Abstract
The cJun NH2-terminal kinase (JNK) stress signaling pathway is implicated in the metabolic response to the consumption of a high fat diet, including the development of obesity and insulin resistance. These metabolic adaptations involve altered liver function. Here we demonstrate that hepatic JNK potently represses the nuclear hormone receptor peroxisome proliferator-activated receptor α (PPARα). JNK therefore causes decreased expression of PPARα target genes that increase fatty acid oxidation / ketogenesis and promote the development of insulin resistance. We show that the PPARα target gene fibroblast growth factor 21 (Fgf21) plays a key role in this response because disruption of the hepatic PPARα - FGF21 hormone axis suppresses the metabolic effects of JNK-deficiency. This analysis identifies the hepatokine FGF21 as a critical mediator of JNK signaling in the liver.
Introduction
The cJun NH2-terminal kinase (JNK) signaling pathway plays an important role in the development of obesity and insulin resistance (Sabio and Davis, 2010). Indeed, JNK1-deficiency in mice prevents the obesity and insulin resistance caused by hyperphagia or the consumption of a high fat diet (HFD) (Hirosumi et al., 2002). Studies of tissue-specific JNK-deficient mice demonstrate that these obesity and insulin resistance phenotypes can be separated. Thus, JNK regulation of the hypothalamic-pituitary hormone axis that regulates energy expenditure is critically required for the development of diet-induced obesity (Belgardt et al., 2010; Sabio et al., 2010a; Vernia et al., 2013). In contrast, JNK function in peripheral tissues, including fat, muscle, and inflammatory cells contributes to diet-induced insulin resistance (Sabio et al., 2008; Sabio et al., 2010b; Han et al., 2013).
Consuming a HFD increases the blood concentration of free fatty acids (Kahn et al., 2006) and causes JNK activation mediated by the mixed-lineage protein kinase pathway (Jaeschke and Davis, 2007; Kant et al., 2013). Activated JNK contributes to obesity development by increasing Activating Protein 1 (AP1)-dependent Dio2 gene expression in the anterior pituitary gland (Vernia et al., 2013). In contrast, targets of JNK signaling that cause insulin resistance are unclear. Early studies suggested that phosphorylation of the insulin receptor adapter protein IRS1 by JNK causes insulin resistance (Aguirre et al., 2000), but subsequent studies have not confirmed this conclusion (Copps et al., 2010). More recently, JNK-mediated regulation of adipokines (Sabio et al., 2008) and inflammatory cytokines (Han et al., 2013) has been implicated in the development of insulin resistance. For example, the promotion of hepatic insulin sensitivity caused by JNK-deficiency in adipocytes and myeloid cells is associated with defects in adipokine/cytokine expression (Sabio et al., 2008; Han et al., 2013). These data indicate that JNK-mediated hepatic insulin resistance may be caused by JNK function in non-hepatic cells (Sabio et al., 2008; Han et al., 2013). This conclusion is consistent with the finding that whole body JNK1-deficiency (Hirosumi et al., 2002), but not hepatic JNK1-deficiency (Sabio et al., 2009), protects against HFD-induced insulin resistance. The function of hepatic JNK is therefore unclear.
The purpose of this study was to re-evaluate the role of hepatic JNK in the metabolic stress response caused by the consumption of a HFD. It is established that JNK is encoded by the Jnk1 and Jnk2 genes in liver (Davis, 2000). We show that mice with compound hepatic ablation of both genes (Jnk1 and Jnk2) exhibit systemic protection against HFD-induced insulin resistance. Moreover, we demonstrate that the peroxisome proliferator-activated receptor α (PPARα) - fibroblast growth factor 21 (FGF21) hormone axis contributes to metabolic regulation by hepatic JNK.
Results
Establishment of mice with JNK-deficiency in the liver
To test the role of hepatic JNK, we compared control mice (LWT) and mice with hepatocyte-specific deficiency of JNK1 (LΔ1), JNK2 (LΔ2), or JNK1 plus JNK2 (LΔ1,2) (Fig. 1A,B). High fat diet (HFD)-induced JNK activation in the liver of LWT mice was partially suppressed in LΔ1 and LΔ2 mice (Figure 1C). In contrast, hepatic JNK activity was not detected in LΔ1,2 mice (Figure 1C). These data indicate that JNK1 and JNK2 may serve partially redundant functions in the liver and confirm the absence of hepatic JNK activity in LΔ1,2 mice. We employed these mouse strains to examine the metabolic consequences of hepatic JNK-deficiency.
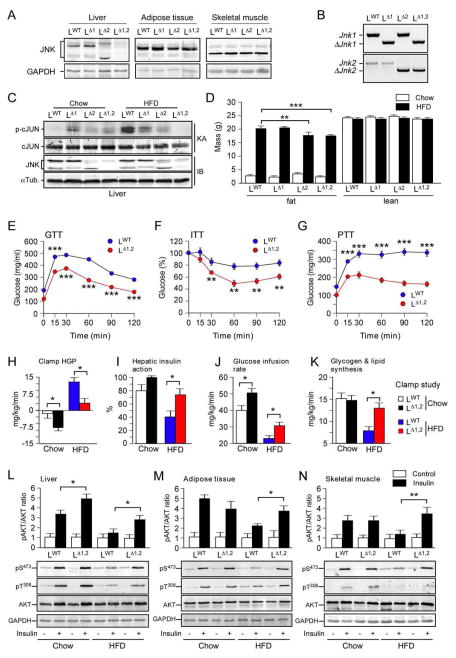
Figure 1. Hepatic JNK contributes to diet-induced obesity and insulin resistance.
(A) The liver, adipose tissue (epididymal), and skeletal muscle (gastrocnemius) of LWT, LΔ1, LΔ2 and LΔ1,2 mice were examined by immunoblot analysis by probing with antibodies to JNK and GAPDH.
(B) Genomic DNA isolated from the liver of LWT, LΔ1, LΔ2 and LΔ1,2 mice was examined by PCR analysis to detect Jnk and ΔJnk alleles.
(C) Liver extracts prepared from mice fed a chow diet or a HFD (16 wk) and starved overnight were examined by immunoblot (IB) analysis by probing with antibodies to αTubulin and JNK. In vitro protein kinase assays (KA) with the substrates GST-cJun and [γ-32P]ATP were performed to measure JNK activity. The amount of cJun and phosphorylated cJun (p-cJun) were detected by staining with Coomassie blue and Phosphorimager (Applied Biosystems) analysis, respectively.
(D) The fat and lean mass of chow-fed and HFD-fed (16 weeks) mice were measured by 1H-MRS analysis (mean ± SEM; n = 8 ~ 10). Significant differences between LWT and LΔ1,2 mice were detected (**, p < 0.01, ***, p < 0.001).
(E,F) Glucose (GTT) and insulin (ITT) tolerance tests were performed on mice fed (16 wk) a HFD (mean ± SEM; n = 35 ~ 50; **, p < 0.01; ***, p < 0.001).
(G) Pyruvate (PTT) tolerance tests were performed on mice fed (16 wk) a HFD (mean ± SEM; n = 20 ~ 30; *, ***, p < 0.001).
(H–K) Insulin sensitivity was measured using hyperinsulinemic-euglycemic clamps with conscious LWT and LΔ1,2 mice fed a chow diet or HFD. The hepatic glucose production (HGP) during the clamp, hepatic insulin action (expressed as insulin-mediated percent suppression of basal HGP), glucose infusion rate, and glycogen plus lipid synthesis (mean ± SEM for 6 ~ 8 experiments) are presented. Statistically significant differences between LWT and LΔ1,2 mice are indicated (* p < 0.05).
(L–N) Chow-fed or HFD-fed LWT and LΔ1,2 mice were starved overnight and then administered insulin (1.5U/kg body mass) by intraperitoneal injection. Liver, epididymal adipose tissue, and gastrocnemius muscle extracts (prepared 15 mins post-injection) were examined (upper panels) by multiplexed ELISA for pS473-AKT and AKT (mean ± SEM; n = 5 ~ 6; *, p < 0.05). Representative extracts were also examined by immunoblot analysis using antibodies to AKT, phospho-AKT (pSer473 and pThr308) and GAPDH (lower panels).
