Abstract
Background
With the advent of the direct-acting antiviral agents (DAAs), significant drug-drug interaction (DDI) potential now exists for patients treated for chronic hepatitis C virus (HCV) infection. However, little is known about how often patients with HCV use medications that may interact with newer HCV treatments, especially those with CYP3A DDI potential.
Methods
Using a large United States commercial insurance database, medication use and comorbidity burden was examined among adult patients with a chronic HCV diagnosis from 2006-2010. Medications were examined by total number of prescription claims, proportion of patients exposed, and DDI potential with prototypical CYP3A DAAs, boceprevir and telaprevir, for which data were available.
Results
Patient comorbidity burden was high and increased over the study period. Medication use was investigated in 53,461 patients with chronic HCV. Twenty-one (53%) of the top 40 most utilized medications were classified as having interaction potential, with 62% of patients received at least one of the top 22 interacting medications by exposure. Of these, 59% and 41% were listed in a common DDI resource but not in medication prescribing information, 77% and 77% had not been investigated in DDI studies, 32% and 27% did not have clear recommendations for DDI management, and only 14% and 23% carried a recommendation to avoid coadministration for boceprevir and telaprevir, respectively.
Conclusion
Practitioners may expect a medication with CYP3A DDI potential in two-thirds of patients with HCV and almost one-half of the most frequently used medications. However, DDI potential may not be reflected in prescribing information.
Keywords: boceprevir, drug interactions, hepatitis C, telaprevir, protease inhibitors, comorbidities
Introduction
Chronic hepatitis C virus infection (HCV) affects an estimated 3.2 million people in the United States (U.S.), while in the European Union HCV affects approximately 8.1 million people and contributes to one-third of deaths in patients with cirrhosis or liver cancer [1-3]. The introduction of direct-acting antivirals (DAAs), beginning with telaprevir (TVR) and boceprevir (BOC) in 2011, has revolutionized the treatment of genotype 1 chronic HCV by improving rates of sustained virologic response dramatically in conjunction with peginterferon and ribavirin (triple therapy) [4]. Currently, there are three classes of DAAs that target different steps in the viral replication cycle: NS3/4A protease inhibitors, NS5B polymerase inhibitors, and NS5A inhibitors. Despite advantages in improving virologic response, many of these new DAAs also carry high drug-drug interaction (DDI) potential due to their metabolism by cytochrome P450 3A (CYP3A) or transport by P-glycoprotein (P-gp) [5].
A number of DDI studies for DAAs have been conducted in both healthy volunteers and diseased populations [6-9] with a focus on medications that have the potential for significant drug interaction or which may be highly utilized in certain patient populations [6, 10, 11]. Recent review articles have provided some guidance for the clinical management of DDIs with the use of NS3/4A protease inhibitors TVR and BOC based on a composite of available literature and theoretical considerations of their clinical pharmacology [5, 12-15]. However, there is a paucity of information on their actual DDI risk in the outpatient setting. Recently, DDI risk with initiation of NS3/4A protease inhibitor therapy was determined to be substantial for about one-half of a small cohort of German HCV patients at a tertiary referral center [16]. Knowledge of the medication use patterns in a larger heterogeneous population would add vital insight to our understanding of the potential for interactions with current and emerging agents [5, 13].
The primary objective of this study was to characterize medication utilization in a representative chronic HCV population. Specific aims included: (1) to assess the most highly utilized medications from 2006-2010 by prescription claims and by exposure, and (2) to evaluate the telaprevir and boceprevir DDI potential of highly utilized medications in patients with HCV. In order to provide a context for interpretation of the medication use data, comorbidities and other demographic and clinical characteristics of the patients with chronic HCV also were explored. The DDI risk for boceprevir and telaprevir, prototypical CYP3A-metabolized DAAs, will be similar to the DDI risk for other DAAs which are substrates or inhibitors of CYP3A such as simeprevir, faldaprevir, or daclatasvir and especially for DAAs that will be co-administered with ritonavir to inhibit their CYP3A-dependent metabolism (ABT-450 and danoprevir). These agents will soon be used extensively in patients with HCV throughout the world.
