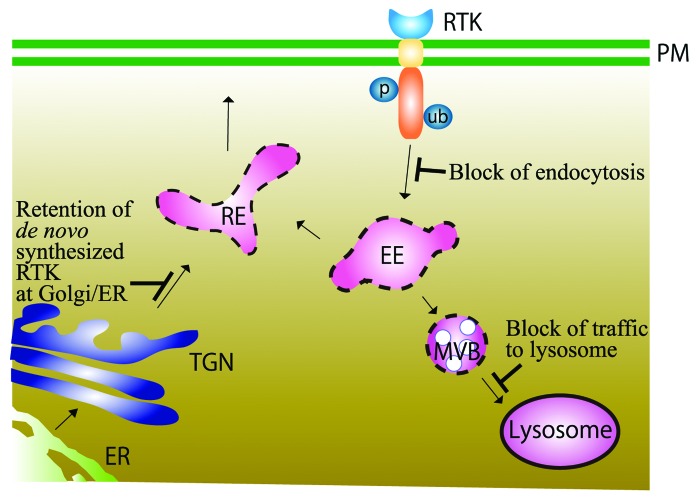
Figure 2 summarizes a view on altered trafficking of RTK and cancer. The view is deduced from the literature survey mentioned above, and is also supported by previous reviews.46-49 In some cases, endocytosis is impaired, and the activated RTK remains on the plasma membrane. In other cases, the activated RTK is incorporated and passed to EE/MVB, but not subsequently sorted to the lysosomes for degradation. Thus, perturbation of downregulating mechanisms of activated RTK can cause persistent signal transduction and contribute to cellular transformation. There are even cases in which mutated RTKs synthesized de novo can initiate signaling at the ER/Golgi before reaching the plasma membrane.
Figure 2. Schematic illustration of RTK trafficking inside the cells. Upon ligand binding, RTKs residing on the plasma membrane (PM) are phosphorylated, ubiquitinated by CBL ubiquitin ligase, endocytosed, and transported to early endosomes (EE). There, RTKs are either sorted to recycling endosomes (RE) or transported to multivesicular bodies (MVB) and eventually to lysosomes, where they are degraded by enzymatic digestion. RTKs are synthesized de novo on the endoplasmic reticulum (ER) and transported through the Golgi apparatus to the RE, and onto the plasma membrane. Blockages or delays of each transport pathway caused by abnormalities of trafficking machineries are shown in blue.
It must be noted that the literature cited above mainly focuses on RTK signaling but not on the trafficking machinery. For example, multiple endocytic pathways exist, including clathrin-dependent, caveolin-dependent and clathrin- and caveolin-independent pathways.50 In the case of deregulation of RTK endocytosis, it is not immediately clear which of the above three pathways is involved, and whether or not the choice of pathway affects the nature of the deregulation. For example, we described that EGF-bound EGFR is ubiquitinated by CBL and then endocytosed. However, according to Sigismund et al.,51 this ubiquitination occurs only at high concentrations of EGF (20 ng/ml in cell culture). At low concentrations of EGF (1.5 ng/ml), almost all (~100%) cell surface EGFR is endocytosed via clathrin-coated vesicles. Ubiquitination of EGFR is dispensable for this type of endocytosis, and the incorporated EGFR is efficiently transported to the EE. Most of the EGFR molecules (~70%) are then recycled back to the plasma membrane via RE, leaving only a minor fraction (~30%) destined for degradation. Since knock-down of clathrin accelerates the decay of phosphorylated AKT, recycling of EGFR via clathrin-coated vesicles is thought to contribute to the sustainability of EGFR signaling. Y1045F (see Figure 1) is an experimentally introduced mutation, and Y1045F-EGFR provides a model for the study of RTKs endocytosed via clathrin-coated vesicles, since this molecule escapes ubiquitination.
By contrast, again according to Sigismund et al.,51 when high concentrations of EGF are used, 60% of the cell surface EGFR molecules undergo endocytosis via clathrin-coated vesicles, as described above, whereas the remaining 40% are endocytosed independently of clathrin, perhaps via a caveolar pathway. Filipin is an experimental reagent used to test the significance of clathrin-independent endocytosis. The reagent interferes with cholesterol, which is enriched in membrane rafts/caveolae. For this type of endocytosis, modification of cargo via mechanisms such as ubiquitination is indispensable, and most ubiquitinated EGFRs are sorted from the EE to the LE/lysosomes for degradation. Therefore, this clathrin-independent endocytosis is involved in downregulating EGFR signaling and is mobilized in response to high levels of ligand stimulation. The differential usage of endocytotic pathways should be taken into consideration when analyzing the fate of mutated and oncogenic RTKs.
