Abstract
The early growth response 1 (Egr-1) transcription factor is rapidly induced by various stimuli and is implicated in the regulation of cell growth, differentiation, and gene expression. The aim of this study was to examine the effect of Helicobacter pylori on the expression of Egr-1 and Egr-1-regulated genes in gastric epithelial AGS cells. Egr-1 expression was assayed by immunoblotting and electrophoretic mobility shift assays using H. pylori-stimulated AGS cells. Transient transfection experiments with promoter-reporter constructs of CD44, ICAM-1, and CD95L were used for expression studies. H. pylori induced the expression of Egr-1 in gastric epithelial cell lines in a dose-dependent manner, with the rapid kinetics that are typical of this class of transcription factors. Immunohistochemical studies of biopsies revealed that Egr-1 expression is more abundant in H. pylori-positive patients than in uninfected individuals. Reporter-promoter transfection studies indicated that Egr-1 binding is required for the H. pylori-induced transcriptional promoter activity of the CD44, ICAM-1, and CD95L (APO-1/Fas) constructs. The blocking of egr-1 with an antisense sequence prevented H. pylori-induced Egr-1 and CD44 protein expression. The MEK1/2 signaling cascade participates in H. pylori-mediated Egr-1 expression, but the p38 pathway does not. The data indicate that H. pylori induces Egr-1 expression in AGS cells in vitro and that the Egr-1 protein is readily detectable in biopsies from H. pylori-positive subjects. These observations suggest that H. pylori-associated Egr-1 expression may play a role, in part, in H. pylori-induced pathology.
Helicobacter pylori is recognized as the major cause of gastritis and duodenal ulceration and as a dominant factor in gastric cancer (28, 36, 39). The exposure of gastric epithelial cells to H. pylori results in the activation of multiple factors, including NF-κB (33), interleukin-8 (IL-8) (43), activator protein 1 (AP-1), and mitogen-activated protein kinases (35), and up-regulates CD44 and ICAM-1 expression (12, 13). However, the exact mechanisms by which H. pylori causes chronic inflammation and gastric pathology remain to be elucidated.
Evidence from recent studies suggests that the early growth response 1 (Egr-1) protein is involved in the regulation of inflammatory and immune responses (14, 30). Egr-1 is an ∼84-kDa protein that binds to GC-rich motifs (32, 46, 47) and is rapidly and transiently induced by a variety of growth factors, cytokines (3, 30), and other stimuli (16, 41, 50) in many cell types (15, 42, 45), and its expression is modulated by the redox environment (11, 20, 21, 37). Egr-1 regulates the tissue-specific expression of growth and coagulation factors, cell surface adhesion molecules, and proteins that can alter cell survival (41, 44). Egr-1 functions as an activator of ICAM-1 and CD44 (14, 26) and as a repressor of the CD23 and Fas/CD95 genes (8). Significantly, CD44 and ICAM-1 are up-regulated on epithelial cells and T cells from H. pylori-infected individuals (12, 13).
The aim of this study was to determine the effect of H. pylori on Egr-1 expression in gastric epithelial cells. We demonstrate here that H. pylori potently induces Egr-1 expression in gastric and colonic cell lines. Also, we show a direct effect of Egr-1 on the regulation of H. pylori-induced expression of CD44, ICAM-1, and CD95L (APO-1/Fas) by using promoter-reporter constructs. As many studies now implicate Egr-1 in pathogenesis, we discuss the ways that this factor may contribute to H. pylori-induced pathology.
MATERIALS AND METHODS
Materials.
The human gastric cancer cell line AGS was obtained from the European Collection of Animal Cell Cultures (Porton Down, Salisbury, United Kingdom). The cell lines KATO-3, HT29, and T84 were obtained from the American Type Culture Collection (Manassas, Va.). All tissue culture media and reagents were obtained from GIBCO BRL (Life Technologies Renfrewshire, Paisley, Scotland). An Egr-1 consensus oligonucleotide and a polyclonal antibody to Egr-1 (588) for use in gel supershift assays were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Poly(dI-dC) was obtained from Pharmacia Biosystems (Milton Keynes, United Kingdom). [γ-32P]ATP (35 pmol; 3,000 Ci/mmol) and d-threo-[dichloroacetyl-1-14C]chloramphenicol (56 mCi/mmol) were from Amersham International (Aylesbury, United Kingdom). The luciferase assay system used was from Promega Inc. (Madison, Wis.). The p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 and the MEK1/2 MAPK inhibitors PD98059 and U0126 were purchased from Calbiochem, Novabiochem Corp., La Jolla, Calif. All other reagents were from Sigma (Poole, Dorset, United Kingdom) unless indicated otherwise in the text.
Cell culture conditions.
AGS cells were grown in RPMI 1640 medium supplemented with 10% (vol/vol) fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM l-glutamine at 37°C in an atmosphere containing 5% CO2. For experiments, AGS cells were seeded at a density of 5 × 105 cells/ml of medium in six-well plates and grown to ∼80% confluence prior to experiments. For some experiments, KATO-3 cells and HT29 cells were grown in RPMI 1640 medium and T84 cells were grown in Dulbecco's modified Eagle medium (DMEM)-Ham’s nutrient mixture-F-12 medium (1:1) and used under similar conditions.
Bacterial strains and growth conditions.
H. pylori reference strains NCTC 11638 and NCTC 11637 (both VacA+ and CagA+) were obtained from the National Collection of Type Cultures, London, United Kingdom. H. pylori strain 60190 (ATCC 49503), which is a CagA+ VacA+ toxigenic strain, its CagA− VacA+ toxigenic isogenic mutant, strain 84-183 (ATCC 53726), which is a CagA+ toxigenic strain, and its VacA− CagA+ nontoxigenic isogenic mutant were kindly provided by J. C. Atherton (Department of Gastroenterology, Nottingham, United Kingdom) and R. Peek (Vanderbilt University School of Medicine, Nashville, Tenn.). The construction of the above mutants has been described in the literature (5, 23). The strains were grown in a microaerobic humidified atmosphere on 7% lysed horse blood Columbia agar at 37°C. After 48 to 72 h, bacteria were harvested in phosphate-buffered saline (PBS) (pH 7.4) containing 8 mM Na2HPO4, 1.5 mM KH2PO4, 137 mM NaCl, and 2.7 mM KCl or in RPMI 1640 medium without antibiotics, resuspended to a concentration of 6 × 108 CFU/ml by using a McFarland standard kit, and used immediately.
Coculturing of AGS cells with bacteria.
Subconfluent AGS cells were cultured alone or with various doses of freshly harvested H. pylori (1 × 104 to 6 × 108 CFU/ml) for various periods of time or were exposed to IL-1β (20 ng/ml) or tumor necrosis factor alpha (TNF-α) (20 ng/ml). For some experiments, Escherichia coli (C-600) and Campylobacter jejuni (clinical isolate) were used as control bacteria. E. coli (C-600) and C. jejuni were harvested as described for H. pylori and incubated with AGS cells at a concentration of 6 × 108 CFU/ml (estimated by the use of McFarland standards). At the end of treatment, AGS cells were harvested and processed for the preparation of whole-cell extracts and Western blotting. In other experiments, AGS cells were cultured for 2 h in the presence of purified H. pylori lipopolysaccharide (LPS) (15 μg/ml) obtained from four different strains (442, 446, M091, and NCTC 11637) or in the presence of E. coli LPS (15 mg/ml; Sigma). Purified H. pylori LPS (from strains 442, 446, M091, and NCTC 11637) was kindly provided by Anthony P. Moran, Department of Microbiology, National University of Ireland, Galway, Ireland.
Preparation of whole-cell extracts.
AGS cells were harvested by scraping and were subsequently washed in ice-cold PBS and collected by centrifugation (1,400 rpm, 5 min; Beckman Allegra 6KR centrifuge with GH-3.8 rotor). The pellet of cells was resuspended in lysis buffer containing 50 mM Tris (pH 6.8), 2% (wt/vol) sodium dodecyl sulfate, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), leupeptin (10 μg/ml), 5% (vol/vol) 2-mercaptoethanol, 0.1% (vol/vol) bromophenol blue, and 10% (wt/vol) glycerol and then solubilized by boiling for 5 min.
