Abstract
Background
The coronary artery calcium score (CAC) predicts future coronary heart disease (CHD) events and could be used to guide primary prevention interventions, but CAC measurement has costs and exposes patients to low-dose radiation.
Methods and Results
We estimated the cost-effectiveness of measuring CAC and prescribing statin therapy based on the resulting score under a range of assumptions using an established model enhanced with CAC distribution and risk estimates from the Multi-Ethnic Study of Atherosclerosis (MESA). Ten years of statin treatment for 10,000 55-year-old women with high cholesterol (10-year CHD risk=7.5%) was projected to prevent 32 myocardial infarctions, cause 70 cases of statin-induced myopathy, and add 1,108 years to total life-expectancy. Measuring CAC and targeting statin treatment to the 2,500 women with CAC>0 would provide 45% of the benefit (+501 life-years), but CAC measurement would cost $2.25 million and cause 9 radiation-induced cancers. Treat All was preferable to CAC screening in this scenario and across a broad range of other scenarios (CHD risk=2.5-15%) when statin assumptions were favorable ($0.13/pill and no quality of life penalty). When statin assumptions were less favorable ($1.00/pill and disutility=0.00384), CAC screening with statin treatment for persons with CAC>0 was cost-effective (<$50,000/quality-adjusted life-year) in this scenario, in 55-year old men with CHD risk=7.5%, and in other intermediate risk scenarios (CHD risk=5-10%). Our results were critically sensitive to statin cost and disutility, and relatively robust to other assumptions. Alternate CAC treatment thresholds (>100 or >300) were generally not cost-effective.
Conclusions
CAC testing in intermediate risk patients can be cost-effective, but only if statins are costly or significantly impact quality of life.
Keywords: coronary, atherosclerosis, economics, calcium, statins
Lowering cholesterol with HMG-CoA Reductase Inhibitors (statins) reduces risk of cardiovascular disease (CVD) in patients who have not yet suffered a CVD event (i.e., primary prevention).1 Using statins in otherwise healthy individuals, however, has financial costs, may be inconvenient or undesirable to patients for a variety of reasons, and exposes patients to risks of adverse drug effects.2-4 Patients at higher CVD risk are more likely to benefit from statins, and current guidelines recommend a more aggressive approach to statin prescribing in these patients,5 but our ability to predict CVD using traditional risk factors (e.g., Framingham-based risk equations) is imperfect.6, 7 Whether additional risk stratification testing might be useful and cost-effective for guiding statin therapy remains controversial3, 8-10.
The coronary artery calcium score (CAC) is an indicator of coronary atherosclerosis measured by computed tomography scanning that could be used to guide statin therapy. While CAC is strongly correlated with traditional risk factors, it provides substantial additional information about an individual's risk for future coronary heart disease (CHD) events, including myocardial infarction, angina, and CHD death,11-13 and helps improve risk classification13 more than other currently available non-traditional risk factors.14 Measurement of the CAC score, however, adds front-end costs and exposes patients to small doses of ionizing radiation.15 It is unclear when using CAC to guide statin therapy might provide significant net health benefits to patients, and whether such use would be cost-effective.
We estimated the costs and effectiveness of using CAC testing to guide primary prevention with statin therapy using an established decision model that was enhanced with CAC parameters from the Multi-Ethnic Study of Atherosclerosis (MESA).11, 16 Unlike previous efforts,17-21 our model carefully accounts for the expected CAC distribution in a range of specific clinical scenarios16, and provides systematic analyses of CAC testing strategies with different treatment thresholds (versus simply prescribing statins or not) under a range of assumptions about statin therapy and CAC scan cost, effectiveness and adverse effects.22
METHODS
Overview and Model Structure
The UNC-RTI (University of North Carolina-Research Triangle Institute) CVD Prevention Model is a state-transition simulation model that can be used to compare incidence of CVD, mortality, quality of life, and costs with and without a prevention intervention.23, 24 In the model, a specific clinical scenario is defined by age, sex, and CVD risk factors. Persons with these characteristics begin in the healthy state then may transition every 12 months. Separate states explicitly model first and subsequent years after myopathy, angina, and myocardial infarction (MI), as well as stroke; costs, quality of life and mortality rates differ in each state. Total costs and quality-adjusted life-years (QALYs) were calculated (discounting at 3%/year for costs and utilities) over a lifetime horizon.
Our primary outcome measure is the incremental cost-effectiveness ratio (ICER), measured in $/QALY. For illustration purposes, we indicate “preferred strategies” under the assumption that society is willing to pay up to $50,000/QALY, but continuous ICER values are provided throughout. Model parameters were varied in one-way and global probabilistic analyses. Online Supplemental Materials describe the model parameters (Supplemental Table 1), and provide additional Methods detail. This research did not involve human subjects or animals and does not require institutional review board approval.
Prevention strategies compared: To scan or not to scan?
The model is used to compare 5 different interventions: 2 strategies where statin prescribing does not depend on results of a coronary artery calcium (CAC) scan (“Treat None”, and “Treat All”), and 3 strategies where a CAC scan is ordered (one time only, with attendant costs and radiation exposure), and statins are prescribed only if the CAC score is above a given threshold (“Treat if CAC>300”, “Treat if CAC>100”, and “Treat if CAC>0”). Statin prescribing is assumed to be differential for 10 years, but accrual of costs and quality-adjusted life-years is simulated across a full lifetime horizon to fully account for the consequences of a life saved or myocardial infarction prevented by statins during those first 10 years of differential treatment.
