Abstract
In addition to the obvious health problems and/or physical limitations associated with HIV, diabetes, and aging, each of these are known to independently affect cognitive functioning. While this relationship to cognition does not necessarily mean frank cognitive impairments are inevitable with HIV, diabetes, and aging, it does entail that each of these conditions may lead to poorer cognitive performance compared to younger adults and individuals without HIV and diabetes. Many individuals may be aware of the physical symptoms associated with these diseases, but may be unaware of the cognitive outcomes associated with HIV and diabetes, especially if not controlled by medication and a healthy lifestyle. Additionally, individuals may be unaware of the significance of maintaining optimal cognitive functioning in order to maintain optimal everyday functioning abilities such as driving, cooking, managing medication regimens, and negotiating finances. Given that highly active antiretroviral therapy (HAART) has allowed individuals with HIV to live to reach older adulthood, and that dysglycemia and/or type 2 diabetes can be a metabolic side effect of these medications (Biron et al., 2012; Norbiato, 2012; Raper, 2010), it is reasonable to assume that there is a subset of individuals aging with HIV and diabetes, which may become more prevalent as individuals continue to age with HIV in the coming decades. Thus, the purpose of this article is to inform healthcare providers and researchers about the cognitive outcomes associated with HIV, diabetes, and aging independently within the context of cognitive reserve, and then to examine the potential synergistic effects of these conditions in individuals living with all three (Figure 1). This article also incorporates potential intervention strategies to protect and possibly improve cognitive functioning, or at the very least mitigate cognitive loss, in this population.
Cognitive Reserve
Cognitive reserve, sometimes referred to as brain reserve, refers to the amount of damage that neurons and neuronal connections can absorb and yet support the physiological function needed to support cognition (Restak, 2009; Stern, 2009; Vance & Wright, 2009). Thus, the more complex and sophisticated the connections between neurons are, the better they are able to communicate and support cognition, even in lieu of disease-related insults. For example, in a simplistic example, the left panel in Figure 2 shows a complex and intricate connection between neurons A – G such that neural communication can be transmitted from A to G from which cognition emerges. In the right panel in Figure 2, the connections between neurons A – G is compromised due to cell death of neurons C, D, and F possibly due to amyloid plaque, neuroinflammation, and oxidative stress, respectively. Yet the remaining connections (i.e., cognitive reserve) allow the neural communication to be transmitted from A to G via paths from A – B – E—G, therefore allowing cognition to emerge despite such damage. Such rerouting of neural communication is similar to other physiological processes. For example, when blood vessels are blocked in occlusive coronary heart disease, the risk of myocardial ischemia is reduced by coronary collateral arteries rerouting blood flow around such blockage (Berry et al., 2007).
Figure 2.
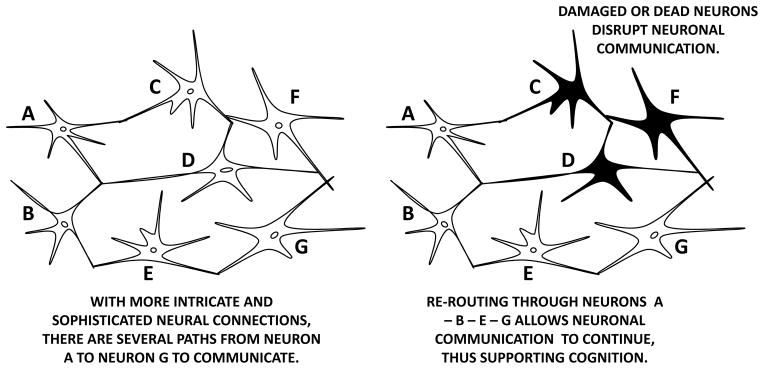
Cognitive reserve and an example of collateral neural communication. Darkened neurons represent damaged or dead neurons that can no longer transmit neural information.
This cognitive reserve is supported or reduced by positive neuroplasticity or negative neuroplasticity, respectively. Positive neuroplasticity refers to those physiological activities in the brain that help form more intricate and sophisticated neural connections. Factors that support positive neuroplasticity are exposure to novel and stimulating stimuli (e.g., education, travel, learning a new language) and conditions that promote good overall health (e.g., physical exercise, good sleep hygiene). Likewise, negative neuroplasticity refers to those physiological activities in the brain that abate or stymie the formation of more intricate and sophisticated neural connections. Factors that support negative neuroplasticity are lack of exposure to novel and stimulating stimuli (e.g., lack of social contact) and conditions that promote poor overall health (e.g., substance abuse, co-morbidities) (Vance & Crowe, 2006; Vance & Wright, 2009).
These processes of positive and negative neuroplasticity have been observed in animal and human studies alike (Restak, 2009; Stern, 2009; Vance & Crowe, 2006; Vance & Wright, 2009). For example, in a seminal study involving 60 community-dwelling older adults (Mage = 60), Boyke and colleagues (2008) conducted brain MRIs to these participants (i.e., Time 1). Then over a 3-month period, these researchers instructed these participants on how to juggle for at least 1 minute in a 3-ball cascade pattern. At which point, brain MRIs of these 25 participants who were able to learn this task were conducted. Finally, after these 25 participants stopped juggling, brain MRIs were conducted again approximately 3 months later (i.e., Time 3). These researchers found that from Time 1 to Time 2 when the participants were challenged with the stimulating activity of learning to juggle, the size of their hippocampi and nucleus accumbens – brain structures important for memory – increased; this change in brain physiology reflects positive neuroplasticity. Also, these researchers found that from Time 2 to Time 3 as participants were no longer challenged with practicing juggling, the size of their hippocampi and nucleus accumbens decreased; this change in brain physiology reflects negative neuroplasticity. From this and several other studies (Brayne et al., 2010; Roe et al., 2008), activities that support cognitive reserve have been shown to delay cognitive impairment or the on-set of dementia, even when the brain experiences neurological insults such as observed with transient ischemic attacks, hypertension, heart disease, and Alzheimer’s disease. It is within this context of cognitive reserve that the cognitive impairments associated with HIV, type 2 diabetes, aging, and the combination thereof are discussed.
HIV and Cognition
HIV not only affects the immune system, it also affects the nervous system and the brain which can deplete cognitive reserve in a number of ways. First, HIV can cross the blood brain barrier and infect and kill glial cells; glial cells are needed to support neuronal health and the death of glial cells produces neurotoxins (Vance, Fazeli, Moneyham, Keltner, & Raper, in press). Second, HIV is also considered an inflammatory disease as well as a neuroinflammatory disease (Kusao et al., 2012). In general, such neuroinflammation has been shown in several clinical populations to reduce cognitive reserve and induce cognitive impairments (Satori, Vance, Slater, & Crowe, 2012). Finally, given the stigmatizing nature of HIV, this can create much stress, depression, and anxiety in those coping with the disease. Such prolonged negative emotion can lead to hypothalamic-pituitary-adrenal axis dysfunction resulting in long-term exposure to the stress hormone cortisol; studies show that excessive exposure to cortisol can be damaging to the brain and also induce cognitive impairments (Karlamangla, Singer, Chodosh, McEwen, & Seeman, 2005; Lupien et al., 2005).
A Several studies have shown that individuals with HIV, as a group, exhibit poorer cognitive performance than their age-matched HIV-negative counterparts (Ettenhofer et al., 2010; Hardy & Hinkin, 2002; Hardy & Vance, 2009). The affected cognitive domains include psychomotor abilities, attention, speed of processing, executive functioning, and memory, all of which reflect dysfunction of frontal-subcortical brain circuitry (Lojek & Bornstein, 2005; Murji et al., 2003; Navia, Jordan, & Price, 1986; Reger, Welsh, Razani, Martin, & Boone, 2002; van Gorp et al., 1993). These cognitive declines mirror the declines seen in normal aging (Craik & Salthouse, 2000) and are particularly relevant as these abilities underlie performance of many necessary everyday activities, such as medication management and driving (Hinkin et al., 2004; Marcotte et al., 2004). The hallmark cognitive impairment is a general slowing in performing cognitive tasks, with those with HIV performing poorer on cognitive tests that include a speeded/timed component (Hardy & Hinkin 2002; Reger et al.). While speed of processing deficits (either frank or subclinical) may be a common symptom of cognitive sequelae in HIV that may be detected on neuropsychological tests, when individuals with HIV are queried about subjective cognitive complaints, many typically report memory problems, as these are more noticeable in interfering with daily functioning (Vance et al., in press; Wilson & Moffat, 1984).
