Abstract
Trypanosoma cruzi is the protozoan parasite that causes Chagas' heart disease, a potentially fatal cardiomyopathy prevalent in Central and South America. Infection with T. cruzi induces cardiac myosin autoimmunity in susceptible humans and mice, and this autoimmunity has been suggested to contribute to cardiac inflammation. To address how T. cruzi induces cardiac myosin autoimmunity, we investigated whether immunity to T. cruzi antigens could induce cardiac myosin-specific autoimmunity in the absence of live parasites. We immunized A/J mice with a T. cruzi Brazil-derived protein extract emulsified in complete Freund's adjuvant and found that these mice developed cardiac myosin-specific delayed-type hypersensitivity (DTH) and autoantibodies in the absence of detectable cardiac damage. The induction of autoimmunity was specific since immunization with extracts of the related protozoan parasite Leishmania amazonensis did not induce myosin autoimmunity. The immunogenetic makeup of the host was important for this response, since C57BL/6 mice did not develop cardiac myosin DTH upon immunization with T. cruzi extract. Perhaps more interesting, mice immunized with cardiac myosin developed T. cruzi-specific DTH and antibodies. This DTH was also antigen specific, since immunization with skeletal myosin and myoglobin did not induce T. cruzi-specific immunity. These results suggest that immunization with cardiac myosin or T. cruzi antigen can induce specific, bidirectionally cross-reactive immune responses in the absence of detectable cardiac damage.
Trypanosoma cruzi is the protozoan parasite that causes Chagas' heart disease (CHD), a potentially fatal cardiomyopathy resulting in dilated tissue. Approximately 16 million people are infected with this protozoan parasite, and 120 million are at risk of infection in Central and South America (28). CHD develops in roughly one-third of T. cruzi-infected individuals as an acute or chronic myocarditis of variable degree (reviewed in reference 39). Among the various mechanisms invoked to explain the pathogenesis of CHD, autoimmunity is one that both has been supported by much experimental evidence and has received much criticism. There is no doubt that autoimmunity results from chronic T. cruzi infection of humans and experimental animals. The questions are (i) whether this autoimmunity is pathogenic and (ii) by what mechanism(s) autoimmunity is induced. The debate surrounding this issue is long-standing (reviewed in references 17, 22, 39, and 40).
T. cruzi infection induces humoral and cellular autoimmunity to a diverse set of autoantigens (reviewed in references 17 and 22), including cardiac myosin. Myosin-specific autoimmunity is induced in both humans (8) and experimental models (24) upon T. cruzi infection. In previous work (24), it was found that within weeks of infection, A/J mice acutely infected with T. cruzi developed severe myocarditis accompanied by myosin-specific delayed-type hypersensitivity (DTH) and antibody production. Autoimmunity to cardiac myosin has also been reported for other instances of cardiac damage, including those occurring as a result of viral infection (32), cardiac transplantation (11), and cardiac surgery (9). Taken together, this information suggests that cardiac damage can lead to the development of cardiac autoimmunity by a bystander activation mechanism in which cardiac damage in the proper setting can activate autoreactive lymphocytes (21).
However, there are a number of reports supporting the theory that a molecular mimicry mechanism is responsible for the development of autoimmunity. Immunization with T. cruzi proteins, in the absence of live parasites, can induce autoimmunity to self-antigens. Immunization of mice with T. cruzi cruzipain induces expansion of T cells and B cells specific to skeletal myosin (13) and autoantibodies to cardiac myosin (14), and immunization of mice with T. cruzi ribosomal proteins induces autoantibodies to mammalian ribosomal proteins (30). Finally, T-cell clones reactive with both myosin and the T. cruzi B13 protein have been isolated from the hearts of humans with CHD (7).
To further investigate whether T. cruzi antigens could induce cardiac autoimmunity in CHD, we tested whether immunization of mice with an extract of T. cruzi in complete Freund's adjuvant (CFA) could induce autoimmunity to the major cardiac autoantigen, cardiac myosin. The T. cruzi extract was produced by using acetone; thus, parasite proteins and no viable parasites were present in the immunogen. We found that mice immunized with this extract developed significant myosin-specific DTH and antibodies, even though the mice did not develop myocarditis as a result. Equally important, immunization of mice with cardiac myosin induced T. cruzi-specific cellular and humoral immune responses, indicating that the immunologic cross-reactivity between T. cruzi and myosin is bidirectional. These results suggest that cardiac damage is not required for the induction of myosin autoimmunity by T. cruzi and that, in the setting of live T. cruzi infection, both bystander activation and antigenic cross-reactivity may contribute to the development of cardiac myosin autoimmunity.
MATERIALS AND METHODS
Mice, parasites, and infection protocol.
Four- to six-week-old male A/J and C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) were housed under specific-pathogen-free conditions. T. cruzi epimastigotes were grown at 26°C in supplemented liver digest-neutralized tryptose (LDNT) medium as described previously (18). Leishmania amazonensis promastigotes (clone LV 78) were maintained in medium 199 (Gibco BRL, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL) and grown at 25°C (27). Mice were infected by intraperitoneal injection of 104 Brazil strain T. cruzi trypomastigotes derived from infection of tissue culture H9C2 rat myoblasts (American Type Culture Collection, Manassas, Va.). Uninfected controls received an intraperitoneal injection of Dulbecco's phosphate-buffered saline (PBS; Gibco BRL) of equal volume. Mice were anesthetized by a single intraperitoneal injection of sodium pentobarbital (60 mg/kg of body weight) for each experimental manipulation. Mice were used and cared for in accordance with the guidelines of the Center for Comparative Medicine at Northwestern University.
Antigens.
