Abstract
Background
The left atrium is a validated marker of clinical and subclinical cardiovascular disease. Left atrial enlargement is often seen among older individuals, however there are few population-based data regarding normal left atrial size among older persons, especially from those who are healthy, and from women. Furthermore, since the left atrium is a three dimensional structure, the commonly used parasternal long axis diastolic diameter often underdiagnoses left atrial enlargement.
Methods and Results
We evaluated left atrial size in 230 healthy participants (mean age 76±5 years) free of prevalent cardiac disease, rhythm abnormality, hypertension, and diabetes selected from the Cardiovascular Health Study, a prospective community based study of risk factors for cardiovascular disease in 5,888 elderly participants. In addition to the standard long axis measurement, we obtained left atrial supero-inferior and lateral diameters and used these dimensions to estimate left atrial volume. These measurements were used to generate reference ranges for determining left atrial enlargement in older men and women, based on the 95% percentiles of the left atrial dimensions in healthy participants, both unadjusted, and after adjustment for age, height and weight. In healthy elderly subjects, indices of left atrial size do not correlate with age or height but with weight and other measures of body build.
Conclusions
These data provide normative reference values for left atrial size in healthy older women and men. The results should be useful for refining diagnostic criteria for left atrial dilation in the older population and may be relevant for cardiovascular risk stratification.
Keywords: echocardiography, atrium, diastole, diagnosis, aging
Left atrial (LA) size is increased in a variety of cardiovascular disorders, and is an indicator of chronically increased left atrial pressure and / or volume (1–5). Since left atrial size can be measured noninvasively by echocardiography, it can be a convenient indicator of overall cardiovascular health (6, 7).
Measurement of LA size is part of the standard echocardiographic examination. Traditionally, maximal end-systolic LA dimension is obtained from the parasternal long axis view, which represents a sagittal view of the heart, and thus evaluates the anterior-posterior dimension of the chamber. However the LA, like all cardiac structures, is three-dimensional and its enlargement may result in an asymmetrical geometry. Therefore measurement of a single LA diameter may underestimate actual LA size. For these reasons, multiple linear dimensions or measurement of LA volume might be preferable to the standard parasternal long axis measurement in view of data which suggest that this single linear dimension may lack sensitivity for diagnosing LA enlargement (7–12). Furthermore, since the prevalence of LA enlargement increases with age, it is critical to have accurate normative reference values to correctly assess cardiovascular risk stratification in the elderly. At present, there is a dearth of such reference data in both population based samples and in women. The population-based Cardiovascular Health Study (CHS) presented an opportunity to obtain such reference values.
METHODS
Study population
The CHS, designed to assess cardiovascular disease, its outcome, and risk factors in older individuals, identified adults from the Health Care Financing Administration Medicare enrollment lists in four U.S. communities, along with other household members aged 65 and older at enrollment (13–15). Recruitment centers were located in Washington County, Maryland; Forsyth County, North Carolina; Sacramento County, California; and Allegheny County, Pennsylvania. Exclusion criteria for the Cardiovascular Health Study included active treatment for cancer, being wheelchair-bound or institutionalized, or being unable to participate in the examination (13–15). Prevalent coronary artery disease, stroke and heart failure did not exclude participants from study enrollment. Including both the original cohort, recruited in 1989–90, and those enrolled in 1992–93 when the study was expanded to include more African-Americans, 5,888 participants were enrolled. Details of the design, sampling, and recruitment, as well as the interview and examination, have been published previously (13–15). Self-report of cardiovascular diseases at baseline was validated according to standardized criteria through assessment of medications, medical records and/or by relevant information obtained during the initial examination. Echocardiography was performed in 1989–90, and again in 1994–5.
In the initial reading of the echocardiograms, only LA anteroposterior diameter was measured (16). In a subset of CHS participants, echocardiogram videotapes recorded in 1994–1995 were re-read by 3 independent readers (GPA, JSG, DK), to obtain additional measures of LA size and geometry. The subset included participants with heart failure at the time of the second echocardiogram (1994–95), those who developed congestive heart failure after the second echocardiogram and prior to June 30, 2000, and controls matched to heart failure cases on age and sex. Participants without an interpretable echocardiogram and those with any grade of significant aortic stenosis (defined as visibly evident decrease in leaflet excursion and/or transaortic peak velocity>1.9 m/sec) or mitral stenosis (identified qualitatively by valve thickening and limitation of leaflet excursion), greater than 2+ mitral regurgitation, or aortic regurgitation greater than 1+ were excluded from analysis. The current report includes participants from the control group who did not have any cardiovascular disease (CVD), defined as transient ischemic attack, stroke, myocardial infarction (MI), angina, revascularization or claudication, heart failure, atrial fibrillation, and who had no history of hypertension or diabetes and were not using beta blockers, angiotensin-converting enzyme (ACE) inhibitors, digitalis, or warfarin at the time of the echocardiography examination in 1994–95. As suggested by current practice guidelines, presence of hypertension was defined as self-report of hypertension and/or taking antihypertensive medications; accordingly incidental high blood pressure values were not used for the diagnosis of hypertension. Diabetes was defined as taking insulin or oral hypoglycemic agents at any time from baseline to the 1994–95 visit, or reporting diabetes at any time in that interval or having a fasting glucose level ≥ 126 mg/dl in 1992–93 when fasting glucose was evaluated. This group of CVD healthy participants included 132 women and 98 men.