It is important to note that not all individuals with HIV exhibit these cognitive impairments. In a recent cluster analytic study of adults with HIV (N = 78; Mage = 46.61), Fazeli and colleagues (under review) found that a majority of the sample (59%) was classified as neurocognitively “normal” compared to a demographically similar HIV-negative reference group (N = 84; Mage = 47.93). Age was the most influential correlate of cluster membership (“normal” cluster versus lower performing cluster). This study suggests that many adults with HIV maintain optimal cognitive functioning. Similarly, in one pre-HAART study, Becker and colleagues (1995) found that in individuals with advanced HIV disease and AIDS, 36% had subcortical impairments, 3% had cortical impairments, and 61% had no impairments. In addition, there is evidence that cognitive functioning in HIV is associated with disease severity, with those in later stages (i.e., symptomatic HIV and AIDS) being more at risk for developing cognitive impairments (Baldewicz et al., 2004; Hardy et al., 1999; Reger et al., 2002). Yet, there remains much inconsistency in the literature about the degree and prevalence of such cognitive impairments. This is likely due to differences in the criteria and operational definitions used to classify such cognitive impairments (Hardy & Vance, 2009).
The most current criteria are the HIV Neurobehavioral Research Center definitions which include three categories of HIV-associated neurocognitive disorders: HIV-associated asymptomatic neurocognitive impairment (performance at least one standard deviation below the mean for age-education-appropriate norms in at least two cognitive domains); HIV-associated mild neurocognitive disorder (performance at least one standard deviation below the mean for age-education-appropriate norms in at least two cognitive domains and this impairment must mildly interfere with daily functioning); and HIV-associated dementia (performance at least two standard deviations below the mean for age-education-appropriate norms in at least two cognitive domains as well as more severe impairment in daily functioning) (Antinori et al., 2007). While the incidence of HIV-associated dementia has decreased significantly in the HAART era, the prevalence and incidence of milder manifestations of HIV-associated neurcognitive disorder seems to be increasing, with an estimated 30% – 50% of individuals with HIV experiencing some form in their lifetime. In a sample of 1,555 adults with HIV from six university clinics across the United States, Heaton and colleagues (2010) found that 52% of the sample had some form of HIV-associated neurocognitive disorder (33% with asymptomatic neurocognitive disorder, 12% with mild neurocognitive disorder, 5% with mixed neurocognitive disorder, and 2% with HIV-associated dementia).
In addition to the heterogeneity in the literature on definitions of cognitive impairment in HIV, there are also confounding factors in this population that make extracting the unique influence of HIV on cognitive functioning cumbersome. There are various factors such as age, education, depression and anxiety, physical and mental exercise, substance use, social support, stress, HIV medication adherence, medical comorbidities (e.g., hepatitis C), and side effects of medications for these comorbidities that may cause some individuals with HIV to be at a greater risk for developing cognitive impairments (Vance et al., in press). Examining these and other factors is imperative in order to avoid overestimating cognitive impairments due to HIV itself. Furthermore, many of these confounding factors are lifestyle factors that may be modifiable. For example, cognitive functioning may be improved by treating anxiety and/or depression, increasing physical exercise, and addressing nutritional deficiencies (Vance et al.). There are also other intervention strategies such as cognitive remediation programs, mnemonic strategies, and certain medications that may help improve or maintain cognitive functioning in individuals with HIV (Vance et al., in press). For example, in a sample of 16 adults with HIV experiencing cognitive slowing, Hinkin and colleagues (2001) used methylphenidate (Ritalin; 30 mg dose/once daily) in a placebo-controlled, single-blind crossover study. These researchers found after 3 days that it was effective in improving cognition. Given that some individuals with HIV may have no cognitive impairments and that there are vast individual differences in factors that may affect cognition, examining cognitive functioning in HIV (including development of screening tools, interventions, and examining factors related to successful aging with HIV) is an important area for those aging with HIV.
Diabetes and Cognition
Compelling evidence, from both cross-sectional and longitudinal studies, link metabolic disorders and type 2 diabetes with increased risk of mild to moderate cognitive impairments. Cross-sectional data demonstrates reduced cognitive performance of patients with type 2 diabetes when compared to the general population across all age groups (Noble, Manly, Schupf, Tang, & Luchsinger, 2012; Stewart & Liolitsa, 1999; Strachan, Deary, Ewing, & Frier, 1997), while longitudinal studies suggest a slow process of developing cognitive impairments over time (Allen, Frier, & Stachan, 2004; Van den Berg et al., 2010). These cognitive impairments are most consistently observed on measures requiring more mental efficiency such as executive functioning, speed of processing, and verbal memory (Awad, Gagnon, & Messier, 2004; Brands et al., 2007; Van den Berg et al.).
Evidence suggests that type 2 diabetes is also associated with either an accelerated cognitive decline or an increased risk of dementia (Allen et al., 2004; Arvanitakis, Wilson, Bienias, Evans, & Bennett, 2004; Umegaki et al., 2012). Individuals with type 2 diabetes over the ages of 65 – 70 are particularly at risk for developing more severe and progressive cognitive impairments (Allen et al.; Biessels, 2011). An increased risk of mild cognitive impairment, a preclinical stage of dementia, has also been found in this population (Luchsinger et al., 2007). Research suggests that the risk of developing vascular dementia and Alzheimer’s disease is increased in people with type 2 diabetes; specifically, the risk of vascular dementia is increased 2 – 3 times, and the risk of Alzheimer’s disease is increased 1.5 – 2 times (Biessels, Staekenborg, Brunner, Brayne, & Scheltens, 2006; Cukierman, Gerstein, & Williamson, 2005).
Furthermore, duration (or chronicity) of type 2 diabetes has also been associated with greater cognitive decline among both men and women (Gregg et al., 2000; Logroscino, Kang, & Grodstein, 2004; Okereke et al., 2008), but findings regarding duration and dementia have been mixed. For example, among 274 adults 80 – 93 years with (n = 36) and without diabetes (n = 238), Hassing and colleagues (2003) found longer duration of type 2 diabetes was related to lower test performance in cognitive domains such as perceptual speed, visuo-spacial ability, episodic memory, semantic memory, inductive reasoning, and global cognition.
In addition to the debate in the literature regarding degree of cognitive impairments related to duration of type 2 diabetes, there are also confounding factors that may cause some individuals with type 2 diabetes to be at a higher risk for cognitive decline. Some studies suggest that impairments in cognitive functions are associated with poorer glycemic control, depression, cardiovascular and cerebrovascular disease, elevated glycohemoglobin, hyperinsulinemia, metabolic dysfunctions, and hypertension (Anderson, Freedland, Clouse, & Lustman, 2001; Awad et al., 2004; Biessels, 2010; Hassing et al., 2004; Kanaya, Barrett-Connor, Gildengorin, & Yaffe, 2004). Subjective health and gate/balance have also been found to mediate the relationship between type 2 diabetes and cognitive functioning (McFall, Feall, Fischer, Dolcos, & Dixon, 2010), which suggests that neuromusculature may also be compromised by these processes.
Brain imaging studies suggest a relationship between cognitive impairments and brain abnormalities in those with and without diabetes. Maschot and colleagues (2006) examined MRI brain scans among 113 participants with type 2 diabetes and 51 controls without type 2 diabetes. These researchers found white matter lesions, cortical and subcortical atrophy, and the presence of cerebral infarcts (adjusted for age, sex, and estimated IQ) were significantly associated with type 2 diabetes. In addition, there was a modest association with A1c (i.e., glycated hemoglobin level which is an indicator of average plasma glucose concentration over 2 to 3 months) and diabetes duration; this is important given that higher A1c was associated with more cognitive impairment. This association was strongest for age, even more so than in control participants, which suggest that the synergistic effects of type 2 diabetes and the aging process can create subtle neurological insults which accumulate overtime and create cognitive impairment. In a related MRI study, Manschot and colleagues (2008) compared 122 participants with type 2 diabetes and 56 matched controls and found more white matter lesions and more atrophy among those with type 2 diabetes compared to controls. Interestingly, 40 patients with type 1 diabetes with a mean duration of 34 years were matched for age and education with 40 patients with type 2 diabetes with a much shorter mean duration of 7 years. Brain MRI showed significantly more white matter lesions and cortical atrophy among those with type 2 diabetes, regardless of their shorter duration (Brands et al., 2007).