Cardiac myosin heavy chains were purified from syngeneic hearts, and skeletal myosin heavy chains were purified from syngeneic quadriceps muscles according to the method of Shiverick et al. (36) with modifications as described previously (24). For immunizations, T. cruzi and L. amazonensis antigens were prepared by washing T. cruzi epimastigotes or L. amazonensis promastigotes three times in PBS and resuspending them in PBS before addition of an equal volume of acetone for extraction. After being washed three times in PBS, these fixed parasites were sonicated and lyophilized prior to quantitation of protein concentration by the method of Bradford (5). For enzyme-linked immunosorbent assays (ELISAs) and DTH assays, T. cruzi and L. amazonensis antigens were prepared by washing T. cruzi epimastigotes or L. amazonensis promastigotes three times in PBS and resuspending them in ice-cold sterile water prior to sonication. The resultant lysate was pelleted at 16,000 × g, and the supernatant was filtered through a 0.2-μm-pore-size filter and lyophilized prior to quantitation of protein concentration by the method of Bradford (5). Antigen was stored at −80°C until further use. Horse cardiac myoglobin was purchased from Sigma (St. Louis, Mo.). LDNT antigen was prepared by lyophilizing 5 ml of LDNT medium (18) and resuspending it in water. Each mouse was immunized with 300 μg of LDNT antigen as described below.
Extraction of T. cruzi proteins, DNA, and RNA.
T. cruzi epimastigotes were separated into protein, DNA, and RNA fractions by using the TRIzol reagent (Life Technologies, Gaithersburg, Md.) according to the manufacturer's instructions.
Immunization.
Mice were immunized with the antigens (300 μg) described above in an emulsion of CFA or incomplete Freund’s adjuvant (IFA; Difco, Detroit, Mich.) in a total volume of 0.1 ml. Three sites in the dorsal flank received subcutaneous injections. Seven days later, mice were given a booster in an identical manner.
Histopathology.
The hearts of the mice were removed, rinsed with PBS, and fixed for 24 h in 10% buffered formalin. Fixed hearts were embedded in paraffin and sectioned, and four sections per heart were stained with hematoxylin-eosin or Masson's trichrome and examined by light microscopy. Each section was examined for evidence of mononuclear and polynuclear cellular inflammation, necrosis, and mineralization, T. cruzi pseudocysts, and fibrosis.
Serologic analysis.
Levels of cardiac myosin-specific, T. cruzi-specific, and L. amazonensis-specific immunoglobulin G (IgG) were determined by ELISA as described previously (24). ELISA plates were coated with 2.5 μg of cardiac myosin, T. cruzi, or L. amazonensis antigen/ml of PBS. Endpoint dilution titers for total IgG were defined as the highest dilution in serum that resulted in an absorbance value at 450 nm of 2 standard deviations above the mean absorbance of a negative control sample (pooled sera from uninfected mice) included on every plate. Levels of creatine kinase-muscle-brain in serum were measured by the Diagnostics Laboratory Animal Resource Center at the University of Chicago by standard methods.
DTH.
Myosin-specific, T. cruzi-specific, and L. amazonensis-specific DTHs were quantitated using a standard ear swelling assay (24). Antigen-induced ear swelling was the result of mononuclear cell infiltration and exhibited typical DTH kinetics (i.e., minimal swelling at 4 h and maximal swelling at 24 to 48 h postinjection).
Statistical analysis.
The statistical significance of DTH, parasitemia, or log (base 2)-transformed serum antibody titer was analyzed by a one-way analysis of variance followed by a two-tailed t test and post hoc Bonferroni's analysis. Titers of 0 were replaced by 1 for logarithmic transformations. P values of <0.05 were considered significant.
RESULTS
Immunization with a T. cruzi protein extract induces cardiac myosin-specific DTH.
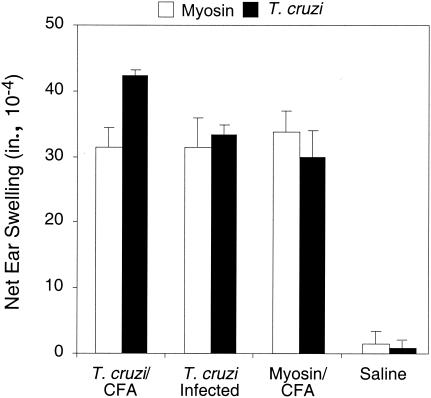
Acute infection of A/J mice with T. cruzi Brazil induces a strong cardiac myosin DTH, high levels of myosin-specific IgG, and severe myocarditis by 21 days postinfection (24). To determine whether immunity to T. cruzi proteins is sufficient to induce cardiac myosin autoimmunity, we immunized A/J mice with an acetone extract of T. cruzi emulsified in CFA. The A/J mouse strain was chosen because it is susceptible to myosin autoimmunity (33), and the acetone extraction method was used because it yields protein and a small amount of nucleic acid. Analysis of immunized animals 21 days later revealed the presence of strong, cardiac myosin-specific DTH (Fig. 1). The magnitudes of myosin DTH were comparable among T. cruzi-immunized, T. cruzi-infected, and myosin-immunized mice (which develop myosin-specific autoimmune myocarditis). To confirm that the myosin autoimmunity resulted from immunity to T. cruzi proteins, we immunized mice with T. cruzi protein, DNA, and RNA in CFA. Only immunization with protein extracts induced myosin DTH (data not shown).
FIG. 1.
Immunization with T. cruzi lysate induces cardiac myosin-specific DTH, and immunization with myosin induces T. cruzi-specific DTH. A/J mice (five per group) were immunized with T. cruzi lysate in CFA (T. cruzi/CFA), infected with T. cruzi (T. cruzi Infected), immunized with cardiac myosin in CFA (Myosin/CFA), or immunized with saline in CFA (Saline). Twenty-one days later, myosin-specific DTH and T. cruzi-specific DTH were measured by a 24-h ear swelling assay. Error bars indicate standard errors of the means.
The development of myosin autoimmunity after immunization with a T. cruzi extract is suggestive of molecular mimicry between a parasite and self-antigens in the host. True molecular mimicry should be bidirectional and also demonstrable at the level of a single T cell or B cell (i.e., a single cell or antibody binds to both a host epitope and a parasite epitope). To further test this notion, we immunized mice with myosin and determined whether immunity to T. cruzi protein(s) developed. Indeed, myosin-immunized mice developed T. cruzi-specific DTH of a magnitude similar to that of acutely T. cruzi-infected and T. cruzi-immunized mice (Fig. 1).