Western blot analysis.
Equivalent amounts of total cell extracts (50 μg of protein/lane) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis essentially as described by Laemmli (24). Proteins were electrotransferred onto polyvinylidene difluoride membranes in a semidry blotting apparatus (Atto). Blots were blocked with 5% (wt/vol) dried skim milk in PBS for 1 h at room temperature (RT) and then incubated for 1 h at RT with a specific polyclonal rabbit anti-human Egr-1 antiserum (diluted 1/1,000). Blots were then incubated with a horseradish peroxidase-conjugated anti-rabbit secondary antibody (diluted 1/1,000) for 1 h at RT. Immunodetection was performed by enhanced chemiluminescence.
Nuclear extract preparation.
Nuclear extracts were prepared from AGS cells as previously described (38). Briefly, the cells were washed twice in ice-cold PBS, harvested by scraping, and transferred to centrifuge tubes on ice. The cells were pelleted by centrifugation at 1,400 rpm for 5 min, washed once in 1 ml of buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM PMSF, and 0.5 mM dithiothreitol), and centrifuged (10,000 rpm, 10 min). The pellet of cells was resuspended in buffer A (20 μl) containing 0.1% (vol/vol) NP-40 and then incubated for 10 min on ice. Lysed cells were centrifuged (10,000 rpm, 10 min). The nuclear pellet was extracted with 15 μl of buffer C (20 mM HEPES [pH 7.9], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% [wt/vol] glycerol, and 0.5 mM PMSF) for 15 min on ice. After incubation, the nuclei were centrifuged (10,000 rpm, 10 min) and the supernatant was diluted with 4 volumes of buffer D (10 mM HEPES [pH 7.9], 50 mM KCl, 0.2 mM EDTA, 25% [wt/vol] glycerol, and 0.5 mM PMSF). The nuclear extracts were used immediately or stored at −70°C until required. The protein concentrations of nuclear extracts were determined by the dye-binding method of Bradford (2).
Electrophoretic mobility shift assays (EMSAs).
For binding assays, nuclear extracts (4 μg of protein) were incubated with 10,000 cpm of an oligonucleotide (27 bp) containing the consensus sequence of the Egr-1 site (5′-GGA TCC AGC GGG GGG GAG CGG GGG CGA-3′) that had been previously labeled with [γ-32P]ATP (10 mCi/mmol) at the 5′ ends with T4 polynucleotide kinase. The assay was performed in 20 μl of binding buffer (10 mM Tris-HCl [pH 7.5], 4% [wt/vol] glycerol, 5 mM dithiothreitol, 1 mM EDTA, 100 mM NaCl, and 0.1 mg of nuclease-free bovine serum albumin/ml) in the presence of 2 μg of poly(dI-dC) as a nonspecific competitor. The reaction mixture was then incubated for 30 min at RT after the addition of the probe DNA. The binding reaction was terminated with loading dye (0.25% bromophenol blue, 0.25% xylene cyanol, and 30% [wt/vol] glycerol in deionized water) prior to electrophoretic separation of the DNA-protein complexes in 5% polyacrylamide gels that had been pre-electrophoresed for 30 min at 80 V. Gels were run at 150 V for 1 to 2 h at RT. After electrophoresis, the gels were dried and autoradiographed at −70°C for 24 to 36 h with intensifying screens.
Immunohistochemical staining studies of Egr-1 expression in gastric biopsies.
The expression of Egr-1 was examined with a polyclonal antibody to Egr-1 by immunohistochemistry of formalin-fixed, paraffin-embedded antral specimens that had been obtained from patients attending the gastroenterology clinic at St. James's Hospital, Dublin, Ireland. Five biopsies with normal antral-type mucosa, 10 biopsies from H. pylori-negative patients with mild chronic gastritis, and 15 biopsies from H. pylori-positive patients with moderate chronic inflammation and focal acute inflammation were studied. The status of H. pylori infection was examined by both histology and a rapid urease test. Patients were considered to be infected with H. pylori if the histology showed characteristic organisms and the Clotest (Ballard Medical Products, Draper, Utah) rapid urease test was positive. Biopsies were snap frozen and used for immunohistochemical studies. Patient groups were classified as follows: normal antral-type mucosa, H. pylori-negative mild gastritis, and H. pylori-positive moderate chronic inflammation with focal acute inflammation.
Specimens (4-μm thick) were deparaffinized and rehydrated through a series of alcohol washes and then incubated with an Egr-1 polyclonal antibody (1:50) overnight at 4°C. Peroxidase-conjugated swine anti-rabbit immunoglobulin G (IgG) (1:50) was used as a secondary antibody and diaminobenzidine tetrachloride was used as the substrate. Bound anti-Egr-1 polyclonal antibody was visualized by the avidin-biotin-peroxidase method (19) by using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.). Counterstaining was performed with Mayer's hematoxylin and mounting was done with Permount. An appropriate polyclonal rabbit Ig control antibody (10 μg/ml) (Z147; Dako) was used for control experiments.
DNA constructs.
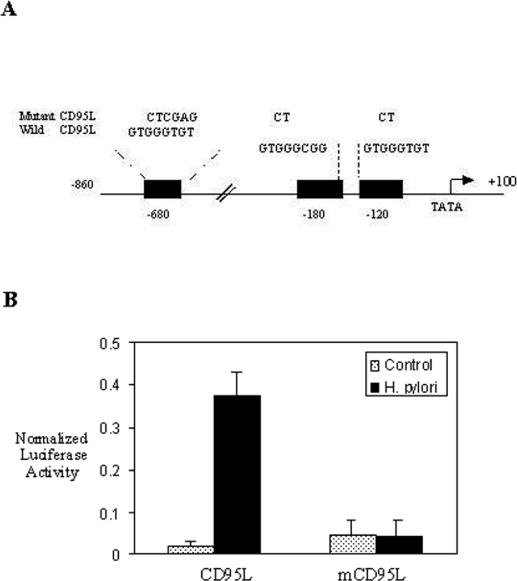
The chloramphenicol acetyltransferase (CAT) promoter plasmid pRb containing 1.7 kb of the CD44 upstream regulatory region was a generous gift from Emma Shtivelman (University of California, San Francisco). John Monroe (Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia) kindly provided the pCAT reporter plasmid containing 1.1 kb of the murine ICAM-1 upstream regulatory region and constructs pBLCD44, pBLmCD44, pBLICAM-1, and pBLmICAM-1. Luciferase reporter plasmids containing the human CD95L promoter spanning the region from −860 to +100 and a mutant CD95L promoter with point mutations at the −680, −180, and −120 Egr-1 sites were previously described (25).
Transient transfection.
AGS cells were seeded in 24-well plates at a density of 5 × 105 cells/ml of medium and grown to ∼80% confluence. The cells were transfected with 1 μg of a plasmid construct carrying the CAT reporter gene (pCD44, pBLCD44, pBLmCD44, pICAM-1, pBLICAM-1, or pBLmICAM-1) by using the GenePORTER transfection reagent (Gene Therapy Systems Inc., San Diego, Calif.) according to the manufacturer's instructions. A transient transfection was also performed with 1 μg of a plasmid carrying the luciferase reporter gene under the control of the wild-type or mutant CD95L promoter. Transfected cells were allowed to recover overnight and then incubated for 24 h in the absence or presence of H. pylori. After stimulation, cell lysates were prepared. For measurements of transfection efficiency, 1 μg of pSV-β-galactosidase control vector (Promega) was simultaneously transfected into the cells and the β-galactosidase activity was measured.
CAT assay.
