Abstract
In the present study, the role of direct procaryote-eucaryote interactions in the virulence of Bacillus cereus was investigated. As a model of human enterocytes, differentiated Caco-2 cells were used. Infection of fully differentiated Caco-2 cells with B. cereus in the exponential phase of growth, in order to minimize the concentration of spores or sporulating microorganisms, shows that a strain-dependent cytopathic effect develops. Interestingly, addition of 3-h-old cultures of some strains resulted in complete detachment of the cultured cells after a 3-h infection whereas no such effect was found after a 3-h infection with 16-h-old cultures. Infection of enterocyte-like cells with B. cereus leads to disruption of the F-actin network and necrosis. Even though the effect of secreted factors cannot be ruled out, direct eucaryote-procaryote interaction seems to be necessary. In addition, we observed that some B. cereus strains were able to be internalized in Caco-2 cells. Our findings add a new insight into the mechanisms of virulence of B. cereus in the context of intestinal infection.
Bacillus cereus is a spore-forming microorganism responsible for different pathological processes. In the context of intestinal infections, this microorganism is associated with emetic and diarrheic syndromes, and its role in extraintestinal infections has been also reported (14, 15). So far, the virulence of B. cereus has been ascribed to the production of exocellular factors such as (i) cereolysin O, a thiol-activated cholesterol binding cytolysin (3, 25); (ii) phospholipases (8, 10, 23); (iii) emetic toxin (cereulide), a heat-stable cyclic dodecadepsipeptide with a molecular mass of around 1.2 kDa (1, 2, 37); (iv) hemolysin BL, a tripartite enterotoxin that requires all the three components (B, L1, and L2) for maximal activity (5, 7, 8, 9, 10, 15, 35); (v) the nonhemolytic enterotoxin complex, a complex of three subunits (34); (vi) hemolysin IV (Hly-IV), which has a strong effect on plasma membranes with a wide range of compositions (8); and (vii) the cytotoxic protein CytK, associated with necrotic enteritis (26, 34).
A cellular response triggered by the interaction between microorganisms and intestinal epithelial cells contributes to the virulence of several pathogens. These biological effects are related to the binding of bacterial surface structures and/or bacterial toxins to cell receptors coupled with cellular signaling pathways. Attack of the host cell cytoskeleton is the main mechanism by which pathogenic microorganisms structurally and functionally alter the host cells, particularly the intestinal epithelial barrier. This interference with the organization of the cytoskeleton can be mediated by enzymatic modification by soluble toxins (12) or by upregulation of depolimerization cascades by direct prokaryote-eucaryote interactions (11, 24, 39). The ability of microorganisms of the genus Bacillus to interact with Caco-2 and HeLa cells has been reported, but no further insight into the role of this interaction in the virulence of this microorganism is available (43). Recently, the effect of spent-culture supernatants of B. cereus on Caco-2 cells, suggesting the production of cytotoxic toxin by the bacteria, was reported. These types of cell damage ranged from microvillus effacement to cell detachment. In addition, translocation of phosphatidylserine from the inner to the outer leaflet of the plasmatic membrane has been reported (38).
Despite the importance of the adhesion and invasion phenomena in the virulence of different microorganisms, the direct interaction of B. cereus with intestinal epithelial cells has not been extensively studied, although the possible role of the adhesion of spores in virulence has been suggested (4). In the present work, we sought to gain knowledge about the ability of B. cereus to interact with enterocyte-like Caco-2 cells and about the structural effects of such interactions on the cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains used in the present study are listed in Table 1. Stock cultures were preserved at −80°C using 0.3 M sucrose as a cryoprotectant. Each strain was reactivated for 16 h at 32°C with agitation in brain heart infusion broth supplemented with 0.1% glucose. The microorganisms were then inoculated (to 4% vol/vol) into 5 ml of brain heart infusion broth supplemented with glucose and incubated with agitation at 32°C for different times as indicated in the tables and figures. They were harvested by centrifugation (900 × g for 10 min).
TABLE 1.
Nomenclature and origin of the strains used in this study
| Straina | Origin | Reference |
|---|---|---|
| M2 | Skim milk powder | 35 |
| 2 | Infant formula | 35 |
| 3 | Infant formula | 35 |
| 253 | Infant formula | 35 |
| 273 | Infant formula | 35 |
| 113 | Infant formula | 35 |
| 114 | Infant formula | 35 |
| ATCC 10873 | Culture collection | |
| ATCC 13061 | Culture collection | |
| T1 | Unknown | 13 |
| T2 | Unknown | 13 |
| E2 | Unknown | 13 |
| Watertown | Unknown | 13 |
| A7 | Unknown | 13 |
| B10502 | Food-poisoning outbreak | —b |
Strain E2 is B. thuringiensis. The remaining strains are B. cereus.
Strain B10502 was kindly provided by the Laboratorio Central de Salud Pública de la Provincia de Buenos Aires, Argentina.
Culture of Caco-2 cells.