Interventions to ameliorate cognitive impairments in those with type 2 diabetes have been investigated, with mixed results. In the Memory in Diabetes Study, researchers compared the effects of intensive versus standard glycemic control on cognitive function and brain volume among 2,977 patients with type 2 diabetes. Participants (55 – 80 years) with type 2 diabetes and elevated cardiovascular risk profiles (i.e., total cholesterol, low-density lipoprotein, high-density lipoprotein) were randomly assigned to receive either intensive or standard glycemic control strategies over 40 months. Results indicated no significant treatment difference in cognitive performance between groups, and an increased mortality among participants in the intensive treatment group (Launer et al., 2011). Albeit, Logroscino, Kang, and Grodstein (2004) investigated the results of treatment for diabetes among 18,999 women aged 70 – 81. Results suggested that participants with type 2 diabetes who were on oral hypoglycaemic agents performed similarly on cognitive tests to women without diabetes at baseline and across 2 years. Women using insulin treatment had a modest increase in odds of poor cognitive performance, and women not using any medication had the greatest odds of poor cognitive performance compared with women without diabetes at both baseline and across 2 years. Clearly, more research is needed to determine the impact of diabetes management (i.e., hypoglycaemic agents, insulin, exercise, diet) exerts on cognitive functioning, but clearly treatment for diabetes can be an important factor in preventing or reducing impairments in cognitive functioning.
Aging and Cognition
Aging is associated with an increased risk for cognitive decline in older adults. In fact, as measured from young to old, many cross-sectional studies demonstrate near linear decline in many areas of cognitive functioning (Hedden et al., 2012; Park et al., 2002). Cognitive impairments have been observed as early as middle age in the domains of attention, memory, psychomotor functioning, and speed of processing (Ball, Vance, Edwards, & Wadley, 2004; Birren, Woods, & Williams, 1980; Caban-Holt, Abner, Kryscio, Crowley, & Schmitt, 2012; Salthouse, 1996). Regardless, it is important to note that cognitive aging is a highly inter-individual process, with considerable individual differences observed in older adults (Glisky, Rubin, & Davidson, 2001; Lee et al., 1994; Piguet et al., 2002; Willis & Schaie, 1986). Thus, cognitive aging in older adults is the norm, but not necessarily universally experienced and obviously not to the same degree in everyone.
Cognitive decline is an important factor to consider in older age health assessment, although it is not considered debilitating unless the loss begins to impact everyday functioning (Ball et al., 2004). Albeit, even with slight cognitive impairment, declines in speed of processing, executive functioning, and memory in particular can negatively affect an older adult’s ability to perform everyday tasks such as driving and managing finances, which can thus decrease quality of life (McGuire, Ford, & Ajani, 2006). In fact, Vance, Ball, Moore, and Benz (2007) proposed that optimal cognitive functioning as the most important component in successful aging. Given that optimal cognition is needed to maintain health behaviors (i.e., remembering to exercise and take medications, negotiating the healthcare system) and to actively engage in life (i.e., social interactions, attending to conversation, remembering details from TV or the news, planning social events), unfortunately cognitive health is often overlooked in medical treatment until it is indicated by serious conditions such as Alzheimer’s disease, stroke, or traumatic brain injury (Vance, Larsen, Eagerton, & Wright, 2011).
Decades of research on cognitive aging have not produced a definitive consensus on the causes and mechanisms of age-related cognitive decline, but several theories have gained prominence. One of the most thoroughly researched is the speed of processing hypothesis (a.k.a., processing speed hypothesis; Ball et al., 2004; Birren, Woods, & Williams, 1980; Salthouse, 1996). This theory posits that slower information processing by the central nervous system is the primary mechanism of cognitive aging. According to Salthouse (1996), cognitive efficiency is determined by an individual’s ability to utilize speed of processing efficiently. As speed of processing slows with increasing age, demands on other cognitive processes are increased, thus less information is being processed which results in poorer overall cognition.
Another widely investigated theory is the common cause hypothesis. This hypothesis suggests that cognitive declines occur as a result of gradual sensory degradation of the physical and biological structures of the brain and the nervous system. As neuronal and synaptic integrity decreases, the brains’ ability to efficiently transmit information between neurons lessens, resulting in system-wide declines in sensory, cognitive, and motor functions (Anstey, 1999; Ball et al., 2004). In fact, in one study with 894 older adults, sensory functioning was found to explain nearly 80% of age-related cognitive variation in verbal memory and speed (Anstey, Luszez, & Sanchez, 2001).
Similarly, Clay and colleagues (2009) examined the sensory deprivation hypothesis, which suggests that sensory decline results in reduced neural processing resources, and compared it to the common cause theory and the speed of processing theory of aging. In a cross-sectional sample of 842 older adults (Mage = 72), these researchers found that visual decline was associated with slower speed of processing, and consequently greater cognitive impairment. Albeit, direct associations between age, memory span, and fluid intelligence were non-significant, as long as age-related declines in vision and processing speed were taken into consideration. Therefore, if visual sensory functioning and processing speed can be maintained, perhaps normal cognitive aging does not necessarily result in a loss of fluid intelligence (Clay et al.). Thus, older adults who develop strategies to cope with these sensory declines may possibly evade the usual age-linked cognitive declines involved in learning new information (Burke & MacKay, 1997; Clay et al.). The results of Clay and colleagues support two theories of age-related cognitive decline – the sensory deprivation hypothesis and speed of processing hypothesis. Regardless of which theory, or combination thereof, is eventually found to completely explain cognitive aging, the importance of ameliorating or mitigating declines is essential in facilitating successful aging.
Synthesis
There is growing concern that as people age with HIV, their exposure to aging processes, HIV, and type 2 diabetes may increase their chances of experiencing cognitive declines that interfere with everyday functioning, medication adherence, safe driving, and quality of life. Rowe and Kahn (1997) posited that there are three components that are necessary to facilitate successful aging and they are: 1) social and active engagement in life, 2) avoidance of disease and disability, and 3) optimal cognitive and physical functioning; however, optimal cognitive functioning may be the most important of these components. If cognitive functioning is compromised, this will undoubtedly impact self-care and disease management; in fact, older adults with HIV who experience cognitive impairments are observed to be less adherent to their HIV medications (Ettenhofer et al., 2009; Hinkin et al., 2004). Likewise, the relationship between social and active engagement in life has also been clearly shown in that those more at-risk of developing cognitive impairment interact less socially (Green, Rebok, & Lyketsos, 2008; Moyle, Kellet, Ballantyne, & Gracia, 2011; Vance, 2012).
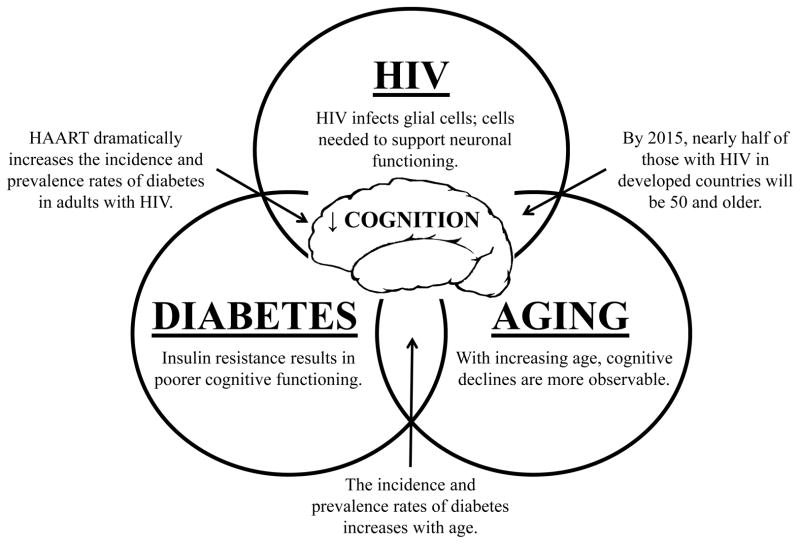
The incidence of type 2 diabetes increases with age and this is also observed in those with HIV. In a chart review of 1,478 patients, the incidence of diabetes was 4.3% in those 18–29 years, 3.2% in those 30–39 years, 9.0% in those 40–49 years, 12.6% in those 50–59 years, and 28.8% in those 60 years and older (Vance, Mugavero et al., 2011). Given what is known about the detrimental effects on cognition associated with HIV, diabetes, and aging, this represents a growing concern for those aging with HIV who are vulnerable to developing diabetes (Figure 1).
Figure 1.
The synergistic effects of HIV, type 2 diabetes, and aging on cognition and cognitive reserve.