See also Figures S1 & S2.
Hepatic JNK-deficiency reduces diet-induced obesity
We found that hepatic JNK-deficiency caused no change in body mass when mice were fed a chow diet (Figure 1D & S1A,B). Similarly, HFD-fed LWT mice and LΔ1 mice developed equal obesity (Figure 1D & S1A,B). In contrast, HFD-fed LΔ2 mice and LΔ1,2 mice gained less fat mass than HFD-fed LWT mice (Figure 1D & S1A,B). These data indicate that JNK2-deficiency, rather than JNK1-deficiency, most closely mimics the phenotype of compound deficiency of JNK1 plus JNK2.
The reduced obesity of LΔ1,2 mice compared with LWT mice was associated with reduced adipose tissue mass (Figure S1B), reduced VLDL triglyceride in serum (Figure S1D,E), reduced total serum triglyceride (31.7 ± 0.03 mg/dl compared with 64.8 ± 0.09 mg/dl; mean ± SEM; n = 8; p < 0.03), reduced hepatic expression of lipogenic genes, including Srebf1 and Fasn (Figure S1F,G), and reduced de novo hepatic lipogenesis (Figure S1H). Together, these data demonstrate that hepatic JNK-deficiency causes dysregulated lipid metabolism.
Hepatic JNK-deficiency increases insulin sensitivity
The HFD-fed LΔ1,2 mice showed improved tolerance to glucose, insulin, and pyruvate (Figure 1E–G). Hyperinsulinemic-euglycemic clamp studies demonstrated reduced hepatic glucose production, increased hepatic insulin action, improved whole body insulin sensitivity (detected by increased glucose infusion rates during the clamps), and increased whole body glycogen plus lipid synthesis (Figure 1H–K). These data demonstrate that hepatic JNK-deficiency causes protection of mice against HFD-induced insulin resistance.
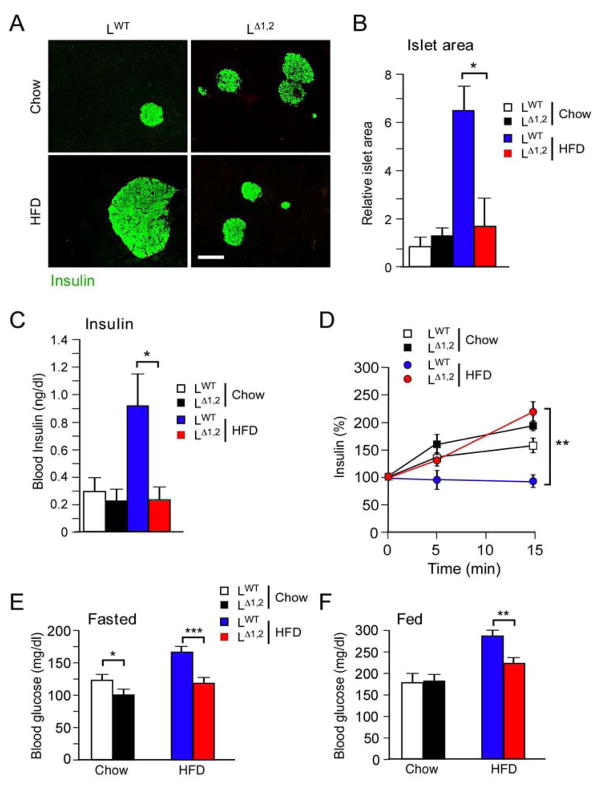
We performed biochemical studies of insulin signaling by measurement of AKT activation in LWT and LΔ1,2 mice. This analysis demonstrated that LΔ1,2 mice were partially protected against the HFD-induced suppression of insulin-stimulated AKT activation in liver, adipose tissue, and skeletal muscle that was detected in LWT mice (Fig. 1L–N). These data confirm the conclusion that HFD-fed LΔ1,2 mice exhibit a systemic increase in insulin sensitivity compared with LWT mice. The increased insulin sensitivity of LΔ1,2 mice correlates with reduced HFD-induced islet hypertrophy, hyperinsulinemia, and suppression of glucose-stimulated insulin secretion (Figure 2A–D). Moreover, HFD-induced hyperglycemia was significantly suppressed in LΔ1,2 mice compared with LWT mice (Figure 2E,F).
Figure 2. Effect of liver-specific JNK-deficiency on hyperinsulinemia.
(A) Mice were fed a chow diet or a HFD (16 wk). Sections of the pancreas were stained with an antibody to insulin. Bar, 100μm.
(B) Relative islet size was measured using Image J64 software (mean ± SEM; n = 30; *, p < 0.05).
(C) The mice were fasted overnight and the blood concentration of insulin was measured (mean ± SEM; n = 16; *, p < 0.05).
(D) Glucose-induced insulin secretion was examined using overnight fasted mice by intraperitoneal injection of glucose and measurement of blood insulin concentration (mean ± SEM; n = 8 ~ 10; **, p < 0.01).
(E,F) Mice were fed a chow diet or a HFD (16 wk). Blood glucose concentration in mice fasted overnight or fed ad libitum was measured (mean ± SEM; n = 35 ~ 50; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
The improved insulin sensitivity of HFD-fed LΔ1,2 mice contrasts with our previous analysis of LΔ1 mice with liver-specific JNK1-deficiency that exhibit increased insulin resistance compared with LWT mice (Sabio et al., 2009). This analysis suggests that hepatic JNK2 may play a critical role in glycemic regulation. Indeed, HFD-fed LΔ2 mice exhibited increased glucose tolerance, reduced islet hypertrophy, and reduced hyperinsulinemia compared with HFD-fed LWT mice (Figure S2A–F). The HFD-fed LΔ2 mice also exhibited increased insulin sensitivity in hyperinsulinemic-euglycemic clamp studies (Figure S2G–M). Nevertheless, these glycemic phenotypes of LΔ2 mice (Figure S2) are modest compared with LΔ1,2 mice (Figures 1 & 2). Together, these data indicate that LΔ1 mice and LΔ2 mice exhibit different glycemic phenotypes, and that LΔ2 mice, and especially LΔ1,2 mice, show improved control of blood glucose concentration compared with LWT mice.
The mechanism that accounts for the differential effects of hepatic JNK1 and JNK2 deficiency is unclear. Previous studies have established that isoforms of JNK with different protein kinase activities are derived by alternative splicing of primary transcripts of the Jnk1 and Jnk2 genes (Davis, 2000). Indeed, JNK substrate specificity is determined by the mutually exclusive inclusion of exons 7a or 7b in Jnk1 and Jnk2 mRNA that encode JNK isoforms α and β (Gupta et al., 1996). Analysis of hepatic Jnk mRNA demonstrated that the major JNK isoforms in liver correspond to JNK1β and JNK2α (Figure S1C). This pattern of alternative splicing of Jnk mRNA may contribute to the differential phenotypes caused by ablation of the hepatic Jnk1 or Jnk2 genes.