The parental Caco-2 cells (19, 40) were routinely grown in Dulbecco's modified Eagle's minimum essential medium (DMEM) plus 25 mM glucose (Life Technologies, Cergy, France), supplemented with 15% heat-inactivated (30 min at 56°C) fetal calf serum and 1% nonessential amino acids (Life Technologies). For maintenance purposes, the cell lines were passaged weekly, using 0.02% trypsin in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 3 mM EDTA. Monolayers were prepared in 24-well tissue culture plates (Iwaki Glass) by seeding 7 × 104 cells per well. Experiments and cell maintenance were carried out at 37°C in a 5% CO2-95% air atmosphere. The culture medium was changed daily. Assays were performed with cells at passages between 49 and 55 for Caco-2 cells. Fully differentiated cells (15 days in culture) were used throughout. Differentiation of the cells was controlled by indirect-immunofluorescence labeling of sucrase-isomaltase, a brush border differentiation marker (data not shown), as previously described (11, 16, 33, 39, 40).
Cell association assays.
Bacterial cultures were centrifuged and the pellet was resuspended in DMEM containing 100 μg of cloramphenicol per ml. Under these conditions, no bacterial growth was observed during the experiment and the bacteria remained viable. After being determined in an hemocytometer, the number of bacterial was adjusted to the required level for cell infection. The bacterial concentration and multiplicity of infection used are indicated in the tables and figures. The cell monolayers were washed twice with PBS before the infection assays were performed, and bacterial suspensions were added to the monolayers and incubated for 2 or 3 h at 37°C in a 5% CO2-95% air atmosphere. The monolayers were then washed three times with PBS and incubated with 1 ml of distilled water per well to lyse the cells. Serial dilutions of the samples were plated onto nutrient agar (3 g of meat extract per liter, 5 g of meat peptone per liter) and incubated at 37° for 16 h.
Cell invasion assays.
To assess the invasion of enterocytes, noninternalized bacteria were killed with the aminoglycoside antibiotic gentamicin as reported previously (25). Briefly, monolayers were washed three times with PBS and then 1 ml of gentamicin (100 μg/ml in PBS) was added per well. After being incubated for 1 h at 37°C, the monolayers were washed twice and lysed with 1 ml of distilled water for 1 h at 37°C. Serial dilutions of the suspensions were plated onto nutrient agar as indicated above.
Transmission electron microscopy.
After the bacterial adhesion assay, cells were fixed and treated. Samples embedded in Epon were reembedded to make sections perpendicular to the bottom of the flask. The specimens were then examined under a JEOL electron microscope.
Immunofluorescence study.
The F-actin cytoskeleton was labeled with fluorescein-labeled phalloidin (33). Briefly, monolayers of Caco-2 cells prepared on glass coverslips were infected with vegetative cells of B. cereus as indicated above. After the infection, the cells were washed with PBS and fixed with 3% paraformaldehyde in PBS for 15 min. Samples were then treated with 50 mM NH4Cl for 10 min to block aldehyde functions, permeabilized for 4 min with Triton X-100, and incubated for 45 min with fluorescein-labeled phalloidin (Sigma Chemical Co., St. Louis, Mo.). They were mounted in 50% (vol/vol) glycerol-0.1% (vol/vol) sodium azide in PBS.
The brush border-associated protein sucrase-isomaltase (SI) was stained by indirect-immunofluorescence labeling. Cells were incubated with anti-human SI antibody (8A9) (diluted 1:200 in 0.2% gelatin-PBS) for 45 min at room temperature (33). After three washes in PBS, incubation with a fluorescein isothiocyanate (FITC)-conjugated second antibody was performed for 45 min at room temperature. No fluorescent staining was observed when the primary antibody was omitted.
Labeling with FITC-annexin V and propidium iodide was performed by a modification of a previously published method (45). Briefly, after infection, cells were washed twice with binding buffer consisting of 25 mM HEPES, 125 mM NaCl, and 2.5 mM CaCl2 (pH 7.2). Then 5 μl of FITC-annexin V, 1 μg of propidium iodide per ml, and 100 μg of RNase per ml were added in 100 μl of binding buffer. The cells were incubated at room temperature for 15 min, and then samples were mounted in 50% glycerol in PBS.
B. cereus bacteria were labeled with a mixture of monoclonal antibodies (MAbs) 113D2b3 and 116A3b3 recognizing lipoteichoic acid (22). Rhodamine isothiocyanate (RITC)-labeled goat anti-mouse antibody (Molecular Probes) was used as secondary antibody. For observation of internalized bacteria by confocal laser-scanning microscopy (CLSM), the cells were permeabilized by incubation with 0.2% Triton X-100 in PBS for 4 min at room temperature. Cell monolayers were then incubated with MAbs 113D2b3 and 116A3b3 (diluted 1:100 in bovine serum albumin-PBS) for 45 min at 22°C and washed three times with PBS. The cells were then incubated with RITC-conjugated secondary antibodies for 60 min on ice and incubated for 30 min at 37°C. The F-actin cytoskeleton was labeled as described above.