Base-case scenarios
Our base-case clinical scenario is a 55-year-old woman with total cholesterol = 221 mg/dl and high density lipoprotein (HDL) cholesterol = 40 mg/dl (“high cholesterol”), and no other CHD risk factors (systolic blood pressure = 120 mmHg without medications, no smoking or diabetes). These risk factors yield an average Framingham-derived 10-year risk for angina, myocardial infarction, or CHD death 7.5%25 (used for state transition probabilities along with a stroke risk of 0.9%), and they also determine the CAC score distribution (see below). Alternate clinical scenarios varying age, sex and overall risk are also considered.
Based on preliminary results showing sensitivity to statin-related parameters, we present two base-case statin scenarios in parallel throughout the manuscript. The first assumes that statins can be obtained at a cost of $0.13/pill (available through $4/month prescribing programs at some large discount retailers26) and that taking a statin pill every day is not associated with any reduction in quality of life (“favorable statin assumptions”); the second scenario assumes a higher cost of $1.00/pill (most statin users do not avail themselves of $4/month programs26) and a quality of life penalty (“disutility”) of .00384 associated with every year of statin use, which is equivalent to trading away 2 weeks of perfect health to avoid 10 years on statins27 (“less favorable statin assumptions”). The quality of life penalty modeled here represents inconvenience, reduction in self-conception of health, and any other reason why a patient might prefer not to take a pill daily; statin-induced myopathy is modeled separately.
Coronary artery calcium score testing parameters
Direct CAC scan costs were obtained from the American Medical Association's Relative-Based Resource Value Scale (RBRVS).28 We added the cost of a physician visit28 for scan interpretation and average workup costs for incidental non-cardiac findings29, 30 (see Supplemental Material).
The prevalence of CAC and expected distribution of the CAC score, conditional on CHD risk factors, is a critical parameter31 that we estimated using a multivariable model derived from the MESA baseline examination.16 We used this model to estimate the proportion of scores falling into categories of 0, 1-100, 101-300, and >300, and then to estimate “post-test” risk for angina, MI and CHD death in these categories using CAC-specific relative risks from MESA11 and previously described methods.16 We assumed that the risk of stroke did not vary with CAC.
We assumed an average radiation dose of 2.3 millisieverts (mSv) from each scan,15 and an excess relative risk (ERR) of 0.001/mSv for all-cause cancer15, 32 (see Supplemental Material).
Statin assumptions
Along with the cost and disutility assumptions described above, we assumed 1) statin use triggers 1 additional physician visit and lipid panel per year, 2) standard dose statins are associated with relative reductions in risk of myocardial infarction (26%; i.e., relative risk = 0.74), angina (26%), stroke (15%), and CHD death (20%)1 (Supplemental Material), 3) statins cause myopathy2 (with associated cost, mortality and disutility for 1 year,27 and which then leads to statin discontinuation), and 4) imperfect statin adherence,33 with discontinuation events occurring immediately. Hepatitis and liver function testing costs were not included.2, 34 Although there is evidence that statins cause diabetes,4 the cardiovascular benefits outweigh the risks, at least in the short term,35 and the statin trial results upon which we base our statin efficacy assumptions account for this effect. Alternate assumptions about statin-associated cost and health impact from these factors or others are easily simulated by increasing overall statin cost and disutility.
RESULTS
Base-case scenario
Ten years of statin treatment for 10,000 55-year-old women with high cholesterol (10-year CHD risk=7.5%, stroke risk=0.9%) was projected to prevent 32 lifetime myocardial infarctions, cause 70 cases of statin-induced myopathy, and add 1,108 years to total life-expectancy (Treat All vs. Treat None). Targeting statin treatment to the 2,500 women with CAC>0 (Treat if CAC>0) would provide 45% of the benefits (+501 life-years), but would require CAC screening for all 10,000, which would cost $2.25 million and cause 9 radiation-induced cancers (Table 1).
Table 1.
