Abstract
Secretory leucoprotease inhibitor (SLPI) is an anti-inflammatory antiprotease which can inhibit lipopolysaccharide-induced NF-κB activation. We examined its ability to inhibit NF-κB activation induced by lipoteichoic acid and investigated the effects of oxidation or complex formation with neutrophil elastase on SLPI's anti-inflammatory properties in U937 myelomonocytic cells and macrophages.
Secretory leucoprotease inhibitor (SLPI) is an 11.7-kDa nonglycosylated serine protease inhibitor produced by the mucosal surfaces of epithelial cells, macrophages, and neutrophils (1, 14, 20, 23). It provides significant protection for the respiratory epithelial surfaces against neutrophil elastase (NE) released from activated or disintegrating neutrophils (6). SLPI also has antibacterial, antiviral, and anti-inflammatory properties (10, 13, 15, 18, 19). Previously we have shown that SLPI, but not oxidized SLPI, can inhibit lipopolysaccharide (LPS)-induced NF-κB activation (26). It does this by inhibiting degradation of IRAK, IκBα, and IκBβ. These findings prompted us to investigate whether SLPI may have broader anti-inflammatory effects, such as the ability to inhibit responses induced by other microbial components, in particular lipoteichoic acid (LTA).
Toll-like receptors (TLRs) belong to a large family of homologous proteins, TLR1 to TLR10 (4), that play an important role in innate immune defenses due to their ability to recognize and discriminate a diverse array of microbial components. Following activation by their cognate ligands, TLRs initiate a conserved intracellular signaling cascade to activate NF-κB and to induce expression of NF-κB-regulated genes (4, 21). LTA signals via TLR2, while TLR4 is the recognized mammalian receptor for LPS (11). TLR2 is also activated by a number of other microbial components, including bacterial lipopeptides and yeast zymosan (17, 22, 24, 28, 29), and can heterodimerize with TLR1 and -6 to enhance its sensitivity to different stimuli (22). Of the known ligands for other TLRs, double-stranded RNA, flagellin, and unmethylated CpG dinucleotides are recognized by TLR3, -5, and -9, respectively. TLR7 and -8 can be regulated by imidazoquinoline compounds, suggesting an antiviral role (for recent reviews see references 2 and 27), while activators of TLR10 have yet to be identified.
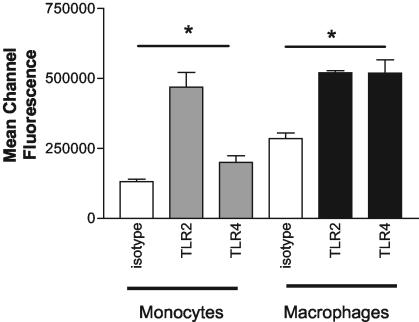
U937 cells express TLR2 and TLR4 on the cell surface.
We evaluated TLR2 and TLR4 cell surface expression on untreated and phorbol myristate acetate (PMA)-differentiated U937 myelomonocytic and macrophage-like cells, respectively, by quantitative fluorescence microscopy (8, 9) with anti-TLR2 and anti-TLR4 monoclonal antibodies and fluorescein isothiocyanate-labeled anti-mouse secondary antibodies. Figure 1 shows that Fc-blocked U937 cells express both TLR2 and TLR4 on their surface, with mean channel fluorescence values (± standard errors of the means) significantly higher for both anti-TLR2 (468,798 ± 52,985) and anti-TLR4 (200,228 ± 24,058) (P ≤ 0.05) than for isotype control antibody-labeled cells (131,390 ± 9,180). Similarly, Fc-blocked macrophages also express TLR2 and TLR4 on the surface (520,231 ± 7,852, 519,260 ± 47,601, and 285,141 ± 19,729 for TLR2, TLR4, and isotype, respectively).
FIG. 1.
U937 cells express TLR2 and TLR4. Untreated monocytic and PMA-differentiated U937 cells were labeled with anti-TLR (gray bars, TLR2; black bars, TLR4) or isotype antibodies (white bars). Binding was quantified by measuring mean channel fluorescence (± standard errors of the means) by laser scanning cytometry. Assays were performed in duplicate or triplicate (n = 4).