AGS cells were washed twice with PBS, collected by scraping, suspended in 100 μl of 0.25 M Tris-HCl (pH 8.0), and lysed by four repeated freeze-thaw cycles. The CAT activity was determined as described previously (14). Briefly, equal amounts of protein from different cell extracts were incubated with 1 mM acetyl coenzyme A and 0.3 μCi of d-threo-[dichloroacetyl-1-14C]chloramphenicol (56 mCi/mmol) in a final volume of 91.5 μl overnight at 37°C. The reaction was terminated by the addition of 350 μl of ethyl acetate and samples were vortexed for 30 s. The samples were then centrifuged (12,000 × g, 1 min), and the upper phase was removed and dried in a vacuum. The pellet was resuspended in 12 μl of ethyl acetate and resolved on a silica thin-layer chromatography plate (0.2-mm thickness) in chloroform-methanol (19:1 [vol/vol]). The plate was dried and autoradiographed to locate the acetylated and nonacetylated species of [14C]chloramphenicol, followed by quantification in a Wallac 1409 DSA liquid scintillation counter.
Luciferase reporter gene assay.
After being stimulated, cells were washed twice with PBS after the medium was discarded by aspiration and were lysed by incubation in 120 μl of 1× cell lysis buffer (Promega) at RT for 15 min. The lysates were cleared by centrifugation (12,000 × g, 5 min). The luciferase activity in cell lysates (20 μl) was determined by using a luciferase assay reagent (Promega), and light emission was measured immediately with a luminometer (Mediators PhL, version 1.6; Diagnostic Systems). The luciferase activity of each sample was normalized for β-galactosidase activity and the results are presented as means ± standard deviations (SD) for three independent experiments.
Antisense Egr-1 oligonucleotides.
An antisense oligonucleotide plasmid was designed and prepared as described previously (9). The insert of the antisense oligonucleotide directed against nucleotides 131 to 149 and 2115 to 2131 of human Egr-1 was subcloned into pcDNA3.1+/zeo at the HindIII and XbaI sites. For control experiments, a sense oligonucleotide with a similar sequence, but in the opposite orientation relative to the promoter, was used. AGS cells were seeded in 24-well plates at a density of 5 × 105 cells/ml of medium and were grown to ∼80% confluence. The cells were transfected with various doses of the antisense Egr-1 oligonucleotide or with the sense Egr-1 oligonucleotide by use of GenePORTER. The transfected cells were allowed to recover overnight and then were incubated for 8 h in the absence or presence of H. pylori (6 × 108 CFU/ml). After stimulation, total cell lysates were prepared and analyzed for Egr-1 protein levels by Western blotting, or the cells were processed for flow cytometric analysis. The primary MAb, an anti-IE (anti-mouse major histocompatibility complex from the American Type Culture Collection [HB 179]) isotype control (10 μl) or an anti-CD44 MAb (D2.1 ascites; 1/100), was incubated with an appropriate aliquot of cells (5 × 105 cells) for 15 min. A fluorescein isothiocyanate (FITC) conjugate (1/50) was incubated with the cells for 10 min, followed by washing and fixation in 0.5% paraformaldehyde. Labeled cells were acquired with a Becton Dickinson FACScan instrument and analyzed with Lysys II software. One microgram of a pSV-β-galactosidase control vector (Promega) was simultaneously transfected as a control for transfection efficiency.
RESULTS
H. pylori activates expression of Egr-1.
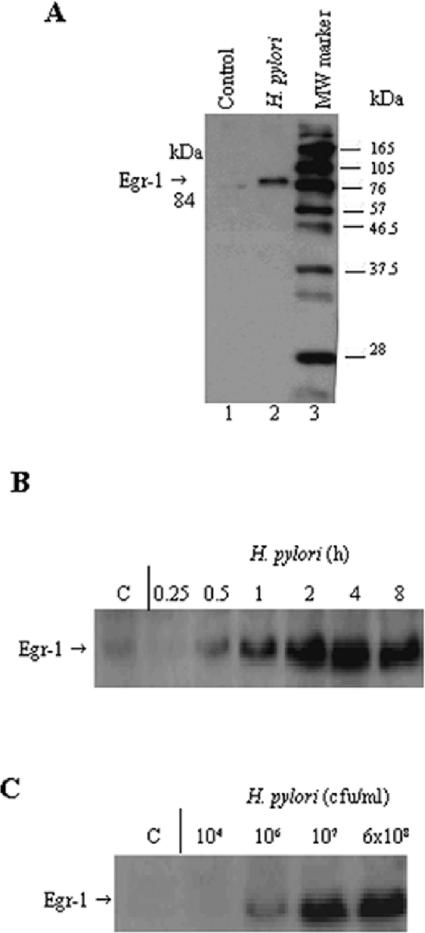
To examine whether Egr-1 expression could be induced by H. pylori, we treated AGS cells with a freshly harvested suspension of H. pylori strains NCTC 11638 and NCTC 11637 (6 × 108 CFU/ml), prepared total cell extracts, and subjected them to Western blot analysis. Figure 1A shows the induction of the 84-kDa Egr-1 protein in AGS cells upon exposure to H. pylori. A transient increase in Egr-1 protein levels was observed in AGS cells cocultured with H. pylori compared to control cells. The induction of Egr-1 expression by H. pylori was observed as early as 30 min after infection, with a significant level of induction seen at 2 h. Egr-1 protein levels were still detected up to 8 h after infection (Fig. 1B). The expression of Egr-1 exhibited a dose-dependent response to H. pylori (Fig. 1C). Moreover, viable H. pylori organisms were required for the induction of Egr-1 expression, as both heat-killed and sonicated preparations of H. pylori did not have an effect (not shown).
FIG. 1.
H. pylori activates Egr-1 expression in AGS cells. (A) Induction of the 84-kDa Egr-1 protein in AGS cells by H. pylori (lane 2). Lane 1, control, untreated cells. MW marker, molecular weight marker. (B) Time course of Egr-1 expression in H. pylori-treated AGS cells. AGS cells were treated with H. pylori (6 × 108 CFU/ml) for various periods of time (as indicated above each lane). (C) Dose response of Egr-1 activation by H. pylori in AGS cells. The cells were treated with different amounts of H. pylori (1 × 104 to 6 × 108 CFU/ml) for 2 h. A Western blot analysis of Egr-1 protein expression was performed on total cell extracts (50 μg of protein/lane). Experiments were performed at least three times with similar results, and a representative experiment is shown.
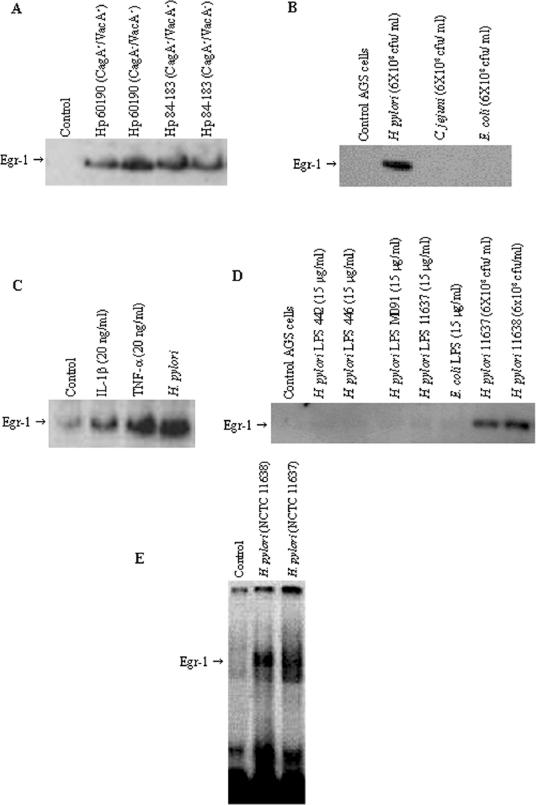
We further examined the effect of H. pylori isogenic mutants that were deficient in CagA or VacA on Egr-1 expression. There were no significant differences in Egr-1 protein levels in AGS cells exposed to isogenic CagA or VacA mutants derived from strains 60190 and 84-183 (Fig. 2A). The activation of Egr-1 appeared to be induced specifically by H. pylori, as no induction of Egr-1 expression was observed when gastric epithelial cells were cocultured with either E. coli or C. jejuni under identical conditions (Fig. 2B).