In addition, although only endocytosis-lysosomal degradation has been reviewed here, autophagy-lysosomal degradation might also be used. In fact, a few cases were reported in which a component of this pathway is involved in human cancers. TSG101, a component of ESCRT-I, is one example. Paradoxically, overexpression of TSG101 is reported in thyroid cancer, suggesting a role as an oncogene,52 whereas deletion of the same gene is found in breast cancer, suggesting a role as a tumor suppressor.53 Thus, the significance of RTK degradation via the autophagy-lysosomal pathway in oncogenesis awaits further analysis.
We have been impressed, in the literature survey described above, by the existence of only a few reports, 13 at the most, in which mutations of RTKs or their associated molecules are oncogenic due to altered trafficking. Thus, we wonder whether the mechanisms by which RTKs escape downregulation might be only weakly oncogenic, compared with RTK overexpression through gene amplification and/or promoter modification, and so less frequently encountered in cancer samples. Alternatively, little attention might have been paid to date to altered trafficking in oncology.
In this review article, altered membrane trafficking was re-visited only from the point of view of RTKs and their associated molecules. We took this approach because RTKs promote cell growth and because we observed aberrant trafficking of KIT in SMAP1-deficient and MDS-bearing mice.34 However, cancer development involves not only tumor initiation but also progression to a malignant invasive/metastatic phenotype. In addition to cell growth, cell motility, cell-cell adhesion, cell de-differentiation, epithelial-mesenchymal transition and mesenchymal-epithelial transition contribute to cancer progression. Thus, analysis of endocytosis, recycling and degradation of molecules involved in these steps, for example E-cadherin and integrin, constitutes a significant field in oncology research that needs to be reviewed separately.49
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- AML
acute myeloid leukemia
- CMML
chronic myelomonocytic leukemia
- EE
early endosomes
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- FLT3
fms-like tyrosine kinase 3
- HGF
hepatocyte growth factor
- ITD
internal tandem duplication
- KIT
tyrosine-protein kinase
- LE
late endosomes
- MDS
myelodysplastic syndrome
- MET
mesenchymal epithelial transition factor
- MVB
multivesicular bodies
- RE
recycling endosomes
- RTK
receptor tyrosine kinase
- T-ALL
T cell acute lymphoblastic lymphoma
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Dikic I, Giordano S. Negative receptor signalling. Curr Opin Cell Biol. 2003;15:128–35. doi: 10.1016/S0955-0674(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 3.Peschard P, Park M. Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell. 2003;3:519–23. doi: 10.1016/S1535-6108(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 4.Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–52. doi: 10.1016/S0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, Citri A, Katz M, Lavi S, Ben-Basat Y, et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–78. doi: 10.1038/sj.onc.1210503. [DOI] [PubMed] [Google Scholar]
- 9.Moscatello DK, Holgado-Madruga M, Godwin AK, Ramirez G, Gunn G, Zoltick PW, Biegel JA, Hayes RL, Wong AJ. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–9. [PubMed] [Google Scholar]
- 10.Okamoto I, Kenyon LC, Emlet DR, Mori T, Sasaki J, Hirosako S, Ichikawa Y, Kishi H, Godwin AK, Yoshioka M, et al. Expression of constitutively activated EGFRvIII in non-small cell lung cancer. Cancer Sci. 2003;94:50–6. doi: 10.1111/j.1349-7006.2003.tb01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olapade-Olaopa EO, Moscatello DK, MacKay EH, Horsburgh T, Sandhu DP, Terry TR, Wong AJ, Habib FK. Evidence for the differential expression of a variant EGF receptor protein in human prostate cancer. Br J Cancer. 2000;82:186–94. doi: 10.1054/bjoc.1999.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD. The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol. 1998;4:148–58. doi: 10.3109/13550289809114515. [DOI] [PubMed] [Google Scholar]
- 13.Grandal MV, Zandi R, Pedersen MW, Willumsen BM, van Deurs B, Poulsen HS. EGFRvIII escapes down-regulation due to impaired internalization and sorting to lysosomes. Carcinogenesis. 2007;28:1408–17. doi: 10.1093/carcin/bgm058. [DOI] [PubMed] [Google Scholar]