Hepatic JNK-deficiency increases fatty acid oxidation
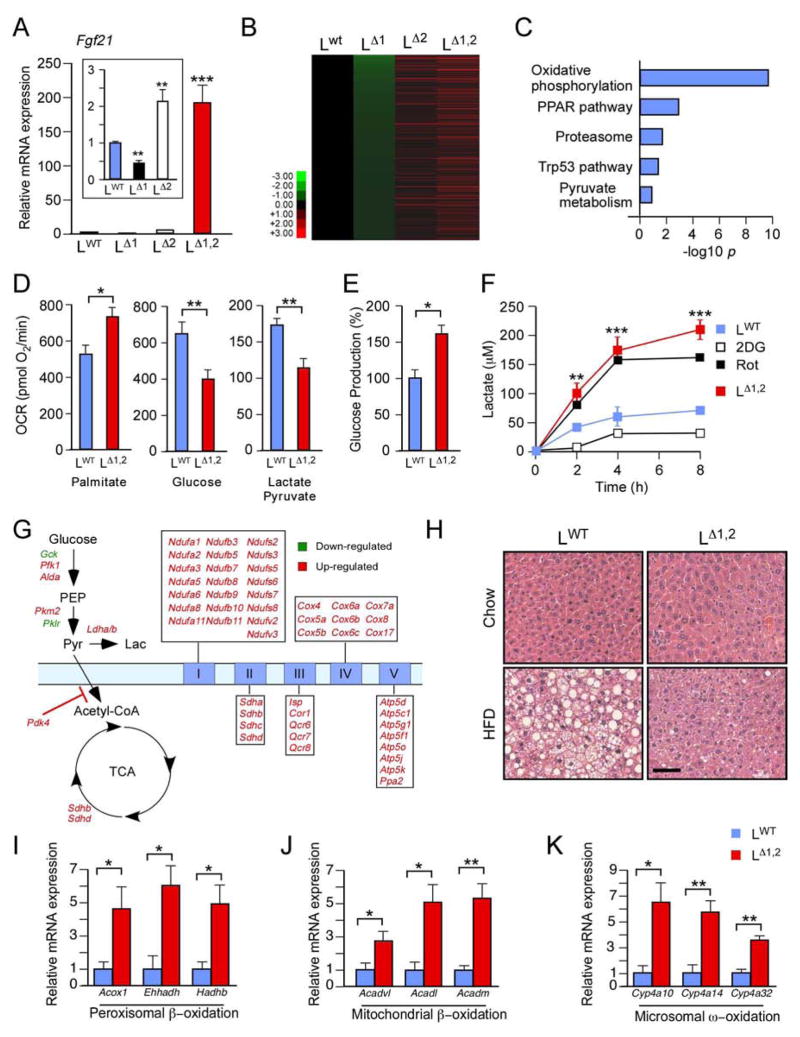
Hepatic gene expression in chow-fed and HFD-fed mice was examined using RNA-seq analysis (Figure S3A,B). JNK-deficiency caused increased gene expression in HFD-fed mice (Figure S3C–E). Gene ontology analysis demonstrated that LΔ1 mice and LΔ2 mice were markedly different and that LΔ2 mice resembled LΔ1,2 mice (Figure S3F–H). Differentially regulated genes that contribute to the hepatic phenotype of the JNK-deficient mice were therefore identified by comparing gene expression patterns with the glycemic phenotype of the mice (LΔ1 < LWT < LΔ2 < LΔ1,2). Genes down-regulated in LΔ1 mice and up-regulated in LΔ1,2 mice to a greater extent than LΔ2 mice compared with LWT mice were identified, including Fgf21 (Figure 3A,B). Gene ontology analysis identified significant (padj < 0.001) association of hepatic JNK1 plus JNK2-deficiency with the PPAR pathway and oxidative metabolism (Figure 3C).
Figure 3. Hepatic JNK suppresses the PPARα pathway and fatty acid oxidation.
(A) Fgf21 mRNA expression by primary hepatocytes obtained from LWT, LΔ1, LΔ2 and LΔ1,2 mice was measured by quantitative RT-PCR assays (mean ± SEM; n = 6; **, p < 0.01; ***, p < 0.001).
(B, C) Heatmap representation of RNA-seq analysis of hepatic genes in overnight fasted HFD-fed mice with the expression profile LΔ1 < LWT < LΔ2 < LΔ1,2. The genes are displayed with lowest expression (top) to highest expression (bottom) in LΔ1 liver. Gene ontology analysis of these genes is presented (C).
(D–F) Seahorse XF24 analysis was performed using primary hepatocytes isolated from LWT and LΔ1,2 mice. Mitochondrial oxygen consumption rate (OCR) in the presence of 200μM palmitate/BSA, 15 mM glucose, or 1 mM pyruvate / 10 mM lactate (D). Glucose production rate per μg protein in the presence of 1 mM pyruvate / 10 mM lactate (E). Lactate production by LWT and LΔ1,2 mice hepatocytes (incubated with 15 mM glucose) and the effect of treatment of LWT hepatocytes with 15 mM 2-deoxyglucose (2DG) or 100 nM rotenone (Rot). (F). The data presented are the mean ± SEM; n=10–15; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
(G) Differentially expressed glycolysis, tricarboxylic acid cycle, and electron transport chain genes identified by RNA-seq analysis in the liver of HFD-fed LΔ1,2 mice compared with HFD-fed LWT are illustrated (padj < 0.05).
(H) LWT and LΔ1,2 mice were fed a chow diet or a HFD (16 weeks). Sections of the liver were stained with hematoxylin and eosin. Bar, 25 μm.
(I–K) Gene expression in the liver of overnight-fasted LWT and LΔ1,2 mice was measured by quantitative RT-PCR assays of mRNA (mean ± SEM; n = 8; **, p < 0.01; ***, p < 0.001).
See also Figures S3 & S4.
To test whether JNK-deficiency increases oxidative metabolism, we investigated mitochondrial oxygen consumption by LΔ1,2 and LWT hepatocytes incubated with different substrates. We found that JNK-deficiency caused increased mitochondrial oxygen consumption in the presence of palmitate (Figure 3D). In contrast, mitochondrial oxygen consumption by LΔ1,2 hepatocytes metabolizing glucose or pyruvate/lactate was decreased (Figure 3D). Moreover, glucose production by LΔ1,2 hepatocytes metabolizing lactate/pyruvate was increased compared with LWT hepatocytes (Figure 3E). This increase in glucose production in vitro was associated with increased expression of the gluconeogenic genes G6pc and Pck1 by LΔ1,2 hepatocytes compared with LWT hepatocytes (Figure S1I). We also found that lactate production by LΔ1,2 hepatocytes metabolizing glucose was increased compared with LWT hepatocytes (Figure 3F). These effects of JNK-deficiency were associated with increased expression of citric acid cycle and respiratory chain genes together with increased expression of Pdk4, an inhibitor of the oxidative metabolism of pyruvate (Figure 3G). These data demonstrate that JNK-deficiency increases the glycolytic conversion of glucose to lactate and selectively promotes the oxidation of fatty acids.
Hepatic JNK-deficiency activates the PPARα pathway
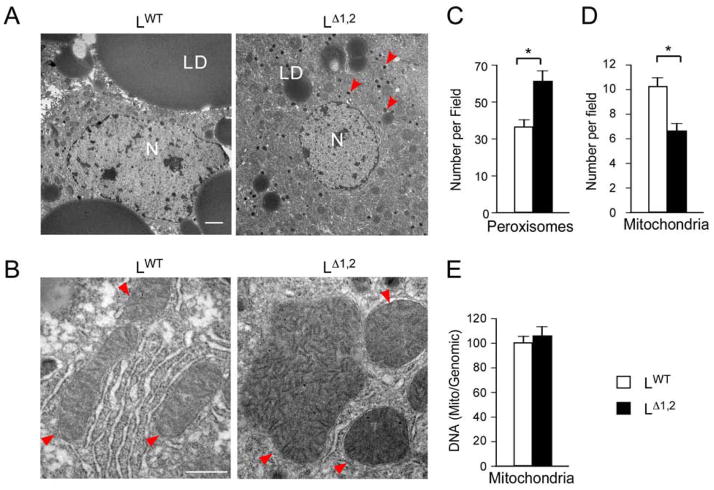
The hepatic PPARα pathway (and PPARβ/δ in fat and muscle) increases fatty acid oxidation by peroxisomes, mitochondria, and the endoplasmic reticulum (Evans et al., 2004; Pyper et al., 2010). PPARα pathway activation and increased fatty acid oxidation in the liver of LΔ1,2 mice are reflected by increased PPARα target gene expression (Figure S3B), including genes required for β-oxidation in peroxisomes (Figure 3I) and mitochondria (Figure 3J) together with ω-oxidation in the endoplasmic reticulum (Figure 3K) compared with LWT mice. The LΔ1,2 mice also exhibited increased numbers of hepatic peroxisomes (Figure 4A,C), increased mitochondrial size (Figure 4B,D,E) (consistent with reduced JNK-promoted mitochondrial fission (Leboucher et al., 2012)), and reduced respiratory exchange ratio (Figure S4) compared with LWT mice. These data are consistent with the presence of increased PPARα pathway-stimulated fatty acid oxidation in the liver of LΔ1,2 mice. The increased in fatty acid oxidation (Figure 3D), together with reduced lipogenesis (Figure S1F–H), contributes to the reduced hepatic steatosis detected in LΔ1,2 mice compared with LWT mice (Figure 3H). Since treatment with PPARα agonists can improve glycemia by increasing fatty acid oxidation (Evans et al., 2004; Pyper et al., 2010), PPARα pathway activation may contribute to the phenotype of LΔ1,2 mice (Figures 1 & 2).
