Abstract
Objective
To identify risk factors for pediatric intensive care unit (PICU) admission and mortality of infants with complete DiGeorge anomaly treated with thymus transplantation. We hypothesized that age at transplantation and presence of congenital heart disease (CHD) would be risk factors for emergent PICU admission, and these factors plus development of septicemia would increase morbidity and mortality.
Design
Retrospective review
Setting
Academic medical-surgical PICU
Patients
All infants with complete DiGeorge anomaly treated with thymus transplantation between January 1, 1993 and July 1, 2010
Interventions
None
Measurements and Main Results
Seventy one infants with complete DiGeorge anomaly were consented for thymus transplantation, and 59 were transplanted. Median age at transplantation was 5.0 (range: 1.1 – 22.1) months. After transplantation, 12/59 infants (20%) required 25 emergent PICU admissions. Seven of 12 (58%) infants survived to PICU discharge with six surviving six months post-transplantation. Forty two of 59 (71%) infants transplanted had CHD, and 9/12 (75%) who were admitted to the PICU had CHD. In 15/25 admissions (60%), intubation and mechanical ventilation were necessary. There was no difference between median ventilation-free days between infants with and without CHD (33 vs. 23 days, p = 0.544). There was also no correlation between ventilation-free days and age of transplantation (R0.17, p=0.423). Age at transplantation and presence of CHD were not associated with risk for PICU admission (OR: 0.95, 95%CI: 0.78 – 1.15 and OR: 1.27, 95%CI: 0.30 – 5.49, respectively) or PICU mortality (OR: 0.98, 95%CI: 0.73 – 1.31 and OR: 0.40, 95%CI: 0.15 – 1.07, respectively).
Conclusions
Most transplanted infants did not require emergent PICU admission. Age at transplantation and presence of CHD were not associated with PICU admission or mortality.
Keywords: DiGeorge anomaly, thymus transplantation, infant, critical illness, pediatric intensive care unit, sepsis, outcome, immunodeficiency, mechanical ventilation
Introduction
Primary immunodeficiencies are a heterogeneous group of rare conditions that predispose children to infection, autoimmune reaction, malignancy, and critical illness.[1] DiGeorge anomaly is a primary immunodeficiency characterized by the presence of congenital heart disease (CHD), hypoparathyroidism, and thymic hypoplasia or athymia.[2, 3] Due to the absence of a functional thymus, children with complete DiGeorge anomaly have primary immunodeficiency that is almost uniformly fatal by two years of age.[4]
Thymus transplantation is an investigational therapy for severely immunocompromised patients with complete DiGeorge anomaly with a one-year survival of 75%.[5-8] Of the survivors, 98% develop naive T cells and T cell function, but immunological reconstitution following thymus transplantation is not immediate.[7, 8] In the first six months following thymus transplantation, infants remain immunodeficient and are at risk for critical illness requiring admission to a pediatric intensive care unit (PICU).[7]
The clinical care required by these infants during the immediate post-transplantation period has not been previously reported. In this study, we described the characteristics and clinical course of infants with complete DiGeorge anomaly who required PICU admission. We hypothesized that age at transplantation and presence of congenital heart disease (CHD) would be risk factors for emergent PICU admission in these infants and these factors plus the development of septicemia would increase mortality and morbidity (e.g., ventilator free days and length of ICU stay) after PICU admission.
Materials and Methods
After Duke University Medical Center Institutional Review Board approval, we retrospectively reviewed the medical records of all infants with complete DiGeorge anomaly referred to our institution for evaluation for thymus transplantation between January 1, 1993 and July 1, 2010. We extracted data pertaining to basic demographics (age, gender, and ethnicity), clinical associations (22q11 hemizygosity, hypoparathyroidism and CHD), and PICU clinical characteristics (e.g., indications for admission, presence of sepsis, and length of PICU stay). We also collected data on PICU interventions including type and degree of respiratory support, presence of central venous lines, use of inotropic support, and continuous renal replacement therapy.