McCutchan and colleagues (2012) examined the effects of metabolic dysfunction, diabetes, and obesity on cognitive functioning in 130 adults with HIV. They found that diabetes was associated with poorer cognitive functioning; however, this relationship was found more strongly in older adults. Likewise, in a small study of 22 older adults with HIV (Mage = 58, SD = 6), Nakamoto and colleagues (2012) examined microstructural changes in the brains of those with and without cerebrovascular risks using diffusion tensor imaging (i.e., a MRI technique). They found abnormal microstructural changes in the caudate and hippocampus (i.e., brain structures associated with memory formation) of these participants; furthermore, theses abnormalities were associated with irregular glucose metabolism which suggest that conditions such as diabetes may play an increased role in poorer cognitive functioning in those aging with HIV. Further evidence from the Hawaii Aging with HIV Cohort supports this relationship between metabolic dysfunction and cognitive loss. Specifically, in this cross-sectional study of 145 adults with HIV, Valcour and colleagues (2006) found that increased levels of insulin resistance was associated with greater levels of cognitive impairment. In addition, hypertension and greater body mass index were associated with such cognitive impairment.
This relationship between HIV, diabetes, and aging is particularly troubling when one considers that HAART, the medications that in many cases are prolonging life and health in those infected with HIV, may also create metabolic side effects that contribute to the development of dysglycemia leading to diabetes (Malaspina et al., 2011; Raper, 2010). For example, in a sample of 1,046 adults with HIV followed over 10 years on HAART (i.e., specifically indinavir, stavudine, and didanosine), Capeau and colleagues (2012) found that 111 (10.61%) patients developed diabetes and that HAART use was a significant factor predicting this outcome. Furthermore, the incidence of diabetes was over 3 times higher in this population (14.1/1,000) compared to those without HIV (4 – 6/1,000).
In considering the synergistic effects of aging, HIV, and type 2 diabetes on cognitive functioning, it is important to also consider several other issues. First, as already mentioned, cognitive aging, even with HIV and type 2 diabetes, is an individual process; while some people will be particularly vulnerable to the effects of all three, others may be more resistant and not experience any observable cognitive impairments (Ball et al., 2004). This could be due to some individuals having more cognitive reserve than others. Second, nurses and healthcare providers need to know how to address these synergistic effects on cognition and make referrals as well as make recommendations for how to prevent, ameliorate, or mitigate such cognitive impairments. (For such recommendations, please see Vance et al., in press.) Finally, nursing and healthcare researchers and other allied healthcare professionals must investigate new ways to address these issues in improving the life of those aging with HIV and type 2 diabetes.
Implications for Practice
In order to prevent or avoid cognitive impairments, healthcare providers must take an active role in assisting patients to decrease their risk of co-morbidities associated with diabetes. A very elementary approach may be taken and has been coined as the Diabetes ABCs of Treatment (Varma, Boyle, Varma, & Piatt, 2008). In this approach, the A stands for A1c. The B stands for blood pressure. The C stands for cholesterol management.
A (A1c)
An A1c test is sometimes called HbA1c, glycated hemoglobin test, or glycohemoglobin. Its relevance to diabetes management is that this blood test used to determine how well diabetes is being controlled (ADA, 2013). In short, hemoglobin A1c provides an average of level blood glucose control over the last 3 months (Makris & Spanou, 2011). Optimally it is used in combination with home blood glucose self-monitoring to make modifications in diabetes medicines.
For euglycemic individuals, the normal range for A1c test is between 4% to 5.6% (American Diabetes Association (ADA), 2013). Whereas, A1c levels between 5.7% to 6.4% designate increased risk of diabetes, and levels of 6.5% or upper indicate diabetes (ADA). Elevated A1c levels indicate out-of-control diabetes and result in complications. For people with diabetes, an A1c less than 7% is optimal.
When determining targets for A1c, the standard goal of < 7% can be individualized when the need arises. For example, functional older adults who are cognitively intact should be held to the same treatment goals as those for younger adults (ADA, 2013). However, for those patients who have limited life expectancies, cognitive impairment, or severe hypoglycemic reactions, the target for the A1c could be relaxed. When less stringent individualized goals are set, they should still avoid acute hyperglycemic extremes.
Nurses and practitioners in healthcare are all well aware that the key to reducing diabetes-related co-morbidities is to optimize glycemic control by lowering the A1c level. Of even greater importance is the early recognition of insulin resistance, screening for lipodystrophy, metabolic syndrome, and pre-diabetes as the key to early prevention of cognitive impairment for the person living with HIV (Robles & Elston, 2011; Valcour et al., 2006). By the point in time when dysglycemic levels reach the diagnostic threshold for frank diabetes, the individual has likely already experienced some degree of neurodegeneration and cognitive impairment (Chen et al., 2012; Roriz-Filho et al., 2009). Insulin resistance is the underlying pathology of metabolic syndrome, pre-diabetes, and early type 2 diabetes.
Insulin resistance is extensively used as a term but is inaccurate at best. The expression “insulin resistance,” refers to the fact that the tissues do not respond physiologically to endogenous insulin and resist its action. Thus, in order to avoid a hyperglycemic state, the pancreas produces a hyperinsulimemic state (Williamson, McNeilly, & Sutherland, 2012). Hyperinsulinemia occurs over prolonged periods of time, which may last for ten or more years until the pancreas finally is unable to keep pace and reduces to the level of insulin produced, resulting in hyperglycemia and ultimately frank type 2 diabetes. A prolonged hyperinsulimemic state can be as deleterious as frank diabetes for development of the traditional co-morbidities as well as fostering cognitive impairment (Williamson et al.).
Although the disturbances in insulin homeostasis are still to be fully elucidated, there are hypotheses to explain the neurodegenerative pathophysiology that occurs with metabolic syndrome, pre-diabetes, and frank type 2 diabetes. The molecular pathophysiology of hyperinsulinemia is also thought to be associated with dysfunction of the cellular post-receptor insulin signaling mechanism (Shulman, 2000); thus, higher levels of insulin are needed to facilitate glucose uptake. Prolonged hyperinsulinemia as found in these fore mentioned metabolic states is believed to cause endothelia proliferation, capillary narrowing, and microinfarcts (Taylor & MacQueen, 2007). Coupling hyperinsulinemia with other known vascular risk factors of the metabolic syndrome (e.g., hypertension, dyslipidemia, and dysglycemia) leads to reduced cerebral perfusion, ischemia, lacunar infarcts, and leukoaraios as well as amyloid angiopathy (Taylor & MacQueen).
Implications for healthcare providers are then to screen for early signs of insulin resistance and metabolic dysfunctions. Euglycemic patients may benefit by having a determination of the Homeostatic Model of Assessment-insulin resistance (HOMA-ir) (Appel, 2005). HOMA-ir is a method used to calculate insulin resistance (Matthews et al., 1985). The formula includes fasting plasma insulin and glucose concentrations to estimate the presence of insulin resistance. A non-invasive clue that an individual may be insulin resistance and benefit from screening is the presence of acanthosis nigricans, central obesity, and lipodystrophy. Acanthosis nigricans is a darkening of skin located primary on the neck, but can also occur on, elbows, knees, groin, or other locations of skin folds. Acanthosis is hypothesized to occur when plasma insulin levels are elevated and stimulate the melanocytes to produce more pigment (Vabres, 2012). Similarly, the presence of an enlarged waist circumference (greater than 40 inches for men and 35 inches for women) is considered the hallmark of the metabolic syndrome. In addition, HIV-drug induced lipodystrophy should not be overlooked. Assessing for subcutaneous tissue depletion in the face, arms, and legs coupled with the presence of a ‘buffalo hump’ and/or a cushinoid appearance is equally imperative (Robles & Elston, 2011). Patients with any of these fore mentioned findings indicative of insulin resistance and or abnormal fat accumulations need to be carefully screened for prediabetes or diabetes.
In order to screen for prediabetes and diabetes, nurses, nurse practitioners, and other healthcare providers can first start by checking either a blood glucose, an A1c, or perform an oral glucose tolerance test (ADA, 2012). Patients meet the criteria for diagnosis of diabetes if their fasting blood glucose level is 126 mg/dL or above or if their random blood glucose levels are greater than 200 (with associated symptoms such as polyuria, polydipsia, or polyphasia). If their blood sugar levels are 100–125 mg/dL, then a diagnosis of prediabetes can be made. Diabetes may also be diagnosed by an A1c level greater than or equal to 6.5%. If a 75-gram oral glucose tolerance test is used for diagnosis, the criterion is met if their blood glucose level is above 200 mg/dL. Regardless of the method initially used, the test method must be repeated at a subsequent visit in order to ensure accuracy in the results.