Echocardiography
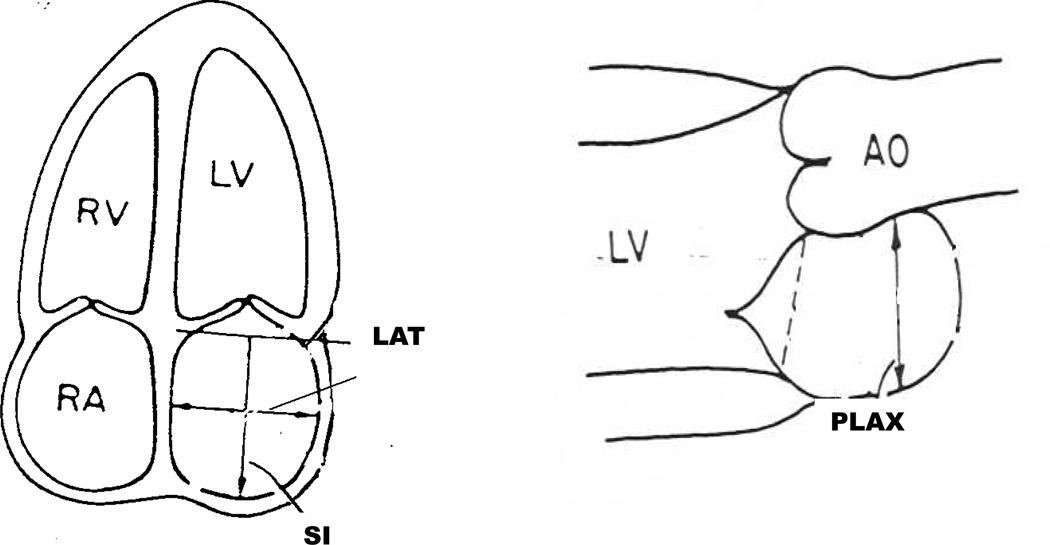
The design for echocardiographic study of participants in the Cardiovascular Health Study has been extensively described (16). All echocardiograms were interpreted, blinded to clinical information, at a centralized core echocardiography laboratory. Linear LA dimensions were measured in three orthogonal planes: parasternal long axis (PLAX), lateral (LAT), and supero-inferior (SI) (see Figure 1). All linear dimensions were measured at the end of ventricular systole determined from the peak of the R wave on the accompanying ECG tracing. The PLAX was taken, in the parasternal long axis view, from the leading edge of the posterior aortic wall just distal to the aortic leaflets perpendicularly to the leading edge of the posterior LA wall. The LAT and SI dimensions were both taken from the apical four chamber view using inner edge to inner edge measurement. The SI dimension was defined by a line bisecting the LA extending from the mid point of the mitral annulus to the midpoint of the superior (cephalad) LA border. The LAT was taken from a perpendicular constructed from the midpoint of the superoinferior dimension extending to the atrial borders. As suggested by current guidelines (17), LA volume (V) was calculated utilizing the length diameter ellipsoid method computed at ventricular end-systole (9,18), applying the following equation: V = 4π/3 × (PLAX/2) × (LAT/2) × (SI/2).
Figure 1.
Schematic representation of the measurement of left atrial volume. The PLAX was taken in the parasternal long axis view. The LAT and SI dimensions were both taken from the apical four chamber view using inner edge to inner edge measurement. LA volume was calculated utilizing the length diameter ellipsoid method, applying the following equation: V = 4π/3 × (PLAX/2) × (LAT/2) × (SI/2).
Statistical Methods
Distributions of the LA size parameters and selected covariates were summarized by their mean, standard deviation, range and the 5th, 50th and 95th percentiles of the observed distributions in the healthy subgroup, separately for men and women. Associations of LA size parameters with age, weight, standing height, heel to knee length, body surface area and body mass index were determined by partial correlation coefficients, adjusted for sex. Regression models were used to test for differences between men and women in their bivariate associations of age or body size with LA measurements, by incorporating an interaction between age or body size and sex. The relationships of PLAX, SI and LA volume to body surface area were explored with bivariate scatterplot smoothers to assess the appropriateness of a linear model. Linear regression was used to estimate the relationships of the size parameters to BSA, and the results plotted along with the 95% prediction intervals.
In order to develop reference ranges that adjust for differences in body size, regression models were created for each LA size parameter in men and women separately, with age, height and weight as independent predictors. For each model, the 95th percentile of the distribution of the residuals, defined as the observed minus the predicted, was used to define the age and body size adjusted upper limit of normal for the LA parameter. The reported reference equations determine the predicted value of the LA parameter, given a participant′s sex, age, weight and height. The width of the interval above that prediction, when added to the predicted value, defines the upper limit of normal.
Results
LAD and LAV relation to gender and body size
Table 1 shows the mean as well as 5th, 50th, and 95th percentiles values for PLAX, SI, and LAT and LAV, for men and women free of CVD, diabetes, and hypertension. As expected, PLAX and LA volume were significantly larger in men than women (p≤ 0.002): LASI was also marginally (but significantly) larger in men (p=0.051). There were no differences in LAT by sex. LA dimensions increased with weight, BMI and BSA (p≤ 0.003 for all). The strongest associations across all dimensions were with weight and its derivative BMI (Table 2). The only significant interaction by sex was the association of age with LASI (p=0.041).
Table 1.