We defined a PICU admission as ‘emergent’ if there was an unexpected clinical deterioration within the first six months after thymus transplantation. Elective admissions (e.g., pre-planned central venous catheter insertion and post-operative admission following elective surgeries) were excluded. We categorized indications for emergent admission to PICU into five categories: respiratory (e.g., severe respiratory distress, severe stridor); cardiovascular (e.g., hemodynamic instability, shock); renal (e.g., severe electrolyte abnormalities, need for renal replacement therapy); neurology (e.g., status epilepticus, altered mental status), and others (e.g., severe bleeding, severe drug reactions). We defined CHD as any congenital heart lesion except patent ductus arteriosus. Septicemia was classified as “early” if a blood culture was positive for bacteria or fungus within the first 24 hours of PICU admission and “late” for all positive blood cultures after this period. We defined mechanical ventilation (MV) as being intubated (or having a tracheostomy) and supported with conventional mechanical ventilation (CMV) or high-frequency ventilation (HFV) at any time during PICU admission. The use of nasal or full face mask continuous positive airway pressure or bi-level positive airway pressure was considered as non-invasive ventilation. To account for death as a competing outcome, we summarized the requirement for MV as ventilator-free days with the maximum of 42 days. We defined central lines, as the presence of any central venous catheter, including surgically inserted central lines and/or peripherally inserted central catheters at any time during PICU admission. For mortality, we included all deaths occurring during PICU admissions or within six months following thymus transplantation.
Statistical Analyses
We present continuous variables as median values and ranges; and categorical variables as counts and percentages. We compared continuous and categorical variables using the Wilcoxon rank sum and Fisher exact tests respectively. Our primary outcomes were PICU admission and mortality. Secondary outcomes for our study were ventilator free days and PICU length of stay (LOS). For risk factors for PICU admission and mortality, we performed univariable exact logistic regression of demographic and clinical risk factors. We evaluated the following potential risk factors for PICU admission: age at transplantation, presence of CHD, presence of associated syndromes, presence of central lines and prior documented viral/bacterial infections. To examine the association between these clinical factors and duration of ventilator-free days, we used the Wilcoxon rank sum and Spearman’s rank correlation tests for categorical (e.g., presence of CHD, associated syndromes) and continuous variables (e.g., age at transplantation) respectively. We evaluated the following risk factors for PICU survival: presence of CHD, immunological status on admission to the PICU, bacteremia after admission to the PICU, and the use of mechanical ventilatory support. Statistical analysis was performed using Stata version 12.1 (College Station, TX). Statistical significance was defined as p < 0.05 for all tests.
Results
Patient characteristics and PICU courses
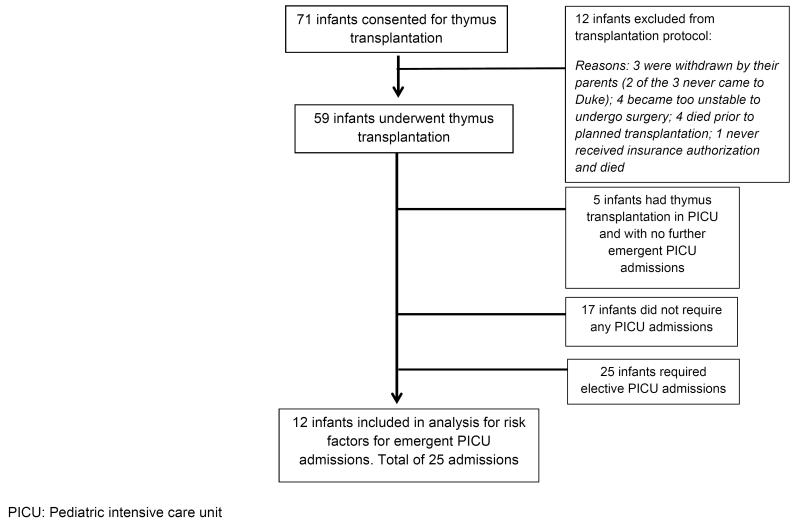
During the study period, 71 infants with complete DiGeorge anomaly were consented for thymus transplantation, and 59 were transplanted (Figure 1). Median age at transplantation was 5.0 (range: 1.1-22.1) months. After transplantation, 12/59 infants (20%) required 25 emergent PICU admissions (Table 1). Median time from transplantation to first PICU admission was 25 (4 to 111) days.
Figure 1.
Flowchart of Cohort of Infants Referred for Thymus Transplantation
Table 1.