T. cruzi-immunized mice develop myosin-specific antibodies.
We hypothesized that if T. cruzi-immunized mice mounted an autoimmune response to a myosin-like protein, the quality of this response would be similar to that seen in myosin-immunized mice. To that end, we compared the myosin-specific antibody isotype profiles of T. cruzi-immunized mice to those of myosin-immunized and T. cruzi-infected mice 21 days after infection or immunization (Table 1). T. cruzi-immunized mice developed myosin-specific IgG but at lower levels than that of either T. cruzi-infected or myosin-immunized mice (Table 1). All immunized and infected mice developed myosin-specific titers of IgG1, a Th2-associated isotype, and IgG2a, a Th1-associated isotype, but the magnitudes of their responses differed. In general, T. cruzi-immunized mice developed lower titers of myosin-specific antibody isotypes than those of myosin-immunized and acutely T. cruzi-infected mice, with the exception of myosin-specific IgM. Overall, the quality of the myosin-specific response, or the myosin-specific antibody profile, of T. cruzi-immunized mice differed from that of myosin-immunized mice or acutely T. cruzi-infected mice.
TABLE 1.
Myosin-specific antibody isotypes produced in myosin-immunized, T. cruzi-immunized, and T. cruzi-infected mice 21 days after infection or immunizationa
| Group | Myosin-specific immunoglobulin isotype
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total IgG
|
IgG1
|
IgG2a
|
IgG2b
|
IgG3
|
IgM
|
|||||||
| GMT | Log2 ± SE | GMT | Log2 ± SE | GMT | Log2 ± SE | GMT | Log2 ± SE | GMT | Log2 ± SE | GMT | Log2 ± SE | |
| T. cruzi-CFA immunized | 141 | 7.1 ± 0.3b | 159 | 7.3 ± 0.5b | 1,600 | 10.6 ± 0.8b | 3 | 1.3 ± 1.3 | 356 | 8.5 ± 0.3b | 126 | 7.0 ± 0.2 |
| T. cruzi infected | 400 | 8.6 ± 0.5b | 400 | 8.6 ± 0.5b | 11,403 | 13.5 ± 0.5b | 2 | 1.1 ± 1.1 | 2,016 | 11.0 ± 0.8b | 178 | 7.5 ± 0.3b |
| Myosin-CFA immunized | 3,676 | 11.8 ± 0.4b | 7,184 | 12.8 ± 0.3b | 2,263 | 11.1 ± 1.3b | 93 | 6.5 ± 1.3b | 4 | 1.9 ± 1.9 | 10 | 3.3 ± 1.5 |
| Saline-CFA immunized | 3 | 1.3 ± 0.9 | 3 | 1.5 ± 1.0 | 3 | 1.5 ± 1.0 | 6 | 2.7 ± 1.1 | 3 | 1.3 ± 0.9 | 3 | 1.3 ± 0.9 |
| Saline injected | 22 | 4.4 ± 1.4 | 10 | 3.3 ± 1.5 | 10 | 3.3 ± 1.5 | 2 | 1.1 ± 1.1 | 5 | 2.2 ± 1.4 | 22 | 4.4 ± 1.4 |
All groups had six mice except the Saline-CFA-immunized group, which had five mice. GMT, geometric mean titer.
P, <0.05 compared to value for the respective saline control.
Development of parasite-induced myosin autoimmunity is specific to T. cruzi, and development of myosin-induced T. cruzi-specific immunity is specific to cardiac myosin.
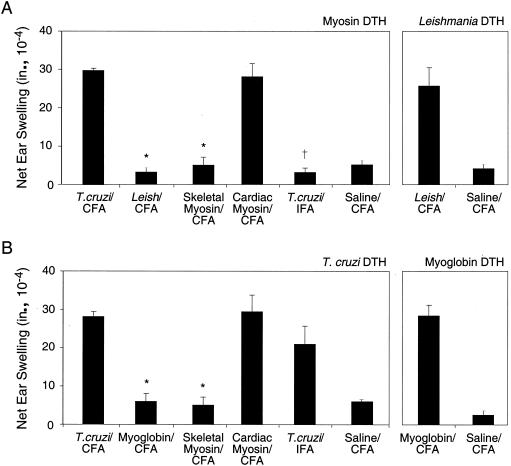
Since we had used only extracts of T. cruzi, it was possible that myosin autoimmunity could result from immunization with any protozoan parasite. We compared the myosin-specific DTH of mice immunized with T. cruzi epimastigotes with that of mice immunized with promastigotes of the related protozoan L. amazonensis. Immunization of mice with T. cruzi, but not L. amazonensis, antigen induced myosin DTH (Fig. 2A) and myosin-specific IgG (Table 2). L. amazonensis-immunized mice did develop Leishmania-specific DTH (Fig. 2A) and Leishmania-specific IgG (data not shown). To set up controls for the test of specificity of cardiac myosin DTH induction, we immunized mice with skeletal and cardiac myosin and found that only cardiac myosin-immunized mice developed cardiac myosin DTH (Fig. 2A). Cardiac myosin-immunized mice also exhibited higher cardiac myosin-specific IgG titers than those of skeletal myosin-immunized mice (Table 2). We then asked whether the presence of the mycobacteria in the form of CFA was required for the induction of myosin autoimmunity after T. cruzi immunization. To address this issue, we immunized mice with the T. cruzi extract emulsified in IFA and found that they did not develop myosin-specific DTH (Fig. 2A) or IgG (Table 2), suggesting that CFA was required for the induction of myosin-specific DTH and IgG.
FIG. 2.