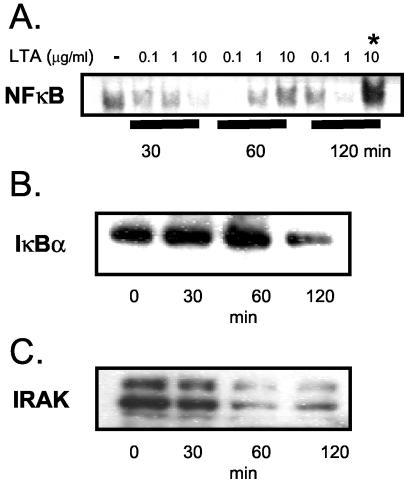
LTA induces NF-κB activation via IRAK and IκBα degradation in U937 cells.
LPS activates NF-κB in U937 cells (26). Figure 2A shows that NF-κB is also activated in response to LTA stimulation (0.1, 1, or 10 μg/ml) in U937 cells (106/ml), with optimal NF-κB nuclear localization and DNA binding activity in 5-μg nuclear extract (5) samples being induced by using an LTA concentration of 10 μg/ml after 2 h. The mechanism by which this occurs involves degradation of IκBα, as shown by decreased IκBα levels detected in cytosolic extracts (5 μg) by Western blotting following 2 h of stimulation with 10 μg of LTA/ml (Fig. 2B). IRAK was degraded by 60 min and up to 2 h in U937 cells in response to stimulation with 10 μg of LTA/ml (Fig. 2C).
FIG. 2.
LTA activates NF-κB via IRAK and IκBα in U937 cells. (A) Electrophoretic mobility shift assay of time course and dose response of LTA-induced NF-κB activation in U937 cells (n = 3). Also shown are Western blots of IκBα (B) and IRAK (C) in cytosolic extracts from untreated cells (t = 0) or cells stimulated with 10 μg of LTA/ml at indicated times (n = 3).
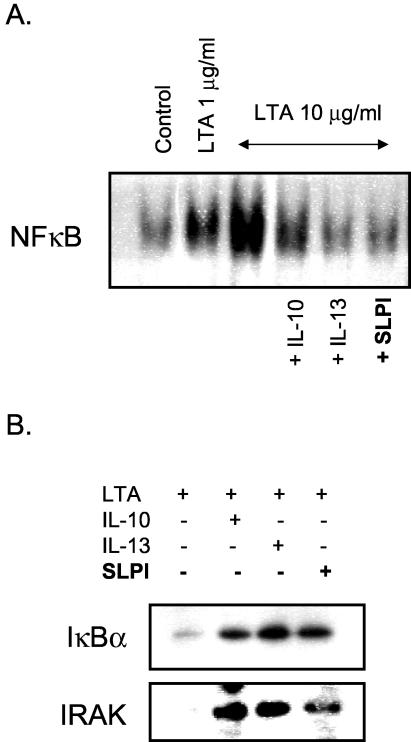
SLPI inhibits LTA-induced NF-κB activation and prevents IκBα and IRAK degradation.
U937 cells (106/ml) were treated with SLPI for 1 h prior to stimulation with 10 μg of LTA/ml for 2 h to determine whether SLPI could inhibit LTA-induced NF-κB activation. Figure 3A shows that SLPI inhibits LTA-induced NF-κB activation. A similar effect was seen by using interleukin-10 (IL-10) and IL-13 as positive controls. Western blot analysis of cytosolic extracts from the same cells (Fig. 3C) revealed that SLPI also prevented LTA-induced IκBα and IRAK degradation. Given that this highly conserved signaling pathway is also activated by other TLRs, it would be interesting to explore whether SLPI can also inhibit NF-κB activation by other TLR agonists (2, 4, 27).
FIG. 3.