FIG. 2.
(A) Effect of H. pylori isogenic mutant strains on Egr-1 expression. AGS cells were incubated for 2 h with CagA+ toxigenic H. pylori strain 60190, its CagA− toxigenic isogenic mutant, CagA+ toxigenic strain 84-183, and its CagA+ VacA− nontoxigenic isogenic mutant. (B) Effect of other bacteria on Egr-1 expression. C. jejuni. E. coli, and H. pylori (NCTC 11638) were cocultured (6 × 108 CFU/ml) with subconfluent AGS cells for 2 h. A Western blot analysis of Egr-1 protein was performed on total cell extracts (50 μg of protein/lane). (C) IL-1β and TNF-α activate Egr-1 expression in AGS cells. AGS cells were treated with IL-1β (20 ng/ml), TNF-α (20 ng/ml), or H. pylori NCTC 11638 (6 × 108 CFU/ml) for 2 h. (D) Effect of H. pylori LPS on Egr-1 expression. AGS cells were incubated with H. pylori LPS (from strains 442, 446, M091, and NCTC 11637) or E. coli LPS at a concentration of 15 μg/ml or with live H. pylori bacteria (NCTC 11637 and NCTC 11638) at 6 × 108 CFU/ml for 2 h. A Western blot analysis of Egr-1 protein was performed on total cell extracts (50 μg of protein/lane). (E) EMSA analysis of Egr-1 activation by H. pylori in AGS cells. AGS cells were incubated with a freshly harvested suspension of H. pylori NCTC 11638 or NCTC 11637 (6 × 108 CFU/ml) for 2 h, and nuclear extracts were prepared and analyzed for Egr-1 DNA binding by EMSA. Control, untreated AGS cells are shown in lane 1. Representative gels of three independent experiments with similar results are shown.
Since IL-1β and TNF-α are known to be elevated in the gastric tissues of H. pylori-infected subjects, the effect of these cytokines on Egr-1 expression was examined. The treatment of AGS cells with IL-1β (20 ng/ml) or TNF-α (20 ng/ml) for 2 h caused increased Egr-1 protein levels comparable to that seen with H. pylori (Fig. 2C). A maximal induction of Egr-1 protein was observed when 10 to 20 ng of either IL-1β and TNF-α/ml was used. No further increase in Egr-1 expression was observed when larger amounts of IL-1β and TNF-α were used (data not shown). Furthermore, the incubation of AGS cells with purified H. pylori LPS (15 μg/ml) and E. coli LPS (15 μg/ml) for 2 h failed to induce Egr-1 expression in AGS cells (Fig. 2D), indicating that Egr-1 expression was not due to a nonspecific innate immune response induced by bacterial endotoxin.
An analysis of Egr-1 expression by EMSAs demonstrated that the incubation of AGS cells with H. pylori strains NCTC 11638 and NCTC 11637 (6 × 108 CFU/ml) for 2 h resulted in a marked Egr-1-induced gel retardation of a DNA-protein complex from the nuclear extracts prepared from stimulated AGS cells compared to unstimulated cells (Fig. 2E).
H. pylori activates Egr-1 in other gastrointestinal epithelial cells.
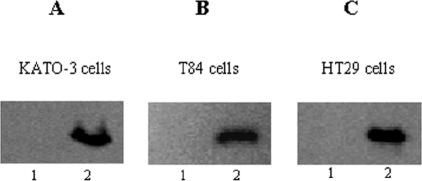
The induction of Egr-1 by H. pylori was further examined with three other gastrointestinal cell lines (KATO-3, T84, and HT29 cells). These cells were cocultured with H. pylori NCTC 11638 (6 × 108 CFU/ml) for 2 h, and after stimulation, total cell extracts were prepared and assayed for Egr-1 expression by Western blotting. H. pylori induced Egr-1 expression in both the gastric epithelial cell line KATO-3 and the colonic epithelial cell lines T84 and HT29 (Fig. 3).
FIG. 3.
Induction of Egr-1 by H. pylori in other gastrointestinal cell lines. KATO-3 (A), T84 (B), and HT29 (C) cells were cocultured with H. pylori NCTC 11638 (6 × 108 CFU/ml) for 2 h (lanes 2) or were left untreated (lanes 1). After stimulation, total cell extracts were prepared and analyzed for Egr-1 expression by immunoblotting. Results are representatives of three independent experiments with similar results.
Involvement of MAPK pathway in H. pylori-induced Egr-1 activation.
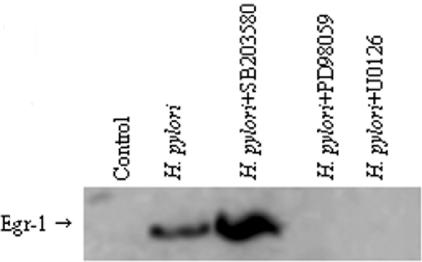
To examine the role of MAPK signaling pathways in H. pylori-induced Egr-1 activation, we pretreated AGS cells with two MEK1/2 MAPK inhibitors, PD98059 (20 μM) and U0126 (10 μM), and the specific p38 MAPK inhibitor SB203580 (20 μM) for 30 min prior to stimulation with H. pylori NCTC 11638 (6 × 108 CFU/ml) for 2 h. A Western blot analysis of Egr-1 expression demonstrated that the MEK1/2 MAPK inhibitors PD98059 (20 μM) and U0126 (10 μM) completely blocked Egr-1 induction by H. pylori but that the p38 MAPK inhibitor SB203580 (10 μM) failed to block this induction (Fig. 4). These observations suggest that H. pylori-induced Egr-1 activity is mediated by the MEK1/2 MAPK signaling cascade.
FIG. 4.
Effect of MAPK inhibitors on H. pylori-induced Egr-1 expression. AGS cells were preincubated for 30 min with the MEK1/2 MAPK inhibitors PD98059 (20 μM) and U0126 (10 μM) and the specific p38 MAPK inhibitor SB 203580 (10 μM) and were then cocultured with H. pylori (6 × 108 CFU/ml) for 2 h.
Immunohistochemical studies.
To assess whether Egr-1 is expressed in the gastric tissues of H. pylori-infected patients, we studied Egr-1 expression in gastric biopsies from healthy donors and H. pylori-negative and -positive patients by immunohistochemistry. The H. pylori infection status of patients was assessed by histology and a rapid urease test (CLOtest). Patient groups were classified as follows: patients with normal antral-type mucosa, H. pylori-negative patients with mild gastritis (noninfected patients), and H. pylori-positive patients with moderate chronic inflammation and focal acute inflammation (H. pylori-infected patients).
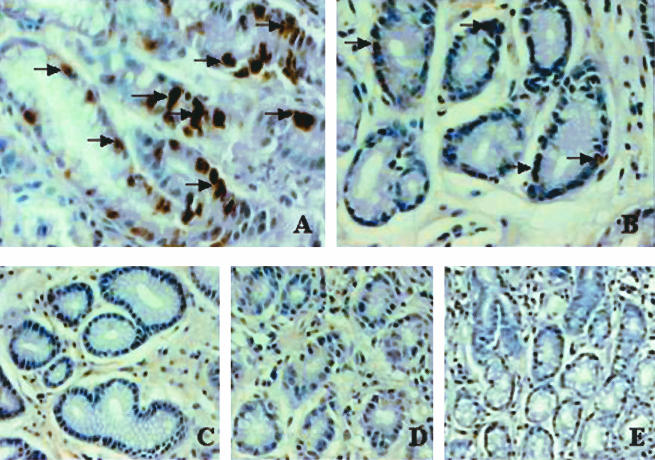
Egr-1 expression was significantly increased in all biopsies obtained from H. pylori-infected patients (n = 15) compared to those from noninfected patients (n = 10) and patients with normal gastric mucosa (n = 5). Egr-1 expression was clearly evident for H. pylori-positive patients (Fig. 5A). In contrast, little or no expression of Egr-1 was observed in antral samples from normal biopsies and H. pylori-negative patients (Fig. 5B and C). In control experiments, antral samples obtained from H. pylori-positive patients were stained with the omission of the primary antibody (Fig. 5D) or with a rabbit polyclonal Ig control (10 μg/ml; Z147) (Fig. 5E).