Preparations were mounted in Vectashield antifade mounting medium (Biosys SA, Compiègne, France). Samples were examined by conventional epifluorescence microscopy using a DMLB microscope coupled to a DC 100 camera (Leica Microscopy Systems Ltd., Heerbrugg, Switzerland). Image analysis was performed by using Image-Pro Plus software (Media Cybernetics, Silver Spring, Md.). When specimens were examined by CLSM, analysis was performed using a confocal laser-scanning microscope (Zeiss model LSM 510, equipped with an air-cooled argon ion laser [488 nm] and a helium neon laser [543 nm]) configured with an Axiovert 100M microscope using a Plan Apochromat 63×/1.40 oil objective lens. Optical sectioning was used to collect en face images 0.40 μm apart. A reconstruction view was obtained by integration of images gathered at a step position of 1 on the x-y axis, using the accompanying Zeiss software with Windows NT. Photographic images were resized, organized, and labeled using Photoshop software (Adobe, San Jose, Calif.). The printed images are representative of the original data.
Statistical analysis.
Statistical analysis of the variations was performed by analysis of variance (Systat, Inc.).
RESULTS
The effect of vegetative B. cereus on enterocyte-like Caco-2 cells depends from the physiological status of the microorganisms.
We first analyzed the association of several B. cereus strains with fully differentiated Caco-2 cells when monolayers were infected at a multiplicity of infection of 140 bacteria per cell. As reported in Table 2, the highest value of cell association with Caco-2 cells was found for strain ATCC 10876. This strain showed values of 6.50 log CFU/well when 3-h-old cultures were coincubated for 2 h with enterocyte-like cells. On the other hand, the lowest value of association was found for strain 273 (5.28 log CFU/well) when 16-h-old cultures were employed. It was noted that the biological activities appeared to be strain dependent. Indeed, some strains, including ATCC 10876, ATCC 13061, T1, Watertown, and A7, promoted cell detachment. The results show that for strains detaching the cells, activity developed only with a 3-h-old culture and that the Caco-2 cells were not detached by 16-h-old cultures of these strains during the same period of infection (3 h). In contrast, B. cereus strains 2, 3, T2, 273, 114 E2, Watertown, and B10502 adhered well to Caco-2 cells without detaching the cells. Analysis of the concentration-dependent adhesion of strains 2 and M2 to Caco-2 cells (Fig. 1) showed that the maximal number of adhering bacteria was reached when around 2 × 107 CFU per well was used (multiplicity of infection, around 30 bacteria per cell). The maximal number of cell-associated bacteria was around 2 × 106 CFU/well (6.30 log CFU/well). This behavior indicates the suitability of expressing association values in terms of total associated bacteria rather than in terms of the percentage of associated bacteria. When bacterial concentrations higher than 2 × 107 CFU/well (7.30 log CFU/well) are added, the percentage of associated microorganisms is lower.
TABLE 2.
Cell association of B. cereus strains with Caco-2 cellsa
| B. cereus strain | Association of B. cereus with Caco-2 cells (log CFU/well)b
|
||
|---|---|---|---|
| 3-h-old culture
|
16-h-old culture, 3-h infection | ||
| 2-h infection | 3-h infection | ||
| M2 | 6.23 ± 0.30 | NDc | 5.73 ± 0.18 |
| 2 | 6.40 ± 0.01 | 6.45 ± 0.01 | 5.75 ± 0.75 |
| 3 | 5.93 ± 0.30 | 5.62 ± 1.00 | 5.76 ± 0.07 |
| T2 | 5.77 ± 0.44 | 5.99 ± 0.07 | 5.91 ± 0.10 |
| 253 | 6.23 ± 0.28 | ND | 5.81 ± 0.12 |
| 273 | 5.71 ± 0.01 | 5.50 ± 0.56 | 5.28 ± 0.47 |
| 113 | 5.84 ± 0.03 | ND | 5.81 ± 0.07 |
| 114 | 6.37 ± 0.42 | 5.42 ± 1.44 | 6.13 ± 0.21 |
| ATCC 10876 | 6.50 ± 0.01 | Detach cells | 6.39 ± 0.18 |
| ATCC 13061 | 5.61 ± 0.18 | Detach cells | 5.51 ± 0.04 |
| E2 | 5.81 ± 0.16 | 6.19 ± 0.17 | 5.97 ± 0.03 |
| T1 | 6.34 ± 0.29 | Detach cells | 6.27 ± 0.02 |
| Watertown | ND | Detach cells | 5.53 ± 0.08 |
| A7 | 5.59 ± 0.29 | Detach cells | 5.34 ± 0.13 |
| B10502 | 5.69 ± 0.30 | ND | ND |
Bacteria were suspended in 1 ml of DMEM with 100 μg of chloramphenicol per ml, and monolayers of differentiated Caco-2 cells cultured in 24-well plates were infected with 108 microorganisms per well. The MOI was 140 bacteria per cell.
Values are means ± standard deviations for three to eight independent experiments performed with successive cellular passages.
ND, not determined.
FIG. 1.
Association of vegetative cells of B. cereus with fully differentiated Caco-2 cells as a function of the bacterial concentration. B. cereus strains 2 and M2 were used. Error bars are not shown when they are smaller than the symbols.
The ability of B. cereus to invade enterocytes is strain dependent.