Health outcomes and costs for statin prescribing strategies with and without coronary calcium screening
| Outcome | Outcome from applying the given strategy to a theoretical cohort of 10,000 55-year-old women with high cholesterol* | ||||
|---|---|---|---|---|---|
| Treat None | Treat if CAC>300 | Treat if CAC>100 | Treat if CAC>0 | Treat All | |
| Cost of CAC screening | $0 | $2.25 million | $2.25 million | $2.25 million | $0 |
| On statins at baseline, n | 0 | 100 | 400 | 2,500 | 10,000 |
| Total lifetime cost of statin therapy, $ | |||||
| - at $0.13/pill† | $1.12 million | $1.21 million | $1.40 million | $2.97 million | $9.02 million |
| - at $1.00/pill† | $3.46 million | $3.74 million | $4.36 million | $9.43 million | $28.91 million |
| Other healthcare costs, $ total | $1,395.8 million | $1,395.1 million | $1,394.5 million | $1,391.6 million | $1,387.9 million |
| Total costs, $ | |||||
| - at $0.13/pill† | $1,396.9 million | $1,398.5 million | $1,398.1 million | $1,396.9 million | $1,396.9 million |
| - at $1.00/pill† | $1,399.3 million | $1,401.1 million | $1,401.1 million | $1,403.3 million | $1,416.8 million |
| Total number of events | |||||
| Angina | 739 | 736 | 731 | 706 | 675 |
| Myocardial infarction | 549 | 548 | 545 | 533 | 517 |
| Stroke | 479 | 479 | 479 | 479 | 473 |
| Statin-induced myopathy | 0 | 1 | 3 | 17 | 70 |
| CT-induced cancer | 0 | 9 | 9 | 9 | 0 |
| Life-years | 249,564 | 249,563 | 249,649 | 250,065 | 250,672 |
| Quality-adjusted life-years | |||||
| - with no statin disutility† | 170,435 | 170,437 | 170,488 | 170,728 | 171,075 |
| - with .00384 statin disutility† | 170,435 | 170,433 | 170,477 | 170,664 | 170,836 |
| Incremental cost, QALY's and $/QALY | |||||
| With favorable statin assumptions† | |||||
| Compared with Treat None | |||||
| - Incremental costs, $ | Reference | + $1.6 million | + $1.2 million | − $0.07 million | − $0.04 million |
| - Incremental QALYs | Reference | + 2 | + 53 | + 292 | + 640 |
| - $/QALY | Reference | $990,000 | $22,000 | Cost-saving | Cost-saving |
| Compared with next cheaper non-dominated strategy | |||||
| - Incremental costs, $ | Dominated | Dominated | Dominated | Least costly | + $0.03 million |
| - Incremental QALYs | Dominated | Dominated | Dominated | Reference | + 347 |
| - $/QALY | Dominated | Dominated | Dominated | Reference | $100‡ |
| With less favorable statin assumptions† | |||||
| Compared with Treat None | |||||
| - Incremental costs, $ | Reference | + $1.8 million | + $1.8 million | + $4.0 million | + $17.5 million |
| - Incremental QALYs | Reference | − 2 | + 41 | + 229 | + 401 |
| - $/QALY | Reference | Dominated | $43,000 | $18,000 | $44,000 |
| Compared with next cheaper non-dominated strategy | |||||
| - Incremental costs, $ | Reference | Dominated | Dominated | + $4.0 million | + $13.5 million |
| - Incremental QALYs | Reference | Dominated | Dominated | + 229 | + 172 |
| - $/QALY | Reference | Dominated | Dominated | $18,000‡ | $78,000 |
The base-case clinical scenario is a 55-year-old woman with total cholesterol = 221 mg/dl, HDL cholesterol = 40 mg/dl, systolic blood pressure = 120 mmHg without medications who does not smoke or have diabetes.
In the favorable statin assumptions scenario, statins cost $0.13/pill and have no disutility. In the less favorable statin assumptions scenario, statins cost $1.00/pill and have disutility = 0.00384, equivalent to 2 weeks of perfect health traded away to avoid 10 years on statins. Note that total statin costs account for discontinuation after myopathy and addition of statin therapy in all strategies for secondary prevention dependent on state membership.
Preferred strategy under the given assumptions if society is willing to pay up to $50,000 per QALY
CAC – Coronary artery calcium; CT – Computed tomography; QALY – Quality-adjusted life-year; Treat All – Treat all persons with statins and do not test for CAC; Treat None – Do not treat with statins and do not test for CAC; Treat if CAC>X – Test for CAC, and treat with statins if the CAC score is over X.
With favorable statin assumptions ($0.13/pill and no quality of life penalty), the costs of statin treatment are low and are almost entirely offset by healthcare savings from prevented events. Targeted statin prescribing, therefore, would not result in substantial savings, and would not be a rational alternative to Treat All (Table 1).
Under less favorable assumptions, statin costs ranged from $3.46-$28.91 million, and these costs were not completely offset by healthcare savings from prevented events; with more persons treated with statins, both costs and QALYs increased. Treat if CAC>100 and >300 identified relatively small numbers of persons to treat with statins and did not generate enough QALYs to offset the testing costs compared with competing strategies. In contrast, Treat if CAC>0 was reasonable (not dominated), producing more QALY's than Treat None at a cost of $18,000/QALY. Treat All produced even more QALYs, but at a much higher price ($78,000/QALY, Table 1).
A parallel analysis for 55-year old men at 7.5% CHD risk (stroke risk=1.2%) yielded very similar results (Supplemental Table 2). Men were more likely to have CAC (39% with CAC>0), more likely to have a myocardial infarction or stroke and less likely to have angina as a first event, and generally had lower life expectancy, but incremental cost-effectiveness ratios were nearly identical ($19,000/QALY for Treat if CAC>0 compared to Treat None; and $80,000/QALY for Treat All compared to Treat if CAC>0).
Sensitivity analyses for base case scenario
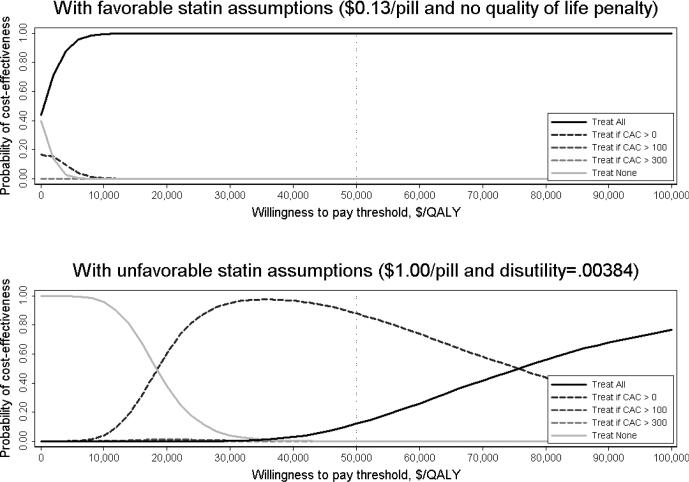
With favorable statin assumptions, Treat All was cost-saving or relatively cost-effective (<$50,000/QALY) in 55-year-old women with high cholesterol even with wide variation of other model parameters in one-way sensitivity analyses (Table 2) and with global probabilistic sensitivity analyses (Figure).