FIG. 5.
Immunohistochemical staining of Egr-1 expression in antral gastric biopsies from H. pylori-infected patients. Immunohistochemical staining was performed on normal biopsies, H. pylori-negative biopsies from patients with mild gastritis, and H. pylori-positive biopsies by the use of an Egr-1 polyclonal antibody (1:50). Patient groups were classified as follows: normal antral-type mucosa, H. pylori negative with mild gastritis (noninfected patients), H. pylori positive with moderate chronic inflammation and focal acute inflammation (H. pylori-infected patients). (A) H. pylori-positive antral gastric biopsy showing increased Egr-1 expression, as indicated by arrows (brown staining). (B) Antral gastric samples from noninfected patients with chronic gastritis showing weak immunostaining (arrows) for Egr-1 expression. (C) Normal antral gastric biopsy showing little or no immunostaining for Egr-1 expression. No primary antibody was used for panel D and an appropriate rabbit polyclonal Ig control was used for panel E. Original magnifications, ×400 (A), ×300 (B and C), and ×200 (D and E).
H. pylori-induced CD44 expression requires Egr-1.
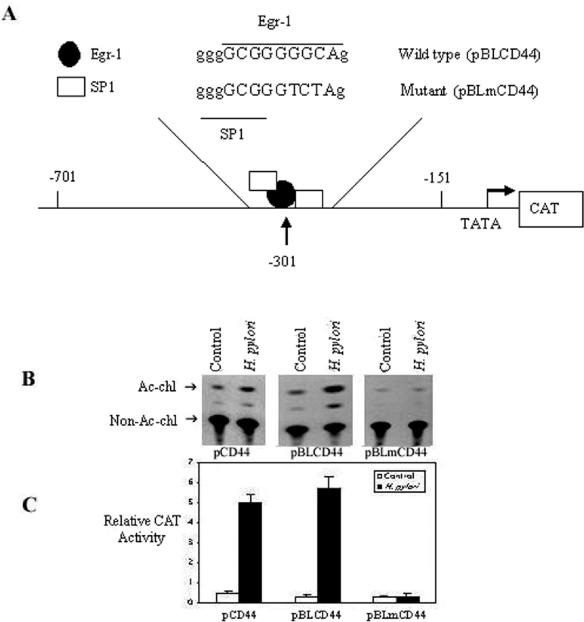
Several studies have shown that the induction of CD44 is dependent on the activation of Egr-1 (14, 26, 30). To test whether the transcription of H. pylori-induced CD44 promoter activity is dependent on the Egr-1 binding site, we used CAT reporter constructs containing a 550-bp region of the CD44 promoter spanning the region from −701 to −151, with (pBLCD44) or without (pBLmCD44) the Egr-1 binding site at position −301 (Fig. 6A), to examine the effect of H. pylori NCTC 11638 (6 × 108 CFU/ml) on the induction of CD44 expression in AGS cells transiently transfected with the appropriate constructs. H. pylori induced CD44 promoter activity 10-fold more than unstimulated cells did (Fig. 6B). The presence of the Egr-1 binding site was required for the observed CD44 promoter activity, as the absence of the −301 Egr-1 binding site eliminated the activity (Fig. 6B and C). In addition, the increase in CD44 transcription in these cells was accompanied by an increase in the amount of CD44 surface expression (see Fig. 10).
FIG. 6.
Regulation of H. pylori-induced CD44 promoter activity by Egr-1. (A) Schematic representation of the CD44 promoter gene constructs. The wild-type CAT reporter construct pBLCD44 and the mutant pBLmCD44 construct differed by the indicated 3-bp mutation, which abolishes Egr-1 binding at position −301 of the CD44 promoter. (B) AGS cells were transfected with the pCAT promoter of pCD44 and the CD44 constructs pBLCD44 and pBLmCD44. After stimulation with H. pylori (6 × 108 CFU/ml) for 24 h, cell lysates were prepared and analyzed for CAT activity. Acetylated (Ac-chl) and nonacetylated (non-Ac-chl) forms of chloramphenicol were separated by thin-layer chromatography, followed by autoradiography (B) and quantitation by scintillation counting (C), with the results expressed as relative CAT activities. All transfection assays were performed three times with similar results, and the data for one experiment are shown as means ± SD. One microgram of pSV-β-galactosidase control vector was simultaneously transfected as a control for transfection efficiency.
FIG. 10.
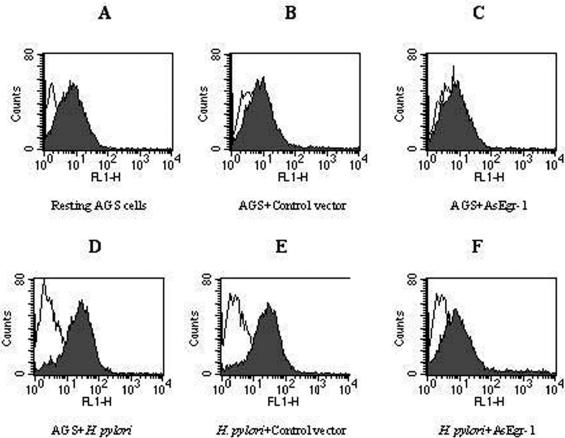
Effect of Egr-1 antisense oligonucleotide on H. pylori-induced CD44 expression on AGS cells. AGS cells (5 × 105 cells/ml) were transfected with 5 μg of antisense Egr-1 oligonucleotide or Egr-1 sense sequence or were left untransfected. After an incubation for 8 h with H. pylori (6 × 108 CFU/ml), the cells were stained with a FITC-labeled anti-CD44 antibody (D2.1 ascites; shaded peaks) or a FITC-labeled isotype control antibody (anti-IE; unshaded peaks). (A) Resting untransfected AGS cells; (B) AGS cells transfected with Egr-1 sense sequence; (C) AGS cells transfected with Egr-1 antisense oligonucleotide; (D) AGS incubated with H. pylori; (E) H. pylori-induced CD44 expression on AGS cells transfected with Egr-1 sense sequence; (F) downregulation of H. pylori-induced CD44 expression on AGS cells by Egr-1 antisense oligonucleotide. Each experiment was repeated three times with similar results, and a representative result is shown.
H. pylori-induced ICAM-1 expression requires Egr-1.
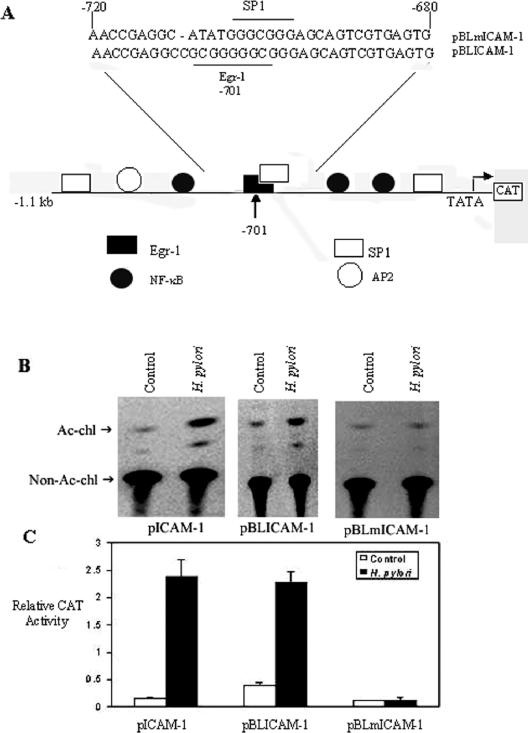
To investigate the relationship between Egr-1 and ICAM-1 induction in AGS cells, we used a plasmid containing 1.1 kb of the murine ICAM-1 promoter (−1091 to +34 bp) upstream of the CAT reporter gene (pBLICAM-1) or the ICAM-1 promoter mutated at the −701 Egr-1 binding site (pBLmICAM-1) (Fig. 7A) for transient transfection experiments. AGS cells were transfected with these constructs (pBLICAM-1 and pBLmICAM-1) and the transfected cells were then cocultured with H. pylori (6 × 108 CFU/ml) or left untreated. The CAT activity was enhanced in H. pylori-treated cells compared to untreated cells (Fig. 7B and C). Furthermore, a significant increase in CAT activity was observed in AGS cells transfected with the wild-type pBLICAM-1 construct compared to those harboring pBLmICAM-1 (Fig. 7B). This reduction in the transcriptional activity of the ICAM-1 promoter as a result of the mutation at the −701 Egr-1 binding site provides additional support for the involvement of the Egr-1 binding site in the activation of the ICAM-1 promoter.