The first step in treating patients with diabetes is lifestyle modification (ADA, 2012). Findings from the Diabetes Prevention Program support the early initiation of lifestyle modification including diet and exercise as it was noted to decrease the progression of prediabetes to diabetes by 58% (Knowler et al., 2002). Patients should be encouraged to include strategies to reduce calories and fat while increasing fiber intake (ADA). Lifestyle changes should also include moderate weight loss (7%) for those who are overweight or obese. Exercise can provide weight loss, but even if weight loss is not achieved, it has been shown to lower A1c in patients with diabetes (Boulé, Haddad, Kenny, Wells, & Sigal, 2001). At times, further optimization of glycemic control is required and the use of insulin sensitizing agents (e.g., metformin, pioglitazone) has been found helpful (Domínguez, Marschoff, González, Repetto, & Serra, 2012). Early introduction of insulin therapy may offer optimal glycemic control and has been correlated with improved cognitive function among Alzheimer’s patients (Morris & Burns, 2012).
As with any disease process, adherence to prescribed treatment measures is always a challenge. In order to improve compliance with diabetes care routines in the HIV patient, teams of healthcare providers should ensure proper education and support for the patients and caregivers. The patient is the most important member of the team so individualizing specific strategies and techniques to encourage compliance are needed. The team members should gather information regarding meal patterns, work schedules, exercise habits, availability of supplies, social situations, and cultural diversity that could potentially impact compliance. Then the team members need to set commonly agreed upon goals for self-care with the patient (ADA, 2013).
B (Blood Pressure)
Another way to prevent cognitive impairments related to diabetes is through the identification and treatment of hypertension. In order to effectively screen and manage hypertension, nurses, nurse practitioners, and other healthcare providers should use the guidelines set forth by the Seventh Report from the Joint National Committee (JNC 7) on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (Chobanian et al., 2003). The JNC 7 recommends that patients with diabetes maintain a blood pressure less that 130/80 mmHg. The JNC 7 uses an algorithmic approach to treatment. Initial treatment for patients diagnosed with hypertension is lifestyle modification including exercise and adherence to the Dietary Approaches to Stop Hypertension Eating Plan (Appel et al., 1997). If blood pressure targets are not met, then pharmacologic agents may be necessary. The ADA (2012) guidelines, the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group, 2002), and the United Kingdom Prospective Diabetes Study (1998) findings support the use of diuretics, beta blockers, angiotensin receptor blockers, and angiotensin converting enzyme inhibitors, or calcium channel blockers in the management of hypertension in patients with diabetes. Furthermore, angiotensin receptor blockers and angiotensin converting enzyme inhibitors are protective against diabetic nephropathy and reduction in albuminuria. Current practice guidelines encourage the use of angiotensin converting enzyme inhibitors as the first line treatment, however, patients may be switched to an angiotensin receptor blockers, if they are intolerant of the angiotensin converting enzyme inhibitor (ADA, 2013). Additionally, angiotensin receptor blockers have been found to reduce macroalbuminuria. Tsukuda and colleagues (2007) found angiotensin II type-1 receptor blocker candesartan to be effective in hypertensive mouse models with type 2 diabetes.
C (Cholesterol)
Medical practitioners should use the Adult Treatment Panel III (ATP III) guidelines for the management of cholesterol (National Institutes of Health (NIH), 2001). The ATP III advises a two-step approach for the management of dyslipidemia. First, the low density lipoprotein (LDL) cholesterol should be targeted initially. Second, once acceptable levels are achieved, focus should be placed on managing the triglycerides when elevated over 150 mg/dL, low HDL and other metabolic syndrome. Commonly a second or third oral dyslipidemic agent may need to be added.
A risk assessment should be performed next to determine the cardiovascular heart disease risk or risk equivalent. Evidence from the United Kingdom Prospective Diabetes Study (1998) supports the concept that patients with diabetes belong in the same category of cardiovascular heart disease as a patient with a previous myocardial infarction; hence, patients with diabetes are considered to have a cardiovascular heart disease risk equivalent (NIH). Therefore, patients with diabetes should be treated as aggressively in order to achieve LDL levels less than 100 mg/dL. Initially, treatment may involve a trial period of diet and exercise. If these efforts fail to achieve an LDL <100 mg/dL, then a statin may be added to the treatment regimen. Likewise, for the lipodystrophic syndrome, optimizing lipid levels by diet and use of dyslipidemic medications (e.g., fibrates and statins) are essential (Moretti, Gorini & Villa, 2011). Although, often a progressive syndrome, lipodystrophy can regress with the cessation of protease inhibitors and this may assist in reversal of the syndrome (Carr et al., 1999; Vigano et al., 2007).
If nurse and nurse practitioners target treatment efforts on the ABCs of diabetes, then the co-morbid conditions associated with diabetes such as stroke, hypertension, and atherosclerosis, cognitive impairment may be lessened or even avoided altogether. Additionally, cognitive function in the person living with HIV can be preserved by actively screening for dysglycemia and dyslipidemia prior to initiating antiretroviral therapy. If levels are noted to be normal, then the patient can be screened in 6 months then yearly afterwards (Robles & Elston, 2011). Albeit, it is important to guard against dysglycemia and dylipidemia to avoid problems later.
Implications for Research
Future research will need to examine not only the cumulative impact of HIV, diabetes, and aging on cognition, it will also be necessary to examine this relationship in lieu of other common co-morbidities highly prevalent in older adults (50+) with HIV such as hypercholesterolemia (51.07%), hypertension (52.67%), coronary artery disease (17.91%), renal disease (12.03%), and hepatitis C (19.14%) (Vance, Mugavero et al., 2011). For instance, Hinkin and colleagues (Hinkin, Castellon, Levine, Barclay, & Singer, 2008) examined 83 adults with HIV and 35 adults with HIV co-infected with hepatitis C to determine the impact of mono-infection and co-infection on cognition. These researchers found that those adults co-infected with hepatitis C performed worse on measures of memory and learning compared to those without hepatitis C. In addition, those co-infected with hepatitis C exhibited a higher prevalence of global cognitive impairments at 63% compared to those mono-infected at 43%. Given that a number of physical co-morbidities (e.g., heart disease, hypertension) and psychiatric co-morbidities are known to promote cognitive decline (Vance, Dodson, Watkins, Kennedy, & Keltner, in press; Vance et al., 2011), it is important to consider the impact these co-morbidities exert on cognitive functioning as adults age with HIV.
In addition, to promote positive neuroplasticity and cognitive reserve in this clinical population, Vance, Eagerton, and colleagues (2011) developed cognitive prescriptions. Cognitive prescriptions are individualized, tailored behavioral programs designed to improve cognition by increasing behaviors known to support cognitive reserve such as intellectual exercise, physical exercise, social stimulation, sleep hygiene, nutrition, reduction of substance use, and mood support. Although it has not been tested in those with HIV or those with diabetes, the principles of cognitive prescriptions may be used in tandem with treatment for HIV and diabetes to support cognitive reserve and general health (Yamamoto et al., 2009).
Finally, fascinating research has emerged on the use of intranasal delivery of neurotrophins, insulin, and insulin-like growth factor-1 as a possible means of addressing cognitive impairment caused by diabetes and HIV as well as in stroke and Alzheimer’s disease. In preliminary studies, such intranasal delivery has been shown to improve short-term and long-term memory as well as heighten mood and odor detection (Craft et al., 2012; Hanson & Frey, 2007; Lindl, Marks, Kolson, & Jordan-Sciutto, 2010). Improvement of odor detection and mood is an added benefit since many adults with HIV experience elevated levels of depression and anxiety as well as reduced levels of olfaction (Vance, 2007; Vance & Burrage, 2006; Vance, Mugavero et al., 2011).
Conclusion
As people age with HIV, metabolic dysfunction, type 2 diabetes, and other comorbidities will become more prevalent, the combination of which will impact neural health and deplete cognitive reserve. This depletion of cognitive reserve will eventually lead to poor cognitive functioning and in more severe cases, result in mild cognitive impairment or even dementia. Therefore, it is important to consider: 1) ways to prevent such metabolic diseases and co-morbidities, 2) ways to decrease the severity of these diseases and co-morbidities through effective treatments and lifestyle choices, 3) ways to bolster cognitive reserve, and then finally, 4) ways to mitigate cognitive losses through mnemonics and other cognitive strategies. This research vector is not only important for people aging with HIV, it is important for everyone given that many of the lifestyle choices that are made today impact cognitive reserve which will be needed as people age normally or with a disease like HIV and diabetes (Vance, 2012).