Reference Ranges for Left Atrial Dimension and Volume and Selected Clinical Variables
| Percentile | ||||||
|---|---|---|---|---|---|---|
| Linear dimensions | Sex | Mean | Range | 0.05 | 0.50 | 0.95 |
| PLAX, cm | Female | 3.0 ± 0.5 | 1.5–4.2 | 2.2 | 3.0 | 3.9 |
| Male | 3.4 ± 0.6 | 2.1–4.8 | 2.4 | 3.3 | 4.4 | |
| SI, cm | Female | 4.6 ± 0.7 | 2.8–6.1 | 3.6 | 4.6 | 5.7 |
| Male | 4.8 ± 0.7 | 3.3–6.7 | 3.7 | 4.7 | 6.1 | |
| LAT, cm | Female | 3.6 ± 0.6 | 1.9–5.2 | 2.4 | 3.6 | 4.6 |
| Male | 3.8 ± 0.6 | 2.4–5.1 | 2.6 | 3.7 | 4.7 | |
| Volume | ||||||
| LAV, mL | Female | 27.4 ± 10.0 | 8.7–53.9 | 12.3 | 26.4 | 46.7 |
| Male | 32.0 ± 10.9 | 13.9–71.5 | 17.2 | 30.1 | 58.2 | |
| Clinical Data | Sex | Mean | Range | 0.05 | 0.50 | 0.95 |
| Age, yrs | Female | 76.5 ± 4.9 | 68–91 | 70 | 76 | 85 |
| Male | 77.3 ± 5.6 | 68–93 | 70 | 75 | 88 | |
| Height, in | Female | 62.8 ± 2.2 | 57.9–68.1 | 58.4 | 63.0 | 66.2 |
| Male | 67.9 ± 2.5 | 60.4–75.2 | 63.8 | 67.8 | 71.7 | |
| Weight | Female | 146 ± 29 | 84–233 | 96 | 144 | 193 |
| Male | 173 ± 26 | 114–254 | 132 | 170 | 222 | |
| BSA | Female | 1.66 ± 0.17 | 1.27–2.07 | 1.37 | 1.66 | 1.93 |
| Male | 1.89 ± 0.16 | 1.48–2.37 | 1.60 | 1.88 | 2.13 | |
| BMI | Female | 25.9 ± 4.6 | 15.2–39.5 | 17.9 | 25.2 | 34.0 |
| Male | 26.3 ± 3.2 | 18.4–37.6 | 21.6 | 26.0 | 31.7 | |
| Systolic BP | Female | 128 ± 18 | 84–181 | 99 | 126 | 158 |
| Male | 130 ± 17 | 88–211 | 109 | 127 | 158 | |
| Diastolic BP | Female | 68.4 ± 9.8 | 42–92 | 51 | 69 | 83 |
| Male | 70.8 ± 9.6 | 49–110 | 56 | 70 | 87 | |
Abbreviations as in text
Table 2.
Partial Correlation coefficients (p-values) of age and body size measures with LA parameters, adjusted for sex.