The induction of bidirectional, cross-reactive immunity between T. cruzi and cardiac myosin is specific. A/J mice (five per group) were immunized with T. cruzi lysate in CFA (T. cruzi/CFA), L. amazonensis lysate in CFA (Leish/CFA), cardiac myoglobin in CFA (Myoglobin/CFA), skeletal myosin in CFA (Skeletal Myosin/CFA), cardiac myosin in CFA (Cardiac Myosin/CFA), T. cruzi lysate in IFA (T. cruzi/IFA), and saline in CFA (Saline/CFA). Twenty-one days later, myosin-specific and L. amazonensis-specific DTH (A) and T. cruzi-specific and myoglobin-specific DTH (B) were measured by a 24-h ear swelling assay. *, P of <0.05 compared to values for the cardiac myosin-CFA group. †, P of <0.01 compared to values for the T. cruzi-CFA group. Error bars indicate standard errors of the means.
TABLE 2.
The development of myosin autoantibodies 21 days postimmunization is specific to T. cruzi immunizationa
| Immunization group | Cardiac myosin-specific IgG
|
|
|---|---|---|
| GMT | Log2 ± SEM | |
| T. cruzi-CFA | 200 | 7.6 ± 0.4b |
| Leishmania-CFA | 6 | 2.7 ± 1.6 |
| Skeletal myosin-CFA | 1,902 | 10.9 ± 1.3b |
| Cardiac myosin-CFA | 9,700 | 13.2 ± 0.4b |
| T. cruzi-CFA | 32 | 5.0 ± 1.7 |
| Saline-CFA | 6 | 2.7 ± 1.6 |
All groups had five mice. GMT, geometric mean titer.
P, <0.05 compared to value for the saline-CFA group.
To determine whether the development of T. cruzi-specific immune responses could be achieved by immunization with other antigens, we analyzed T. cruzi DTH and T. cruzi-specific IgG in mice immunized with cardiac myoglobin, skeletal myosin, or cardiac myosin at 21 days postimmunization. Only mice immunized with cardiac myosin developed T. cruzi DTH (Fig. 2B) and T. cruzi-specific IgG (Table 3). Myoglobin-immunized mice did develop myoglobin-specific DTH (Fig. 2B) and IgG (data not shown). Interestingly, mice immunized with T. cruzi in IFA developed lower magnitudes of T. cruzi-specific DTH (Fig. 2B) and IgG (Table 3) than mice immunized with T. cruzi in CFA.
TABLE 3.
The induction of T. cruzi-specific antibodies 21 days postimmunization is specific to immunization with cardiac myosina
| Immunization group |
T. cruzi-specific IgG
|
|
|---|---|---|
| GMT | Log2 ± SEM | |
| T. cruzi antigen-CFA | 172,216 | 17.4 ± 0.5b |
| Myoglobin-CFA | 7 | 2.9 ± 1.8 |
| Skeletal myosin-CFA | 141 | 7.1 ± 0.3 |
| Cardiac myosin-CFA | 348 | 8.4 ± 0.6b |
| T. cruzi antigen-IFA | 89,144 | 16.4 ± 1.5b |
| Saline-CFA | 115 | 6.8 ± 0.2 |
All groups had five mice. GMT, geometric mean titer.
P, <0.05 compared to value for the saline-CFA group.
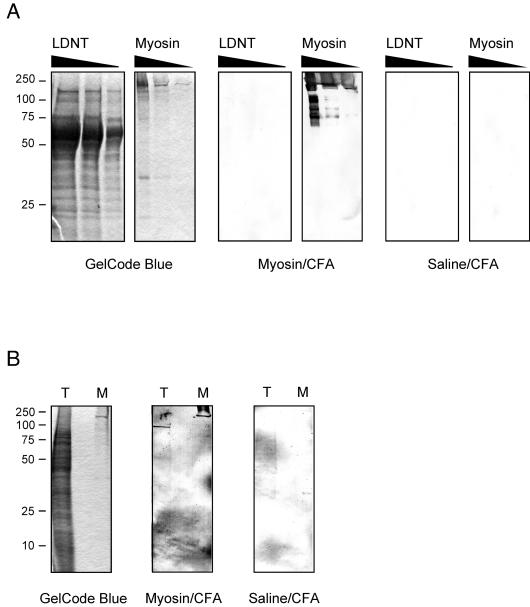
Finally, to eliminate the possibility that the development of myosin DTH was due to myosin contamination from the medium used to grow the parasite, we immunized mice with LDNT antigen as described in Materials and Methods and found that these mice did not develop either myosin-specific DTH or IgG (data not shown). In addition, we analyzed LDNT antigens by Western blotting using sera from myosin-immunized mice and found no IgG reactivity with any protein (Fig. 3A). Interestingly, when we performed the Western blotting of the T. cruzi lysate used in DTH assays with sera from myosin-immunized mice, we found reactivity to a protein migrating between 75 and 100 kDa (Fig. 3B). Sera from some myosin-immunized mice did not detect this protein. Both the LDNT and T. cruzi Western blots did not reveal a protein migrating at around 200 kDa, supporting the idea that the development of myosin DTH by T. cruzi lysate in myosin-immunized mice was not due to a contaminating myosin from the medium used to grow the parasite.
FIG. 3.
IgG antibodies from myosin-immunized mice do not react with medium proteins but do react with proteins in a T. cruzi lysate. Serum samples from five myosin-immunized or saline-immunized mice obtained 21 days postimmunization were pooled for use in a Western blot analysis. (A) LDNT homogenate (40, 20, and 10 μg) and Sigma myosin proteins (30, 3, and 0.3 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the same gel, stained with GelCode Blue or transferred to nitrocellulose, and blotted with a 1:200 dilution of sera from myosin-immunized mice (Myosin/CFA) or saline-immunized mice (Saline/CFA). (B) T. cruzi lysate (T; 10 μg) used for DTH assays and Sigma myosin (M; 0.3 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, stained with GelCode Blue or transferred to nitrocellulose, and blotted with a 1:200 dilution of sera from myosin-immunized mice (Myosin/CFA) or saline-immunized mice (Saline/CFA). The positions of molecular mass standards (in kilodaltons) are indicated at the left.