Table 2.
One-Way Sensitivity Analyses
| Parameter (base-case assumption) | Preferred strategy for a 55-year old woman with high cholesterol* if society is willing to pay up to $50,000/QALY |
|
|---|---|---|
| Favorable statin assumptions: $0.13/pill and no disutility† | Less favorable statin assumptions: $1.00/pill and disutility = .00384† | |
| Base-case | Treat All | Treat if CAC>0 |
| Statin price ($0.13 or $1.00/pill)† | ||
| - $0.10/pill | Treat All | Treat All |
| - $0.13 | Treat All† | Treat All |
| - $0.50 | Treat All | Treat All |
| - $1.00 | Treat All | Treat if CAC>0† |
| - $2.00 | Treat if CAC>0 | Treat if CAC>0 |
| - $4.00 | Treat None | Treat None |
| Statin disutility (0 or .00384)† | ||
| -0 | Treat All† | Treat All |
| - .001 | Treat All | Treat All |
| - .00384 | Treat All | Treat if CAC>0† |
| - .0075 | Treat All | Treat if CAC>0 |
| - .01 | Treat if CAC>0 | Treat if CAC>0 |
| - .015 | Treat if CAC>0 | Treat None |
| - .02 | Treat None | Treat None |
| Statin myopathy rate, per person-year (.001) | ||
| - .005 | Treat All | Treat if CAC>0 |
| - .01 | Treat All | Treat if CAC>0 |
| - .05 | Treat All | Treat if CAC>0 |
| Statin discontinuation (31%) | ||
| - 0% | Treat All | Treat if CAC>0 |
| - 50% | Treat All | Treat if CAC>0 |
| - 75% | Treat All | Treat All |
| Statin efficacy (0% reduction)‡ | ||
| 25% increase | Treat All | Treat if CAC>0 |
| 25% reduction | Treat All | Treat if CAC>0 |
| 50% reduction | Treat None | Treat None |
| 75% reduction | Treat None | Treat None |
| CAC scan direct cost ($114.37) | ||
| - $100 | Treat All | Treat if CAC>0 |
| - $250 | Treat All | Treat if CAC>0 |
| - $400 | Treat All | Treat if CAC>0 |
| - $600 | Treat All | Treat All |
| Average cost of working up incidental findings from CAC scan ($40.19) | ||
| - $0 | Treat All | Treat if CAC>0 |
| - $100 | Treat All | Treat if CAC>0 |
| - $250 | Treat All | Treat if CAC>0 |
| CAC scan relative risks (0% reduction from Detrano 200811)‡ | ||
| 10% reduction | Treat All | Treat if CAC>0 |
| 25% reduction | Treat All | Treat if CAC>0 |
| 50% reduction | Treat All | Treat All |
| 75% reduction | Treat All | Treat All |
| CAC scan radiation exposure (2.3 mSv) | ||
| 1 mSv | Treat All | Treat if CAC>0 |
| 5 mSv | Treat All | Treat if CAC>0 |
| 10.5 mSv15 | Treat All | Treat if CAC>0 |
| All-cause mortality relative risks after CVD events (0% reduction)‡ | ||
| 25% reduction | Treat All | Treat if CAC>0 |
| 50% reduction | Treat All | Treat if CAC>0 |
| 75% reduction | Treat All | Treat if CAC>0 |
The base-case clinical scenario is a 55-year-old woman with total cholesterol = 221 mg/dl, HDL cholesterol = 40 mg/dl, systolic blood pressure = 120 mmHg without medications who does not smoke or have diabetes.
Indicates base-case scenarios. Statin price and disutility were varied in one-way sensitivity analyses within each scenario to show the independent contribution of each factor. For one-way analyses where a different parameter is varied, we show results for both favorable and unfavorable statin assumption scenarios. In the favorable statin assumptions scenario, statins cost $0.13/pill and have no disutility. In the less favorable statin assumptions scenario, statins cost $1.00/pill and have disutility = .00384, equivalent to 2 weeks of perfect health traded away to avoid 10 years on statins.
Statin efficacy (relative risk reductions for myocardial infarction, angina, CHD death, and stroke), CAC scan relative risks (for CHD events based on the CAC score), and all cause mortality relative risks after CVD events (risk of death multipliers in post-CHD and stroke event states) were varied from the base-case assumptions (see Supplemental Table 1) simultaneously by the % reduction shown, after conversion to the log scale. Statin efficacy relative risks were also increased in sensitivity analyses given that larger relative risks were observed for lower risk participants in our meta-analysis source for statin efficacy1.
CHD – Coronary heart disease; CVD events – Cardiovascular disease events, including; CAC – Coronary artery calcium; Treat if CAC>X – Test for CAC, and treat with statins if the CAC score is over X.
Figure. Probability of cost-effectiveness of Treat All, Treat None and CAC screening strategies at different willingness to pay thresholds.