| Variable | PLAX | LAT | SI | LAV |
|---|---|---|---|---|
| N: | 220 | 202 | 207 | 197 |
| Age | .09 (.19) |
−.06 (.39) |
−.11 (w)1 (.23) .17 (m) (.11) |
.02 (.79) |
| Weight | .31 (<.001) |
.23 (.001) |
.33 (<.001) |
.41 (<.001) |
| Height | .01 (.85) |
.07 (.29) |
.06 (.42) |
.08 (.27) |
| Heel-knee length | .11 (.09) |
.14 (.047) |
.22 (.002) |
.22 (.002) |
| BSA | .26 (<.001) |
.20 .003 |
.29 (<.001) |
.35 (<.001) |
| BMI | .35 (<.001) |
.24 .003 |
.36 (<.001) |
.44 (<.001) |
p-values are in parentheses below the correlation coefficients.
bivariate correlations for women (w) and men (m) separately due to significant interaction.
Linear models accounted for 10–24% of the variability in LA dimensions, with the combination of height and weight equivalent to that of BMI, and each explaining more of the variability in LA linear dimension than BSA.
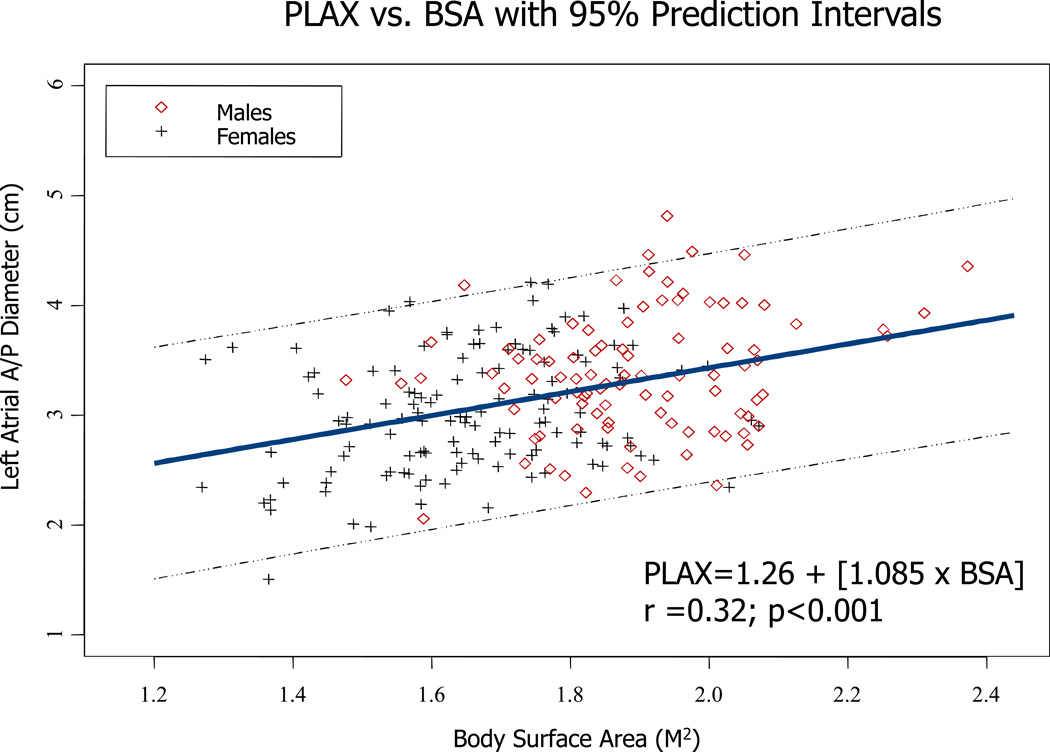
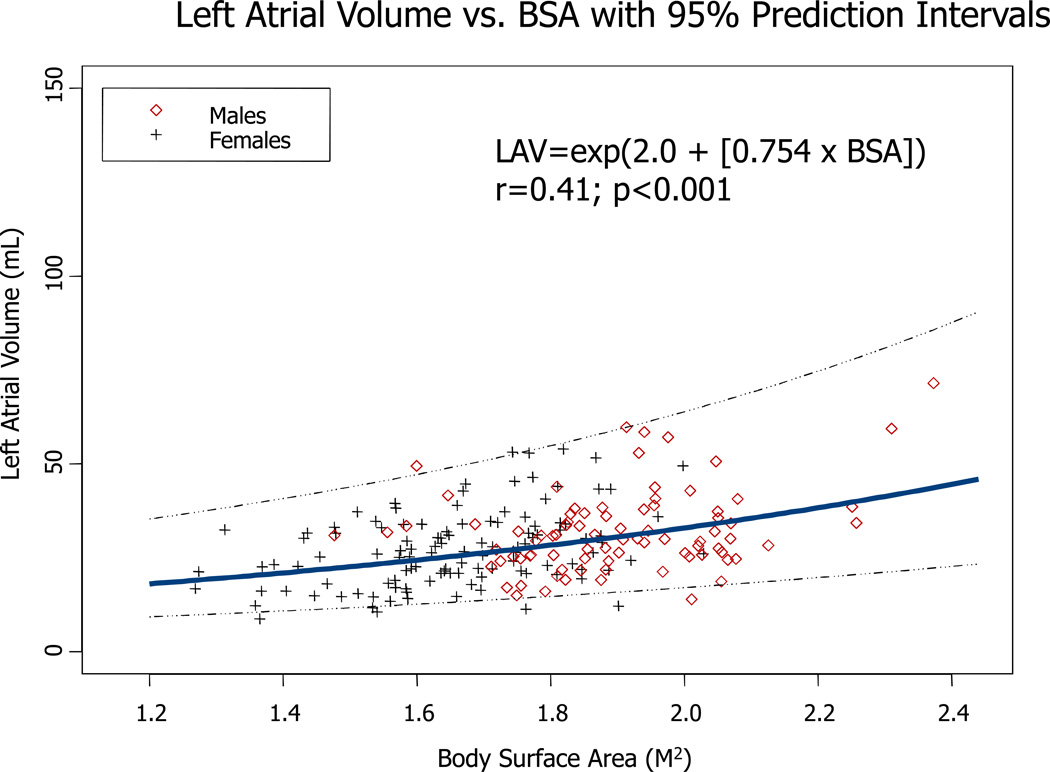
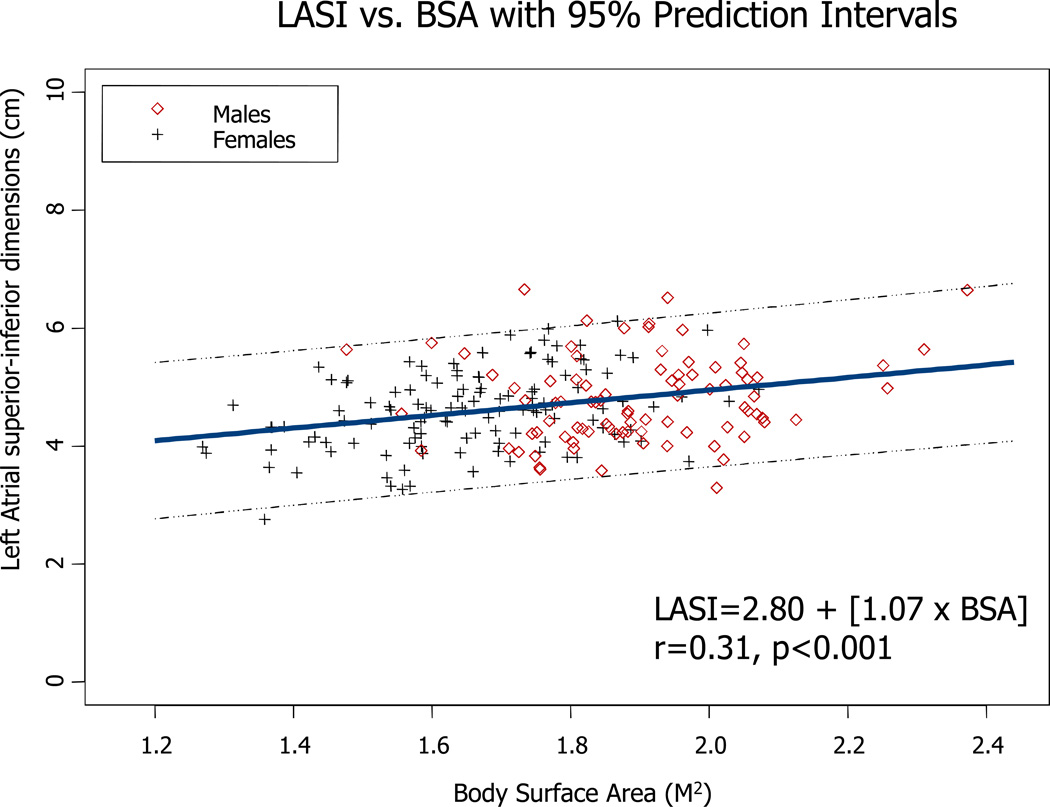
For LAV, models incorporating body size, age, and sex accounted for 18% to 24% of the variability in this parameter. Similar to linear dimensions, models using BSA had the lowest R2 values. Figures 2–4 illustrate the direct relationship between LA PLAX dimension, LA SI dimension, and LA volume and body surface area. Regression equations with both r and p values are provided.
Figure 2.
Regression between LA PLAX dimension and body surface area in both genders. Regression equation and 95% prediction interval are presented.
Figure 4.
Regression between LA volume and body surface area in both genders Regression equation and 95% prediction interval are presented.