FIG. 7.
Regulation of H. pylori ICAM-1 promoter activity by Egr-1. (A) Model of ICAM-1 constructs. pBLICAM-1 contains 1.1 kb of 5′ flanking sequence from the murine ICAM-1 gene cloned upstream of a CAT reporter, and the mutant construct pBLmICAM-1 contains the depicted 5-bp mutation at position −701 of the ICAM-1 promoter. AGS cells were transfected with the pCAT promoter of ICAM-1 and the pBLICAM-1 or pBLmICAM-1 construct, and after stimulation with H. pylori (6 × 108 CFU/ml) for 24 h, cell lysates were prepared and assayed for CAT activity. Acetylated (Ac-chl) and nonacetylated (non-Ac-chl) forms of chloramphenicol were separated by thin-layer chromatography, followed by autoradiography (B) and quantitation by scintillation counting (C), with the results expressed as relative CAT activities. One microgram of pSV-β-galactosidase control vector was simultaneously transfected as a control for transfection efficiency. Each experiment was performed three times with similar results, and the results of one experiment are shown as means ± SD.
H. pylori-induced CD95L promoter activity requires Egr-1.
We next examined the effect of H. pylori on Egr-1-induced CD95L promoter activity by using AGS cells transfected with a luciferase reporter construct bearing the wild-type CD95L promoter spanning the region from −860 to +100, with or without mutations at the −680, −180, and −120 Egr-1 binding sites (Fig. 8A). Coculturing of these AGS cells with H. pylori NCTC 11638 (6 × 108 CFU/ml) resulted in an enhancement of the wild-type CD95L promoter activity, as demonstrated by an increased luciferase activity. This activation was attributed to the induction of the transcription factor Egr-1, as mutation of the consensus Egr-1 binding sites markedly diminished the CD95L promoter activity (Fig. 8B).
FIG. 8.
Regulation of H. pylori CD95L promoter activity by Egr-1. (A) Schematic representation of the luciferase reporter of the wild-type CD95L promoter from −860 to +100, with or without a mutation at the −680, −180, and −120 Egr-1 binding sites. (B) CD95L constructs were transfected into AGS cells as equivalent amounts of plasmids bearing wild-type CD95L and mutant CD95L promoters. After stimulation with H. pylori (6 × 108 CFU/ml) for 24 h, cell lysates were prepared and assayed for luciferase activity. Normalized luciferase activity was defined as the activity of the luciferase reporter gene relative to the activity of the β-galactosidase plasmid promoter. All transfection assays were performed three times with similar results, and the results for one experiment are shown as means ± SD.
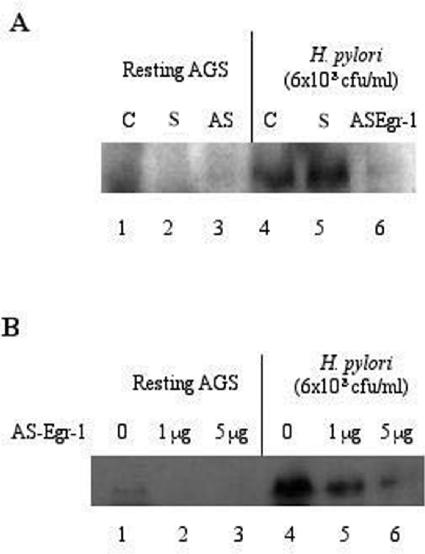
Egr-1 antisense oligonucleotide inhibits H. pylori-induced Egr-1 activation.
AGS cells were transfected with the Egr-1 antisense construct (Egr-1/PcDNA3.1+/Zeo) or the Egr-1 sense oligonucleotide. After 48 h, the cells were incubated for an additional 8 h with H. pylori NCTC 11638. After treatment, total cell lysates were prepared and analyzed for Egr-1 expression by immunoblotting. H. pylori-induced Egr-1 expression was inhibited by the Egr-1 antisense sequence in a dose-dependent manner (Fig. 9).
FIG. 9.
Effect of Egr-1 antisense oligonucleotide on H. pylori-induced Egr-1 expression. (A) AGS cells were transfected with 5 μg of antisense Egr-1 oligonucleotide (Egr-1/PcDNA3.1+/Zeo) or the Egr-1 sense sequence or were left alone for 48 h. After an incubation of AGS cells with H. pylori (6 × 108 CFU/ml) for 8 h, total cell lysates were prepared and analyzed for Egr-1 protein expression by immunoblotting. Lanes 1 and 4, nontransfected AGS cells; lanes 2 and 5, AGS cells transfected with Egr-1 sense sequence; lanes 3 and 6, AGS cells transfected with Egr-1 antisense oligonucleotide. (B) Dose-dependent inhibition of H. pylori-induced Egr-1 expression in AGS cells by Egr-1 antisense oligonucleotide. Three independent experiments were performed with similar results, and a representative gel is shown. S, Egr-1 sense; AS Egr-1, antisense Egr-1.
Egr-1 antisense oligonucleotide downregulates H. pylori-induced CD44 expression.
The transfection of AGS cells with an Egr-1 antisense oligonucleotide resulted in the downregulation of H. pylori-induced CD44 expression, while the Egr-1 sense sequence had no significant effect on H. pylori-induced CD44 expression in these cells (Fig. 10).
DISCUSSION
This study investigated the influence of H. pylori on the induction of Egr-1 in gastric epithelial cells. Since the initial H. pylori-mediated processes that result in primary transcriptional events in gastric epithelial cells in response to infection are not fully understood, we were interested in determining the effect of this pathogen on a representative member of the immediate-early gene family of transcription factors (Egr-1). Given that Egr-1 regulates the early expression of factors associated with diverse, physiologically important events, including cell differentiation, proliferation, and apoptosis (30, 44), we reasoned that the transcriptional activation of this factor by H. pylori could represent a potential mechanism whereby the bacterium augments, amplifies, or perpetuates, via the induction of Egr-1 activity, the expression of products known to be closely associated with gastric pathology.
In this study, the in vitro exposure of AGS cells to H. pylori strains NCTC 11638 and NCTC 11637 induced rapid Egr-1 expression. The rapid kinetics of Egr-1 induction are typical of this family of early response transcription factors. Significantly, immunohistochemical studies demonstrated that Egr-1 expression was elevated in gastric biopsies obtained from H. pylori-positive subjects compared to those from uninfected individuals with either normal antral-type mucosa or mild chronic gastritis. Also, the treatment of AGS cells with the cytokines TNF-α and IL-1β increased the expression of Egr-1. The levels of these cytokines were elevated in the gastric tissues of H. pylori-infected subjects. The activation of Egr-1 expression in AGS cells observed in this study was apparently specific for H. pylori, as both E. coli and C. jejuni failed to induce Egr-1 expression. Purified H. pylori LPS (15 μg/ml) from three clinical isolates (442, 446, and M091) and one reference strain (NCTC 11637) or E. coli LPS had no effect on Egr-1 expression in gastric epithelial cells. Others have also demonstrated that intestinal epithelial cells are unresponsive to LPS exposure (7, 22, 23), presumably to prevent intestinal inflammation from occurring in the host in response to nonpathogenic commensal gut flora. However, other types of cells express Egr-1 in response to LPS (4, 51).