To assess the ability of B. cereus strains to invade epithelial cells, we performed the aminoglycoside antibiotic assay. Strains ATCC 10876, T1, 2, M2, and B10502 were examined after 2 h of infection. Since spores are not killed by gentamicin, the presence of sporulating microorganisms could mimic invasion events. All strains tested showed a minimum of heat-resistant microorganisms (60°C for 30 min) between 3 and 4 h of incubation (data not shown). On the basis of these findings, the ability of B. cereus to invade Caco-2 cells was determined with 3-h-old cultures. Invasion was considered positive only when the number of survivors after the gentamicin treatment was significantly larger than the number of survivors after heat treatment (60°C for 30 min). It is important to point out that under the conditions of the infection assay, bacteria neither grow nor sporulate. The values for heat-resistant microorganisms stated in Table 3 are probably due to the carryover of spores from the reactivation steps. Strains 2 and T1 showed the largest numbers of internalized bacteria in Caco-2 cells (Table 3). For these strains, both the number of microorganisms gaining entry into the cells (around 4.80 log CFU/well) and the percentage of invading bacteria in relation to associated microorganisms were significantly greater than those observed for the remaining strains being studied. In contrast, lower values were observed for strain M2 and ATCC 10876, and strain B10502 did not internalized at all.
TABLE 3.
Cell association and internalization of B. cereus in Caco-2 cellsa
| Strain | No. of bacteria (log CFU/ml)b
|
No. of survivors after heat treatment (log CFU/ml)b,c | |
|---|---|---|---|
| Associated | Internalized | ||
| M2 | 6.17 ± 0.34 | 3.86 ± 0.19a | 2.90 ± 0.34 |
| 2 | 6.44 ± 0.35 | 4.86 ± 0.25b | 1.40 ± 0.38 |
| ATCC 10876 | 5.95 ± 0.80 | 2.72 ± 0.72 | 1.90 ± 0.54 |
| T1 | 6.26 ± 0.35 | 4.80 ± 0.28b | 1.42 ± 0.22 |
| B10502 | 5.77 ± 0.36 | 1.72 ± 0.59 | <1.1 |
Bacteria were suspended in 1 ml of DMEM with 100 μg of chloramphenicol per ml, and monolayers of differentiated Caco-2 cells cultured in 24-well plates were infected with 108 microorganisms per well. The multiplicity of infection was 140 bacteria per cell. Experiments were performed with 3-h-old cultures, and the infection periods were 2 h.
Values are means ± standard deviations of triplicate determinations in at least two independent experiments performed with successive cellular passages. Letters indicates significant differences. a, P < 0.05; b, P ≤ 0.01.
Survivors after heat treatment (60°C for 30 min) are the number of sporulated or sporulating microorganisms.
B. cereus organisms residing within infected Caco-2 cells were observed by labeling the bacteria in permeabilized cells with MAbs 113D2b3 and 116A3b3 recognizing lipoteichoic acid (22), followed by a CLSM analysis. As observed in Fig. 2A and B, bacteria were observed intracellularly in cells in which F-actin labeling was not present. In addition, as observed by transmission electron microscopy, B. cereus interacted with shortened and disorganized microvilli (Fig. 2D) and resided intracellularly (Fig. 2E).
FIG. 2.
Observation of internalized B. cereus bacteria within fully differentiated Caco-2 cells infected with B. cereus strain 2. (A and B) Examination by immunofluorescence labeling and CLSM analysis of internalized B. cereus bacteria. Bacteria were labeled with a mixture of MAbs 113D2b3 and 116A3b3 recognizing lipoteichoic acid (22) and RITC-labeled goat anti-mouse antibody as the secondary antibody. No fluorescent staining was observed when the primary antibody was omitted. CLSM analysis (horizontal x-y optical sections, one section every 0.40 μm) was conducted to collect 57 en face images (22.8 μm). Labeling of brush border-associated SI determining the apical domain was observed at 0.8 to 1.6 μm (data not shown). High-magnification micrographs (reconstructed images from 57 successive sections obtained by CLSM analysis) show red-labeled bacteria at 9.2 to 12.2 μm (A) and 1.2 to 5.6 μm (B). Note that the red-labeled bacteria were observed in cells in which no labeling of F-actin develops. Identical results have been obtained with strain T1 (data not shown). (C to E) Examination by transmission electron microscopy of cultured fully differentiated human Caco-2 cells infected with B. cereus strain 2. (C) A high-magnification micrograph shows a transverse ultrathin section of control cells. Note the well-ordered microvilli. (D) A high-magnification micrograph shows a B. cereus bacterium interacting with microvilli that are shortened and disorganized. (E) A low-magnification micrograph shows B. cereus bacteria residing within the Caco-2 cells; the microvilli are not well-ordered and are disorganized and enlarged. Identical results have been obtained with strain T1 (data not shown). The micrographs are representative of two experiments conducted with successive cell passages.
Direct interaction between B. cereus and fully differentiated Caco-2 cells induces F-actin rearrangements and necrosis of eucaryotic cells.