Materials and Methods
Study Population and Data
A retrospective observational study design was employed using the Truven Health Analytics Marketscan® Commercial Claims and Encounters Research Database for the years 2006 to 2010. The database includes de-identified medical inpatient and outpatient claims, outpatient pharmaceutical claims, and enrollment data files and provides demographic information, medical diagnoses, health care procedures, and pharmacy claims for approximately 20 million enrollees from over 100 nationwide U.S. insurers.
Five different 1-year cross-sectional cohorts were constructed for each year from 2006 to 2010 to examine demographic characteristics, medication use, and comorbid health conditions in chronic HCV patients. Within each 1-year cross-section, patients were selected for inclusion if they 1) had at least 1 inpatient or 2 outpatient International Classification of Diseases, 9th edition (ICD-9) codes for chronic HCV (070.54 or 070.44) occurring on separate days, 2) were ≥18 years of age, 3) and had continuous enrollment for the entire 1-year period. This cohort was used to investigate demographic and clinical characteristics. A subcohort of these patients with prescription insurance benefits filling at least one prescription per year was further selected to examine concomitant medication use. This subcohort was identified to ensure adequate capture of prescription claims information. Given the prevalent cross-sectional nature of this study, patients could contribute to multiple cross-sections if meeting eligibility criteria for multiple years.
Demographic and Clinical Characteristics
To characterize patients with HCV eligible to receive telaprevir or boceprevir, information was captured for each 1-year calendar period including demographic (age and gender), type of insurance coverage, and region of residence (e.g., Northeast, North Central, South or West). In addition, comorbidities that were reported previously as highly prevalent in chronic HCV patients or otherwise believed to be clinically relevant were identified for each 1-year cross-sectional cohort in either the inpatient or outpatient files using compiled ICD-9 code definitions from the medical literature (Appendix A) [17, 18]. Compensated cirrhosis was defined directly by ICD-9 code definition; advanced liver disease was a composite definition representing decompensated disease and included codes for ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, portal hypertension, esophageal varices, hepatorenal syndrome, or hepatocellular carcinoma. The Charlson Comorbidity Index (CCI), a general measure of comorbidity and predicted mortality, was calculated for each 1-year cross-sectional cohort [19, 20].
Medication Use
Medication use was characterized among the subcohort of chronic HCV patients with prescription insurance benefits. Prescription drug use was identified through national drug codes (NDCs) in the outpatient pharmaceutical files merged with the REDBOOK supplement, which identifies specific medications and therapeutic categories. As standard of care during the study period was dual therapy with interferon and ribavirin, a ‘treated’ patient was defined as having ≥ 1 prescription claim for ribavirin plus ≥1 prescription claim for peginterferon alfa-2a/2b or interferon alfacon-1 during each 1-year eligible calendar period. The average number of distinct medications per individual (drug name without regard to strength or form) also was calculated within the 1-year calendar period for eligible enrollees.
Drug-Drug Interaction Potential
To identify highly utilized medications in the chronic HCV cohort, the 200 most commonly used medications were identified and ranked according to the total number of prescription claims. These medications were then assessed for the potential to interact with telaprevir or boceprevir using the University of Liverpool Hepatitis DDI website (as of September 2013), a recommended international resource from the American Association for the Study of Liver Diseases (AASLD) HCV treatment guidelines [21]. This resource was used as prior data, because it has been suggested to be a more comprehensive resource than the prescribing information [22, 23]. Drug interactions with telaprevir and boceprevir are designated on this site as: “should not be coadministered”, “potential interaction”, or “no clinically significant interaction expected” [21].
To identify the annual proportion of HCV patients exposed to medications with known telaprevir and boceprevir DDI potential, medications were selected from the Liverpool resource's printable charts (as of April 2013) if classified as either “should not be coadministered” or “potential interaction” [21]. For each of these medications, the proportion of patients with ≥1 outpatient prescription claim during each 1-year period of eligibility was determined. Medications were then ranked by average annual proportion of patients exposed within each 1-year cross-sectional cohort. Medications were queried by individual drug ingredients such that one query could span multiple products, identifying usage by total exposure rather than through individually marketed products. For example, codeine was not ranked on the top 40 medications by claims when comparing individual drug products, but its total population exposure was 8.9% across multiple drug products.