Infant Characteristics
| Demographics | Infants who received a transplant and had emergent PICU admission (n=12), N (%) |
Infants who received a transplant without emergent PICU admission (n=47), N (%) |
p value* |
|---|---|---|---|
| Gender | |||
| Male | 8 (67) | 29 (62) | 0.99 |
| Ethnicity | |||
| White | 6 (50) | 31 (66) | 0.39 |
| Black | 4 (33) | 6 (13) | |
| Asian | 1(8) | 2 (4) | |
| Hispanic | 1 (8) | 6 (13) | |
| Others | 0 (0) | 2 (4) | |
| Median age at time of transplantation, months (range) |
3.7 (1.7 – 14.4) | 5.3 (1.1 – 21.8) | 0.32 |
| Genetic or syndromic associations | |||
| 22q11 hemizygosity | 5 (42) | 18 (38) | 0.99 |
| CHARGE | 3 (25) | 12 (26) | 0.99 |
| Infant of diabetic mother | 3 (25) | 8 (17) | 0.68 |
| Other clinical conditions | |||
| Hypocalcemia | 9 (75) | 36 (77) | 0.99 |
| Congenital heart disease | 9 (75) | 33 (70) | 0.99 |
| Cyanotic congenital heart disease | 1 (8) | 13 (28) | 0.31 |
Statistical tests performed: Fisher exact test for categorical variables and Wilcoxon rank-sum test for continuous variables.
PICU: Pediatric intensive care unit
Of the 25 admissions, 16 (64%) were for respiratory distress (Table 2). The majority of these admissions [10 (62%)] were due to lower airway symptoms; 3 (19%) had upper airway symptoms (e.g., stridor and superior vena cava obstruction); 3 (19%) presented with apnea. Among these 16 admissions, two were supported with non-invasive ventilation; 11 (69%) required intubation and mechanical ventilation, with 8/11 (73%) of these admissions being supported with high frequency ventilation (HFV). For non-respiratory indications for PICU admission, 4/9 (44%) required intubation. Of all PICU admissions requiring HFV, 4/8 (50%) survived. In comparison, in PICU admissions in which HFV was not required, survival was 16/17 (94%) (p = 0.023). Nine patients in these 25 admissions had CHD: there were three infants with aortic “lesions” (aortic narrowing, right aortic arch, aberrant right subclavian), three with pulmonary stenosis, and three others (ventricular septal defect, atrio-ventricular canal defect, tetralogy of Fallot). Two of these nine infants (atrio-ventricular canal defect and tetralogy of Fallot) had their CHD corrected prior to their respective emergent PICU admissions.
Table 2.
Clinical characteristics and course of emergent PICU admissions (n = 25)
| Number, N (%) | |
|---|---|
| Indications for admission | |
| Respiratory | 16 (64) |
| Cardiovascular | 3 (12) |
| Neurology | 2 (8) |
| Others | 4 (16) |
| Mechanical ventilation | |
| Conventional | 15 (60) |
| High frequency | 8 (32) |
| Inotropic support within first five days of PICU admission | 1 (4) |
| Seizures | 2 (8) |
| Significant clinical bleeding* | 1 (4) |
| Central line present on admission to PICU | 13(52) |
| Culture-proven bacteremia within first 24 hours | 2 (8) |
| Culture-proven bacteremia after 24 hours of PICU admission | 6 (24) |
| PICU admission mortality | 5 (20) |
PICU: Pediatric intensive care unit
Requiring blood product transfusion or surgical intervention
Median duration of PICU LOS was 10 (1 to 63) days. Of the 12 infants admitted to the PICU emergently, seven (58%) infants survived to PICU discharge with six surviving to six months post-transplantation. Among the five PICU deaths, only one occurred during the first PICU admission, with the other four occurring during subsequent admissions. 45/47 (95%) infants who did not require emergent PICU admissions survived to six months post-transplantation.
Immunological Profile
None of the infants showed evidence of thymic function at the time of PICU admission. The T cell counts at that time ranged from 0 to 24,146 cells/mm3. The T cells were either third party T cells causing graft-versus-host-disease (n=1), low numbers of recipient uneducated T cells present in patients with typical complete DiGeorge anomaly (n=6), thymus donor T cells (n=1), or recipient T cells causing atypical DiGeorge anomaly (n=7). There was no difference in the median T cell counts at the time of PICU admission between PICU survivors and non-survivors [295 (24, 1500) vs. 143 (129, 449), p = 0.734]. The most common immunosuppressive drugs utilized in our infants were steroids (n=13) and cyclosporine (n=7).