Cellular damage triggered in Caco-2 cells by B. cereus and alteration of the F-actin distribution reported above prompted us to study whether B. cereus induces this detrimental effect. Infection of Caco-2 cells with B. cereus strains ATCC 10876, 2, T1, B10502, and M2 leads to different extents of cellular damage, characterized by observation of areas in cell monolayers showing a dramatic alteration in F-actin distribution (Fig. 3). These results raised the question of the requirement for direct procaryote-eucaryote contact to trigger these biological effects. Infection of Caco-2 cells with either 1 × 108 or 5 × 107 CFU/ml of B. cereus strain 2 led to a dose-response effect (Fig. 4A). It was noteworthy that cell culture medium obtained from cell monolayers infected for 3 h with 108 microorganisms did not cause appreciable changes to the cytoskeleton. In addition, a time-dependent study showed that the F-actin disassembly developed as a function of the time (Fig. 4B). Taken together, these data show that infection with B. cereus bacteria alone is sufficient to produce the F-actin disorganization in Caco-2 cells.
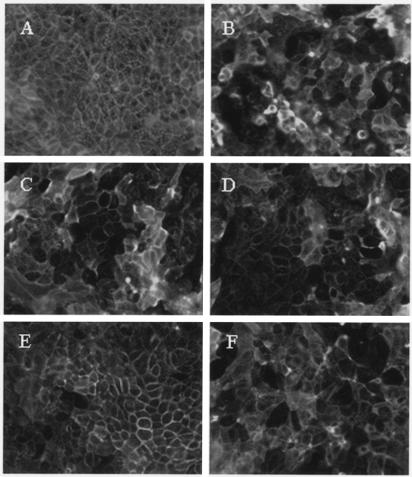
FIG. 3.
Effect of adhering B. cereus strains on the F-actin network of human enterocyte-like Caco-2 cells. Monolayers were infected with 108 CFU per well and incubated for 2 h in the presence of 100 μg of chloramphenicol per ml at 37°C. Fixed and permeabilized cells were labeled with FITC-phalloidin. (A) Control cells; (B) ATCC 10876; (C) 2; (D) T1; (E) B 10502; (F) M2. (A) An en face micrograph shows the typical mosaic pattern of the distribution of brush border-associated F-actin that results from the different patterns of microvilli on the apical surface of the cells, i.e., cells with well-ordered microvilli and cells with dense and packed microvilli (11, 33). (B to F) Cells infected by different B. cereus strains develop different patterns of F-actin alteration. Strains ATCC 10876 (B), 2 (C), T1 (D), and M2 (F) promote in place a dramatic disorganization of F-actin distribution, showing large translucent zones, whereas cells in other zones conserved the mosaic pattern of F-actin distribution. For strain B10502 (E), cells showed a marked rounding with a conserved punctuated F-actin expression in the center of the cells. Micrographs are representative of three experiments conducted with successive cell passages.
FIG. 4.
Effect of B. cereus strain 2 on the F-actin distribution in fully differentiated Caco-2 cells. (A) Effect of different concentrations of B. cereus strain 2 bacteria and culture supernatant on the F-actin distribution. En face micrographs of control uninfected cells (a), cells infected with 5 × 108 CFU/ml (b), cells infected with 1 × 107 CFU/ml (c), and culture medium of primarily infected monolayers (d) are shown. The culture medium was recovered from cells infected for 2 h with 1 × 108 CFU/ml, centrifuged, filtered, and added to a fresh cell monolayer. (B) Time course of F-actin disassembly shows the progressive decrease of F-actin immunofluorescence in cells infected with strain 2. (a) T0, (b) 15 min, (c) 1 h, (d) 2 h. Results are representative of three experiments conducted with successive cell passages.
In an attempt to gain further insight in to the effect of B. cereus infection in Caco-2 cells, we performed experiments in which cells were labeled with FITC-annexin V and propidium iodide after the infection period (Fig. 5). Whereas some apoptotic cells were normally found even in untreated control monolayers (Fig. 5A), infection of enterocyte-like cells with B. cereus led to different extents of cell necrosis. Interestingly, a higher ratio of apoptotic cells was observed in monolayers infected with strain B10502 (Fig. 5E).
FIG. 5.
Necrosis in fully differentiated Caco-2 cells infected with B. cereus strains. After 2 h of infection with 108 CFU/ml, cells were doubly labeled with FITC-annexin V and propidium iodide. (A) control cells; (B) ATCC 10876; (C) 2; (D) T1; (E) B 10502; (F) M2. Red labeling shows nuclei from necrotic cells, whereas green labeling is indicative of translocation of phosphatidylserine from the inner to the outer leaflet of the cells. apo, apoptotic cell; nec: necrotic cell. A necrotic cell showing double labeling is shown in panel D. Micrographs are representative of three experiments conducted with successive cell passages.
DISCUSSION
Interaction with enterocytes is a major component of the virulence of intestinal pathogens. Indeed, adhesion of microorganisms to the apical surface of enterocytes prevents microorganisms from being eliminated by the cleansing mechanisms of the intestine. In addition, attached bacteria can trigger a biological response from the eucaryotic cell by inducing signaling pathways associated with surface receptors. A further hallmark of virulence is the ability of some microorganisms for forced internalization into nonphagocytic cells. Several pathogens produce dramatic changes in cellular anatomy and function through a series of mechanisms mediated by proteinaceous exocellular factors and/or direct procaryotic-eucaryotic interaction (for reviews, see references 18 and 45).