SLPI inhibits LTA-induced NF-κB activation and degradation of IκBα and IRAK. SLPI (10 μg/ml), IL-10 (10 ng/ml), or IL-13 (10 ng/ml) inhibits NF-κB activation (A) and IκBα and IRAK degradation (B) by LTA in U937 cells (n = 3).
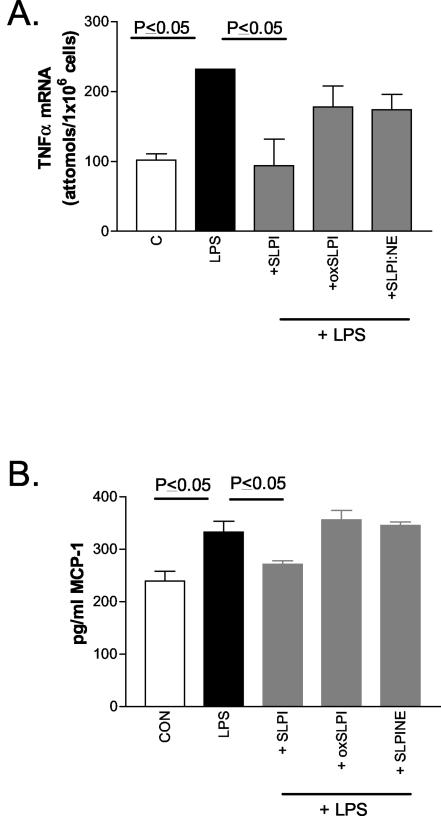
SLPI, but not oxidized or elastase-complexed SLPI, can inhibit TLR-induced cytokine expression.
Inactivation of SLPI by oxidation (oxSLPI) or complex formation with NE (SLPI:NE), in addition to inhibiting its antiprotease activity, is also likely to decrease its anti-inflammatory properties. oxSLPI cannot inhibit LPS-induced NF-κB activation and IκBα or IκBβ degradation (26). Here we examined whether the ability of SLPI to inhibit TLR-induced proinflammatory gene expression is also lost by oxidation or complex formation with NE.
Similar to results of another report, we found that SLPI could downregulate LPS-induced tumor necrosis factor α (TNF-α) expression by showing that it had a direct effect on TNF-α gene transcription (16). Figure 4A shows that SLPI (10 μg/ml), but not oxSLPI (10 μg/ml) or SLPI:NE (34 μg/ml), can inhibit TNF-α gene transcription induced by 4 h of stimulation with LPS (1 μg/ml) (P ≤ 0.05) in U937 cells (106/ml) as measured by Quantikine mRNA enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc., Minneapolis, Minn.). SLPI also impaired MCP-1 production in response to LPS. MCP-1, a CC chemokine, is a regulator of adhesion molecule expression and cytokine production in monocytes (12). LPS-induced (0.01 μg/ml for 24 h) MCP-1 production in cell supernatants as measured by protein ELISA (Quantikine ELISA) was inhibited by SLPI, but neither oxSLPI nor SLPI:NE was able to impair the LPS effect (Fig. 4B).
FIG. 4.
SLPI inhibits LPS-induced TNF-α gene transcription and MCP-1 production. SLPI, but not oxSLPI or SLPI:NE, inhibits LPS-induced cellular TNF-α mRNA levels (A) and MCP-1 production (B) in U937 cells. Assays were performed in duplicate (n = 3). C and CON, control.
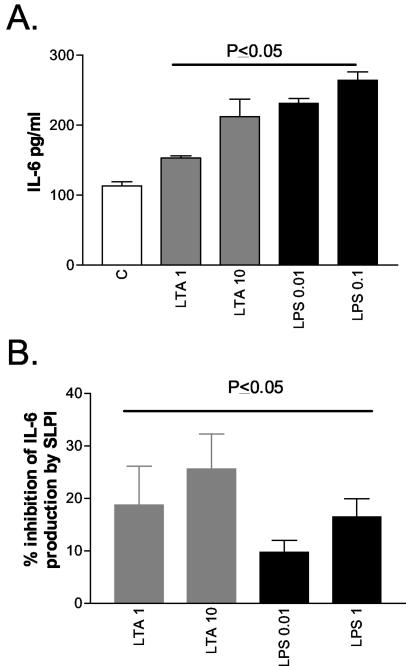
SLPI decreases LTA- and LPS-induced IL-6 production from PMA-differentiated U937 cells.