Risk factors for PICU admission
CHD had no impact on risk for PICU admission (OR: 1.27 95%CI: 0.30 – 5.49) or mortality (OR 0.40, 95%CI: 0.15 – 1.07). After leaving out infants who had corrective surgeries performed, CHD remained a non-significant risk factor. Age at transplantation was also not a risk factor for PICU admission (OR: 0.95, 95%CI: 0.78 – 1.15) or mortality (OR: 0.98, 95%CI: 0.73 – 1.31). The remaining clinical demographics (presence of 22q11 hemizygosity, CHARGE syndrome, presence of central lines, and prior infections) were not found to be significant risk factors for PICU admissions.
Risk factors and duration of mechanical ventilation
Median ventilator-free duration in our cohort of infants was 32 (0 – 42) days. There was no association between the presence of 22q11, CHARGE syndrome, and prior infections with ventilator-free days. There was also no correlation between the age at transplantation and duration of ventilator-free days (R = 0.17, p = 0.423). There was no difference in median ventilator free days between infants with and without CHD [33 (0, 42) vs. 23 (0, 42), p = 0.544].
Bacteremia during PICU admission
Bacteremia was present in 8/25 (32%) admissions in seven separate infants. In two of the eight admissions, bacteremia developed within the first 24 hours of admission. Beyond the first 24 hours, there were six episodes of bacteremia and one episode of fungemia among seven infants in seven separate admissions. Of the eight episodes of bacteremia, the most common pathogens were coagulase negative Staphylococcus aureus (50%) and Enterococcus fecalis (25%). There was no difference in median ventilator-free days between infants with and without sepsis (bacteremia and fungemia) [23 (0, 42) vs. 33 (0, 42) days, p = 0.727)]. Bacteremia after 24 hours was associated with increased LOS [29 (14, 63) vs. 5 (1, 32) days, p = 0.004]. Although infants with late bacteremia stayed in the PICU longer, they did not have an increased odds of mortality (OR: 0.58, 95% CI: 0.05 – 6.37).
Discussion
Less than 25% of our infants with complete DiGeorge anomaly required intensive care following thymus transplantation with neither age at time of thymus transplantation nor presence of CHD impacting the need for PICU admission, ventilator-free days, or mortality. We found that bacteremia that developed after 24 hours of PICU admission increased PICU LOS but did not impact mortality.
There is a paucity of data on the effect of CHD on the overall prognosis of children with DiGeorge anomaly. To our knowledge, there is only one previous study that demonstrated the association between pulmonary stenosis/atresia and worse outcome after cardiac surgery in children with DiGeorge anomaly.[9] In contrast, our current investigation did not demonstrate any association between CHD and need for PICU admission, need for MV, or mortality in children with complete DiGeorge anomaly. This may be due to the fact that the majority of our infants (29/43) had acyanotic congenital heart lesions and we excluded elective, post-operative admissions in our study.
In children with primary immunodeficiencies, age has been demonstrated to be an important factor associated with survival after hematopoietic stem cell transplantation (HSCT). [10] We did not find that age at thymus transplantation was an important factor in the determining the need for PICU admission, morbidity or mortality. Our findings are consistent with a previous study of 26 infants who underwent HSCT for complete DiGeorge anomaly where investigators found that neither age at transplantation nor underlying genetics (22q11.2 and chromodomain helicase 7) were associated with mortality. Of note, this study did not evaluate the association between bacteremia and survival.[11]
The incidence of bacteremia in our series was relatively high with bacteremia developing in more than 25% of the emergent PICU admissions. Previous publications on nosocomial infections in the general PICU population have reported an overall bloodstream infection rate of approximately 4-7%.[12-14] In our study, the relatively high incidence of bacteremia after PICU admission is most likely accounted for by age and underlying immunodeficiency, [12] , and we noted that bacteremia was associated with increased PICU LOS but not ventilator-free days or mortality. Our findings are consistent with other published studies demonstrating that bacteremia is not independently associated with mortality in immunodeficient children (e.g., those with malignancies having undergone HSCT).[15-20]