In Table 3 we provide reference equations for estimating whether LA dilation is present for linear dimensions and volume, where dilation is defined as a value above the 95th percentile in healthy older adults, after adjusting for age, height and weight. Although height was not associated with the LA parameters in bivariate models, once adjusted for weight, height become significant in several of the models, so was included in the reference equations. Similarly, age was not significantly correlated with LA parameters in bivariate models, but once stratified by sex and adjusted for body size, age was significant in all of the models for men, and for consistency, was included in all of the reference equations. Additional analysis was also performed to test the effect of adjustment for body size on the diagnosis of LA enlargement. Based on our reference equations, we identified participants who would be classified as having elevated LA parameters and compared them against those whose values exceeded the sex-specific 95th percentiles of the LA parameter distributions, unadjusted for body size. Of the 10 healthy participants exceeding the sex-specific 95th percentile for PLAX, 4 were classified normal after using our reference equations to adjust for height and weight. For LASI, 2/10 were reclassified normal, and for volume 2/9 reclassified normal. Conversely, of the 213 participants below the sex-specific 95th percentile of the PLAX distribution, 4 (1.9%) were reclassified as having high PLAX when body size was considered. For LASI, 2/200 (1%) were reclassified as high and for LA volume, 2/191 (1%) were reclassified as high. Perhaps more informative, considering all 848 participants with any LA measures, of those classified normal by the 95th percentile, 4.3%, 3.2% and 5.7% were reclassified as high for PLAX, LASI and LA volume, respectively once body size was considered. Of those classified high by the 95th percentile, 15.5%, 21.3% and 8.1% were reclassified as normal for PLAX, LASI and LA volume, respectively, once body size was considered.
Table 3.
Reference Equations for Selected LA Parameters
| Predicted Value | Interval width of Normal 1 |
R2 | |
|---|---|---|---|
| PLAX | |||
| women | 3.82 + 0.015 age – 0.051 height(in) + 0.0085 weight(lb) 3.82 + 0.015 age – 1.995 height(m) + 0.019 weight(kg) |
0.86 | 0.16 |
| men | 1.79 + 0.024 age – 0.028 height(in) + 0.0095 weight(lb) 1.79 + 0.024 age – 1.095 height(m) + 0.021 weight(kg) |
0.82 | 0.15 |
| LASI | |||
| women | 4.95 + 0.0006 age – 0.030 height(in) + 0.0105 weight(lb) 4.95 + 0.0006 age – 1.198 height(m) + 0.023 weight(kg) |
0.95 | 0.17 |
| men | 4.01 + 0.035 age – 0.059 height(in) + 0.012 weight(lb) 4.01 + 0.035 age – 2.33 height(m) + 0.27 weight(kg) |
1.30 | 0.14 |
| LAV | |||
| women | 47.7 + 0.150 age – 1.02 height(in) + 0.223 weight(lb) 47.7 + 0.150 age – 40.20 height(m) + 0.492 weight(kg) |
15.1 | 0.28 |
| men | −20.6 + 0.524 × age – 0.32 height(in) + 0.195 weight(lb) −20.6 + 0.524 × age –12.60height(M) + 0.431 weight(kg) |
20.6 | 0.18 |
Value to add to the predicted value to reach the upper limit of the normal range.
Discussion
In a subgroup of the CHS without cardiovascular disease, hypertension, or diabetes, indices of LA size and volume increased with increasing body size and were larger in men than in women. The variability in body size, age, and sex explained up to 24% of the variability of LA size in the reference subjects. As the correlation matrix provided in Table 2 shows, the correlation between LA dimensions and either BSA or BMI were stronger than those seen between LA dimensions and height.
Relationship to Prior Studies
There have been many studies of LA dimension and LA volume in a variety of subject groups (19–31). Some of the relevant studies are summarized in Tables 4 and 5. Only three prior studies have established normal reference ranges for apical four chamber long axis dimension (23,30–31), but none of these studies contained data on substantial numbers of older individuals, nor were the data broken down by gender. Thus the data presented herein are likely to be relevant to diagnosis of LA enlargement in the current population of patients encountered by clinicians.
Table 4.
Left Atrial Linear Dimensions: Prior Studies.
| Author | Year | N |
Mean Age |
Population | LA-PLAX | LASI** | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| men | women | total | men | women | total | |||||
| Triulzi (2D) | 1984 | 84 | 39 | volunteers | - | - | 3.0±0.3 | - | - | 4.1±0.6 |
| Tsang (M-mode) | 2001 | 1655 | - | cross sectional - AF patients excluded | - | - | 4.1±0.8 | - | - | - |
| Schnittger(2D) | 1983 | 35 | 18–60 | - | 2.7–4.5 (3.6)* | - | - | 5.1 (4.1–6.1) | ||
| Gottdiener (M-mode) | 1995 | - | - | VA population | - | - | - | - | - | - |
| Lauer (M-mode) | 1995 | men=288 women=524 | 20–45 36±10 | “healthy restricted” | 3.8±0.4 (3.0–4.4) | 3.3±0.3 (2.7–4.0) | ||||
| Vasan (M-mode) | 1997 | 1099 | 20–45 | healthy cohort | 4.2–4.5 | 3.7–4.0 | - | - | ||
| Gardin (M-mode) | 1979 | 136 | 20–97 | healthy population | - | - | 2.7–4.2 | - | - | |
| Henry (M-mode) | 1978 | 105 | 1d-23 | normal | 4.3 | |||||
| Manolio | 2002 | 3882 | CHS population>65 | 3.9* | 4.2* | - | ||||
| (2D–directed M-mode) | without heart disease | up to 5.2 | up to 4.8 | |||||||
| Pearlman | 1990 | 72 | NA | hospital employees | 4 | |||||
| Pritchett (2D-directed M-mode) | 2003 | 767 | Olmstead County | up to 4.2 | up to 4.6 | 3.7±0.51 | ||||
| current study (2-D) | 2008 | 230 | 2D | CHS population>65 | 3.4±0.6 (2.5–4.8) | 3.0±0.5 (1.5–4.2) | 4.8±0.7 | 4.6±0.7 | ||
Mean values are reported
measurements obtained in the short axis view
no prior study gives gender-specific values for this parameter
Table 5.