- 14.Abella JV, Peschard P, Naujokas MA, Lin T, Saucier C, Urbé S, Park M. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol Cell Biol. 2005;25:9632–45. doi: 10.1128/MCB.25.21.9632-9645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt L, Duh FM, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 16.Joffre C, Barrow R, Ménard L, Calleja V, Hart IR, Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol. 2011;13:827–37. doi: 10.1038/ncb2257. [DOI] [PubMed] [Google Scholar]
- 17.Beghini A, Larizza L, Cairoli R, Morra E. c-kit activating mutations and mast cell proliferation in human leukemia. Blood. 1998;92:701–2. [PubMed] [Google Scholar]
- 18.Kanakura Y, Furitsu T, Tsujimura T, Butterfield JH, Ashman LK, Ikeda H, Kitayama H, Kanayama Y, Matsuzawa Y, Kitamura Y. Activating mutations of the c-kit proto-oncogene in a human mast cell leukemia cell line. Leukemia. 1994;8(Suppl 1):S18–22. [PubMed] [Google Scholar]
- 19.Xiang Z, Kreisel F, Cain J, Colson A, Tomasson MH. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signaling. Mol Cell Biol. 2007;27:267–82. doi: 10.1128/MCB.01153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 21.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–65. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 22.Choudhary C, Olsen JV, Brandts C, Cox J, Reddy PN, Böhmer FD, Gerke V, Schmidt-Arras DE, Berdel WE, Müller-Tidow C, et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell. 2009;36:326–39. doi: 10.1016/j.molcel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 24.Sargin B, Choudhary C, Crosetto N, Schmidt MH, Grundler R, Rensinghoff M, Thiessen C, Tickenbrock L, Schwäble J, Brandts C, et al. Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood. 2007;110:1004–12. doi: 10.1182/blood-2007-01-066076. [DOI] [PubMed] [Google Scholar]
- 25.Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, Tamura A, Honda H, Sakata-Yanagimoto M, Kumano K, et al. Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature. 2009;460:904–8. doi: 10.1038/nature08240. [DOI] [PubMed] [Google Scholar]
- 26.Kahn RA, Bruford E, Inoue H, Logsdon JM, Jr., Nie Z, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML, et al. Consensus nomenclature for the human ArfGAP domain-containing proteins. J Cell Biol. 2008;182:1039–44. doi: 10.1083/jcb.200806041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn RA. GAPs: Terminator versus effector functions and the role(s) of ArfGAP1 in vesicle biogenesis. Cell Logist. 2011;1:49–51. doi: 10.4161/cl.1.2.15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaineau M, Danglot L, Proux-Gillardeaux V, Galli T. Role of HRB in clathrin-dependent endocytosis. J Biol Chem. 2008;283:34365–73. doi: 10.1074/jbc.M804587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng AP, Ferrando AA, Lee W, Morris JP, 4th, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–71. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 30.Khwaja SS, Liu H, Tong C, Jin F, Pear WS, van Deursen J, Bram RJ. HIV-1 Rev-binding protein accelerates cellular uptake of iron to drive Notch-induced T cell leukemogenesis in mice. J Clin Invest. 2010;120:2537–48. doi: 10.1172/JCI41277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanabe K, Torii T, Natsume W, Braesch-Andersen S, Watanabe T, Satake M. A novel GTPase-activating protein for ARF6 directly interacts with clathrin and regulates clathrin-dependent endocytosis. Mol Biol Cell. 2005;16:1617–28. doi: 10.1091/mbc.E04-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kon S, Tanabe K, Watanabe T, Sabe H, Satake M. Clathrin dependent endocytosis of E-cadherin is regulated by the Arf6GAP isoform SMAP1. Exp Cell Res. 2008;314:1415–28. doi: 10.1016/j.yexcr.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Sangar F, Schreurs AS, Umaña-Diaz C, Clapéron A, Desbois-Mouthon C, Calmel C, Mauger O, Zaanan A, Miquel C, Fléjou JF, et al. Involvement of small ArfGAP1 (SMAP1), a novel Arf6-specific GTPase-activating protein, in microsatellite instability oncogenesis. Oncogene. 2013 doi: 10.1038/onc.2013.211. [DOI] [PubMed] [Google Scholar]
- 34.Kon S, Minegishi N, Tanabe K, Watanabe T, Funaki T, Wong WF, Sakamoto D, Higuchi Y, Kiyonari H, Asano K, et al. Smap1 deficiency perturbs receptor trafficking and predisposes mice to myelodysplasia. J Clin Invest. 2013;123:1123–37. doi: 10.1172/JCI63711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legendre-Guillemin V, Metzler M, Charbonneau M, Gan L, Chopra V, Philie J, Hayden MR, McPherson PS. HIP1 and HIP12 display differential binding to F-actin, AP2, and clathrin. Identification of a novel interaction with clathrin light chain. J Biol Chem. 2002;277:19897–904. doi: 10.1074/jbc.M112310200. [DOI] [PubMed] [Google Scholar]