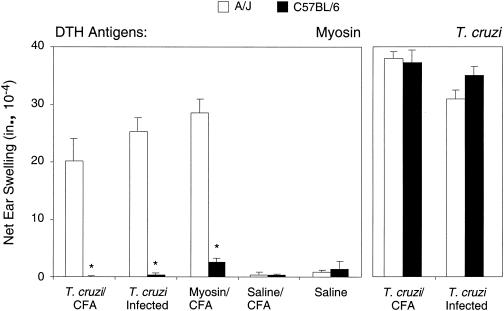
Immunization with T. cruzi induces myosin autoimmunity in susceptible mice.
Cardiac myosin DTH can be induced in A/J mice but not in C57BL/6 mice when the mice are acutely infected with the Brazil strain of T. cruzi or immunized with cardiac myosin (24, 33). We hypothesized that the development of myosin DTH by T. cruzi immunization is likewise restricted by the host strain. A/J mice, but not C57BL/6 mice, developed myosin-specific DTH upon T. cruzi immunization, acute T. cruzi infection, or myosin immunization, confirming this hypothesis (Fig. 4). A/J mice also developed significantly higher levels of myosin-specific IgG than did C57BL/6 mice upon T. cruzi immunization, infection, or myosin immunization (Table 4), which is in agreement with previous findings (24).
FIG. 4.
The induction of myosin-specific DTH upon immunization with T. cruzi lysate is mouse strain specific. A/J and C57BL/6 mice were immunized with T. cruzi lysate in CFA (T. cruzi/CFA), infected with T. cruzi (T. cruzi Infected), immunized with myosin in CFA (Myosin/CFA), immunized with saline in CFA (Saline/CFA), or injected with saline (Saline). Twenty-one days later, myosin-specific DTH and T. cruzi-specific DTH were measured by a 24-h ear swelling assay. *, P of <0.01 compared to values for the A/J group. Error bars indicate standard errors of the means.
TABLE 4.
Myosin antibody titers in A/J and C57BL/6 mice immunized with cardiac myosin, immunized with T. cruzi extract, or infected with T. cruzi 21 days after infection or immunization
| Groupa | A/J mice
|
C57BL/6 mice
|
||
|---|---|---|---|---|
| GMTb | Log2 ± SE | GMT | Log2 ± SE | |
| T. cruzi antigen-CFA immunized | 152 | 7.2 ± 0.2c | 11 | 3.5 ± 1.8 |
| T. cruzi infected | 303 | 8.2 ± 0.3c,d | 115 | 6.8 ± 0.2 |
| Myosin-CFA immunized | 1,600 | 10.6 ± 0.3c,d | 117 | 6.9 ± 1.4 |
| Saline injected | 5 | 2.2 ± 1.4 | 46 | 5.5 ± 1.1 |
All groups had five A/J and five C57BL/6 mice.
GMT, geometric mean titer.
P < 0.05 compared to the value for the respective saline control group.
P < 0.05 compared to the value for the T. cruzi-CFA-immunized group.
Myosin autoimmunity induced by T. cruzi immunization is not associated with myocarditis.
Myosin-immunized and T. cruzi-infected A/J mice develop myosin autoimmunity associated with myocarditis (24). Since the magnitudes of myosin DTH were similar in T. cruzi-immunized and myosin-immunized mice, we examined the hearts of mice 21 days postimmunization with a T. cruzi lysate. These mice did not develop cardiac inflammation or damage as assessed by histopathology or analysis of serum creatine kinase-muscle-brain levels (data not shown), indicating that the mere presence of cardiac myosin autoimmune responses in these mice is not sufficient for the development of cardiac inflammation.
DISCUSSION
It was previously found that A/J mice acutely infected with the Brazil strain of T. cruzi develop acute myocarditis accompanied by strong myosin-specific DTH and IgG production (24). In this study, it was found that myosin-specific autoimmunity could be induced in mice simply by immunization with a protein extract of T. cruzi emulsified in CFA. Since there was no cardiac damage, this autoimmunity seems to result from cross-reactive immunity between parasite antigen(s) and cardiac myosin. This response was specific with respect to parasite antigen (T. cruzi extract versus L. amazonensis extract), host antigen (cardiac myosin versus skeletal myosin or cardiac myoglobin), and host strain (susceptible A/J mice versus resistant C57BL/6 mice) and was bidirectional. Immunization with cardiac myosin led to elaboration of T. cruzi-specific immune responses.
These results indicate that cardiac damage is not required for the induction of myosin autoimmunity and point to T. cruzi-cardiac myosin immunologic cross-reactivity as the basis for the response. Although the issues of specificity and bidirectionality were not addressed as thoroughly as in the present paper, other investigators have reported instances in which immunization with T. cruzi antigens induces autoimmunity. Immunization with T. cruzi ribosomal proteins induces antibodies to mammalian ribosomal proteins (30), immunization with T. cruzi cruzipain induces T cells and autoantibodies specific to skeletal myosin (13) and autoantibodies to cardiac myosin (14), immunization with T. cruzi soluble antigens induces T cells and antibodies against myelin basic protein (2), and immunization of mice with T. cruzi-shed acute-phase protein induces T cells against Cha, a novel mammalian antigen (15). There are also examples from other disease models in which immunization with proteins or lysates from infectious organisms, including Chlamydia trachomatis (42), Helicobacter pylori (3, 31), hepatitis B virus (37), and group A streptococci (34), among others, induces autoimmune responses.