These acceptability curves illustrate probabilistic results from 10,000 model runs of the base case clinical scenario (a 55-year-old woman with high cholesterol), under both favorable and unfavorable statin assumption scenarios, that account for the uncertainty in parameter estimates described in Supplemental Table 1. The decision to measure CAC is sensitive to the willingness to pay threshold when unfavorable assumptions about statins are used. A disutility of .00384 is equivalent to 2 weeks of perfect health traded away to avoid 10 years on statins. CAC – Coronary artery calcium score; QALY – Quality-adjusted life-years
With unfavorable statin assumptions, the Treat if CAC>0 strategy remained relatively cost-effective (≤$50,000/QALY; see Supplemental Table 3 for ICER values) in one-way sensitivity analyses that varied the statin myopathy rate, the statin discontinuation rate (unless it is 75% or greater), statin efficacy (unless it is reduced by 50% or more), CAC scan costs (unless direct costs are $600 or more), CAC-specific relative risk estimates (unless they are reduced by 50% or more), the degree of radiation exposure from the CAC scan (up to 10.5 mSv), or assumptions about the downstream mortality risk after CVD events (Table 2). Preference for the Treat if CAC>0 strategy was somewhat sensitive, however, to global probabilistic parameter variation, and very sensitive to society's willingness-to-pay threshold (Figure).
When statin costs are even higher ($4.00/pill) or disutility larger (0.02, equivalent to trading away over 10 weeks of perfect health to avoid 10 years on statins), Treat None is preferred (Table 2).
Alternate clinical scenarios
With favorable statin assumptions and using a willingness-to-pay threshold of $50,000/QALY, the Treat All strategy was preferred in every clinical scenario that we simulated, including men and women at lower age and lower risk (Table 3). With less favorable statin assumptions, the Treat if CAC>0 strategy was preferred in most scenarios where CHD risk was intermediate (5%-10%), but with some variation by age and sex (Table 3). Supplemental Tables 4a and 4b provide clinical characteristics, CAC prevalence (and thus the “yield” of CAC screening in each clinical scenario), and continuous ICER values for each of these alternate clinical scenarios. Supplemental Tables 5-8 demonstrate shifts in the window of cost-effectiveness for CAC testing with altered assumptions about statin efficacy, cost and disutility. A CAC threshold higher than 0 (Treat if CAC>100) was cost-effective only in edge cases, usually when statin cost or disutility was assumed to be quite high (see Supplemental Tables 5-8); otherwise the CAC=0 threshold was preferred. Treat if CAC>300 was dominated in every scenario we analyzed.
Table 3.
Preferred strategy for different age, sex and 10-year CHD risk scenarios if society is willing to pay up to $50,000/QALY
| Preferred strategy* assuming society is willing to pay up to $50,000/QALY |
||||||
|---|---|---|---|---|---|---|
| Sex | Favorable statin assumptions: $0.13/pill and no disutility† |
Less favorable statin assumptions: $1.00/pill and disutility = .00384† |
||||
| - 10-year CHD Risk‡ | Age=45 years | Age=55 years | Age=65 years | Age=45 years | Age=55 years | Age=65 years |
| Women | ||||||
| - 2.5% risk | Treat All | Treat All | Treat All | Treat None | Treat None | Treat None |
| - 5% risk | Treat All | Treat All | Treat All | Treat if CAC>0 | Treat if CAC>0 | Treat if CAC>0 |
| - 7.5% risk | Treat All | Treat All¶ | Treat All | Treat if CAC>0 | Treat if CAC>0¶ | Treat if CAC>0 |
| - 10% risk | Treat All | Treat All | Treat All | Treat All | Treat All | Treat if CAC>0 |
| - 15% risk | Treat All | Treat All | Treat All | Treat All | Treat All | Treat All |
| Men | ||||||
| - 2.5% risk | Treat All | Treat All | --- | Treat None | Treat None | --- |
| - 5% risk | Treat All | Treat All | Treat All | Treat if CAC>0 | Treat if CAC>0 | Treat if CAC>0 |
| - 7.5% risk | Treat All | Treat All | Treat All | Treat if CAC>0 | Treat if CAC>0 | Treat if CAC>0 |
| - 10% risk | Treat All | Treat All | Treat All | Treat All | Treat if CAC>0 | Treat if CAC>0 |
| - 15% risk | Treat All | Treat All | Treat All | Treat All | Treat All | Treat All |
The preferred strategy is either cheaper than less effective alternatives, or costs less than $50,000 per additional QALY gained over the less effective alternatives; and, if there is a more effective alternative, it costs more than $50,000 per additional QALY gained. Test-and-Treat strategies are bolded.
In the favorable statin assumptions scenario, statins cost $0.13/pill and have no disutility. In the less favorable statin assumptions scenario, statins cost $1.00/pill and have disutility = .00384, equivalent to 2 weeks of perfect health traded away to avoid 10 years on statins.
The 10-year Framingham-derived risk for angina, myocardial infarction, CHD death or stroke, estimated using Framingham-based equations25, was manipulated for different scenarios by modifying conventional risk factors, particularly total and high density lipoprotein cholesterol. The base-case clinical scenario is indicated¶. For the risk factor levels used in each scenario and all relevant incremental cost-effectiveness ratios, see Supplemental Tables 4a and 4b.
Base-case clinical scenario. Results from this scenario are shown in detail in Tables 1 and 2.
QALY – Quality-adjusted life-years; CHD – Coronary heart disease; CAC – Coronary artery calcium; Treat All – Treat all persons with statins and do not test for CAC; Treat None – Do not treat with statins and do not test for CAC; Treat if CAC>X – Test for CAC, and treat with statins if the CAC score is over X.