Medications with drug interaction potential in the Liverpool resources' complete recommendations were selected for subanalysis if they were listed in the top 40 most utilized medications by prescription claims or had high patient exposure. The prescribing information was compared with the Liverpool resource charts for the selected medications. Drug interaction details in the Liverpool resource were used to further analyze the proportion of these drugs with DDI potential formally investigated in studies, the proportion with clear and actionable recommendations for DDI management, and the proportion not recommended for coadministration. A recommendation for DDI management was considered clear and actionable if there was a statement advising an action such as avoiding, adjusting dose, monitoring, or therapeutic drug monitoring.
Statistical Analysis
For each 1-year cross-sectional cohort, descriptive statistics were performed to assess each demographic and clinical characteristic, including the proportion of patients ‘treated’ with PEG-interferon and ribavirin and comorbid conditions. An average from 2006-2010 across each characteristic was weighted by the total number of patients in each year. In addition, the annual proportions of patients utilizing medications present in the top 200 drug list and those defined by our team as clinically relevant in the chronic HCV population were also described.
All analyses were performed using SAS 9.2 (SAS institute, Cary, NC). This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Results
Of 197,381 individuals aged ≥18 with any HCV diagnosis in the study period, 71,584 patients (106,283 1-year cross-sections) received a diagnosis of chronic HCV in at least one inpatient or two outpatient visits and maintained continuous enrollment for at least one 1-year period. Demographic and clinical comorbidity characteristics are reported in Table 1. There were fewer eligible individuals in 2006 and 2007 relative to the successive years. Average overall age of the study sample was 51.2 ± 7.5 years with an increasing trend over the 5-year period from age 49.7 in 2006 to age 52.4 in 2010; 62.2% were male. The majority of the study patients resided in the Southern region of the U.S. (48.6%) and had a preferred provider organization insurance plan (65.7%). Comorbid conditions prevalent in ≥5% of patients (in order of decreasing prevalence) were hypertension, lipid metabolism disorders, compensated cirrhosis, advanced liver disease, type 2 diabetes, depression, non-alcoholic fatty liver disease (NAFLD), and chronic obstructive pulmonary disease (COPD)/asthma. Prevalence increased over the study period for the majority of conditions queried, with the highest increases observed for obesity, hepatocellular carcinoma, and alcohol abuse/dependence. Average rates of liver transplant and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) in the cohort were 3.9% and 2.8%, respectively.
Table 1. Demographic and clinical characteristics of a U.S. chronic HCV population in a commercial insurance database, 2006-2010.
| 2006 | 2007 | 2008 | 2009 | 2010 | Weighted Average | |
|---|---|---|---|---|---|---|
| Enrollees, n | (71,584 individuals, 106,283 cross-sections) | |||||
| 16,216 | 16,235 | 25,380 | 24,683 | 23,769 | N=106,283 | |
|
| ||||||
| Sociodemographics, % | ||||||
|
| ||||||
| Age in years, mean | 49.7 | 50.4 | 51.2 | 51.8 | 52.4 | 51.2 |