Due to the common theme of severe immunodeficiency, it is interesting to compare complete DiGeorge anomaly patients to HSCT patients. Data from a previous study demonstrated that HSCT patients with underlying non-malignant conditions had an increased risk for PICU admission.[21] In a large study of 111 critically ill children with primary immunodeficiency undergoing HSCT, 39 (35%) children required a total of 58 PICU admissions.[21] This is in contrast with only 12 (20%) of our 59 infants requiring emergent PICU admissions. One possible reason for this difference is that in the prior study, 104 (94%) children received chemotherapy prior to their HSCT, whereas less than half of our patients received immunosuppression (generally consisting of calcineurin inhibitor and/or rabbit anti thymocyte globulin therapy).[21]
Consistent with previous findings of critically ill children treated with HSCT [17, 22], respiratory distress was the most common indication for PICU admission in our study. In the current investigation, we found an association between the need for HFV and increased mortality, as has been demonstrated in prior studies involving children treated with HSCT. [17, 18, 20] Other investigators found increased mortality in those who required high positive end-expiratory pressure or ventilated with a permissive hypercapnia strategy.[18] However, this relationship is not surprising as the use of HFV, high positive end-expiratory pressure, and permissive hypercapnia are indicators of severity of respiratory illness, rather than independent risk factors for mortality. The association between these respiratory strategies and mortality should not be viewed as a cause and effect relationship. Despite a general trend toward improvement in clinical outcomes in immunosuppressed children requiring MV, those with more severe respiratory failure still have an overall poor prognosis. [23]
This single-center retrospective review has limitations. We defined sepsis based on patients with positive blood cultures rather than the international pediatric sepsis consensus conference definition which includes the presence of systemic inflammatory response (based on changes in heart rate, respiratory rate, temperature and white cell count) in presence of suspected or proven infection. [24] This definition was chosen based on the substantial risk and concern for sepsis in all of these profoundly immunodeficient patients at the time of PICU admission. Using bacteremia allowed for analysis including only those patients with true bacterial infection. Studies on immunodeficient patients are made challenging by the fact that individual primary immunodeficient conditions are rare, even when considered collectively. Our sample population is heterogeneous with different underlying genetic and syndromic conditions. Care must be taken not to over-extrapolate our results to other congenital immunodeficient children and acquired immunodeficient states. Our cohort of children was recruited over a span of 17 years. As such, changes in clinical practice (e.g., ventilator strategies with lower tidal volumes, changes in immunosuppression strategies during the peri-transplant period, adaptation of the transplantation protocol to include more advanced diagnostic and therapeutic techniques) could have potentially influenced outcomes over time.
Conclusion
This is the first published description of the critical care course of infants with complete DiGeorge anomaly treated with thymus transplantation. Most transplanted infants did not require emergent PICU admission, and we did not identify clear predictors for PICU admission after thymus transplantation. Our study adds to the limited medical literature on the critical care course and outcomes of children with primary immunodeficiencies. To improve the overall care of these children, a collaborative registry among international PICUs should be considered to allow for more consistent data collection in critically ill infants across the wide spectrum of primary immunodeficiencies.
Acknowledgments
Christoph P. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001117).
Footnotes
Disclosures: Part of this study was presented in an abstract format at the Society of Critical Care Medicine’s 42nd Critical Care Congress in January 2013.