DISCUSSION
Statins are effective at preventing CHD events across a very broad range of CHD risk1, including risk levels well below current statin treatment thresholds5, and are considered to be very safe2, 34. Our results indicate that when they are also inexpensive ($0.13/pill = $4/month) and easy to use (i.e., no quality of life penalty), obtaining a CAC scan in order to prevent some patients from having to take a statin is not a cost-effective approach. Under these assumptions, which represent a likely scenario in the current generic statin era, our model suggests that it is better not to order the test and instead simply to treat with statins (“Treat All”).
When statins cost more and/or substantially diminish quality of life, our analyses show that CAC testing can be a cost-effective way of identifying patients who are at very low CHD risk and therefore have little to gain from these medications. Our base-case scenario illustrates such a situation: for a 55-year-old woman with high cholesterol and 10-year CHD risk of 7.5%, whose insurance would pay $1/pill for her statin prescription and who would much prefer not to take them (disutility=.00384; i.e., she would be willing to trade 2 weeks of perfect health to avoid 10 years of statin therapy), our analysis demonstrates that a strategy of obtaining a CAC scan first and only treating her with statins if the scan demonstrates some evidence of calcified atherosclerotic plaque (i.e., CAC>0) is relatively cost-effective. This decision would only be rational, however, if society is willing to pay at least $18,000/QALY but not over $78,000/QALY. These threshold values, and thus the potential cost-effectiveness of CAC testing, depend critically on statin assumptions (cost and disutility) and on the specifics of the clinical scenario (age, sex, risk factor profile), which drive both CHD risk and CAC prevalence.
Our results are generally consistent with prior cost-effectiveness analyses. Two recent analyses20, 21 simulated CAC testing and reclassification of a population of intermediate risk persons (using two different population/community-based cohort studies) with statin20, 21 and antihypertensive21 treatment intensity based on post-test risk. One assumed inexpensive statins ($0.13/pill) and found results very consistent with our favorable statin assumptions scenario (i.e., “Treat All” dominated),20 and the other used higher statin costs ($1.56/pill), and found cost-effectiveness of CAC screening, at least in men,21 consistent with our unfavorable statin assumptions scenario. Unlike either of these analyses, ours considered more focused, specific clinical scenarios instead of averaging results over the entire intermediate risk population. Our results suggest that the cost-effectiveness of CAC testing varies, even among persons at intermediate risk, depending on the specifics of the clinical scenario. Older cost-effectiveness analyses incorporated follow-up ischemia testing after a positive CAC scan18, 19 (which is not currently recommended12) and used assumptions about CAC risk stratification that predate MESA and other modern cohort studies,17-19 and are therefore not directly comparable.
Our results are also consistent with prior cost-effectiveness analyses showing that statin therapy can be cost-effective even in lower-risk patients, assuming it remains efficacious, if it is inexpensive and does not significantly reduce quality of life.27, 36, 37 While $4/month programs available at discount pharmacy outlets should provide access to very low cost statins, most Americans do not avail themselves of these programs,26 and the costs of even generic statins are substantially higher at retail pharmacies.27, 36 The decrement in quality of life associated with statin use is similarly hard to parameterize for cost-effectiveness modeling. Patients often express a preference not to take statins, which presumably reflects an expected impact on quality of life; but it is difficult to quantify that impact for any given individual, and there is wide variation between individuals and within individuals over time (and with experience and education). Our approach, though imperfect, is consistent with prior work38 and current guidelines5 that suggest that patient preferences and quality of life are important to consider when making decisions about statin prescribing and CAC testing.
The cost-effectiveness of CAC testing is also somewhat sensitive to assumptions about the effectiveness of risk stratification provided by the CAC test. According to our best estimates from MESA11 (used for these analyses), a previous meta-analysis,39 and recent results from other cohort studies,40-42 the relative risks associated with high CAC scores are large. With degradation in those relative risk estimates, CAC testing is no longer cost-effective (Table 2). This degradation might occur if scanning technique and interpretation are lower quality in practice than they have been in research studies, or if research estimates of CAC prediction were otherwise over-optimistic, such as might occur from the lack of blinding to CAC scores that has been common in cohort studies. This sensitivity to assumptions creates a powerful rationale for real-world effectiveness testing with a clinical trial, as has been proposed43; such a trial, however, would be very expensive and would take many years to complete.
Our finding that the “>0” CAC threshold strategy dominates higher CAC score thresholds is somewhat surprising. Higher CAC scores are associated with substantially higher relative risk for CHD (Supplemental Table 1), and anecdotes suggest that higher scores are often used in clinical practice for decision-making. The low prevalence of high CAC scores, however, make these high treatment threshold strategies inefficient (i.e., the “number needed to scan” is too high). While surprising, our findings are consistent with prior commentary on this issue44.