Having shown that SLPI can inhibit both LTA- and LPS-induced NF-κB activation (26) and can also impair LPS-induced TNF-α and MCP-1 expression in myelomonocytic cells, we next determined whether SLPI could also interfere with LTA- and LPS-induced cytokine expression by macrophages. U937 cells were treated with PMA (100 ng/ml for 72 h) to induce their differentiation into macrophages. The effects of LTA and LPS on IL-6 protein production from these cells were quantified by ELISA (R&D Systems). Increasing doses of either LTA (1 and 10 μg/ml) or LPS (0.01 and 0.1 μg/ml) increased IL-6 protein production in a dose-dependent fashion (P ≤ 0.05) (Fig. 5A). Preincubation with SLPI impaired both responses (Fig. 5B), decreasing the LTA effects by 19% ± 7% and 26% ± 7% (P ≤ 0.05) when 1 and 10 μg of LTA/ml was used, respectively. oxSLPI could not inhibit this effect (data not shown). SLPI also inhibited LPS-induced IL-6 production by 10% ± 2% and 17% ± 4% when 0.01 and 0.1 μg of LPS/ml was used, respectively (P ≤ 0.05).
FIG. 5.
SLPI inhibits LTA- and LPS-induced IL-6 expression in macrophages. (A) LTA- and LPS-induced IL-6 protein production by PMA-differentiated U937 cells; (B) percent inhibition of IL-6 by SLPI (10 μg/ml). All assays were performed in duplicate (n = 7). C, control.
Taken together, the data show that SLPI can impair LTA- and LPS-induced proinflammatory gene expression in monocytes and macrophage in vitro; however, oxidation and elastase complex formation of SLPI diminish its anti-inflammatory properties. These findings add to our understanding of what is presently known regarding the anti-inflammatory properties of SLPI (3, 7, 15, 16, 18, 19, 25, 30).
Acknowledgments
This work was supported by the Health Research Board (grant RP/198/2002 awarded to C. Taggart), The Alpha One Foundation, The Programme for Research in Third Levels Institutes administered by HEA, and The Royal College of Surgeons in Ireland.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abe, T., N. Kobayashi, K. Yoshimura, B. C. Trapnell, H. Kim, R. C. Hubbard, M. T. Brewer, R. C. Thompson, and R. G. Crystal. 1991. Expression of the secretory leukoprotease inhibitor gene in epithelial cells. J. Clin. Investig. 87:2207-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S. 2003. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15:5-11. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft, G. S., K. Lei, W. Jin, G. Longenecker, A. B. Kulkarni, T. Greenwell-Wild, H. Hale-Donze, G. McGrady, X. Y. Song, and S. M. Wahl. 2000. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat. Med. 6:1147-1153. [DOI] [PubMed] [Google Scholar]
- 4.Bowie, A., and L. A. O'Neill. 2000. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 67:508-514. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Gauthier, F., U. Fryksmark, K. Ohlsson, and J. G. Bieth. 1982. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim. Biophys. Acta 700:178-183. [DOI] [PubMed] [Google Scholar]
- 7.Gipson, T. S., N. M. Bless, T. P. Shanley, L. D. Crouch, M. R. Bleavins, E. M. Younkin, V. Sarma, D. F. Gibbs, W. Tefera, P. C. McConnell, W. T. Mueller, K. J. Johnson, and P. A. Ward. 1999. Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J. Immunol. 162:3653-3662. [PubMed] [Google Scholar]
- 8.Greene, C., C. Taggart, G. Lowe, P. Gallagher, N. McElvaney, and S. O'Neill. 2003. Anti-neutrophil elastase capacity is impaired locally in community acquired pneumonia. J. Infect. Dis. 188:769-776. [DOI] [PubMed] [Google Scholar]
- 9.Greene, C. M., G. Meachery, C. C. Taggart, C. P. Rooney, R. Coakley, S. J. O'Neill, and N. G. McElvaney. 2000. Role of IL-18 in CD4+ T lymphocyte activation in sarcoidosis. J. Immunol. 165:4718-4724. [DOI] [PubMed] [Google Scholar]