Left Atrial Volumes: Prior Studies.
| Author | Year | n | Age | Population | Formula | Mean | Men | Women |
|---|---|---|---|---|---|---|---|---|
| Lester | 1999 | 98 98 98 |
59 59 59 |
random patients; prosthetic valve excluded |
M-mode/sphere V=4/3 Π r3 from M-mode LA LA dimension (PLA) single plane A4C MOD biplane MOD |
39±20 58±30 64±30 |
- - - |
- - - |
| Tsang | 2001 | 1655 | 75±7 no AF | retrospective | modified biplane A-L method |
62±25 | ||
| Wang | 1984 | 51 | 37 | normal | biplane MOD |
35±10 | 38±10 | 32±10 |
| Ren | 1983 | 12 | 53±12 (23–65) |
normal | area-length | 43±13 | 46±16 | 40±10 |
| Pearlman | 1990 | 72 | "adults" | Hospital employees |
V=4/3 π r1r2r3 | 42 upper limit of normal |
- | - |
| Pritchett (2D directed M-mode) |
2003 | 56±7 | Olmstead County “reference group” |
V=4/3 π r1r2r3 | 42±11 | |||
| Current Study (2-D) |
2008 | 230 | 77 | CHS population V=4 π /3 r1r2r3 |
V=4/3 π r1r2r3 | 32±11 | 27±10 |
LA dimensions
For PLAX LA dimension, the 95th percentile values were 3.9 and 4.4 cm for women and men, somewhat smaller than those reported by Pritchett (9) and others (Table 5). There are several potential explanations for why our values for PLAX dimensions are smaller. We believe that the principal reasons are that, first, we employed direct, on-screen 2-dimensional measurements, rather than M-mode strip-chart measurements (9). Second, since our population was extremely well characterized, we had more of an opportunity to exclude comorbid conditions, common preclinical risk factors, and any evidence of prevalent cardiovascular disease. Third, most of the studies summarized in Table 5 included younger participants than those in our study.
By contrast, there are very few data in the literature with which to compare our results for SI dimensions. Our 95th percentile values of 5.6 cm in women and 6.1 cm in men are considerably higher than the 95th percentile value of 5.3 cm reported by Triulzi (30) in his study of 84 normal individuals, mean age 39 (range 15–76) and in a subsequent paper from the same institution, which gave a similar value for 72 normal individuals. Differences were likely due to the younger age of the participants in the Triulzi study, and likely due to differences in body size as well, since most adults gain weight throughout middle age. For this reason, we have also provided reference equations to identify upper limits of normal values adjusted for age and body size.
As has been shown in prior studies, we found a direct relationship between linear LA dimensions and body surface area in both men and women. As has been previously demonstrated in the Framingham study (24,26), parameters of LA size do not correlate strongly with height. Nevertheless, it is interesting to highlight the significant correlation found between LA size and heel-to-knee length (Table 2), suggesting that the association of height and LA size may be partially masked by the probable age-related height reduction in our sample of elderly individuals.
LA Volume
In previous studies which have examined LA volumes, biplane area-length (8,9,11), Simpson′s rule (8,23) and other methods have been employed. The method employed in this study was first established in an angiographic study (5), which models the LA as an ellipse. Given the three dimensional nature of the LA, volume measurements are a more desirable approach to quantitation, in that they integrate linear dimensions (8,9). Moreover, in disease states, it is possible that the atrium enlarges asymmetrically, since enlargement in the anteroposterior dimension (represented by the PLAX) may be limited by the spine. Lester (8) has shown a substantial underestimation of LA volumes when the single M-mode PLA dimension is used in the ellipsoid volume formula, compared with results when either an apical single or biplane method of disks is used (Table 5). More recent studies have also confirmed these findings by three dimensional echocardiography or cardiac MRI (12). Our results confirm these findings; using median values for men, on average, the LA volume would be 42% smaller than that obtained when the three different linear dimensions are used.
As in previous studies (9), we used simple linear dimensions to obtain LA volume, and we believe that the ease of measurement would enable widespread clinical use. Our 95th percentile values for LA volume were, respectively, 46.7 ml in women and 58.2 ml in men, which is somewhat larger than the values obtained by Wang (31) in a series of normal individuals, mean age of 51 (26). These investigators also showed a gender difference in LA volumes, as our data confirm. The 50th percentile value reported by Tsang et al. (10) in the subgroup of older individuals without atrial fibrillation was 62 ml (no gender-based values given) which is larger than the values reported in the present study. However in this study, hypertensive patients as well as patients with mitral regurgitation and/or prevalent MI or CAD were included. In our study, by contrast, we performed a very strict selection of healthy patients which, as noted above, likely explains the lower mean LA diameters and volumes reported. In addition, body size differences across studies could explain different 95th percentile values, which is why we also present reference equations in terms of age and body size.
It is interesting to highlight that the reference values of the elderly population provided by the present study somewhat overlap the reference values for adults provided by current guidelines (17). In particular we found that partition values for LA diameter in women (3.8 vs 3.9 cm) and for LA volume in men (58 vs 58.2mL) were virtually identical. However, some interesting differences are observed in LA diameter for men (4.6 vs 4.0 cm) and in LA volume in women (47 vs 52 mL), possibly showing that in the elderly a different geometrical adaptation of the LA over time might occur. Our data results suggest that with aging, while the LA in women tends to enlarge its superior-inferior diameter, in men the geometric shape of the normal LA becomes spherical, as suggested by larger reference LA AP diameter at similar normal LA volume.
It was not the purpose of this study to determine which parameter of LA size maximizes the ability to diagnose LA enlargement. From the work of Pritchett (9), it appears that LA enlargement by LAV might identify a larger population. Surprisingly, in Pritchett′s study, the agreement between linear dimensions and volume indices was only fair, suggesting that these two parameters identify different populations.