Our analysis was limited in a number of ways. We used estimates of statin efficacy from a meta-analysis that included patients with moderately high CHD risk (Supplemental Material),1 but we applied those efficacy estimates in scenarios where risk is lower and the evidence for statin therapy effectiveness is less established. Our results were robust to substantial degradation in statin efficacy, but clearly would not hold if statins were ineffective in any given scenario. The excess cancer caused by radiation may vary with age, sex, scan technique and other factors15, 32; and radiation may also induce a small increase in CHD rates.45 We did not model these intricacies, but our analyses reveal little sensitivity to this parameter. Given the lower prevalence of CAC in non-White populations46, we would expect some differences in cost-effectiveness across race/ethnicity, but did not attempt these analyses. We illustrated our results by indicating which strategy would be preferred if a simple (and traditional/”mythical”47) willingness-to-pay threshold of $50,000/QALY were used by policymakers. Some have suggested that a higher threshold is more consistent with societal values,48 or that using any simple threshold is unrealistic47. We agree, and have provided continuous ICER values throughout to enable alternate illustrations and interpretation. We did not attempt to model the possibility that CAC testing might stimulate statin adherence, or to analyze other uses of CAC testing, such as to guide aspirin or antihypertensive therapy, to screen symptomatic patients, repeated use over time, etc.
Our cost-effectiveness analysis supports a limited role for CAC testing in asymptomatic persons. When statins are expected to be effective, safe, and inexpensive, and the patient does not have a strong preference against taking the medication, our analyses suggest that the decision to prescribe a statin is relatively straightforward and that CAC testing is neither necessary nor cost-effective. The decision to use a statin, however, is often difficult for clinicians and patients. In these settings, the additional information about CHD risk that CAC testing provides can be worth the additional expense and radiation exposure that comes with the test.
Supplementary Material
ACKNOWLEDGEMENTS
None
FUNDING
This work was funded by the National Heart, Lung and Blood Institute (R21HL112256). Dr. Phillips was also supported by the National Cancer Institute (P01CA130818), and Dr. Auer by the Swiss National Science Foundation (PBLAP3-142761). None of the funders had any role in design and conduct of the study, interpretation of the data, preparation, review or approval of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
DISCLOSURES
No conflicts of interest are reported.
REFERENCES
- 1.Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering ldl cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law M, Rudnicka AR. Statin safety: A systematic review. Am J Cardiol. 2006;97(Supplement) doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Redberg RF, Katz MH. Healthy men should not take statins. JAMA. 2012;307:1491–1492. doi: 10.1001/jama.2012.423. [DOI] [PubMed] [Google Scholar]
- 4.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW. acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2013 doi: 10.1016/j.jacc.2013.11.002. 2013. [DOI] [PubMed] [Google Scholar]
- 6.Brindle P, Beswick A, Fahey T, Ebrahim S. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: A systematic review. Heart. 2006;92:1752–1759. doi: 10.1136/hrt.2006.087932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM. Cardiovascular risk prediction: Basic concepts, current status, and future directions. Circulation. 2010;121:1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 8.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ, Wilson PW. acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2013 doi: 10.1016/j.jacc.2013.11.005. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaha MJ, Nasir K, Blumenthal RS. Statin therapy for healthy men identified as “increased risk”. JAMA. 2012;307:1489–1490. doi: 10.1001/jama.2012.425. [DOI] [PubMed] [Google Scholar]
- 10.Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, Humphrey LL. Emerging risk factors for coronary heart disease: A summary of systematic reviews conducted for the u.S. Preventive services task force. Ann Intern Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 11.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr., Stein JH, Tracy CM, Vogel RA, Wesley DJ. Accf/aha 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the american college of cardiology foundation clinical expert consensus task force (accf/aha writing committee to update the 2000 expert consensus document on electron beam computed tomography). Circulation. 2007;115:402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 13.Polonsky TS, McClellan RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KP, Einstein AJ, Berrington de Gonzalez A. Coronary artery calcification screening: Estimated radiation dose and cancer risk. Arch Intern Med. 2009;169:1188–1194. doi: 10.1001/archinternmed.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pletcher MJ, Sibley C, Pignone M, Vittinghoff E, Greenland P. Interpretation of the coronary artery calcium score in combination with conventional cardiovascular risk factors: The multi-ethnic study of atherosclerosis (mesa). Circulation. 2013;128:1076–1084. doi: 10.1161/CIRCULATIONAHA.113.002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, Fayad Z, Budoff MJ, Rumberger J, Naqvi TZ, Shaw LJ, Faergeman O, Cohn J, Bahr R, Koenig W, Demirovic J, Arking D, Herrera VL, Badimon J, Goldstein JA, Rudy Y, Airaksinen J, Schwartz RS, Riley WA, Mendes RA, Douglas P, Shah PK. From vulnerable plaque to vulnerable patient--part iii: Executive summary of the screening for heart attack prevention and education (shape) task force report. Am J Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 18.O'Malley PG, Greenberg BA, Taylor AJ. Cost-effectiveness of using electron beam computed tomography to identify patients at risk for clinical coronary artery disease. Am Heart J. 2004;148:106–113. doi: 10.1016/j.ahj.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Shaw LJ, Raggi P, Berman DS, Callister TQ. Cost effectiveness of screening for cardiovascular disease with measures of coronary calcium. Prog Cardiovasc Dis. 2003;46:171–184. doi: 10.1016/s0033-0620(03)00085-9. [DOI] [PubMed] [Google Scholar]