The virulence of B. cereus has so far been ascribed to the production of extracellular factors (6, 8, 9, 15). However, the ability of this microorganism to attach to or invade enterocytes could significantly increase its pathogenic potential. We show here that vegetative cells of B. cereus can associate with differentiated enterocyte-like cells. Even though 100 μg of chloramphenicol per ml was used in all the assays performed and even though this concentration is sufficient to prevent bacterial growth of all strains under study (data not shown), some B. cereus strains completely detached monolayers after a 3-h incubation period. In addition, some B. cereus strains were able to enter the cells.
It is well known that expression and localization of surface molecules in eucaryotic cells depends on differentiation status (25, 28) and that the surface properties of microorganisms undergoing exponential growth are very different from those of spores or sporulating microorganisms (30, 42). In addition, the ability of microorganisms to interact with eucaryotic cells depends on the growth phase of the bacterial cultures (31). That is why our experimental conditions differed from those employed in previous studies (4, 43) in two main aspects. First, we used differentiated cultured enterocyte-like cells rather than undifferentiated cells from overnight cell culture (42). Second, we used bacterial cultures in the late exponential phase of growth instead of freeze-thawed 18-h-old cultures (43). The aminoglycoside antibiotic gentamicin assay can be used, as with other microorganisms (14, 29), to assess the invasion of enterocyte-like cells by spore-forming microorganisms. However, care must be taken to minimize the concentration of both spores and sporulating bacteria. Otherwise, spores surviving the antibiotic treatment may be considered internalized microorganisms. When 16-h-old cultures were used, the ratio of microorganisms surviving the gentamicin treatment was not significantly different from the ratio of heat-resistant microorganisms (data not shown). Study of the internalization of B. cereus by using fresh cultures harvested in the exponential growth phase constitutes a substantial improvement over methods previously employed (43).
Interestingly, in the present study we observed that the dose-dependent association of strains 2 and M2 of B. cereus with enterocyte-like Caco-2 cells shows a saturation around 2 × 107 CFU/well. These findings could indicate that sites for B. cereus adhesion are saturating on the apical domain of cells, suggesting the presence of a brush border-associated specific receptor(s) for B. cereus adhesins.
Concerning the effect of the association of B. cereus on the F-actin cytoskeleton, our results are the first to be reported that show the ability of this microorganism to disrupt the F-actin network of enterocyte-like cells. In addition, B. cereus induced severe damage in cell membrane permeability, as evidenced by double labeling with propidium iodide and FITC-annexin V. The extent of necrosis did not correlate with the ability of microorganisms to invade enterocytes. Both invasive and noninvasive strains produced necrosis of cells. Noninvasive strain B10502 elicited both necrosis and apoptosis, whereas its effect on the actin network was indistinguishable from that of the remaining strains (compare Fig. 2 and 4D). Although our experiments are not conclusive on whether adhesion and/or invasion of B. cereus can trigger cellular responses without the participation of secreted products, we could hypothetize that attachment of microorganisms to the apical domain of the enterocyte-like cells might facilitate the development of microenvironments with high concentrations of biologically active factors. In addition, when bacteria are in contact with a host cell, effectors could be translocated across the eucaryotic cell membrane by means of a series of secretion systems that have been well studied in gram-negative microorganisms (18, 29, 41, 44). However, some kind of secretion mechanism seems to be present in gram-positive bacteria. Indeed, streptolysin O can mediate the injection of effector proteins into host cells (36). This cytolysin belongs to the family of cholesterol-binding thiol-activated cytolytic toxins. It is well known that B. cereus is able to produce cereolysin O, a cytolysin belonging to the same group of pore-forming toxins (27). Thus, the contact between B. cereus and the eucaryotic cell could enhance the virulence of B. cereus through different mechanisms, (i) by inducing signaling cascades either by interaction with surface receptors on eucaryotic cells or by internalization into host cells, or (ii) by creating a microenvironment with a high concentration of toxic factors or by means of direct translocation of proteinaceous factors through pores in the cytoplasmic membrane.
Expression of genes related to the production of virulence factors in the B. cereus group is under the control of the plcR regulon, which, in turn, is activated at the onset of the stationary phase of growth (20, 32). It is worth nothing that the maximal biological activity found in our experimental system was observed with mid-log-phase cultures. These findings may indicate that release of exocellular factors to the external milieu is not sufficient to elicit biological responses in epithelial cells. Another possible hypothesis may be that cell-cell contact is necessary for the release or translocation of protein virulence factors. Interestingly, activation of the expression of the plcR regulon is related to a signaling peptide acting as a quorum-sensing effector (21). Adhesion of microorganisms to epithelial cells could contribute to the creation of zones of high bacterial concentrations that, in turn, facilitates events related to bacterium-bacterium interactions.
Summarizing, we show for the first time the relationship between B. cereus association with enterocyte-like cells and cytopathic effects. Our findings could support the role of adhesion-invasion events in the virulence of this microorganism. Further work is needed to gain further knowledge about the mechanisms involved in this interaction.
Acknowledgments
We are grateful to R. Amsellem for her expert assistance in cell culture. We are also indebted to V. Nicolas for her kind help in image analysis (Imagerie Cellulaire, IFR75-ISIT, Faculté de Pharmacie Paris XI).