T. cruzi-immunized mice developed myosin-specific DTH and autoantibodies. The induction of myosin autoimmunity was T. cruzi specific because immunization with Leishmania did not induce myosin DTH or IgG (Fig. 2). The magnitudes of myosin DTH were similar for T. cruzi-immunized, myosin-immunized, and T. cruzi-infected mice, suggesting that T-cell tolerance to myosin was effectively overcome in all three groups. On the other hand, myosin-specific IgG levels were weakest in T. cruzi-immunized mice and strongest in myosin-immunized mice (Table 3). We also observed across multiple experiments that some T. cruzi-immunized mice did not produce myosin-specific IgG but that all myosin-immunized and T. cruzi-infected mice produced myosin-specific IgG. These findings suggest that immunization with T. cruzi extract in CFA does not overcome host B-cell tolerance as effectively as does T. cruzi infection or myosin immunization. Interestingly, mice immunized with T. cruzi in IFA failed to develop myosin autoimmunity (Fig. 2A and Table 3). The presence of the mycobacterial antigens may be important for activating the innate immune response and driving the development of a Th1 response to overcome tolerance to cardiac myosin (reviewed in reference 4). The same effect may be achieved during live infection with the parasite, which induces development of a cardiac immune environment rich in proinflammatory cytokines and chemokines (1, 10, 38) and other inflammatory intermediates, as suggested by others (26, 35). We are currently working to identify additional cardiac autoantigens induced by T. cruzi immunization or infection as well as to explore the specific qualitative and quantitative differences in autoimmunity resulting from infection versus immunization.
The induction of cardiac myosin-specific DTH by T. cruzi infection and myosin immunization is dependent on the host strain; A/J mice develop myosin DTH, and C57BL/6 mice do not (24). We found the same pattern in A/J and C57BL/6 mice immunized with T. cruzi proteins. A/J mice also developed significantly higher levels of myosin-specific IgG than did C57BL/6 mice upon T. cruzi immunization, T. cruzi infection, or myosin immunization, in agreement with previous findings (24). The simplest explanation for these results is that there is an immunogenetic component that mediates the induction of DTH and antibodies to self-antigens in different strains of mice. We are further exploring this question by testing other mouse strains for susceptibility and resistance to autoimmunity induced by T. cruzi.
Most interestingly, myosin-immunized mice developed T. cruzi-specific DTH and IgG (Fig. 2B; Table 2), indicating that the cross-reactive immunity is bidirectional. Another group reported that myosin-immunized mice produce antibodies that bind to T. cruzi, as detected by immunofluorescence microscopy (6). In addition, immunization with other host proteins induces T. cruzi-specific immune responses: myelin basic protein immunization induces proliferation of T cells against T. cruzi antigens (2), immunization with a peptide of mammalian Cha induces proliferation of T cells against a peptide from T. cruzi-shed acute-phase protein (15), and immunization with a peptide from mammalian ribosomes induces antibodies against a peptide from T. cruzi ribosomes (30). The induction of T. cruzi responses was specific to cardiac myosin because immunization with cardiac myoglobin or skeletal myosin did not induce T. cruzi responses. This result may be interpreted one of two ways: (i) the cardiac myosin shares peptide epitopes with the T. cruzi antigen while myoglobin and skeletal myosin do not or (ii) the T. cruzi antigen is not immunologically cross-reactive with cardiac myosin but rather to another autoantigen released in myosin-immunized mice. Myocarditis or myositis was not induced in skeletal myosin-immunized or myoglobin-immunized mice. The induction of T. cruzi responses in myosin-immunized mice may occur as a result of cardiac damage or inflammation or other reasons, such as epitope spreading, bystander activation, and cryptic epitope expression (reviewed in reference 23). We are currently attempting to distinguish between these two hypotheses and identify the target host and T. cruzi antigens. A potential antigen under investigation is the antigen from the T. cruzi lysate used in DTH assays that is recognized by sera from myosin-immunized mice (Fig. 3B). The size of this antigen is different from the sizes of other published cross-reactive antigens, including B13 (8), ribosomal proteins (12, 29), Cha (15), cruzipain (13), and Fl-160 (41). We have attempted to do searches for A/J mouse alpha heavy-chain cardiac myosin homologs in T. cruzi DB but have found only unknown-clone sequences with low scores (smallest sum probability, <10−7).
Finally, T. cruzi-immunized mice did not develop myocarditis despite vigorous cardiac myosin-specific T-cell and antibody responses. This finding suggests that the development of myosin autoimmunity does not necessarily lead to cardiac inflammation. In the CHD field, it is important to stress the difference between autoimmune responses and pathogenic autoimmunity because, as our results show, the presence of strong cellular autoimmunity does not necessarily imply pathogenicity. This point is of particular significance to groups researching autoimmunity in CHD. Other groups have reported that immunization with T. cruzi proteins, including T. cruzi ribosomal proteins (30) and cruzipain (14), induces cardiac alterations. We are currently varying our immunization regimen to test whether the additional stimulus provided by live infection can be afforded by addition of immune stimulants such as interleukin-1, tumor necrosis factor alpha, and lipopolysaccharide. It is notable that inclusion of each of these with coxsackieviral infection of normally resistant mice promoted cardiac inflammation (19, 20).
In conclusion, we have shown that immunization with T. cruzi antigens induced cardiac myosin autoimmunity in the absence of cardiac pathology. We have also found that induction of peripheral immune tolerance to either cardiac myosin or T. cruzi decreases the DTH response to both antigens (25). This method, which involves administration of antigen-coupled syngeneic splenocytes, is effective at preventing myosin-induced myocarditis (16, 25). The simplest explanation for these findings is that of molecular mimicry between T. cruzi and cardiac antigens (reviewed in references 23 and 35). However, we have not directly proven the molecular mimicry hypothesis (i.e., a single cell or antibody binds to both a host epitope and a parasite epitope) because we have not demonstrated it at the level of a single T cell or B cell. The molecular mimicry hypothesis needs to be proven through the identification of T-cell clones or monoclonal antibodies that react with epitopes of both parasite and heart antigens. What does all this mean for live T. cruzi infection? While cardiac damage may be sufficient to induce an autoimmune response in a susceptible host like the A/J mouse, an immune response to T. cruzi antigens may also induce an autoimmune response, provided that the right inflammatory environment exists. The major question that remains, not only for T. cruzi infection but also for other infection-induced inflammatory diseases exhibiting autoimmune responses, is What are the relative contributions of pathogen-directed immunity, autoimmune responses, and other mechanisms to tissue damage and inflammation?