Figure 4. Hepatic JNK-deficiency increases peroxisome number and mitochondrial size.
(A, B) LWT and LΔ1,2 mice were fed a HFD (16 wks). Sections of the liver were examined by transmission electron microscopy. Representative images are presented. Key: nucleus (N); lipid droplet (LD). Arrow heads indicate peroxisomes (A) and mitochondria (B). Bar, 2 μm (A) and 0.5 μm (B).
(C, D) The number of peroxisomes (C) and mitochondria (D) per field was measured (mean ± SEM; n = 30; *, p < 0.05).
(E) Relative mitochondrial DNA copy number was measured (mean ± SEM; n = 3).
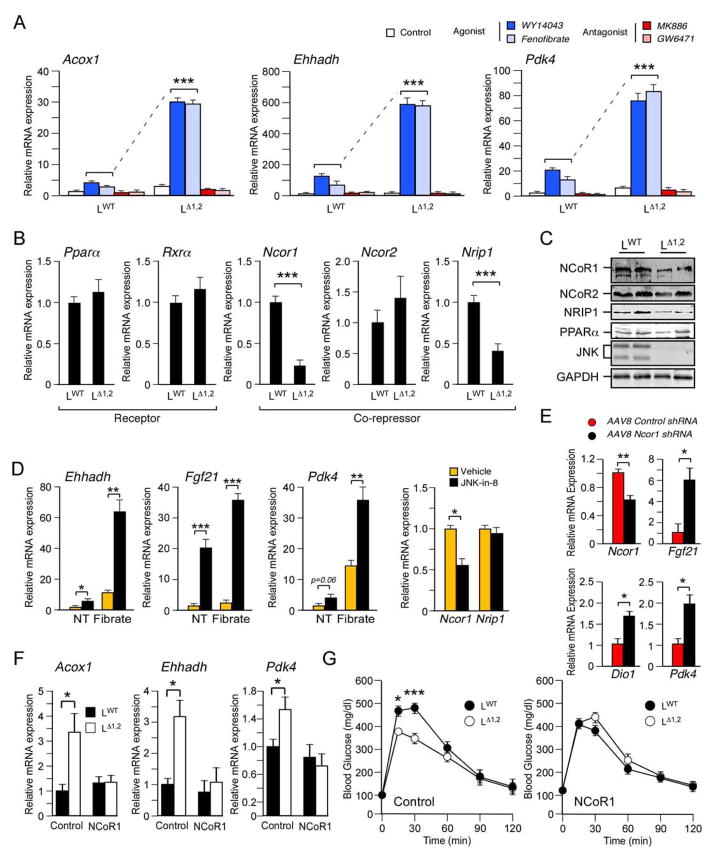
The mechanism that mediates PPARα pathway activation in JNK-deficient liver is unclear. We therefore examined PPARα-dependent gene expression in primary hepatocytes. Studies of three PPARα target genes (Acox1, Ehhadh, and Pdk4) demonstrated no major differences in the amount of basal expression between LΔ1,2 and LWT hepatocytes. Gene expression was suppressed by treatment with the PPARα antagonists GW6471 or MK886 (Figure 5A). Treatment with the PPARα agonists WY14043 or Fenofibrate caused increased gene expression by LWT hepatocytes that was greatly potentiated in LΔ1,2 hepatocytes (Figure 5A). This increase in PPARα-dependent gene expression was not caused by changes in the expression of PPARα or its heterodimeric partner RXRα (Figure 5B). Reduced expression of co-repressors could contribute to increased PPARα function (Perissi et al., 2010; Mottis et al., 2013) and previous studies have established that decreased NCoR1 expression can cause increased nuclear hormone receptor activity (Li et al., 2011; Yamamoto et al., 2011). We found that hepatic JNK-deficiency decreased the expression of the PPARα co-repressors NCoR1 and NRIP1 (Figure 5B,C). The reduced expression of these co-repressors may contribute to the metabolic phenotype of LΔ1,2 mice because it is established that reduced expression of NCoR1 (Mottis et al., 2013) or NRIP1 (Nautiyal et al., 2013) is sufficient to promote insulin sensitivity.
Figure 5. Hepatic JNK inhibits PPARα-dependent gene expression.
(A) Primary hepatocytes were obtained from LWT and LΔ1,2 mice and incubated with solvent (DMSO, Control), PPARα agonists (50 μM WY14043 or 100 μM Fenofibrate), or PPARα antagonists (10 μM GW6471 or 20 μM MK886) for 16h. PPARα target gene (Acox1, Ehhadh, and Pdk4) expression was examined by measurement of mRNA by quantitative RT-PCR analysis (mean ± SEM; n = 6; ***, p < 0.001).
(B) The expression of Pparα, Rxrα, Ncor1, Ncor2, and Nrip1 mRNA by LWT and LΔ1,2 primary hepatocytes was measured by quantitative RT-PCR analysis (mean ± SEM; n = 6; ***, p < 0.001).
(C) LWT and LΔ1,2 primary hepatocytes were examined by immunoblot analysis by probing with antibodies to PPARα, NCoR1, NCoR2, NRIP1, JNK, and GAPDH.
(D) Primary wild-type hepatocytes were treated (12 h) with DMSO (vehicle) or 1μM JNK-in-8 (a small molecule JNK inhibitor) prior to measurement of mRNA expression by quantitative RT-PCR analysis (mean ± SEM; n = 6; *, p < 0.05; **, p < 0.01; ***, p < 0.001). The effect of treatment (8 h) of the hepatocytes with DMSO (NT) or 100 μM Fenofibrate (Fibrate) was examined.
(E) Recombinant AAV8 vectors were employed to express Control shRNA or Ncor1 shRNA in the liver of HFD-fed (4 wks.) of LWT and LΔ1,2 mice. Hepatic mRNA expression at 10 ~ 16 days post-infection was examined by quantitative RT-PCR analysis (mean ± SEM; n = 14 ~ 15; *, p < 0.05; **, p < 0.01).
(F, G) NCoR1 or GFP (Control) were expressed in the liver of HFD-fed (4 wks.) of LWT and LΔ1,2 mice using recombinant adenovirus vectors (10 ~ 16 days). The hepatic expression of Acox1, Ehhadh & Pdk4 mRNA was measured by quantitative RT-PCR analysis (F). The mice were examined by glucose tolerance tests (G). The data presented are the mean ± SEM (n = 6; *, p < 0.05; ***, p < 0.001).
See also Figures S5 & S6.
To confirm the conclusion that loss of JNK protein kinase activity causes increased PPARα pathway activity, we examined the effect of the selective inhibitor JNK-in-8 (Zhang et al., 2012). This analysis demonstrated that inhibition of JNK protein kinase activity caused increased PPARα target gene expression (Ehhadh, Fgf21 & Pdk4) by primary hepatocytes treated with the PPARα-agonist Fenofibrate (Figure 5D). The JNK inhibitor also caused decreased expression of Ncor1 mRNA (Figure 5D). This observation indicates that the JNK inhibitor and Jnk gene ablation cause a similar decrease in Ncor1 expression and increase in PPARα pathway activity. However, the decrease in Nrip1 expression detected in LΔ1,2 hepatocytes (Figure 5B,C) was not detected when wild-type hepatocytes were treated with the JNK inhibitor (Figure 5D). The mechanism that accounts for this differential regulation of Nrip1 expression is unclear, but it is possible that short-term pharmacological JNK inhibition may be insufficient to phenocopy the effects of chronic JNK loss-of-function in LΔ1,2 hepatocytes. These data suggest that Ncor1, rather than Nrip1, represents a key target of hepatic JNK.