J. Minnaard is a fellow of the Universidad Nacional de La Plata, and P. F. Pérez is a member of the Carrera de Investigador Cientifico y Tecnológico del Consejo Nacional de Investigaciones Cientificas y Técnicas (CONICET). This work was partially financed by the International Foundation for Science, Sweden (E/2977-2), and the Agencia Nacional de Promoción Cientifica y Tecnológica, Argentina (PICT:09-08810).
Editor: F. C. Fang
REFERENCES
- 1.Agata, N., M. Mori, M. Otha, S. Suwan, I. Ohtani, and M. Isobe. 1994. A novel dodecadepsipeptide cereulide, isolated from Bacillus cereus causes vacuole formation in Hep-2 cells. FEMS Microbiol. Lett. 121:31-34. [DOI] [PubMed] [Google Scholar]
- 2.Agata, N., M. Ohta, M. Mori, and M. Isobe. 1995. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol. Lett. 129:17-20. [DOI] [PubMed] [Google Scholar]
- 3.Alouf, J. E. 2000. Bacterial protein toxins. An overview. Methods Mol. Biol. 145:1-26. [DOI] [PubMed] [Google Scholar]
- 4.Andersson, A., P. E. Granum, and U. Rönner. 1998. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int. J. Food Microbiol. 39:93-99. [DOI] [PubMed] [Google Scholar]
- 5.Beecher, D. J., and A. C. Lee Wong. 1994. Identification of hemolysin BL-producing Bacillus cereus isolates by a discontinuous hemolytic pattern in blood agar. Appl. Environ. Microbiol. 60:1646-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beecher, D. J., and A. C. Lee Wong. 2000. Cooperative, synergistic and antagonistic haemolytic interactions between haemolysin BL, phosphatidylcholine phospholipase C and sphingomyelinase from Bacillus cereus. Microbiology 146:3033-3039. [DOI] [PubMed] [Google Scholar]
- 7.Beecher, D. J., and A. C. Lee Wong. 2000. Tripartite haemolysin BL: isolation and characterization of two distinct homologous sets of components from a single Bacillus cereus isolate. Microbiology 146:1371-1380. [DOI] [PubMed] [Google Scholar]
- 8.Beecher, D. J., T. W. Olsen, E. B. Somers, and A. C. Lee Wong. 2000. Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C and collagenase to virulence of Bacillus cereus endophthalmitis. Infect. Immun. 68:5269-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beecher, D. J., J. S. Pulido, N. P. Barney, and A. C. Lee Wong. 1995. Extracellular virulence factors in Bacillus cereus endophthalmitis: methods and implication of involvement of hemolysin BL. Infect. Immun. 63:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beecher, D. J., J. L. Schoeni, and A. C. Lee Wong. 1995. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 63:4423-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect. Immun. 64:1918-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boquet, P., P. Munro, C. Fiorentini, and I. Just. 1998. Toxins from anaerobic bacteria: specificity and molecular mechanisms of action. Curr. Opin. Microbiol. 1:66-74. [DOI] [PubMed] [Google Scholar]
- 13.Burns, J. L., A. Griffith, J. J. Barry, M. Jonas, and E. Y. Chi. 2001. Transcytosis of gastrointestinal epithelial cells by Escherichia coli K1. Pediatr. Res. 49:30-37. [DOI] [PubMed] [Google Scholar]
- 14.Callegan, M. C., M. C. Booth, B. D. Jett, and M. S. Gilmore. 1999. Pathogenesis of gram-positive bacterial endophthalmitis. Infect. Immun. 67:3348-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callegan, M. C., B. D. Jett, L. E. Hancock, and M. S. Gilmore. 1999. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect. Immun. 67:3357-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chantret, I., A. Rodolosse, A. Barbat, E. Dussaulx, E. Brot-Laroche, A. Zweibaum, and M. Rousset. 1994. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence of glucose-dependent negative regulation. J. Cell Sci. 107:213-225. [DOI] [PubMed] [Google Scholar]
- 17.Christie, P. J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cossart, P., P. Boquet, S. Normark, and R. Rappuoli (ed.). 2000. Cellular microbiology. ASM Press, Washington, D.C. [DOI] [PubMed]
- 19.Fogh, J., J. M. Fogh, and T. Orfeo. 1977. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 59:221-226. [DOI] [PubMed] [Google Scholar]
- 20.Gohar, M., O. A. Okstad, N. Gilois, V. Sanchis, A. B. Kolsto, and D. Lereclus. 2002. Two dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2:784-791. [DOI] [PubMed] [Google Scholar]
- 21.Gominet, M., L. Slamti, N. Gilois, M. Rose, and D. Lereclus. 2001. Oligopeptide permease is required for the expression of the Bacillus thuringiensis plcR regulon and for virulence. Mol. Microbiol. 40:963-975. [DOI] [PubMed] [Google Scholar]
- 22.Granato, D., F. Perotti, I. Masserey, M. Rouvet, M. Golliard, A. Servin, and D. Brassart. 1999. Cell surface-associated lopiteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl. Environ. Microbiol. 65:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granum, P. E., and H. Nissen. 1993. Sphingomyelinase is part of the "enterotoxin complex' produced by Bacillus cereus. FEMS Microbiol. Lett. 110:97-100. [DOI] [PubMed] [Google Scholar]