Acknowledgments
We thank A. W. Rademaker for advice on statistical analysis and S. J. Anderson, W. J. Karpus, and S. D. Miller and members of their laboratories for advice and guidance throughout this project.
This work was supported in part by grants from the U.S. Public Health Service. J. S. Leon was supported by a predoctoral fellowship from the American Heart Association, Midwest Affiliate. D. M. Engman is an Established Investigator of the American Heart Association.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aliberti, J. C., J. T. Souto, A. P. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 158:1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sabbagh, A., C. A. Garcia, B. M. Diaz-Bardales, C. Zaccarias, J. K. Sakurada, and L. M. Santos. 1998. Evidence for cross-reactivity between antigen derived from Trypanosoma cruzi and myelin basic protein in experimental Chagas disease. Exp. Parasitol. 89:304-311. [DOI] [PubMed] [Google Scholar]
- 3.Appelmelk, B. J., I. Simoons-Smit, R. Negrini, A. P. Moran, G. O. Aspinall, J. G. Forte, T. De Vries, H. Quan, T. Verboom, J. J. Maaskant, P. Ghiara, E. J. Kuipers, E. Bloemena, T. M. Tadema, R. R. Townsend, K. Tyagarajan, J. M. Crothers, Jr., M. A. Monteiro, A. Savio, and J. De Graaff. 1996. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect. Immun. 64:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billiau, A., and P. Matthys. 2001. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 70:849-860. [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chambo, J. G., P. M. Cabeza Meckert, and R. P. Laguens. 1990. Presence of anti-Trypanosoma cruzi antibodies in the sera of mice with experimental autoimmune myocarditis. Experientia 46:977-999. [DOI] [PubMed] [Google Scholar]
- 7.Cunha-Neto, E., V. Coelho, L. Guilherme, A. Fiorelli, N. Stolf, and J. Kalil. 1996. Autoimmunity in Chagas' disease: identification of cardiac myosin-B13 Trypanosoma cruzi protein crossreactive T cell clones in heart lesions of a chronic Chagas' cardiomyopathy patient. J. Clin. Investig. 98:1709-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha-Neto, E., M. Duranti, A. Gruber, B. Zingales, I. de Messias, N. Stolf, G. Bellotti, M. E. Patarroyo, F. Pilleggi, and J. Kalil. 1995. Autoimmunity in Chagas' disease cardiomyopathy: biological relevance of a cardiac myosin-specific epitope crossreactive to an immunodominant Trypanosoma cruzi antigen. Proc. Natl. Acad. Sci. USA 92:3541-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Scheerder, I. K., M. L. de Buyzere, J. R. Delanghe, D. L. Clement, and R. J. Wieme. 1989. Anti-myosin humoral immune response following cardiac injury. Autoimmunity 4:51-58. [DOI] [PubMed] [Google Scholar]
- 10.dos Santos, P. V., E. Roffe, H. C. Santiago, R. A. Torres, A. P. Marino, C. N. Paiva, A. A. Silva, R. T. Gazzinelli, and J. Lannes-Vieira. 2001. Prevalence of CD8(+)alpha beta T cells in Trypanosoma cruzi-elicited myocarditis is associated with acquisition of CD62L(Low)LFA-1(High)VLA-4(High) activation phenotype and expression of IFN-gamma-inducible adhesion and chemoattractant molecules. Microbes Infect. 3:971-984. [DOI] [PubMed] [Google Scholar]
- 11.Fedoseyeva, E. V., F. Zhang, P. L. Orr, D. Levin, H. J. Buncke, and G. Benichou. 1999. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J. Immunol. 162:6836-6842. [PubMed] [Google Scholar]
- 12.Ferrari, I., M. J. Levin, G. Wallukat, R. Elies, D. Lebesgue, P. Chiale, M. Elizari, M. Rosenbaum, and J. Hoebeke. 1995. Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human beta 1-adrenergic receptor. J. Exp. Med. 182:59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giordanengo, L., R. Fretes, H. Diaz, R. Cano, A. Bacile, E. Vottero-Cima, and S. Gea. 2000. Cruzipain induces autoimmune response against skeletal muscle and tissue damage in mice. Muscle Nerve 23:1407-1413. [DOI] [PubMed] [Google Scholar]
- 14.Giordanengo, L., C. Maldonado, H. W. Rivarola, D. Iosa, N. Girones, M. Fresno, and S. Gea. 2000. Induction of antibodies reactive to cardiac myosin and development of heart alterations in cruzipain-immunized mice and their offspring. Eur. J. Immunol. 30:3181-3189. [DOI] [PubMed] [Google Scholar]
- 15.Girones, N., C. I. Rodriguez, E. Carrasco-Marin, R. F. Hernaez, J. L. de Rego, and M. Fresno. 2001. Dominant T- and B-cell epitopes in an autoantigen linked to Chagas' disease. J. Clin. Investig. 107:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godsel, L. M., K. Wang, B. A. Schodin, J. S. Leon, S. D. Miller, and D. M. Engman. 2001. Prevention of autoimmune myocarditis through the induction of antigen-specific peripheral immune tolerance. Circulation 103:1709-1714. [DOI] [PubMed] [Google Scholar]
- 17.Kierszenbaum, F. 1999. Chagas' disease and the autoimmunity hypothesis. Clin. Microbiol. Rev. 12:210-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhoff, L. V., and F. A. Neva. 1985. Chagas' disease in Latin American immigrants. JAMA 254:3058-3060. [PubMed] [Google Scholar]
- 19.Lane, J. R., D. A. Neumann, A. Lafond-Walker, A. Herskowitz, and N. R. Rose. 1992. Interleukin 1 or tumor necrosis factor can promote Coxsackie B3-induced myocarditis in resistant B10. A mice. J. Exp. Med. 175:1123-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane, J. R., D. A. Neumann, A. Lafond-Walker, A. Herskowitz, and N. R. Rose. 1991. LPS promotes CB3-induced myocarditis in resistant B10. A mice. Cell. Immunol. 136:219-233. [DOI] [PubMed] [Google Scholar]
- 21.Leon, J. S., and D. M. Engman. 2001. Autoimmunity in Chagas heart disease. Int. J. Parasitol. 31:554-560. [DOI] [PubMed] [Google Scholar]
- 22.Leon, J. S., and D. M. Engman. 2003. The contribution of autoimmunity to Chagas disease?, p. 97-106. In K. M. Tyler and M. A. Miles (ed.), World class parasites: American trypanosomiasis, vol. 7. Kluwer Academic Publishers, Boston, Mass.