The mechanism whereby H. pylori induces Egr-1 expression is not clear at present. The expression of Egr-1 in H. pylori-infected AGS cells did not appear to be related to the CagA or VacA status of the strains used. In contrast to our data, previous animal studies demonstrated that the presence of both markers (CagA and VacA) influenced the ability of H. pylori to colonize the mouse gastric mucosa (70% for CagA+ Tox+ strains versus 33% for CagA− Tox− strains) (10). However, several virulence factors other than the Cag pathogenicity island may contribute to the outcome of an H. pylori infection. Zheng et al. showed a correlation between ulcer formation and strains expressing Lewis antigens but not the CagA, VacA, or IceA phenotype (54). Other studies did not find any correlation between Lewis antigen or Cag pathogenicity island expression and the severity of the disease (29). Taken together, our results demonstrate that other virulence factors distinct from the CagA molecule could be responsible for increased Egr-1 protein expression, which is in accordance with the hypothesis that other components in H. pylori strains which carry the cagA gene, distinct from CagA itself, are involved in eliciting the inflammatory response (17).
Taken together, these observations support the view that there is an association between Egr-1 expression and H. pylori-induced phenotypic alterations to gastric epithelial cells in vitro. It is not known, however, whether Egr-1 expression levels are sustained throughout the course of chronic H. pylori infections. The prolonged exposure of gastric tissue to H. pylori provokes a host inflammatory response and consequent gastritis. The documented ability of H. pylori (31, 35) and other bacteria (18, 48, 49) to activate MAPK cascades in host cells provides a potential mechanism whereby Egr-1 induction may be facilitated. Consistent with this, we demonstrated a role for the MEK1/2 MAPK pathway in H. pylori-induced Egr-1 activation.
Egr-1 is known to regulate the expression of genes encoding TNF-α (53), IL-2 (6), ICAM-1 (26), p53 (34), and CD44 (14, 27), and these gene products have been documented to be elevated in H. pylori-positive subjects. Consistent with these findings, we demonstrated that H. pylori induced the expression of CD44 and ICAM-1 by using AGS cells that were transiently transfected with the respective promoter and with a CAT or luciferase reporter gene construct. The transcriptional induction of the promoter and reporter constructs was dependent on the presence of intact Egr-1 binding sites, as no stimulation-dependent increase in CAT or luciferase activity was found for constructs lacking Egr-1 sites. Importantly, the pBLmCD44 and pBLmICAM1 constructs, which harbor mutations in the Egr-1 binding site, retained the ability to bind Sp1 but not Egr-1 (14, 26, 27). Therefore, the elimination of CD44 and ICAM1 promoter activity in the respective mutant constructs was due solely to the disruption of Egr-1 binding and not to a disruption of Sp1 binding. Moreover, the functional relevance of these observations was provided by the finding that an egr-1 antisense oligonucleotide blocked H. pylori-induced Egr-1 activation in a dose-dependent manner and prevented the H. pylori-stimulated surface expression of CD44.
Finally, the induction of apoptosis by H. pylori is linked to Fas receptor and ligand overexpression in H. pylori-infected patients (40). Here we demonstrated a role for Egr-1 in the regulation of CD95L promoter activity in gastric cells. The CD95L (APO-1/Fas) ligand, a type II transmembrane protein of the TNF family (46), plays an essential role in apoptosis (1). Mutations at the Egr-1 binding site in the CD95L promoter prevented activation, indicating a role for Egr-1 in the regulation of CD95L promoter activity, an observation that agrees with published data (25).
In summary, we report that H. pylori induces Egr-1 protein expression in gastric epithelial cells in vitro. Given the ubiquitous expression of Egr-1 and its documented cell-specific and tissue-restricted pleiotropic roles in differentiation and proliferation, it is probable that the activation of this transcription factor represents its participation in H. pylori-induced pathogenesis. However, we appreciate that the increased expression of Egr-1 seen in gastric biopsies from H. pylori-infected subjects may be due to the more severe pathology associated with these samples than with those obtained from uninfected individuals. Nevertheless, the presence of Egr-1 in gastric biopsies of H. pylori-infected subjects, combined with our in vitro functional studies demonstrating a strict requirement for egr-1 expression, lends support to the view that this transcription factor may have a role in the infection process. An interesting, but loose, precedent for this is provided by the recent demonstration that murine Citrobacter rodentium infections are accompanied by an augmented colonic expression of egr-1 (7). However, considerable further work is required to establish a causal relationship between Egr-1 expression or activity and H. pylori-induced pathogenesis.
Acknowledgments
We thank John G. Monroe (Department of Pathology and Laboratory Medicine, University of Pennsylvania) for providing the CD44 and ICAM-1 promoter constructs. We also thank Emma Shtivelman (University of California, San Francisco) for the CD44 promoter plasmid and Timothy McCaffrey (Department of Biochemistry and Molecular Biology, The George Washington University, Washington, D.C.) for kindly providing the antisense Egr-1 oligonucleotides.
Editor: F. C. Fang
REFERENCES
- 1.Alderson, M. R., T. W. Tough, T. Davis-Smith, S. Braddy, B. Falk, K. A. Schooley, R. G. Goodwin, C. A. Smith, K. Ramsdell, and D. H. Lynch. 1995. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J. Exp. Med. 181:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Cao, X., G. R. Guy, V. P. Sukhatme, and Y. H. Tan. 1992. Regulation of Egr-1 by tumor necrosis factor and interferon in primary human fibroblasts. J. Biol. Chem. 267:1345-1349. [PubMed] [Google Scholar]
- 4.Coleman, D. L., A. H. Bartiss, V. P. Sukhatme, J. Liu, and H. D. Rupprecht. 1992. Lipopolysaccharide induces Egr-1 mRNA and protein in murine peritoneal macrophages. J. Immunol. 149:3045-3051. [PubMed] [Google Scholar]
- 5.Cover, T. L., M. K. Tummuru, P. Cao, S. A. Thompson, and M. J. Blaser. 1994. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori species. J. Biol. Chem. 269:10566-10573. [PubMed] [Google Scholar]
- 6.Decker, E. L., C. Sherka, and P. F. Zipfel. 1998. The early growth response protein (Egr-1) regulates interleukin-2 transcription by synergistic interaction with the nuclear factor of activated T cells. J. Biol. Chem. 273:26923-26930. [DOI] [PubMed] [Google Scholar]
- 7.De Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 69:6217-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinkel, A., W. K. Aicher, C. Haas, P. F. Zipfel, H. H. Peter, and H. Eibel. 1997. Transcription factor Egr-1 activity down-regulates Fas and CD23 expression in B cells. J. Immunol. 159:2678-2684. [PubMed] [Google Scholar]
- 9.Du, B., C. Fu, K. C. Kent, H. Bush, Jr., A. H. Schulick, K. Kreiger, T. Collins, and T. A. McCaffrey. 2000. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-β type II receptor. J. Biol. Chem. 275:39039-39047. [DOI] [PubMed] [Google Scholar]
- 10.Elizalde, J. I., J. Gómez, J. Panés, M. Lozano, M. Casadevall, J. Ramírez, P. Pizcueta, F. Marco, F. D. de Rojas, D. N. Granger, and J. M. Piqué. 1997. Platelet activation in mice and human Helicobacter pylori infection. J. Clin. Investig. 100:996-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esposito, F., V. Agosti, G. Morrone, F. Morra, C. Cuomo, T. Russo, S. Venuta, and F. Cimino. 1994. Inhibition of the differentiation of human myeloid cell lines by redox changes induced through glutathione depletion. Biochem. J. 1301:649-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan, X. J., A. Long, M. Goggins, X. G. Fan, P. W. N. Keeling, and D. Kelleher. 1996. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonization. Gut 38:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan, X. J., A. Long, X. G. Fan, W. N. Keeling, and D. Kelleher. 1995. Adhesion molecule expression on gastric intraepithelial lymphocytes of patients with Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 7:541-546. [PubMed] [Google Scholar]