- 36.Metzler M, Legendre-Guillemin V, Gan L, Chopra V, Kwok A, McPherson PS, Hayden MR. HIP1 functions in clathrin-mediated endocytosis through binding to clathrin and adaptor protein 2. J Biol Chem. 2001;276:39271–6. doi: 10.1074/jbc.C100401200. [DOI] [PubMed] [Google Scholar]
- 37.Mishra SK, Agostinelli NR, Brett TJ, Mizukami I, Ross TS, Traub LM. Clathrin- and AP-2-binding sites in HIP1 uncover a general assembly role for endocytic accessory proteins. J Biol Chem. 2001;276:46230–6. doi: 10.1074/jbc.M108177200. [DOI] [PubMed] [Google Scholar]
- 38.Rao DS, Chang JC, Kumar PD, Mizukami I, Smithson GM, Bradley SV, Parlow AF, Ross TS. Huntingtin interacting protein 1 Is a clathrin coat binding protein required for differentiation of late spermatogenic progenitors. Mol Cell Biol. 2001;21:7796–806. doi: 10.1128/MCB.21.22.7796-7806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waelter S, Scherzinger E, Hasenbank R, Nordhoff E, Lurz R, Goehler H, Gauss C, Sathasivam K, Bates GP, Lehrach H, et al. The huntingtin interacting protein HIP1 is a clathrin and alpha-adaptin-binding protein involved in receptor-mediated endocytosis. Hum Mol Genet. 2001;10:1807–17. doi: 10.1093/hmg/10.17.1807. [DOI] [PubMed] [Google Scholar]
- 40.Rao DS, Hyun TS, Kumar PD, Mizukami IF, Rubin MA, Lucas PC, Sanda MG, Ross TS. Huntingtin-interacting protein 1 is overexpressed in prostate and colon cancer and is critical for cellular survival. J Clin Invest. 2002;110:351–60. doi: 10.1172/JCI0215529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao DS, Bradley SV, Kumar PD, Hyun TS, Saint-Dic D, Oravecz-Wilson K, Kleer CG, Ross TS. Altered receptor trafficking in Huntingtin Interacting Protein 1-transformed cells. Cancer Cell. 2003;3:471–82. doi: 10.1016/S1535-6108(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 42.Daly RJ. Cortactin signalling and dynamic actin networks. Biochem J. 2004;382:13–25. doi: 10.1042/BJ20040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hui R, Campbell DH, Lee CS, McCaul K, Horsfall DJ, Musgrove EA, Daly RJ, Seshadri R, Sutherland RL. EMS1 amplification can occur independently of CCND1 or INT-2 amplification at 11q13 and may identify different phenotypes in primary breast cancer. Oncogene. 1997;15:1617–23. doi: 10.1038/sj.onc.1201311. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigo JP, García LA, Ramos S, Lazo PS, Suárez C. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:3177–82. [PubMed] [Google Scholar]
- 45.Timpson P, Lynch DK, Schramek D, Walker F, Daly RJ. Cortactin overexpression inhibits ligand-induced down-regulation of the epidermal growth factor receptor. Cancer Res. 2005;65:3273–80. doi: 10.1158/0008-5472.CAN-04-2118. [DOI] [PubMed] [Google Scholar]
- 46.Bache KG, Slagsvold T, Stenmark H. Defective downregulation of receptor tyrosine kinases in cancer. EMBO J. 2004;23:2707–12. doi: 10.1038/sj.emboj.7600292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crosetto N, Tikkanen R, Dikic I. Oncogenic breakdowns in endocytic adaptor proteins. FEBS Lett. 2005;579:3231–8. doi: 10.1016/j.febslet.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 48.Lanzetti L, Di Fiore PP. Endocytosis and cancer: an ‘insider’ network with dangerous liaisons. Traffic. 2008;9:2011–21. doi: 10.1111/j.1600-0854.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 49.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–50. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]
- 50.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 51.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–19. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Liu RT, Huang CC, You HL, Chou FF, Hu CCA, Chao FP, Chen CM, Cheng JT. Overexpression of tumor susceptibility gene TSG101 in human papillary thyroid carcinomas. Oncogene. 2002;21:4830–7. doi: 10.1038/sj.onc.1205612. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Li X, Francke U, Cohen SN. The TSG101 tumor susceptibility gene is located in chromosome 11 band p15 and is mutated in human breast cancer. Cell. 1997;88:143–54. doi: 10.1016/S0092-8674(00)81866-8. [DOI] [PubMed] [Google Scholar]