- 20.Sniderman AD, Thanassoulis G, Lawler PR, Williams K, Furberg CD. Comparison of coronary calcium screening versus broad statin therapy for patients at intermediate cardiovascular risk. Am J Cardiol. 2012;110:530–533. doi: 10.1016/j.amjcard.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 21.van Kempen BJ, Spronk S, Koller MT, Elias-Smale SE, Fleischmann KE, Ikram MA, Krestin GP, Hofman A, Witteman JC, Hunink MG. Comparative effectiveness and cost-effectiveness of computed tomography screening for coronary artery calcium in asymptomatic individuals. J Am Coll Cardiol. 2011;58:1690–1701. doi: 10.1016/j.jacc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 22.Pletcher MJ, Pignone M. Evaluating the clinical utility of a biomarker: A review of methods for estimating health impact. Circulation. 2011;123:1116–1124. doi: 10.1161/CIRCULATIONAHA.110.943860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pignone M, Earnshaw S, Pletcher MJ, Tice JA. Aspirin for the primary prevention of cardiovascular disease in women: A cost-utility analysis. Arch Intern Med. 2007;167:290–295. doi: 10.1001/archinte.167.3.290. [DOI] [PubMed] [Google Scholar]
- 24.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: A cost-utility analysis. Ann Intern Med. 2006;144:326–336. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Gellad WF, Zhou L, Lin YJ, Lave JR. Access to and use of $4 generic programs in medicare. J Gen Intern Med. 2012;27:1251–1257. doi: 10.1007/s11606-012-1993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pletcher MJ, Lazar L, Bibbins-Domingo K, Moran A, Rodondi N, Coxson P, Lightwood J, Williams L, Goldman L. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150:243–254. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 28.Ingenix, Inc. A comprehensive listing of rbrvs values for cpt and hcpcs codes. St. Anthony's Publishing; 2011. [Google Scholar]
- 29.Machaalany J, Yam Y, Ruddy TD, Abraham A, Chen L, Beanlands RS, Chow BJ. Potential clinical and economic consequences of noncardiac incidental findings on cardiac computed tomography. J Am Coll Cardiol. 2009;54:1533–1541. doi: 10.1016/j.jacc.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Hlatky MA, Iribarren C. The dilemma of incidental findings on cardiac computed tomography. J Am Coll Cardiol. 2009;54:1542–1543. doi: 10.1016/j.jacc.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Kooter AJ, Kostense PJ, Groenewold J, Thijs A, Sattar N, Smulders YM. Integrating information from novel risk factors with calculated risks: The critical impact of risk factor prevalence. Circulation. 2011;124:741–745. doi: 10.1161/CIRCULATIONAHA.111.035725. [DOI] [PubMed] [Google Scholar]
- 32.Einstein AJ. Effects of radiation exposure from cardiac imaging how good are the data? J Am Coll Cardiol. 2012;59:553–565. doi: 10.1016/j.jacc.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: A meta-analysis. Can J Cardiol. 2012;28:574–580. doi: 10.1016/j.cjca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Tolman KG. The liver and lovastatin. Am J Cardiol. 2002;89:1374–1380. doi: 10.1016/s0002-9149(02)02355-x. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the jupiter trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–153. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell AP, Simpson RJ. Statin cost effectiveness in primary prevention: A systematic review of the recent cost-effectiveness literature in the united states. BMC Res Notes. 2012;5:373. doi: 10.1186/1756-0500-5-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 39.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: A systematic review and meta-analysis. Arch Intern Med. 2004;164:1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 40.Elias-Smale SE, Proenca RV, Koller MT, Kavousi M, van Rooij FJ, Hunink MG, Steyerberg EW, Hofman A, Oudkerk M, Witteman JC. Coronary calcium score improves classification of coronary heart disease risk in the elderly: The rotterdam study. J Am Coll Cardiol. 56:1407–1414. doi: 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 41.Mohlenkamp S, Lehmann N, Moebus S, Schmermund A, Dragano N, Stang A, Siegrist J, Mann K, Jockel KH, Erbel R. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J Am Coll Cardiol. 2011;57:1455–1464. doi: 10.1016/j.jacc.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 42.Kavousi M, Elias-Smale S, Rutten JH, Leening MJ, Vliegenthart R, Verwoert GC, Krestin GP, Oudkerk M, de Maat MP, Leebeek FW, Mattace-Raso FU, Lindemans J, Hofman A, Steyerberg EW, van der Lugt A, van den Meiracker AH, Witteman JC. Evaluation of newer risk markers for coronary heart disease risk classification: A cohort study. Ann Intern Med. 2012;156:438–444. doi: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 43.Ambrosius WT, Polonsky TS, Greenland P, Goff DC, Jr., Perdue LH, Fortmann SP, Margolis KL, Pajewski NM. Design of the value of imaging in enhancing the wellness of your heart (view) trial and the impact of uncertainty on power. Clin Trials. 2012;9:232–246. doi: 10.1177/1740774512436882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blaha MJ, Blumenthal RS, Budoff MJ, Nasir K. Understanding the utility of zero coronary calcium as a prognostic test: A bayesian approach. Circ Cardiovasc Qual Outcomes. 2011;4:253–256. doi: 10.1161/CIRCOUTCOMES.110.958496. [DOI] [PubMed] [Google Scholar]
- 45.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, Harrison JD, Hildebrandt G, Ivanov V, Kashcheev VV, Klymenko SV, Kreuzer M, Laurent O, Ozasa K, Schneider T, Tapio S, Taylor AM, Tzoulaki I, Vandoolaeghe WL, Wakeford R, Zablotska LB, Zhang W, Lipshultz SE. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–1511. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: Results from the multi-ethnic study of atherosclerosis (mesa). Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 47.Weinstein MC. How much are americans willing to pay for a quality-adjusted life year? Med Care. 2008;46:343–345. doi: 10.1097/MLR.0b013e31816a7144. [DOI] [PubMed] [Google Scholar]
- 48.Braithwaite RS, Meltzer DO, King JT, Jr., Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.