REFERENCES
- 1.Geha RS, Notarangelo LD, Casanova JL, et al. Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee. J Allergy Clin Immunol. 2007;120(4):776–94. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conley ME, Beckwith JB, Mancer JF, et al. The spectrum of the DiGeorge syndrome. J Pediatr. 1979;94(6):883–90. doi: 10.1016/s0022-3476(79)80207-3. [DOI] [PubMed] [Google Scholar]
- 3.Barrett DJ, Ammann AJ, Wara DW, et al. Clinical and immunologic spectrum of the DiGeorge syndrome. J Clin Lab Immunol. 1981;6(1):1–6. [PubMed] [Google Scholar]
- 4.Markert ML, Hummell DS, Rosenblatt HM, et al. Complete DiGeorge syndrome: persistence of profound immunodeficiency. J Pediatr. 1998;132(1):15–21. doi: 10.1016/s0022-3476(98)70478-0. [DOI] [PubMed] [Google Scholar]
- 5.Cleveland WW, Fogel BJ, Brown WT, et al. Foetal thymic transplant in a case of Digeorge’s syndrome. Lancet. 1968;2(7580):1211–4. doi: 10.1016/s0140-6736(68)91694-2. [DOI] [PubMed] [Google Scholar]
- 6.Markert ML, Boeck A, Hale LP, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341(16):1180–9. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 7.Markert ML, Alexieff MJ, Li J, et al. Postnatal thymus transplantation with immunosuppression as treatment for DiGeorge syndrome. Blood. 2004;104(8):2574–81. doi: 10.1182/blood-2003-08-2984. [DOI] [PubMed] [Google Scholar]
- 8.Markert ML, Devlin BH, Alexieff MJ, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109(10):4539–47. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michielon G, Marino B, Formigari R, et al. Genetic syndromes and outcome after surgical correction of tetralogy of Fallot. Ann Thorac Surg. 2006;81(3):968–75. doi: 10.1016/j.athoracsur.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Fischer A, Landais P, Friedrich W, et al. Bone marrow transplantation (BMT) in Europe for primary immunodeficiencies other than severe combined immunodeficiency: a report from the European Group for BMT and the European Group for Immunodeficiency. Blood. 1994;83(4):1149–54. [PubMed] [Google Scholar]
- 11.Janda A, Sedlacek P, Honig M, et al. Multicenter survey on the outcome of transplantation of hematopoietic cells in patients with the complete form of DiGeorge anomaly. Blood. 2010;116(13):2229–36. doi: 10.1182/blood-2010-03-275966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Pediatrics. 1999;103(4):e39. doi: 10.1542/peds.103.4.e39. [DOI] [PubMed] [Google Scholar]
- 13.Zuschneid I, Schwab F, Geffers C, et al. Reducing central venous catheter-associated primary bloodstream infections in intensive care units is possible: data from the German nosocomial infection surveillance system. Infect Control Hosp Epidemiol. 2003;24(7):501–5. doi: 10.1086/502236. [DOI] [PubMed] [Google Scholar]
- 14.Elward AM, Fraser VJ. Risk factors for nosocomial primary bloodstream infection in pediatric intensive care unit patients: a 2-year prospective cohort study. Infect Control Hosp Epidemiol. 2006;27(6):553–60. doi: 10.1086/505096. [DOI] [PubMed] [Google Scholar]
- 15.Hallahan AR, Shaw PJ, Rowell G, et al. Improved outcomes of children with malignancy admitted to a pediatric intensive care unit. Crit Care Med. 2000;28(11):3718–21. doi: 10.1097/00003246-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Yogaraj JS, Elward AM, Fraser VJ. Rate, risk factors, and outcomes of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2002;110(3):481–5. doi: 10.1542/peds.110.3.481. [DOI] [PubMed] [Google Scholar]
- 17.Jacobe SJ, Hassan A, Veys P, et al. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit Care Med. 2003;31(5):1299–305. doi: 10.1097/01.CCM.0000060011.88230.C8. [DOI] [PubMed] [Google Scholar]
- 18.Kache S, Weiss IK, Moore TB. Changing outcomes for children requiring intensive care following hematopoietic stem cell transplantation. Pediatr Transplant. 2006;10(3):299–303. doi: 10.1111/j.1399-3046.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 19.Lakshmi KS, Jayashree M, Singhi S, et al. Study of nosocomial primary bloodstream infections in a pediatric intensive care unit. J Trop Pediatr. 2007;53(2):87–92. doi: 10.1093/tropej/fml073. [DOI] [PubMed] [Google Scholar]
- 20.van Gestel JP, Bollen CW, Bierings MB, et al. Survival in a recent cohort of mechanically ventilated pediatric allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2008;14(12):1385–93. doi: 10.1016/j.bbmt.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Cole TS, Johnstone IC, Pearce MS, et al. Outcome of children requiring intensive care following haematopoietic SCT for primary immunodeficiency and other non-malignant disorders. Bone Marrow Transplant. 2012;47(1):40–5. doi: 10.1038/bmt.2011.26. [DOI] [PubMed] [Google Scholar]
- 22.Diaz MA, Vicent MG, Prudencio M, et al. Predicting factors for admission to an intensive care unit and clinical outcome in pediatric patients receiving hematopoietic stem cell transplantation. Haematologica. 2002;87(3):292–8. [PubMed] [Google Scholar]
- 23.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9(3):270–7. doi: 10.1097/PCC.0b013e31816c7260. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]