To test whether decreased Ncor1 expression is sufficient to account for PPARα pathway activation, we used shRNA to knock-down hepatic Ncor1 expression with an adenovirus-associated virus serotype 8 (AAV8) vector. Control studies demonstrated that the AAV8-shNcor1 vector reduced the expression of hepatic Ncor1 mRNA (Figure 5E). Expression of PPARα target genes (Fgf21 and Pdk4) was increased in the mice treated with the AAV8-shNcor1 vector compared with mice treated with the control AAV8-shLuc vector (Figure 5E). These data confirm that reduced NCoR1 expression is sufficient to increase hepatic PPARα pathway activity.
We performed complementation assays to test whether restoration of NCoR1 expression suppressed the effect of hepatic JNK-deficiency to increase PPARα target gene expression. Indeed, ectopic NCoR1 expression in the liver of LΔ1,2 mice reduced expression of the PPARα target genes Acox1, Ehhadh, and Pdk4 (Figure 5F). Moreover, restoring NCoR1 prevented the effect of JNK-deficiency to cause improved glucose tolerance in HFD-fed mice (Figure 5G). Together, these data confirm the conclusion that reduced NCoR1 expression contributes to the effects of JNK-deficiency on the hepatic PPARα signaling pathway.
A reduction in co-repressor expression in JNK-deficient hepatocytes (Figure 5B,C) may cause not only increased PPARα activity, but also increased activity of other nuclear hormone receptors, including the thyroid hormone receptor that binds NCoR1 (Horlein et al., 1995). Indeed, reduced NCoR1 expression in the liver caused increased expression of the thyroid hormone receptor target gene Dio1 (Figure 5E). To test this prediction, we examined triiodothyronine (T3) -stimulated gene expression in primary hepatocytes isolated from LWT and LΔ1,2 mice. We found that JNK-deficiency caused significantly increased T3-stimulated expression of thyroid hormone receptor target genes (Figure S5A). Increased expression of these genes in the liver of LΔ1,2 mice compared with LWT mice in vivo was detected in the absence of significant changes in the amount of blood thyroid hormone (Figure S5B,C). These data support the conclusion that hepatic JNK-deficiency may increase the activity of multiple nuclear hormone receptors by decreasing co-repressor activity.
Hepatic JNK-deficiency and the PPARγ pathway
The PPARγ pathway plays an important role in AP1-dependent regulation of hepatic lipid metabolism (Hasenfuss et al., 2014). The mechanism is mediated by increased expression of PPARγ2 that is promoted by cFos heterodimers with cJun/JunB/JunD and is antagonized by Fra1/2 heterodimers with cJun (Hasenfuss et al., 2014). Since AP1 proteins are key targets of the JNK signaling pathway (Davis, 2000), we anticipated that hepatic JNK-deficiency may regulate the expression of both AP1 proteins and PPARγ2. Indeed, hepatic JNK-deficiency caused reduced expression of cJun, JunD, Fra2, cFos, and FosB in HFD-fed mice (Figure S6A), but hepatic JNK-deficiency caused no significant change in the HFD-induced expression of PPARγ2 (Figure S6B). The absence of a PPARγ2 expression phenotype caused by hepatic JNK-deficiency was an unexpected finding that may reflect the balance of positive and negative regulation of PPARγ2 expression by different heterodimeric AP1 transcription factor complexes (Hasenfuss et al., 2014). Nevertheless, compromised hepatic AP1 function in LΔ1,2 mice (Figure S6A) may contribute to decreased expression of Ncor1 and Nrip1 (Figure S6C,D), and indirectly increase PPARγ pathway activity by reducing co-repressor expression.
Hepatic JNK-deficiency increases expression of the PPARα target gene Fgf21
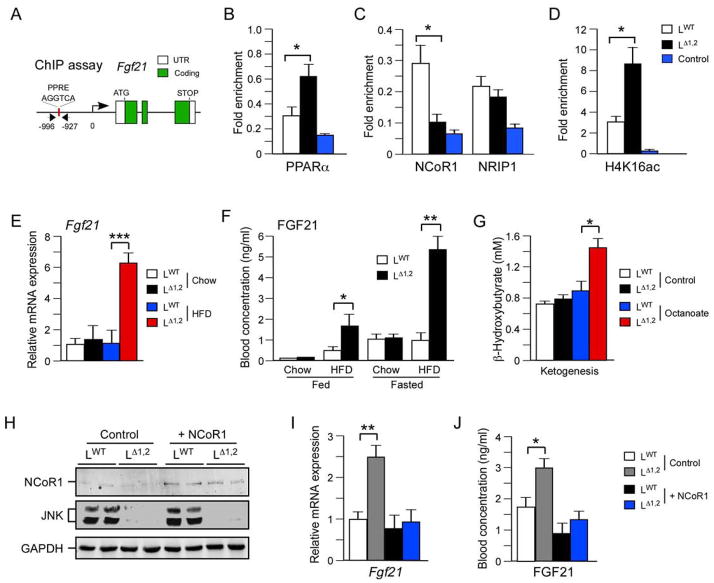
We performed chromatin immunoprecipitation assays to examine proteins bound to the promoter of the PPARα target gene Fgf21 (Figure 6A). This analysis demonstrated that JNK-deficiency increased the amount of PPARα bound to the Fgf21 promoter (Figure 6B). We also found that JNK-deficiency reduced the amount of bound NCoR1 (Figure 6C). In contrast, JNK-deficiency did not change the interaction of NRIP1 with the Fgf21 promoter (Figure 6C). As expected, the reduced interaction with NCoR1 was associated with increased histone acetylation on the Fgf21 promoter (Figure 6D). Together, these data indicate that reduced NCoR1 function contributes to increased PPARα pathway activation caused by JNK-deficiency in the liver.
Figure 6. The FGF21 pathway is repressed by JNK.
(A–D) Chromatin immunoprecipitation (ChIP) assays were performed using liver isolated from overnight fasted LWT and LΔ1,2 mice with antibodies to non-immune immunoglobulin (Control), PPARα, NCoR1, NRIP1, and acetyl-lysine16 histone H4 (H4K16ac). The fold enrichment of a fragment of the Fgf21 promoter with a PPRE site was measured by quantitative PCR analysis (mean ± SEM; n = 6; p < 0.05).
(E) The hepatic expression of Fgf21 mRNA in overnight fasted mice was examined by quantitative RT-PCR analysis (mean ± SEM; n = 8; ***, p < 0.001).
(F) The concentration of FGF21 in the blood of chow-fed and HFD-fed LWT and LΔ1,2 mice was measured by ELISA. Blood was collected from mice fed ad libitum or fasted overnight (mean ± SEM; n=6 *, p < 0.05; **, p < 0.01).
(G) Ketone body production was measured in overnight-fasted LWT and LΔ1,2 mice challenged (6 h) without (Control) and with Octanoate (mean ± SEM; n = 5; *, p < 0.05).
(H–J) NCoR1 or GFP (Control) were expressed in the liver of HFD-fed (4 wks.) LWT and LΔ1,2 mice using recombinant adenovirus vectors (10 ~ 16 days). The mice were fasted overnight and hepatic expression of NCoR1 and GAPDH were examined by immunoblot analysis (H). The amount of Fgf21 mRNA was measured by quantitative RT-PCR analysis (I) and the concentration of FGF21 in the blood was measured by ELISA (J). The data presented are the mean ± SEM; n = 6; *, p < 0.05; **, p < 0.01.
See also Figure S7.
The hepatic PPARα pathway plays a major role in ketogenesis (Evans et al., 2004; Pyper et al., 2010). The mechanism is mediated, in part, by PPARα-dependent expression of Fgf21 (Badman et al., 2007; Inagaki et al., 2007; Badman et al., 2009; Potthoff et al., 2009), a gene that is dysregulated by hepatic JNK-deficiency (Figure 3A). Comparison of LWT and LΔ1,2 mice demonstrated that JNK-deficiency caused increased hepatic expression of Fgf21 mRNA (Figure 6E), increased circulating amounts of FGF21 in the blood (Figure 6F), and increased ketogenesis (Figure 6G). Complementation assays demonstrated that restoration of hepatic NCoR1 expression (Figure 6H) suppressed the effect of JNK-deficiency to cause increased expression of Fgf21 mRNA in the liver (Figure 6I) and the amount of FGF21 circulating in the blood (Figure 6J). Moreover, shRNA-mediated knock-down of Ncor1 caused increased hepatic expression of FGF21 (Figure 5E). Together, these data confirm that JNK-regulated NCoR1 expression contributes to the effects of JNK to inhibit the PPARα - FGF21 hormone axis.