- 10.Hiemstra, P. S., R. J. Maassen, J. Stolk, R. Heinzel-Wieland, G. J. Steffens, and J. H. Dijkman. 1996. Antibacterial activity of antileukoprotease. Infect. Immun. 64:4520-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 12.Jiang, Y., D. I. Beller, G. Frendl, and D. T. Graves. 1992. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J. Immunol. 148:2423-2428. [PubMed] [Google Scholar]
- 13.Jin, F. Y., C. Nathan, D. Radzioch, and A. Ding. 1997. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 88:417-426. [DOI] [PubMed] [Google Scholar]
- 14.Kramps, J. A., C. Franken, C. J. Meijer, and J. H. Dijkman. 1981. Localization of low molecular weight protease inhibitor in serous secretory cells of the respiratory tract. J. Histochem. Cytochem. 29:712-719. [DOI] [PubMed] [Google Scholar]
- 15.Lentsch, A. B., J. A. Jordan, B. J. Czermak, K. M. Diehl, E. M. Younkin, V. Sarma, and P. A. Ward. 1999. Inhibition of NF-κB activation and augmentation of IκBβ by secretory leukocyte protease inhibitor during lung inflammation. Am. J. Pathol. 154:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lentsch, A. B., H. Yoshidome, R. L. Warner, P. A. Ward, and M. J. Edwards. 1999. Secretory leukocyte protease inhibitor in mice regulates local and remote organ inflammatory injury induced by hepatic ischemia/reperfusion. Gastroenterology 117:953-961. [DOI] [PubMed] [Google Scholar]
- 17.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 18.McNeely, T. B., M. Dealy, D. J. Dripps, J. M. Orenstein, S. P. Eisenberg, and S. M. Wahl. 1995. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J. Clin. Investig. 9:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeely, T. B., D. C. Shugars, M. Rosendahl, C. Tucker, S. P. Eisenberg, and S. M. Wahl. 1997. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood 90:1141-1149. [PubMed] [Google Scholar]
- 20.Mihaila, A., and G. M. Tremblay. 2001. Human alveolar macrophages express elafin and secretory leukocyte protease inhibitor. Z. Naturforsch. 56:291-297. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill, L. A., K. A. Fitzgerald, and A. G. Bowie. 2003. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 24:286-290. [DOI] [PubMed] [Google Scholar]
- 22.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallenave, J. M., M. Si-Ta har, G. Cox, M. Chignard, and J. Gauldie. 1997. Secretory leukocyte proteinase inhibitor is a major leukocyte elastase inhibitor in human neutrophils. J. Leukoc. Biol. 61:695-702. [DOI] [PubMed] [Google Scholar]
- 24.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 25.Song, X., L. Zeng, W. Jin, J. Thompson, D. E. Mizel, K. Lei, R. C. Billinghurst, A. R. Poole, and S. M. Wahl. 1999. Secretory leukocyte protease inhibitor suppresses the inflammation and joint damage of bacterial cell wall-induced arthritis. J. Exp. Med. 190:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taggart, C. C., C. M. Greene, N. G. McElvaney, and S. J. O'Neill. 2002. Secretory leucoprotease inhibitor prevents lipopolysaccharide-induced IκBα degradation without affecting phosphorylation or ubiquitination. J. Biol. Chem. 277:33648-33653. [DOI] [PubMed] [Google Scholar]
- 27.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 28.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 29.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, Y., D. L. DeWitt, T. B. McNeely, S. M. Wahl, and L. M. Wahl. 1997. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. J. Clin. Investig. 99:894-900. [DOI] [PMC free article] [PubMed] [Google Scholar]