Footnotes
There are no conflicts of interest with any of the authors.
Contributor Information
David E. Vance, Email: devance@uab.edu, School of Nursing, NB 456, 1701 University Boulevard, University of Alabama at Birmingham (UAB), Birmingham, AL 35294-1210, Office: 205-934-7589, Fax: 205-996-7183.
Pariya L. Fazeli, Email: pfazeli@ucsd.edu, HIV Neurobehavioral Research Program 220 Dickinson Street, Suite B (8231), University of California, San Diego, CA 92103, Office: 619-543-6584.
Joan E. Dodson, Email: joan3d@uab.edu, Department of Psychology & Center for Translational Research in Aging and Mobility, Holly Mears Building, Room 130, 924 19th Street South, University of Alabama at Birmingham (UAB), Birmingham, AL 35294, Office: 205-934-2551.
Michelle Ackerman, Email: mlynnack@uab.edu, Department of Psychology, University of Alabama at Birmingham (UAB), Birmingham AL 35294, Office: 334-467-8864.
Michele Talley, Email: talleym@uab.edu, School of Nursing, NB 543, 1701 University Boulevard, University of Alabama at Birmingham (UAB), Birmingham, AL 35294, Office: 205-934-6647.
Susan J. Appel, Email: sappel@ua.edu, Capstone College of Nursing, The University of Alabama, Tuscaloosa, AL PO Box 870358, Tuscaloosa, AL 3578-0358, Office: 205-348-1026.
References
- Allen KV, Frier BM, Strachan MWJ. The relationship between type 2 diabetes and cognitive dysfunction: Longitudinal studies and their methodological limitations. European Journal of Pharmacology. 2004;490:169–175. doi: 10.1016/j.ejphar.2004.02.054. [DOI] [PubMed] [Google Scholar]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blockers versus diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Journal of the American Medical Association. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(supplement 1):s11–s63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36(supplement 1):s11–s66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Anstey K. Construct overlap in resource theories of memory aging: Commentary. Gerontology. 1999;45:348–350. doi: 10.1159/000022119. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszez MA, Sanchez L. A reevaluation of the common factor theory of shared variance among age, sensory, function, and cognitive function in older adults. The Journals of Gerontology: Series B. Psychological Sciences and Social Sciences. 2001;6B(1):3–11. doi: 10.1093/geronb/56.1.p3. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SJ. Calculating insulin resistance in the primary care setting: Why should we worry about insulin levels in euglycemic patients? Journal of the American Academy of Nurse Practitioners. 2005;17(8):331–336. doi: 10.1111/j.1745-7599.2005.0052.x. [DOI] [PubMed] [Google Scholar]
- Appel LJ, Moore TJ, Obarzaniek E, Vollmer WM, Svetkey LP, Sacks FM The DASH Collaborative Research Group. A clinical trial of the effects of dietary patterns on blood pressure. The New England Journal of Medicine. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Archives of Neurology. 2004;61(5):661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. Journal of Clinical and Experimental Neuropsychology. 2004;26(8):1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Baldewicz TT, Leserman J, Silva SG, Petitto JM, Golden RN, Perkins DO, Evans DL. Changes in neuropsychological functioning with progression of HIV-1 infection: Results of an 8-year longitudinal investigation. AIDS and Behavior. 2004;8(3):345–355. doi: 10.1023/B:AIBE.0000044081.42034.54. [DOI] [PubMed] [Google Scholar]
- Ball KK, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M Advanced Cognitive Training for Independent and Vital Elderly Study Group. Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Vance DE, Edwards JE, Wadley VG. Aging and the brain. In: Rizzo M, Eslinger PJ, editors. Principles and practice of behavioral neurology and neuropsychology. Philadelphia, PA: Saunders; 2004. pp. 795–809. [Google Scholar]
- Becker JT, Caldararo R, Lopez OL, Dew MA, Dorst SK, Banks G. Qualitative features of the memory deficit associated with HIV infection and AIDS: Cross-validation of a discriminant function classification scheme. Journal of Clinical and Experimental Neuropsychology. 1995;17:134–142. doi: 10.1080/13803399508406588. [DOI] [PubMed] [Google Scholar]
- Berry C, Balachandran KP, L’Allier PL, Lesperance J, Bonan R, Oldroyd KG. Importance of collateral circulation in coronary heart disease. European Heart Journal. 2007;28:278–291. doi: 10.1093/eurheartj/ehl446. [DOI] [PubMed] [Google Scholar]
- Biessels GJ. Intensive glucose lowering and cognition in type 2 diabetes. The Lancet Neurology. 2011;10:949–950. doi: 10.1016/S1474-4422(11)70199-5. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes: A systematic review. Lancet Neurology. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- Biron A, Bobin-Dubigeon C, Volteau C, Piroth L, Perré P, Leport C, Biron C. Metabolic syndrome in French HIV-infected patients: Prevalence and predictive factors after 3 years of antiretroviral therapy. AIDS Research and Human Retroviruses. 2012;28(12):1672–1678. doi: 10.1089/AIDS.2012.0048. [DOI] [PubMed] [Google Scholar]
- Birren JE, Woods AM, Williams MV. Behavioral slowing with age: Causes, organization, and consequences of slowing. In: Poon LW, editor. Aging in the 1980’s psychological issues. Washington, D. C: American Psychological Association; 1980. pp. 293–308. [Google Scholar]
- Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. Journal of American Medical Association. 2001;286:1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Büchel C, May A. Training-induced brain structure changes in the elderly. Journal of Neuroscience. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brands AMA, Biessels GJ, Kappelle LJ, de Haan EHF, de Valk HW, Algra K, Kessels RP. Cognitive functioning and brain MRI in patients with type 1 and type 2 diabetes mellitus: A comparative study. Dementia and Geriatric Cognitive Disorders. 2007;23(5):343–350. doi: 10.1159/000100980. [DOI] [PubMed] [Google Scholar]
- Brands A, Van den Berg E, Manschot SM, Biessels GJ, Kappelle LJ, De Haan EHF, Kessels RPC. A detailed profile of cognitive dysfunction and its relation to psychological distress in patients with type 2 diabetes mellitus. Journal of the International Neuropsychological Society. 2007;13:288–297. doi: 10.1017/S1355617707070312. [DOI] [PubMed] [Google Scholar]
- Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, Povikoski T, Sulkava R. Education, the brain and dementia: Neuroprotection or compensation? Brain. 2010;133(Pt. 8):2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- Burke DM, MacKay DG. Memory, language, and ageing. Philosophical Translations: Biological Sciences. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychological Bulletin. 1995;117(2):285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Caban-Holt A, Abner E, Kryscio RJ, Crowley JJ, Schmitt FA. Age-expanded normative data for the ruff 2&7 selective attention test: Evaluating cognition in older males. The Clinical Neuropsychologist. 2012;26(5):751–768. doi: 10.1080/13854046.2012.690451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capeau J, Bouteloup V, Katlama C, Bastard JP, Guiyedi V, Chêne G. Ten-year diabetes incidence of 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26(3):303–314. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: A cohort study. Lancet. 1999;353(9170):2093–2099. doi: 10.1016/S0140-6736(98)08468-2. [DOI] [PubMed] [Google Scholar]
- Chen RH, Jiang XZ, Zhao XH, Qin YL, Gu Z, Gu PL, Zou YF. Risk factors of mild cognitive impairment in middle aged patients with type 2 diabetes: A cross-section study. Annales d’Endocrinologie. 2012;73(3):208–212. doi: 10.1016/j.ando.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. Journal of American Medical Association. 2003;289(19):2560–2572. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [Google Scholar]
- Clay OJ, Edwards JD, Ross LA, Okonkwo O, Wadley VG, Roth DL, Ball KK. Visual function and cognitive speed of processing mediate age-related decline in memory span and fluid intelligence. Journal of Aging Health. 2009;21(4):547–566. doi: 10.1177/0898264309333326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Gerton B. Intranasal insulin therapy for Alzheimer’s disease and amnestic mild cognitive impairment: A pilot clinical trial. Archives of Neurology. 2012;69(1):29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Salthouse TA. Handbook of aging and cognition. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes – systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- Domínguez RO, Marschoff ER, González SE, Repetto MG, Serra JA. Type 2 diabetes and/or its treatment leads to less cognitive impairment in Alzheimer’s disease patients. Diabetes Research and Clinical Practice. 2012;98(1):68–74. doi: 10.1016/j.diabres.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Ettenhofer ML, Foley J, Behdin N, Levine AJ, Castellon SA, Hinkin CH. Reaction time variability in HIV-positive individuals. Archives of Clinical Neuropsychology. 2010;25(8):891–798. doi: 10.1093/arclin/acq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, Lam M, Foley J. Aging, neurocognition, and medication adherence in HIV infection. American Journal of Geriatric Psychiatry. 2009;17(4):281–290. doi: 10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Crowe M, Ross LA, Wadley V, Ball KK, Vance DE. Cognitive functioning in adults aging with HIV: Exploring cognitive subtypes and influential factors. Journal of Clinical and Experimental Neuropsychology. doi: 10.14302/issn.2324-7339.jcrhap-13-191. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology, Learning, Memory, and Cognition. 2001;27:131–146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Green AF, Rebok G, Lyketsos CG. Influence of social network characteristics on cognition and functional status with aging. International Journal of Geriatric Psychiatry. 2008;23(9):972–978. doi: 10.1002/gps.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg EW, Yaffe K, Cauley JA, Rolka DB, Blackwell TL, Nerayan KMV, Cummings SR. Is diabetes associated with cognitive impairment and cognitive decline among older women? Archives of Internal Medicine. 2000;160(2):174–180. doi: 10.1001/archinte.160.2.174. 10-1001/pubs.Arch Intern Med.-ISSN-0003-9926-160-2-ioi90108. [DOI] [PubMed] [Google Scholar]