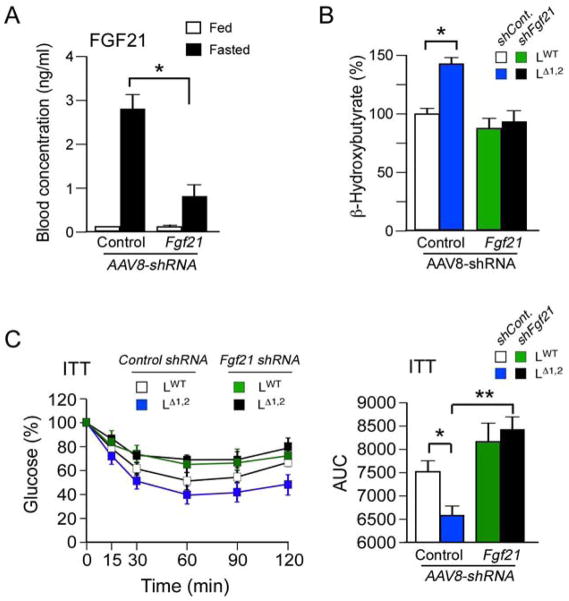
To test whether increased Fgf21 expression contributes to the phenotype of LΔ1,2 mice, we used shRNA to knock-down hepatic Fgf21 expression. Control studies demonstrated that an AAV8-shFgf21 vector reduced hepatic Fgf21 mRNA expression by 75 ± 7% (mean ± SD; n = 6; p < 0.05). Moreover, the amount of FGF21 in the blood was reduced in mice treated with the AAV8-shFgf21 vector compared with mice treated with the control AAV8-shLuc vector (Figure 7A). We found that the shFgf21 vector prevented the increased ketogenesis (Figure 7B) and suppressed the improved insulin tolerance (Figure 7C) caused by hepatic JNK-deficiency. These data support the conclusion that the PPARα - FGF21 hormone axis contributes to metabolic regulation by hepatic JNK.
Figure 7. FGF21 mediates metabolic effects of hepatic JNK-deficiency.
(A) AAV8 vectors were employed to express Control shRNA and Fgf21 shRNA in the liver. The mice were fed a HFD (4 wk). FGF21 in the blood of mice fed ad-libitum or fasted overnight was measured by ELISA (mean ± SEM; n = 6; *, p < 0.05).
(B) Ketogenesis was examined in HFD-fed mice that were fasted overnight and then challenged with octanoate (6 h) by measurement of blood β-hydroxybutyrate (mean ± SEM; n = 15 ~ 30; p < 0.05). The data are normalized to blood β-hydroxybutyrate in control mice at 0 h (100%).
(C) Insulin tolerance tests (ITT) were performed on HFD-fed mice. The time course of blood glucose clearance and the area under the curve (AUC) are presented (mean ± SEM; n = 15 ~ 30; *, p < 0.05; **, p < 0.01).
FGF21 contributes to the systemic metabolic effects of hepatic JNK-deficiency
FGF21 regulates adipose tissue metabolism, in part, by reducing PPARγ inhibitory sumoylation (Dutchak et al., 2012) and increasing PGC1α expression (Potthoff et al., 2009; Fisher et al., 2012). We therefore examined LWT and LΔ1,2 mice to investigate whether hepatic JNK might regulate a FGF21/PPARγ - PGC1α axis in adipose tissue. Analysis of epididymal adipose tissue sections demonstrated that the HFD-induced hypertrophy and macrophage infiltration of LWT adipose tissue were reduced in LΔ1,2 mice (Figure S7A). Gene expression analysis demonstrated decreased expression of macrophage genes, including genes associated with both M1 and M2 polarization, and reduced expression of the adipokine Leptin (Figure S7B). These observations are consistent with the decreased obesity of HFD-fed LΔ1,2 mice compared with HFD-fed LWT mice (Figure 1D & S1A,B). We found that LΔ1,2 mice exhibited increased expression of the adipose tissue PPARγ target genes Adiponectin (Holland et al., 2013; Lin et al., 2013) and Fgf21 (Muise et al., 2008; Wang et al., 2008) compared with LWT mice (Figure S7B). FGF21-stimulated Pgc1α expression has been implicated in the brown-like adaptation of sub-cutaneous white adipose tissue (Fisher et al., 2012). Indeed, we found increased expression of brown-like genes in the inguinal adipose tissue of HFD-fed LΔ1,2 mice compared with HFD-fed LWT mice (Figure S7C). Together, these data indicate that FGF21 contributes to the systemic metabolic effects of hepatic JNK-deficiency.
Discussion
The liver is a critical organ that orchestrates metabolism within the body. In the fed state, hepatic lipogenesis is increased and the liver can export triglycerides in the form of very low density lipoprotein to peripheral tissues. In contrast, fasting increases hepatic fatty acid oxidation and the delivery of ketone bodies to peripheral tissues. The re-programming of liver function between these two metabolic states is mediated, in part, by endocrine hormones (insulin, glucagon, FGF15/19, and FGF21) (Potthoff et al., 2012). The metabolic transition also involves key signal transduction pathways that are differentially activated during feeding and starvation. For example, the PPARα pathway plays an important role during starvation by increasing fatty acid oxidation / ketogenesis and promoting insulin sensitivity (Evans et al., 2004; Pyper et al., 2010). In contrast, the cJun NH2-terminal kinase (JNK) signaling pathway is active in the fed state and is implicated in hepatic steatosis and insulin resistance (Davis, 2000; Sabio and Davis, 2010). These opposing actions of the PPARα and JNK signaling pathways contribute to a metabolic switch that can determine liver function.
This study demonstrates that JNK plays a major role in the regulation of hepatic metabolism by inhibiting nuclear hormone receptor pathways, including PPARα, that increase fatty acid oxidation and ketogenesis. Multiple PPARα target genes likely contribute to these actions of JNK, but Fgf21 plays a key role in ketogenesis (Badman et al., 2007; Badman et al., 2009), insulin sensitivity (Holland et al., 2013; Lin et al., 2013), glycemia (Kharitonenkov et al., 2005; Xu et al., 2009a; Xu et al., 2009b), and obesity (Kharitonenkov et al., 2005; Coskun et al., 2008). Indeed, suppression of FGF21 expression in the liver prevents the effects of hepatic JNK-deficiency on insulin sensitivity and ketogenesis (Figure 7). The PPARα - FGF21 hormone axis is therefore an important target of the hepatic JNK signaling pathway that regulates metabolism.
Our analysis establishes that JNK inhibits PPARα target gene expression in the liver. This effect of JNK is not mediated by changes in the expression of PPARα (or its partner RXRα), but is associated with reduced expression of the co-repressors NCoR1 and NRIP1 (Figure 5B,C). ChIP assays demonstrated that JNK-deficiency changes the interaction of PPARα (increased) and NCoR1 (reduced) with a PPRE in the promoter of the PPARα target gene Fgf21 (Figure 6A–C). This reduction in co-repressor binding is sufficient to account for PPARα pathway activation (Perissi et al., 2010; Mottis et al., 2013) and may be caused by the reduced co-repressor expression detected in mice with hepatic JNK-deficiency (Figure 5B,C). Indeed, shRNA studies demonstrated that Ncor1 knock-down was sufficient to cause increased hepatic FGF21 expression (Figure 5E). Moreover, restoration of Ncor1 expression in the liver prevented PPARα pathway activation (Figure 5F), FGF21 expression (Figure 6I,J), and improved glucose tolerance (Figure 5G) caused by JNK-deficiency. These data demonstrate that JNK promotes repression of PPARα target gene expression by regulating co-repressor function.