- 23.Leon, J. S., and D. M. Engman. 2003. The significance of autoimmunity in the pathogenesis of Chagas heart disease. Front. Biosci. 8:315-322. [DOI] [PubMed] [Google Scholar]
- 24.Leon, J. S., L. M. Godsel, K. Wang, and D. M. Engman. 2001. Cardiac myosin autoimmunity in acute Chagas' heart disease. Infect. Immun. 69:5643-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leon, J. S., K. Wang, and D. M. Engman. 2003. Myosin autoimmunity is not essential for cardiac inflammation in acute Chagas' disease. J. Immunol. 171:4271-4277. [DOI] [PubMed] [Google Scholar]
- 26.Malkiel, S., A. P. Kuan, and B. Diamond. 1996. Autoimmunity in heart disease: mechanisms and genetic susceptibility. Mol. Med. Today 2:336-342. [DOI] [PubMed] [Google Scholar]
- 27.McGwire, B. S., W. A. O'Connell, K. P. Chang, and D. M. Engman. 2002. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J. Biol. Chem. 277:8802-8809. [DOI] [PubMed] [Google Scholar]
- 28.Moncayo, A. 1999. Progress towards interruption of transmission of Chagas disease. Mem. Inst. Oswaldo Cruz 94(Suppl. 1):401-404. [DOI] [PubMed] [Google Scholar]
- 29.Motran, C. C., F. M. Cerban, W. Rivarola, D. Iosa, and E. Vottero de Cima. 1998. Trypanosoma cruzi: immune response and functional heart damage induced in mice by the main linear B-cell epitope of parasite ribosomal P proteins. Exp. Parasitol. 88:223-230. [DOI] [PubMed] [Google Scholar]
- 30.Motran, C. C., R. E. Fretes, F. M. Cerban, H. W. Rivarola, and E. Vottero de Cima. 2000. Immunization with the C-terminal region of Trypanosoma cruzi ribosomal P1 and P2 proteins induces long-term duration cross-reactive antibodies with heart functional and structural alterations in young and aged mice. Clin. Immunol. 97:89-94. [DOI] [PubMed] [Google Scholar]
- 31.Negrini, R., A. Savio, C. Poiesi, B. J. Appelmelk, F. Buffoli, A. Paterlini, P. Cesari, M. Graffeo, D. Vaira, and G. Franzin. 1996. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology 111:655-665. [DOI] [PubMed] [Google Scholar]
- 32.Neu, N., K. W. Beisel, M. D. Traystman, N. R. Rose, and S. W. Craig. 1987. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3-induced myocarditis. J. Immunol. 138:2488-2492. [PubMed] [Google Scholar]
- 33.Neu, N., N. R. Rose, K. W. Beisel, A. Herskowitz, G. Gurri-Glass, and S. W. Craig. 1987. Cardiac myosin induces myocarditis in genetically predisposed mice. J. Immunol. 139:3630-3636. [PubMed] [Google Scholar]
- 34.Quinn, A., S. Kosanke, V. A. Fischetti, S. M. Factor, and M. W. Cunningham. 2001. Induction of autoimmune valvular heart disease by recombinant streptococcal M protein. Infect. Immun. 69:4072-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose, N. R. 2001. Infection, mimics, and autoimmune disease. J. Clin. Investig. 107:943-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiverick, K. T., L. L. Thomas, and N. R. Alpert. 1975. Purification of cardiac myosin: application to hypertrophied myocardium. Biochim. Biophys. Acta 393:124-133. [DOI] [PubMed] [Google Scholar]
- 37.Singh, V. K., H. K. Kalra, K. Yamaki, T. Abe, L. A. Donoso, and T. Shinohara. 1990. Molecular mimicry between a uveitopathogenic site of S-antigen and viral peptides. Induction of experimental autoimmune uveitis in Lewis rats. J. Immunol. 144:1282-1287. [PubMed] [Google Scholar]
- 38.Talvani, A., C. S. Ribeiro, J. C. Aliberti, V. Michailowsky, P. V. Santos, S. M. Murta, A. J. Romanha, I. C. Almeida, J. Farber, J. Lannes-Vieira, J. S. Silva, and R. T. Gazzinelli. 2000. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-gamma as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes Infect. 2:851-866. [DOI] [PubMed] [Google Scholar]
- 39.Tanowitz, H. B., L. V. Kirchhoff, D. Simon, S. A. Morris, L. M. Weiss, and M. Wittner. 1992. Chagas' disease. Clin. Microbiol. Rev. 5:400-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarleton, R. L. 2001. Parasite persistence in the aetiology of Chagas disease. Int. J. Parasitol. 31:550-554. [DOI] [PubMed] [Google Scholar]
- 41.Van Voorhis, W. C., and H. Eisen. 1989. Fl-160. A surface antigen of Trypanosoma cruzi that mimics mammalian nervous tissue. J. Exp. Med. 169:641-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi, Y., X. Yang, and R. C. Brunham. 1997. Autoimmunity to heat shock protein 60 and antigen-specific production of interleukin-10. Infect. Immun. 65:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]