- 14.Fitzgerald, K. A., and L. A. O'Neill. 1999. Characterization of CD44 induction by IL-1: a critical role for Egr-1. J. Immunol. 162:4920-4927. [PubMed] [Google Scholar]
- 15.Fu, Z. F., E. Weihe, Y. M. Zheng, M. K. Schafer, H. Sheng, S. Corisdeo, F. J. Rauscher III, H. Koprowshi, and B. Dietzschold. 1993. Differential effects of rabies and borna disease viruses on immediate-early- and late-response gene expression in brain tissues. J. Virol. 67:6674-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gashler, A., and V. P. Sukhatme. 1995. Early growth response protein 1 (Egr-1): prototype of a zinc finger family of transcription factors. Prog. Nucleic Acids Res. Mol. Biol. 50:191-224. [DOI] [PubMed] [Google Scholar]
- 17.Ghiara, P., M. Marchetti, M. J. Blaser, M. K. Tummuru, T. L. Cover, E. D. Segal, L. S. Tompkins, and R. Rappuoli. 1995. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect. Immun. 63:4154-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 19.Hsu, S. M., L. Raine, and H. Fanger. 1981. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 29:577-580. [DOI] [PubMed] [Google Scholar]
- 20.Huang, R. P., and E. D. Adamson. 1993. Characterization of the DNA-binding properties of the early growth response-1 (egr-1) transcription factor: evidence for modulation by a redox mechanism. DNA Cell Biol. 12:265-273. [DOI] [PubMed] [Google Scholar]
- 21.Jin, N., N. D. Hatton, M. A. Harrington, X. Xia, S. H. Larsen, and R. A. Rhoades. 2000. H2O2-induced egr-1, fra-1, and c-jun gene expression is mediated by tyrosine kinase in aortic smooth muscle cells. Free Radic. Biol. Med. 29:736-746. [DOI] [PubMed] [Google Scholar]
- 22.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1997. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalia, N., K. D. Bardhan, J. C. Atherton, and N. J. Brown. 2002. Toxigenic Helicobacter pylori induces changes in the gastric mucosal microcirculation in rats. Gut 51:641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 297:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Li-Weber, M., O. Laur, and P. H. Krammer. 1999. Novel Egr/NF-AT composite sites mediate activation of the CD95 (APO-1/Fas) ligand promoter in response to T cell stimulation. Eur. J. Immunol. 29:3017-3027. [DOI] [PubMed] [Google Scholar]
- 26.Maltzman, J. S., J. A. Carmen, and J. G. Monroe. 1996. Transcriptional regulation of the Icam-1 gene in antigen receptor-and phorbol-stimulated B lymphocytes: role for transcription factor Egr-1. J. Exp. Med. 83:1747-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maltzman, J., J. Carman, and J. Monroe. 1996. Role for Egr-1 in regulation of stimulus-dependent CD44 transcription in B lymphocytes. Mol. Cell. Biol. 16:2283-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall, B. J. 1994.Helicobacter pylori. Am. J. Gastroenterol. 89:S116-S128. [PubMed] [Google Scholar]
- 29.Marshall, D. G., S. O. Hynes, D. C. Coleman, C. A. O'Morain, C. J. Smyth, and A. P. Moran. 1999. Lack of a relationship between Lewis antigen expression and cagA, CagA, vacA and VacA status of Irish Helicobacter pylori isolates. FEMS Immunol. Med. Microbiol. 24:79-90. [DOI] [PubMed] [Google Scholar]
- 30.McMahan, S. B., and J. G. Monroe. 1996. The role of early growth response gene 1 (Egr-1) in regulation of the immune response. J. Leukoc. Biol. 60:159-166. [DOI] [PubMed] [Google Scholar]
- 31.Meyer-ter-Vhen, T., A. Covacci, M. Kist, and H. L. Pahl. 2000. Helicobacter pylori activates mitogen-activated protein kinase cascades and induces expression of the proto-oncogenes c-Fos and c-Jun. J. Biol. Chem. 275:16064-16072. [DOI] [PubMed] [Google Scholar]
- 32.Milbrandt, J. A. 1987. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science 238:797-799. [DOI] [PubMed] [Google Scholar]
- 33.Munzenmaier, A., C. Lange, E. Glockr, A. Covacci, A. Moran, S. Bereswill, A. P. Baeuerele, M. Kist, and H. L. Phal. 1997. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J. Immunol. 159:6140-6147. [PubMed] [Google Scholar]
- 34.Nair, P., S. Muthukkumar, S. F. Sells, S. S. Han, V. P. Sukhatme, and V. M. Rangnekar. 1997. Early growth response-1-dependent apoptosis is mediated by p53. J. Biol. Chem. 272:20131-20138. [DOI] [PubMed] [Google Scholar]
- 35.Naumann, M., S. Wessler, C. Bartsch, B. Wieland, A. Covacci, R. Haas, and T. F. Meyer. 1999. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J. Biol. Chem. 274:1655-1662. [DOI] [PubMed] [Google Scholar]
- 36.Nomura, A., G. N. Stemmermann, P. Chyou, I. Kato, G. I. Perez-Perez, and M. J. Blaser. 1991. Helicobacter pylori infection and gastric carcinoma in a population of Japanese-Americans in Hawaii. N. Engl. J. Med. 325:1132-1136. [DOI] [PubMed] [Google Scholar]
- 37.Nose, K., and M. Ohba. 1996. Functional activation of the egr-1 (early growth response-1) gene by hydrogen peroxide. Biochem. J. 316:381-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osborn, L., S. Kunkel, and G. J. Nabel. 1989. Tumor necrosis factor alpha and interleukin-1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc. Natl. Acad. Sci. USA 86:2336-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 40.Rudi, J., D. Kuck, S. Strand, A. von Herbay, S. M. Mariani, P. H. Krammer, P. R. Galle, and W. Stremmel. 1998. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J. Clin. Investig. 102:1506-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto, K. M., S. D. Nimer, J. D. Rosenblatt, and J. C. Gasson. 1992. HTLV-1 and HTLV-11 tax trans-activate the human Egr-1 promoter through different cis-acting sequences. Oncogene 7:2125-2130. [PubMed] [Google Scholar]
- 42.Seyfert, V. L., V. P. Sukhatme, and J. G. Monroe. 1989. Differential expression of a zinc finger-encoding gene in response to positive versus negative signaling through receptor immunoglobulin in murine B lymphocytes. Mol. Cell. Biol. 9:2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma, S. A., M. K. R. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 63:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman, E. S., and T. Collins. 1999. Pathways of Egr-1 mediated gene transcription in vascular biology. Am. J. Pathol. 154:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sukhatme, V. P. 1990. Early transcriptional events in cell growth: the Egr family. J. Am. Soc. Nephrol. 1:859-866. [DOI] [PubMed] [Google Scholar]
- 46.Sukhatme, V. P., X. Cao, L. C. Chang, C. H. Tsai-Morris, D. Stamenkovich, P. C. Ferreira, D. R. Cohen, S. A. Edwards, T. B. Shows, and T. Curran. 1988. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell 53:37-43. [DOI] [PubMed] [Google Scholar]
- 47.Swirnoff, A. H., and J. Milbrandt. 1995. NGF1-A and related zinc-finger transcription factors. Mol. Cell. Biol. 15:2275-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warny, M. A., A. C. Keates, S. Keates, I. Castagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, T. LaMont, and C. P. Kelly. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu, Z., R. Dziarski, Q. Wang, K. Swartz, K. M. Sakamoto, and D. Gupta. 2001. Bacterial peptidoglycan-induced TNF-α transcription is mediated through the transcription factor Egr-1, Elk-1 and NF-κB. J. Immunol. 167:6975-6982. [DOI] [PubMed] [Google Scholar]
- 51.Yao, J., N. MacKman, T. S. Edgington, and S. T. Fan. 1997. Lipopolysaccharide induction of the tumor necrosis factor-α promoter in human monocyte cells: regulation by Egr-1, c-Jun, NF-kappa B transcription factors. J. Biol. Chem. 272:17795-17801. [DOI] [PubMed] [Google Scholar]