- 24.Guignot, J., I. Peiffer, M. F. Bernet-Camard, D. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering Escherichia coli family infecting the human polarized intestinal Caco-2/TC7. Infect. Immun. 68:3554-3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guignot, J., M.-F. Bernet-Camard, C. Poüs, L. Plançon, C. Le Bouguenec, and A. L. Servin. 2001. Polarized entry into human epithelial cells of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128: evidence for α5β1-integrin recognition, and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 69:1856-1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy, S. P., T. Lund, and P. E. Granum. 2001. CytK toxin of Bacillus cereus forms pores in planar lipid bilayers and is cytotoxic to intestinal epithelia. FEMS Microbiol. Lett. 197:47-51. [DOI] [PubMed] [Google Scholar]
- 27.Henderson, B., M. Wilson, R. McNab, and A. J. Lax. 1999. Bacterial protein toxins, p. 273-310. In B. Henderson, M. Wilson, R. McNab, and A. J. Lax (ed.), Cellular microbiology. Bacteria-host interactions in health and disease. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 28.Hess, D. J., M. J. Henry-Stanley, E. A. Moore, and C. L. Wells. 2001. Integrin expression, enterocyte maduration and bacterial internalization. J. Surg. Res. 98:116-122. [DOI] [PubMed] [Google Scholar]
- 29.Jacob-Dubuisson, F., C. Locht, and R. Antoine. 2001. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol. Microbiol. 40:306-313. [DOI] [PubMed] [Google Scholar]
- 30.Kotiranta, A., M. Haapasalo, K. Kari, E. Kerosuo, I. Olsen, T. Sorsa, J. H. Meurman, and K. Lounatmaa. 1998. Surface structure, hydrophobicity, phagocytosis and adherence to matrix proteins of Bacillus cereus cells with and without the crystalline surface protein layer. Infec. Immun. 66:4895-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusters, J. G., G. A. W. M. Mulders-Kremers, C. E. M. van Doornik, and B. A. M. van der Zeijst. 1993. Effects of multiplicity of infection, bacterial protein synthesis and growth phase on adhesion to and invasion of human cell lines by Salmonella typhimurium. Infect. Immun. 61:5013-5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lereclus, D., H. Agaisse, M. Gominet, S. Salamitou, and V. Sanchis. 1996. Identification of a Bacillus thuringiensis gene that positively regulates transcription of the phosphatidylinositol-specific phospholipase C gene at the onset of the stationary phase. J. Bacteriol. 178:2749-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liévin-Le Moal, V., R. Amsellem, A. L. Servin, and M.-H. Coconnier. 2002. Lactobacillus acidophilus (strain LB) from the resident adult human gastrointestinal microflora exerts activity against brush border damage promoted by a diarrhoeagenic Escherichia coli in human enterocyte-like cells. Gut 50:803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund, T., M.-L. De Buyser, and P. E. Granum. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254-261. [DOI] [PubMed] [Google Scholar]
- 35.Lund, T., and P. E. Granum. 1997. Comparison of biological effect of the two different enterotoxin complexes isolated from three different strains of Bacillus cereus. Microbiology 143:3329-3336. [DOI] [PubMed] [Google Scholar]
- 36.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 37.Mikami, T., T. Horikawa, T. Murakami, T. Matsumoto, A. Yamakawa, S. Murayama, S. Katagiri, K. Shinagawa, and M. Susuki. 1994. An improved method for detecting cytostatic toxin (emetic toxin) of Bacillus cereus and its application to food samples. FEMS Microbiol. Lett. 119:53-58. [DOI] [PubMed] [Google Scholar]
- 38.Minnaard, J., M. Humen, and P. F. Pérez. 2001. Effect of Bacillus cereus exocellular factors on human intestinal epithelial cells. J. Food Prot. 64:1535-1541. [DOI] [PubMed] [Google Scholar]
- 39.Peiffer, I., A. L. Servin, and M. F. Bernet-Camard. 1998. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect. Immun. 66:4036-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto, M., S. Robine-Leon, M. Appay, M. Kedinger, N. Triadou, E. Dussaulx, B. Lacroix, P. Simon-Assmann, K. Haffen, J. Fogh, and A. Zweibaum. 1983. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 47:323-330. [Google Scholar]
- 41.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 42.Rönner, U., U. Husmark, and A. Henriksson. 1990. Adhesion of Bacillus spores in relation to hydrophobicity. J. Appl. Bacteriol. 69:550-556. [DOI] [PubMed] [Google Scholar]
- 43.Rowan, N. J., K. Deans, J. G. Anderson, C. G. Gemmell, I. S. Hunter, and T. Chaithong. 2001. Putative virulence factor expression by clinical and food isolates of Bacillus spp. after growth in reconstituted infant milk formulae. Appl. Environ. Microbiol. 67:3873-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 45.Sears, C. L., and J. B. Kaper. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60:167-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Engeland, M., B. Schutte, A. H. N. Hopman, F. C. S. Ramaekers, and C. P. M. Reutelingsperger. 1999. Cytochemical detection of cytoskeletal and nucleoskeletal changes during apoptosis, p. 430-463. In G. P. Studzinski (ed.), Apoptosis: a practical approach. Oxford University Press, Oxford, United Kingdom.