The presence of AP1 sites in the Ncor1 and Nrip1 promoters (Figure S6C,D) suggests that these genes may be direct targets of a hepatic JNK signaling pathway that activates AP1 (Figure S6A), but other transcription factors or indirect mechanisms (e.g. miRNA pathways) may also contribute to JNK-regulated expression of NCoR1 and NRIP1. Additional analysis will be required to define the mechanism of co-repressor regulation by hepatic JNK. Nevertheless, it is established that Ncor1 mRNA expression is dynamically regulated by exposure of cells to fatty acids and other stimuli that can activate JNK (Yamamoto et al., 2011). Indeed, JNK-stimulated Ncor1 gene expression may contribute to this dynamic regulation and cause JNK-mediated repression of the PPARα pathway.
Further studies will be required to test whether PPARα pathway repression is mediated exclusively by JNK-regulated expression of the co-repressors NCoR1 and NRIP1. Our analysis does not exclude the possibility that additional factors may contribute to co-repressor regulation. Examples include roles for alternative splicing, phosphorylation, nuclear/cytoplasmic transport, protein stability (sumoylation), and protein instability (ubiqutination) (Perissi et al., 2010; Mottis et al., 2013). The possible regulation of NCoR1 degradation by JNK is intriguing because studies of AP1 regulation in macrophages have established that cJun phosphorylation triggers the dissociation and subsequent degradation of NCoR1 (Ogawa et al., 2004). Whether this mechanism is relevant to the PPARα pathway is unclear. Nevertheless, a recent study has highlighted NCoR1 protein turnover as an important regulatory mechanism for gene expression related to energy metabolism (Catic et al., 2013).
The regulation of NCoR1 and NRIP1 expression by JNK suggests that mice with hepatic JNK-deficiency may exhibit altered function of multiple nuclear receptors. For example, hepatic NCoR1 functions as a potent inhibitor of thyroid hormone signaling (Feng et al., 2001; Astapova et al., 2008; Fozzatti et al., 2011) and we found that hepatic JNK-deficiency caused increased thyroid hormone-dependent gene expression (Figure S5). Similarly, both NCoR1 and NRIP1 inhibit the nuclear hormone receptor LXR (Herzog et al., 2007; Astapova et al., 2008). JNK-deficiency may therefore also regulate LXR, cross-talk with the PPARα pathway (Boergesen et al., 2012), and contribute to the regulation of hepatic lipid metabolism (Beaven et al., 2013). These considerations indicate that there is potential for significant complexity in the hepatic nuclear receptor signaling network that is regulated by JNK. Further studies will be required to determine the roles of these nuclear receptors (including FXR, GR, HNF4, LXR & TR) in the metabolic response to JNK activation. Nevertheless, our analysis establishes that the PPARα -FGF21 hormone axis is an important mediator of metabolic regulation by hepatic JNK.
Conclusions
The activity of hepatic JNK is regulated by starvation and feeding. These changes in JNK activity regulate the hepatic PPARα - FGF21 hormone axis. Studies of mice with hepatic JNK-deficiency demonstrate an exaggerated starvation response (increased ketogenesis) and a reduced response to feeding (protection against hyperglycemia and insulin resistance). These responses are mediated, in part, by the hepatic PPARα - FGF21 hormone axis. Hepatic JNK therefore plays a key role in diet-mediated metabolic regulation.
EXPERIMENTAL PROCEDURES
Mice
We have previously described Jnk1LoxP/LoxP mice (Das et al., 2007) and Jnk2LoxP/LoxP mice (Han et al., 2013). C56BL/6J mice (stock number 000664), and B6.Cg-Tg(Alb-cre)21Mgn/J mice (stock number 003574) (Postic et al., 1999) were obtained from The Jackson Laboratory. The mice were backcrossed to the C57BL/ 6J strain (ten generations) and housed in a facility accredited by the American Association for Laboratory Animal Care (AALAC). The mice employed in this study were LWT (Cre+), LΔ1 (Cre+ Jnk1LoxP/LoxP), LΔ2 (Cre+ Jnk2LoxP/LoxP), and LΔ1,2 (Cre+ Jnk1LoxP/LoxP Jnk2LoxP/LoxP). All studies were performed using male mice (8–24 wk old). Male mice (8 weeks old) were fed a standard Control (chow) diet or a high fat diet (HFD) (Iso Pro 3000, Purina and F3282, Bioserve, Inc.). Whole body fat and lean mass were non-invasively measured using 1H-MRS (Echo Medical Systems). The animal studies were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Viral transduction studies
The adeno-associated virus (AAV) vector pAAVscCB6siFluc EGFP that expresses an shRNA that targets Luciferase was modified by exchanging the Luciferase shRNA sequence (BamH1 sites) with a sequence that encodes an Fgf21 shRNA (5′-GATCAAAAAAGGGATTCAACACAG GAGAAACTTCGGTTTCTCCTGTGTTGAATCCC-3′). The vector pAAV-RFP-U6 used to express Ncor1 shRNA (target sequence 5′-CGGCATAATCTTGACAACCTT-3′) was obtained from Vector Biolabs. Recombinant AAV serotype 8 (AAV8) were prepared and amplified by the University of Massachusetts Gene Therapy Vector Core (Mueller et al., 2012; Ahmed et al., 2013). Male mice (8 weeks old) were treated by intravenous injection (tail vein) with 3×1011 genomic copies/mouse of AAV8-shLuc (Control), AAV8-shFgf21, or AAV8-shNcor1 (200 μl final volume). Studies were performed 30 ~ 90 days post-infection.
Complementation studies were performed using Ad-GFP (Control) and Ad-NcoR1 (NM_001252313) adenovirus stocks obtained from Applied Biological Materials. Male mice (8 weeks old) were fed a HFD (4 wks.) and then treated by intravenous injection (tail vein; 200 μl final volume) with 8×109 genomic copies/mouse of recombinant adenovirus. Studies were performed 10 ~ 16 days post-infection.
Blood analysis
Blood glucose was measured with an Ascensia Breeze 2 glucometer (Bayer). β-Hydroxybutyrate was measured by colorimetric assays (Wako). Insulin and TSH in plasma were measured by multiplexed ELISA using a Luminex 200 machine (Millipore). T3 and T4 (Calbiotech) and FGF21 (Millipore) in plasma were measured using ELISA kits. Serum triglyceride was measured using a kit (Sigma). Lipoprotein analysis was performed by the University of Cincinatti Mouse Metabolic Phenotyping Center.
Glucose, insulin and pyruvate tolerance tests
Glucose and insulin tolerance tests were performed by intraperitoneal injection of mice with glucose (1g/kg), insulin (0.5 U/kg) or pyruvate (1g/kg) using methods described previously (Sabio et al., 2008).
Hepatic lipogenesis
Mice were starved overnight and re-fed (3h). Lipogenesis was examined in vivo by injecting mice with 20 μCi/g 3H2O and measurement of radioactivity incorporation by scintillation counting with adjustments for 3H2O specific activity and tissue mass (Stansbie et al., 1976; Zhang et al., 2006).
Ketogenesis assays
Ketone body formation in vivo was measured using the ketogenic substrate octanoate (McGarry and Foster, 1971). Briefly, mice starved overnight were subjected to intraperitoneal injection of 10 μL/g of 250 mM sodium octanoate in sterile water prior to measurement of β-hydroxybutyrate in the blood (Potthoff et al., 2009).
Hyperinsulinemic-euglycemic clamp studies
The clamp studies were performed by the UMASS Mouse Metabolic Phenotyping Center. Following an overnight fast, a 2-hr hyperinsulinemic-euglycemic clamp was conducted in conscious mice with a primed and continuous infusion of human insulin (150 mU/kg body weight priming followed by 2.5 mU/kg/min; Humulin; Eli Lilly), and 20% glucose was infused at variable rates to maintain euglycemia (Kim et al., 2004).
Metabolic cage analysis
The analysis was performed by the Mouse Metabolic Phenotyping Center at the University of Massachusetts Medical School. The mice were housed under controlled temperature and lighting with free access to food and water using metabolic cages (TSE Systems).
Statistical analysis
Differences between groups were examined for statistical significance using the Student’s test or analysis of variance (ANOVA) with the Fisher’s test.