Study Limitations
We believe that the strengths of our study include use of a well-defined cohort to identify healthy participants and to adjust for body size measures. However, the study is not without limitations. The major limitation is that the study sample is small. With 132 women and 98 men, our numbers were too small to split the sample in order to perform a validation analysis of the 95th percentile limits; furthermore the truncated age range of the population might reduce the chance to observe a relationship between age and LA size. In addition, the reference equations in Table 3 have not yet been validated prospectively. However, some limited validation is suggested by the finding that 38% of CHS patients with prevalent systolic heart failure have LA enlargement by our 95th percentile method, and 45.6% when the prediction equations are used (32).
In the present manuscript we have not excluded otherwise healthy obese participants, which might suggest that the reported association between LA size and measures of body build could be influenced by the presence of obese individuals. In order to see if this were so, we examined scatter-plot smoothers of the LA parameters across the range of BMI, and found no deviation from a linear model, with the possible exception of PLAX for BMI values < 16 or > 35kg/m2. We reran all models excluding the few individuals in this range, and there were no meaningful changes in the classification of participants with high LA parameters based on the reference equations. We note further, that although the risk associated with uncomplicated obesity is well established in children and adults, previous reports from the Cardiovascular Health Study have shown that while being underweight at age 65 or older is associated with worse outcomes than being normal weight, overweight or frank obesity is rarely associated with worse outcomes than normal weight and may even be associated with significantly better outcomes (33, 34, 35). However, because of the relatively high BMI of our participants and the direct association of BMI with LA paramaters, we encourage use of the reference equations rather than the 95th percentiles to identify individuals with high LA parameters.
It is a potential limitation that participants in the study might have had atrial fibrillation that was not detected on the regularly scheduled CHS follow up visits, and that this arrhythmia might have contributed to LA enlargement independent of the demographic factors explored in our analysis. Also, the study does not take into account the potential impact of diastolic dysfunction, as assessed by transmitral E and A velocities and their ratios. To account for this potential confounder, we have performed several additional analyses on diastolic filling in our population of normal elderly patients. There was no significant relationship between early peak flow velocity and LA volume for either men or women, in a linear regression adjusting for age, height and weight, p=0.10 for women and 0.28 for men. In addition, the E/A ratios by sex-specific quartiles of LA volume were identical in all groups. In fact, the p-value for a linear trend across quartiles was 0.094, and there was no evidence of a deviation from linearity (p=0.67). Thus, at least by the standard transmitral velocity analysis, there did not appear to be a significant interaction between diastolic filling and LA size. Whether we would reach the same conclusion if either pulmonary vein velocities or tissue Doppler data were available is unanswerable within the framework of CHS.
It has been suggested that the biplane-area might estimate LA volume more accurately as compared to the prolate ellipse model using three different orthogonal planes (36). Unfortunately in the CHS database, only data for the computation of left atrial volume through the use of three orthogonal axis linear measurements were available and therefore a comparison with other methods could not be performed. However, according to the current ASE guidelines (17), LA can be adequately represented as a prolate ellipse and therefore we believe that our methodology is acceptable as well and widely used in clinical practice.
Clinical Implications
We have established a series of reference equations and normative values for LA dimensions and volumes in a well characterized, older, and cardiovascular-healthy individuals. LA size is more strongly related to BSA and BMI than to height, as has been previously shown in younger populations with heart disease (1, 10). Given that the prevalence of heart disease increases with age, we believe that these equations may be useful in helping to better integrate quantitative tools into everyday clinical echocardiography. The development of these reference standards for the elderly might facilitate future study into whether and which parameters better predict cardiovascular complications or help identify preclinical cardiovascular disease.
Left atrial (LA) size is increased in a variety of cardiovascular disorders, and its enlargement is usually an indicator of chronically increased left atrial pressure and / or volume. Since the prevalence of LA enlargement increases with age, it is critical to have accurate normative reference ranges for heart sizes to correctly predict cardiovascular risk in the elderly. At present, there is a dearth of such reference data in both population based samples and in women. Accordingly, we have evaluated parameters of LA size in 230 healthy men and women (mean age 76±5 years) who were judged to be free of heart disease, hypertension, and diabetes. These subjects were a subset of the Cardiovascular Health Study, a prospective, community-based study of risk factors for cardiovascular disease conducted in 5,888 elderly participants. We found that LA size is more strongly related to measures of body weight than to body height and developed a series of reference equations that will predict what the normal LA dimensions and volumes should be, based on the subject’s age, height and weight. We have also created some nomograms which will enable clinicians to easily diagnose LA enlargement. We hope that these data can be used to help better determine what a normal LA size should be. An earlier diagnosis of LA enlargement might permit earlier preventive cardiology strategies in, for example, hypertensive disease.
Figure 3.
Regression between LA SI dimension and body surface area in both genders. Regression equation and 95% prediction interval are presented.
Acknowledgement
The authors which to acknowledge Cheryl Egher, Karen Fowle, and Florentina Petillo for their technical assistance and Jacqueline Jolie for her assistance with manuscript preparation.
Funding Sources
The research reported in this article was supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, and N01 HC-15103 from the National Heart, Lung, and Blood Institute. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Footnotes
Disclosures
None.