- Hanson LR, Frey WH., 2nd Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. Journal of Neuroimmune Pharmacology. 2007;2(1):81–86. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH. Reaction time slowing in adults with HIV: Results of a meta-analysis using brinley plots. Brain and Cognition. 2002;50(1):25–34. doi: 10.1016/s0278-2626(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Moore LH. Age differences and neurocognitive performance in HIV-infected adults. New Zealand Journal of Psychology. 1999;28(2):94–101. [Google Scholar]
- Hardy D, Vance D. The neuropsychology of HIV/AIDS in older adults. Neuropsychology Review. 2009;19(2):263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- Hassing LB, Johansson B, Pedersen NL, Nilsson SE, Berg S, McClearn G. Type 2 diabetes mellitus and cognitive performance in a population-based sample of the oldest old: Impact of comorbid dementia. Aging, Neuropsychology, and Cognition. 2003;10(2):99–107. 10.10170S1355617704104165. [Google Scholar]
- Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn GM, Johansson B. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: Evidence from a longitudinal study. Age and Ageing. 2004;33:355–361. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F The CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Rentz DM. Cognitive profile of amyloid burden and white matter hyperintensitivies in cognitively normal older adults. Journal of Neuroscience. 2012;32(46):16233–15242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Farinpour R, Newton T, Singer E. Methylphenidate improves HIV-1-associated cognitive slowing. Journal of Neuropsychiatry and Clinical Neuroscience. 2001;113(2):248–254. doi: 10.1176/appi.neuropsych.13.2.248. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. Journal of Addictive Disease. 2008;27(2):11–17. doi: 10.1300/J069v27n02-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: A 4-year prospective study of the Rancho Bernardo Study cohort. Archives of Internal Medicine. 2004;164(12):1327–1333. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Chodosh J, McEwen BS, Seeman TE. Urinary cortisol excretion as a predictor of incident cognitive impairment. Neurobiology of Aging. 2005;26S:S80–S84. doi: 10.1016/j.neurobiolaging.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Valcour V. Cognitive performance related to HIV-1-infected monocytes. Journal of Neuropsychiatry and Clinical Neuroscience. 2012;24(1):71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, Bryan RN. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): A randomised open- label substudy. The Lancet Neurology. 2011;10(11):969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM, Ross ER, Gower A, Paris IM, Martenson R, Lorenns SA. Spatial learning deficits in the aged rat: Neuroanatomical and neurochemical correlates. Brain Research Bulletin. 1994;33:489–500. doi: 10.1016/0361-9230(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DA, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: Pathogenesis and therapeutic opportunities. Journal of Neuroimmune Pharmacology. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. British Medical Journal. 2004;328(7439):548. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lojek E, Borstein RA. The stability of neurocognitive patterns in HIV-infected men: Classification considerations. Journal of Clinical and Experimental Neuropsychology. 2005;27:665–682. doi: 10.1081/13803390490918426. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang M, Manly JJ, Mayeaux R. Relation of diabetes to mild cognitive impairment. Archives of Neurology. 2007;64(4):570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psycneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Makris K, Spanou L. Is there a relationship between mean blood glucose and glycated hemoglobin? Journal of Diabetes Science and Technology: From Basic Science to Clinical Practice. 2011;5(6):1572–1583. doi: 10.1177/193229681100500634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp C, Letendre SL, Jeste D, Grant I. Successful cognitive aging in persons living with HIV infection. Journal of Neurovirology. 2011;17:110–119. doi: 10.1007/s13365-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschot SM, Biessels GJ, Rutten GEHM, Kessels RCP, Gispen WH, Kappelle LJ. Peripheral and central neurologic complications in type 2 diabetes mellitus: No association in individual patients. Journal of the Neurological Sciences. 2008;264(1/2):157–162. doi: 10.1016/j.jns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Manschot SM, Brands AM, Van der Grond J, Kessels RPC, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55(4):1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- Marcotte TD, Wolfson T, Rosenthal TJ, Heaton RK, Gonzalez R, Ellis RJ The HNRC Group. A multimodal assessment of driving performance in HIV infection. Neurology. 2004;63:1417–1422. doi: 10.1212/01.wnl.0000141920.33580.5d. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McCutchan JA, Marquie-Beck JA, Fitzsimons CA, Letendre SL, Ellis RJ, Grant I. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Geall BP, Fischer AL, Dolcos S, Dixon RA. Testing covariates of type 2 diabetes-cognition associations in older adults: Moderating or mediating effects? Neuropsychology. 2010;24(5):547–562. doi: 10.1037/a0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire LC, Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life. American Journal of Geriatric Psychiatry. 2006;14(1):36–42. doi: 10.1097/01.JGP.0000192502.10692.d6. [DOI] [PubMed] [Google Scholar]
- Mogi M, Tsukuda K, Li JM, Iwanami J, Min LJ, Sakata A, Horiuchi M. Inhibition of cognitive decline in mice fed a high-salt and cholesterol diet by the angiotensin receptor blocker, olmesartan. Neuropharmacology. 2007;53(8):899–905. doi: 10.1016/j.neuropharm.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Moretti A, Gorini A, Villa RF. Pharmacotherapy and prevention of vascular dementia. CNS & Neurological Disorders Drug Targets. 2011;10(3):370–390. doi: 10.2174/187152711794653832. [DOI] [PubMed] [Google Scholar]
- Morris JK, Burns JM. Insulin: An emerging treatment for Alzheimer’s disease dementia? Current Neurology and Neuroscience Reports. 2012 doi: 10.1007/s11910-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle W, Kellett U, Ballantyne A, Gracia N. Dementia and loneliness: An Australian perspective. Journal of Clinical Nursing. 2011;20(9–10):1445–1453. doi: 10.1111/j.1365-2702.2010.03549.x. [DOI] [PubMed] [Google Scholar]
- Murji S, Rourke SB, Donders J, Carter SL, Shore D, Rourke BP. Theoretically derived CVLT subtypes in HIV-1 infection: Internal and external validation. Journal of the International Neuropsychological Society. 2003;9:1–16. doi: 10.1017/s1355617703910010. [DOI] [PubMed] [Google Scholar]
- Nakamoto BK, Jahanshad N, McMurtay A, Kallianpur KJ, Chow DC, Vaclour VG, Shikuma CM. Cerebrovascular risk factors and brain microstructural abnormalities on diffusion tensor images in HIV-infected individuals. Journal of Neurovirology. 2012;18(4):303–314. doi: 10.1007/s13365-012-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (NIH) Third Report on the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive Summary. Bethesda, MD: National Institutes of Health; 2001. [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Annals of Neurology. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Noble JM, Manly JJ, Schupf N, Tang MX, Luchsinger JA. Type 2 diabetes and ethnic disparities in cognitive impairment. Ethnic and Disease. 2012;22(1):38–44. [PMC free article] [PubMed] [Google Scholar]
- Norbiato G. Endocrine, metabolic, and immunologic components of HIV infection. Annals of the New York Academy of Sciences. 2012;1262:51–55. doi: 10.1111/j.1749-6632.2012.06620.x. [DOI] [PubMed] [Google Scholar]
- Okereke OI, Kang JH, Cook NR, Gaziano JM, Manson JE, Buring JE, Grodstein F. Type 2 diabetes mellitus and cognitive decline in two large cohorts of community-dwelling older adults. Journal of the American Geriatrics Society. 2008;56(6):1028–1036. doi: 10.1111/j.1532-5415.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davison NS, Smith AD, Smith PK. Models of visospatial and verbal memory across the adult life span. Psychology of Aging. 2002;17:299–320. doi: 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Piguet O, Grayson DA, Broe GA, Tate RL, Bennett HP, Lye TC, Ridley L. Normal aging and executive functions in “old-old” community dwellers: Poor performance is not an inevitable outcome. International Psychogeriatrics. 2002;14(2):139–159. doi: 10.1017/s1041610202008359. [DOI] [PubMed] [Google Scholar]
- Raboud JM, Diong C, Carr A, Grinspoon S, Mulligan K, Sutinen J Glitazone and Lipoatrophy Meta-Analysis Working Group. A meta-analysis of six placebo- controlled trials of thiazolidinedione therapy for HIV lipoatrophy. HIV Clinical Trials. 2010;11(1):39–50. doi: 10.1310/hct1101-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JL. The medical managment of HIV disease. In: Durham JD, Lashley FR, editors. The person with HIV/AIDS: Nursing perspective. 4. New York, NY: Springer Publishing Company; 2010. pp. 221–291. [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Restak R. Think smart: A neuroscientist’s prescription for improving brain’s performance. New York, NY: Penguin; 2009. [Google Scholar]
- Robles DT, Elston DM. Lipodystrophy in HIV. 2011 Retrieved November 15, 2012 at http://emedicine.medscape.com/article/1082199-overview#showall.
- Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: Variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of Neurology. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roriz-Filho SJ, Sá-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochimica Et Biophysica Acta. 2009;1792(5):432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. The Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Satori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. Journal of Neuroscience Nursing. 2012;44(4):206–217. doi: 10.1097/JMM.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. The Journal of Clinical Investigation. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2018. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabetic Medicine. 1999;16(2):93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- Strachan MWJ, Deary I, Ewing FME, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20(3):438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- Taylor VH, MacQueen GM. Cognitive dysfunction associated with metabolic syndrome. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity. 2007;8(5):409–418. doi: 10.1111/j.1467-789X.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- Tsukuda K, Mogi M, Li JM, Iwanami J, Min LJ, Sakata A, Horiuchi M. Amelioration of cognitive impairment in the type-2 diabetic mouse by the angiotensin II type-1 receptor blocker candesartan. Hypertension. 2007;50(6):1099–1105. doi: 10.1161/HYPERTENSIONAHA.107.099374. [DOI] [PubMed] [Google Scholar]
- Umegaki H, Hayashi T, Nomura H, Yanagawa M, Nonogaki Z, Nakshima H, Kuzuya M. Cognitive dysfunction: An emerging concept of a new diabetic complication in the elderly. Geriatrics and Gerontology International. 2012 doi: 10.1111/j.1447-0594.2012.00922.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. British Medical Journal. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- Vabres P. A hypothesis on the pathogeny of rounded and linear epidermal nevi (nevoid acanthosis nigricans) [Naevus epidermiques lineaires et arrondis: une hypothese pathogenique] Annales De Dermatologie Et De Venereologie. 2012;139(3):177–179. doi: 10.1016/j.annder.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, Shikuma CM. Insulin resistance is associated with cognition among HIV-1-infected patients: The Hawaii Aging with HIV Cohort. Journal of Acquired Immune Deficiency Syndromes. 2006;43(4):405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- Van den Berg E, Reijmer YD, de Bresser J, Kessels RP, Kappelle LJ, Biessels GJ. A 4-year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:58–65. doi: 10.1007/s00125-009-1571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp WG, Hinkin C, Satz P, Miller EN, Weisman J, Holston S, Dixon W. Subtypes of HIV-related neuropsychological functioning: A cluster analysis approach. Neuropsychology. 1993;7(1):62–72. [Google Scholar]
- Vance DE. Olfactory and psychomotor symptoms in HIV and aging. Medical Science Monitor. 2007;13(10):1–3. [PubMed] [Google Scholar]
- Vance DE. Potential factors that may promote successful cognitive aging. Nursing: Research and Reviews. 2012;2:27–32. [Google Scholar]
- Vance DE, Ball KK, Moore BS, Benz RL. Cognitive remediation therapies for older adults: Implications for nursing practice and research. Journal of Neuroscience Nursing. 2007;39:226–231. doi: 10.1097/01376517-200708000-00007. [DOI] [PubMed] [Google Scholar]
- Vance DE, Burrage J., Jr Chemosensory declines in older adults with HIV: Identifying interventions. Journal of Gerontological Nursing. 2006;32(7):42–48. doi: 10.3928/00989134-20060701-06. [DOI] [PubMed] [Google Scholar]
- Vance DE, Crowe M. A proposed model of neuroplasticity and cognitive reserve in older adults. Activities, Adaptation and Aging. 2006;30(3):61–79. doi: 10.1300/J016v30n03_04. [DOI] [Google Scholar]
- Vance DE, Dodson JE, Watkins J, Kennedy BH, Keltner NL. Neurological and psychiatric diseases and their unique cognitive profiles: Implications for nursing practice and research. Journal of Neuroscience Nursing. doi: 10.1097/JNN.0b013e3182829038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Eagerton G, Harnish B, McKie-Bell P, Fazeli P. Cognitive prescription across the lifespan: A nursing approach to increasing cognitive reserve. Journal of Gerontological Nursing. 2011;37(4):22–29. doi: 10.3928/00989134-20101202-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Fazeli PL, Moneyham L, Keltner NL, Raper JL. Assessing and treating forgetfulness and cognitive problems in adults with HIV. Journal of the Association of Nurses in AIDS Care. doi: 10.1016/j.jana.2012.03.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Larsen KI, Eagerton G, Wright MA. Comorbidities and cognitive functioning: Implications for nursing research and practice. Journal of Neuroscience Nursing. 2011;43(4):215–224. doi: 10.1097/JNN.Ob013e3182212a04. [DOI] [PubMed] [Google Scholar]
- Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: A cross-sectional study of co-morbidity prevalence and clinical characteristics across decades of life. Journal of the Association of Nurses in AIDS Care. 2011;22(1):17–25. doi: 10.1016/j.jana.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Vance DE, Wright MA. Positive and negative neuroplasticity: Implications for age-related cognitive declines. Journal of Gerontological Nursing. 2009;35(6):11–17. doi: 10.3928/00989134-20090428-02. [DOI] [PubMed] [Google Scholar]
- Varma S, Boyle L, Varma M, Piatt G. Controlling the ABCs of diabetes in clinical practice: A community-based endocrinology practice experience. Diabetes Research and Clinical Practice. 2008;80:89–95. doi: 10.1016/j.diabres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Vigano A, Brambilla P, Cafarelli L, Giacomet V, Borgonovo S, Zamproni I, Mora S. Normalization of fat accrual in lipoatrophic, HIV-infected children switched from stavudine to tenofovir and from protease inhibitor to efavirenz. Antiviral Therapy. 2007;12(3):297–302. [PubMed] [Google Scholar]
- Williamson R, McNeilly A, Sutherland C. Insulin resistance in the brain: An old- age or new-age problem? Biochemical Pharmacology. 2012;84(6):737–745. doi: 10.1016/j.bcp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Willis SL, Schaie KW. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychology of Aging. 1986;1:239–247. doi: 10.1037//0882-7974.1.3.239. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Moffat N. Introduction. In: Wilson BA, Moffat N, editors. Clinical management of memory problem. Rockville, MD: Aspen Systems Corporation; 1984. pp. 1–4. [Google Scholar]
- Yamamoto N, Yamanaka G, Takasugi E, Ishikawa M, Yamanaka T, Murakami S, Otsuka K. Lifestyle intervention reversed cognitive function in aged people with diabetes mellitus: Two-year follow up. Diabetes Research and Clinical Practice. 2009;85(3):343–346. doi: 10.1016/j.diabres.2009.05.014. [DOI] [PubMed] [Google Scholar]