Supplementary Material
Acknowledgments
We thank Alfredo Giménez-Cassina and Marc Liesa for critical discussions, Shmulik Motola for assistance with RNA-seq analysis, Lara Strittmatter and Greg Hendricks for assistance with electron microscopy, Vicky Benoit for expert technical assistance, and Kathy Gemme for administrative assistance. These studies were supported by a grant from the National Institutes of Health (DK090963). The UMASS Mouse Metabolic Phenotyping Center is supported by grant DK093000. The UMASS Electron Microscopy Core is supported by grants from the National Center For Research Resources (S10RR021043 and S10RR027897). G.S. was supported by the Ramón y Cajal Program and grants ERC 260464, EFSD 2030, MICINN SAF2010-19347 and Comunidad de Madrid S2010/BMD-2326. CNIC is supported by the Ministry of Economy and Competitiveness and the Pro-CNIC Foundation. S.V. was supported by a fellowship from the Ramon Areces Foundation. R.J.D. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
ACCESSION NUMBERS
Gene expression data were deposited (accession number GSE55190) in the Gene Expression Omnibus: http://www.ncbi.nlm.nih.gov/geo
Supplemental Information includes Supplemental Experimental Procedures and 7 figures and can be found with the article online at http:
AUTHOR CONTRIBUTIONS
S.V., G.S., & R.J.D. designed the study; experimental analysis was performed by S.V., J.C-K., L.G-H., & T.B.; metabolic cage & clamp studies were performed by D.Y.J. & J.K.K.; viral vectors were amplified by G.G.; bioinformatic analysis was performed by J.X., H.P.S. & M.G.; and S.V. & R.J.D. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Ahmed SS, Li J, Godwin J, Gao G, Zhong L. Gene transfer in the liver using recombinant adeno-associated virus. Curr Prot Micro. 2013;Chapter 14(Unit14D):16. doi: 10.1002/9780471729259.mc14d06s29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci USA. 2008;105:19544–19549. doi: 10.1073/pnas.0804604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinol. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalphα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Beaven SW, Matveyenko A, Wroblewski K, Chao L, Wilpitz D, Hsu TW, Lentz J, Drew B, Hevener AL, Tontonoz P. Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab. 2013;18:106–117. doi: 10.1016/j.cmet.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Bronneke HS, Brodesser S, Hampel B, Schauss AC, et al. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci USA. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boergesen M, Pedersen TA, Gross B, van Heeringen SJ, Hagenbeek D, Bindesboll C, Caron S, Lalloyer F, Steffensen KR, Nebb HI, et al. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor α in mouse liver reveals extensive sharing of binding sites. Mol Cell Biol. 2012;32:852–867. doi: 10.1128/MCB.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catic A, Suh CY, Hill CT, Daheron L, Henkel T, Orford KW, Dombkowski DM, Liu T, Liu XS, Scadden DT. Genome-wide map of nuclear protein degradation shows NCoR1 turnover as a key to mitochondrial gene regulation. Cell. 2013;155:1380–1395. doi: 10.1016/j.cell.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copps KD, Hancer NJ, Opare-Ado L, Qiu W, Walsh C, White MF. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 2010;11:84–92. doi: 10.1016/j.cmet.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinol. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- Das M, Jiang F, Sluss HK, Zhang C, Shokat KM, Flavell RA, Davis RJ. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci USA. 2007;104:15759–15764. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Feng X, Jiang Y, Meltzer P, Yen PM. Transgenic targeting of a dominant negative corepressor to liver blocks basal repression by thyroid hormone receptor and increases cell proliferation. J Biol Chem. 2001;276:15066–15072. doi: 10.1074/jbc.m011027200. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzatti L, Lu C, Kim DW, Park JW, Astapova I, Gavrilova O, Willingham MC, Hollenberg AN, Cheng SY. Resistance to thyroid hormone is modulated in vivo by the nuclear receptor corepressor (NCOR1) Proc Natl Acad Sci USA. 2011;108:17462–17467. doi: 10.1073/pnas.1107474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss SC, Bakiri L, Thomsen MK, Williams EG, Auwerx J, Wagner EF. Regulation of steatohepatitis and PPARγ signaling by distinct AP-1 dimers. Cell Metab. 2014;19:84–95. doi: 10.1016/j.cmet.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B, Hallberg M, Seth A, Woods A, White R, Parker MG. The nuclear receptor cofactor, receptor-interacting protein 140, is required for the regulation of hepatic lipid and glucose metabolism by liver X receptor. Mol Endocrinol. 2007;21:2687–2697. doi: 10.1210/me.2007-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, et al. An FGF21-Adiponectin-Ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013;17:790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27:498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kant S, Barrett T, Vertii A, Noh YH, Jung DY, Kim JK, Davis RJ. Role of the mixed-lineage protein kinase pathway in the metabolic stress response to obesity. Cell reports. 2013;4:681–688. doi: 10.1016/j.celrep.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- Leboucher GP, Tsai YC, Yang M, Shaw KC, Zhou M, Veenstra TD, Glickman MH, Weissman AM. Stress-induced phosphorylation and proteasomal degradation of mitofusin 2 facilitates mitochondrial fragmentation and apoptosis. Mol Cell. 2012;47:547–557. doi: 10.1016/j.molcel.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, et al. Adiponectin Mediates the Metabolic Effects of FGF21 on Glucose Homeostasis and Insulin Sensitivity in Mice. Cell Metab. 2013;17:779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Foster DW. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971;246:1149–1159. [PubMed] [Google Scholar]
- Mottis A, Mouchiroud L, Auwerx J. Emerging roles of the corepressors NCoR1 and SMRT in homeostasis. Genes Dev. 2013;27:819–835. doi: 10.1101/gad.214023.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C, Ratner D, Zhong L, Esteves-Sena M, Gao G. Production and discovery of novel recombinant adeno-associated viral vectors. Curr Prot Micro. 2012;Chapter 14(Unit14D):11. doi: 10.1002/9780471729259.mc14d01s26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muise ES, Azzolina B, Kuo DW, El-Sherbeini M, Tan Y, Yuan X, Mu J, Thompson JR, Berger JP, Wong KK. Adipose fibroblast growth factor 21 is upregulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–412. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- Nautiyal J, Christian M, Parker MG. Distinct functions for RIP140 in development, inflammation, and metabolism. Trends Endocrinol & Metab. 2013;24:451–459. doi: 10.1016/j.tem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Jepsen K, Sawka-Verhelle D, Perissi V, Sasik R, Rose DW, Johnson RS, Rosenfeld MG, Glass CK. A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci USA. 2004;101:14461–14466. doi: 10.1073/pnas.0405786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: evolving models of co-repressor action. Nat Rev Genet. 2010;11:109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nuc Recept Signal. 2010;8:e002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Barrett T, Jung DY, Ko HJ, Ong H, Morel C, Mora A, Reilly J, Kim JK, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010a;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Mora A, Kim JK, Davis RJ. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Davis RJ. c-Jun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010;35:490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Kennedy NJ, Cavanagh-Kyros J, Jung DY, Ko HJ, Ong H, Barrett T, Kim JK, Davis RJ. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010b;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansbie D, Brownsey RW, Crettaz M, Denton RM. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976;160:413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernia S, Cavanagh-Kyros J, Barrett T, Jung DY, Kim JK, Davis RJ. Diet-induced obesity mediated by the JNK/DIO2 signal transduction pathway. Genes Dev. 2013;27:2345–2355. doi: 10.1101/gad.223800.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol. 2008;28:188–200. doi: 10.1128/MCB.00992-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009a;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. Am J Physiol Endocrinol & Metab. 2009b;297:E1105–1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Williams EG, Mouchiroud L, Canto C, Fan W, Downes M, Heligon C, Barish GD, Desvergne B, Evans RM, et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell. 2011;147:827–839. doi: 10.1016/j.cell.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Inesta-Vaquera F, Niepel M, Zhang J, Ficarro SB, Machleidt T, Xie T, Marto JA, Kim N, Sim T, et al. Discovery of potent and selective covalent inhibitors of JNK. Chem Biol. 2012;19:140–154. doi: 10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.