References
- 1.Gottdiener JS, Reda DJ, Williams DW, Materson BJ. Left atrial size in hypertensive men: influence of obesity race age Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1997;29:651–658. doi: 10.1016/s0735-1097(96)00554-2. [DOI] [PubMed] [Google Scholar]
- 2.Henry WL, Morganroth J, Pearlman AS, Clark CE, Redwood DR, Itscoitz SB, Epstein SE. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation. 1976;53:273–279. doi: 10.1161/01.cir.53.2.273. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population- based cohort The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 4.Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 5.Sauter HJ, Dodge HT, Johnston RR, Graham TP. The relationship of left atrial pressure and volume in patients with heart disease. Am Heart J. 1964;67:635–642. doi: 10.1016/0002-8703(64)90334-5. [DOI] [PubMed] [Google Scholar]
- 6.Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1206–1207. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 7.Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42:1206–1207. doi: 10.1016/s0735-1097(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 8.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 9.Pritchett Am, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 10.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 11.Khankirawatana B, Khankirawatana S, Porter T. How should left atrial size be reported? Comparative assessment with use of multiple echocardiographic methods. Am Heart J. 2004;147:369–374. doi: 10.1016/j.ahj.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Tops FT, van der Wall EE, Schalij MJ, Bax JJ. Multi-modality imaging to assess left atrial size, anatomy and function. Heart. 2007;93:1461–1470. doi: 10.1136/hrt.2007.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CHS Collaborative Research Group. Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Tell G, Fried L, Hermanson B, Manolio T, Newman A, Borhani N for the Cardiovascular Health Study Collaborative Research Group. Recruitment of adults 65 years and older as participants in The Cardiovascular Health Study. Ann Epidemiol. 1993:3358–3366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free- living elderly subjects: the cardiovascular health study [published erratum appears in j am soc echocardiogr 1992 sep-oct; 5(5):550] J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Murray JA, Kennedy JW, Figley MM. Quantitative angiocardiography 11 The normal left atrial volume in man. Circulation. 1968;37:800–804. doi: 10.1161/01.cir.37.5.800. [DOI] [PubMed] [Google Scholar]
- 19.Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size: the Framingham Heart Study. Hypertension. 1995;25:1155–1160. doi: 10.1161/01.hyp.25.6.1155. [DOI] [PubMed] [Google Scholar]
- 20.Toutouzas K, Trikas A, Pitsavos C, Barbetseas J, Androulakis A, Stefanadis C, Toutouzas P. Echocardiographic features of left atrium in elite male athletes. Am J Cardiol. 1996;78:1314–1317. doi: 10.1016/s0002-9149(96)00622-4. [DOI] [PubMed] [Google Scholar]
- 21.Lauer MS, Larson MG, Levy D. Gender-specific reference M-mode values in adults: population-derived values with consideration of the impact of height. J Am Coll Cardiol. 1995;26:1039–1046. doi: 10.1016/0735-1097(95)00275-0. [DOI] [PubMed] [Google Scholar]
- 22.Manolio TA, Gottdiener JS, Tsang TS, Gardin JM. The Cardiovascular Health Study Collaborative Research Group Left atrial dimensions determined by M-mode echocardiography in black and white older (> or = 65 years) adults (The Cardiovascular Health Study) Am J Cardiol. 2002;90:983–987. doi: 10.1016/s0002-9149(02)02665-6. [DOI] [PubMed] [Google Scholar]
- 23.Pearlman JD, Triulzi M, King ME, Abascal V, Newell J, Weyman AE. Left atrial dimensions in growth and development: normal limits for two-dimensional echocardiography. J Am Coll Cardiol. 1990;16:1168–1174. doi: 10.1016/0735-1097(90)90549-5. [DOI] [PubMed] [Google Scholar]
- 24.Vasan RS, Larson MG, Levy D, Evans JC, Benjamin EJ. Distribution and categorization of echocardiographic measurements in relation to reference limits: the Framingham Heart Study: formulation of a height and sex-specific classification and its prospective validation. Circulation. 1997;96:1863–1873. doi: 10.1161/01.cir.96.6.1863. [DOI] [PubMed] [Google Scholar]
- 25.Gardin JM, Henry WL, Savage DD, Ware JH, Bum C, Borer JS. Echocardiographic measurements in normal subjects: evaluation of an adult population without clinically apparent heart disease. J Clin Ultrasound. 1979;7:439–447. doi: 10.1002/jcu.1870070606. [DOI] [PubMed] [Google Scholar]
- 26.Varizi SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation: the Framingham Heart Study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin EJ, D′Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death: the Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 28.Thomas L, Levett K, Boyd A, Leung DY, Schiller NB, Ross DL. Compensatory changes in atrial volumes with normal aging: is atrial enlargement inevitable? J Am Coll Cardiol. 2002;40:1630–1635. doi: 10.1016/s0735-1097(02)02371-9. [DOI] [PubMed] [Google Scholar]
- 29.Schnittger I, Gordon EP, Fitzgerald PJ, Meese, Popp RL. Standardized intracardiac measurements of two-dimensional echocardiography. J AM Coll Cardiol. 1983;2:934–938. doi: 10.1016/s0735-1097(83)80242-3. [DOI] [PubMed] [Google Scholar]
- 30.Triulzi M, Gillam LD, Gentile F, Newell JB, Weyman AE. Normal adult cross-sectional echocardiographic values: linear dimensions and chamber areas. Echocardiography. 1984;1:403–426. [Google Scholar]
- 31.Wang Y, Gutman JM, Heilbron D, Wahr D, Schiller NB. Atrial volume in a normal adult population by two-dimensional echocardiography. Chest. 1984;86:595–601. doi: 10.1378/chest.86.4.595. [DOI] [PubMed] [Google Scholar]
- 32.Gottdiener J, Kitzman D, Aurigemma G, Arnold A, Manolio T. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons >/= 65 years of age: The Cardiovascular Health Study. Am J Cardiol. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 33.Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity. 2007;15:1827–1840. doi: 10.1038/oby.2007.217. [DOI] [PubMed] [Google Scholar]
- 34.Diehr P, Bild DE, Harris TB, Duxbury A, Siscovick D, Rossi M. Body massindex and mortality in nonsmoking older adults: the Cardiovascular HealthStudy. Am J Public Health. 1998;88:623–629. doi: 10.2105/ajph.88.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diehr P, O'Meara ES, Fitzpatrick A, Newman AB, Kuller L, Burke G. Weight, mortality, years of healthy life, and active life expectancy in older adults. J Am Geriatr Soc. 2008;1:76–83. doi: 10.1111/j.1532-5415.2007.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ujino K, Barnes ME, Cha SS, Langins AP, Bailey KR, Seward JB, Tsang TS. Two-dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol. 2006;98:1185–1188. doi: 10.1016/j.amjcard.2006.05.040. [DOI] [PubMed] [Google Scholar]