| (±SD) | (7.3) | (7.3) | (7.5) | (7.5) | (7.6) | (7.5) |
| Gender, male | 61.5 | 61.4 | 62.1 | 62.6 | 62.8 | 62.2 |
|
| ||||||
| Health plan type | ||||||
| Comprehensive | 4.9 | 2.0 | 2.7 | 2.3 | 2.8 | 2.9 |
| HMO | 14.7 | 15.1 | 23.1 | 18.5 | 16.7 | 18.1 |
| POS | 10.6 | 11.0 | 9.3 | 8.4 | 8.0 | 9.3 |
| PPO | 67.3 | 69.4 | 62.1 | 67.7 | 64.0 | 65.7 |
|
| ||||||
| Region | ||||||
| Northeast | 8.8 | 7.5 | 18.6 | 12.5 | 16.2 | 13.5 |
| North Central | 19.8 | 18.7 | 15.5 | 16.3 | 15.6 | 16.8 |
| South | 51.5 | 52.5 | 48.1 | 50.6 | 42.6 | 48.6 |
| West | 19.5 | 20.9 | 17.6 | 20.5 | 24.8 | 20.7 |
|
| ||||||
| Comorbidities, % | ||||||
|
| ||||||
| Metabolic | ||||||
| Hypertension | 32.0 | 34.8 | 37.5 | 43.2 | 44.7 | 39.2 |
| Lipid metabolism disorders | 19.2 | 20.8 | 22.4 | 26.7 | 27.5 | 23.8 |
| Type II diabetes | 16.4 | 17.3 | 18.7 | 20.0 | 20.3 | 18.8 |
| Clinically-defined obesity | 2.1 | 2.8 | 3.2 | 5.9 | 6.5 | 4.3 |
|
| ||||||
| Psychiatric | ||||||
| Depression | 12.2 | 13.0 | 12.6 | 16.2 | 16.7 | 14.4 |
| Drug abuse/dependence | 3.3 | 3.1 | 3.7 | 5.3 | 5.6 | 4.3 |
| Alcohol abuse/dependence | 2.9 | 3.1 | 3.8 | 5.3 | 5.8 | 4.3 |
| Bipolar disorders | 2.2 | 2.4 | 2.5 | 2.9 | 2.9 | 2.6 |
|
| ||||||
| Hepatic | ||||||
| Compensated cirrhosis | 18.0 | 19.9 | 20.2 | 23.6 | 26.5 | 22.0 |
| Advanced liver disease | 9.9 | 10.7 | 11.1 | 13.8 | 15.5 | 12.5 |
| NAFLD | 5.0 | 5.7 | 6.5 | 8.1 | 8.8 | 7.0 |
| Alcoholic liver disease | 3.9 | 4.0 | 4.2 | 5.0 | 5.9 | 4.7 |
| Liver transplant | 3.2 | 3.4 | 3.7 | 4.3 | 4.7 | 3.9 |
| Viral hepatitis B | 3.6 | 3.5 | 4.0 | 3.5 | 3.6 | 3.7 |
| Hepatocellular carcinoma | 1.8 | 2.3 | 3.1 | 3.3 | 4.0 | 3.0 |
|
| ||||||
| Other | ||||||
| COPD or asthma | 8.0 | 8.8 | 9.4 | 11.1 | 11.6 | 10.0 |
| Rheumatoid arthritis | 3.1 | 3.0 | 3.1 | 3.4 | 3.4 | 3.2 |
| HIV/AIDS | 1.9 | 2.2 | 3.0 | 3.2 | 3.2 | 2.8 |
Abbreviations: SD, standard deviation; chronic HCV, chronic Hepatitis C virus infection; HMO, Health Maintenance Organization; POS, Point of Service; PPO, Preferred Provider Organization; NAFLD: non-alcoholic fatty liver disease; COPD, chronic obstructive pulmonary disease; HIV/AIDS: human immunodeficiency virus/acquired immunodeficiency syndrome
A subcohort of 53,461 individuals (79,185 cross-sections) had prescription insurance benefits and was followed to examine medication use patterns. HCV treatment rates and comorbidity measures for this subcohort are reported in Table 2. On average, 31.2% of patients were classified as ‘treated’, having received both interferon and ribavirin. The mean Charlson Comorbidity Index score was 2.2 and increased over the study period; the average for the treated group was lower than the untreated group (1.9 vs 2.4, respectively). The average number of unique medications filled per patient for the treated group was higher than the untreated group (11.5 vs 8.9, respectively).
Table 2. Comorbidity and medication use characteristics of a U.S. chronic HCV population in a commercial insurance database, 2006-2010.
| 2006 | 2007 | 2008 | 2009 | 2010 | Weighted Average | |
|---|---|---|---|---|---|---|
| Enrollees with pharmacy benefit, n | (53,461 individuals, 79,185 cross-sections) | |||||
| 11,424 | 11,517 | 19,379 | 18,882 | 17,983 | (sum) 79,185 | |
|
| ||||||
| HCV Treated | ||||||
| Ribavirin + interferon, % | 24.8 | 21.9 | 19.1 | 18.4 | 15.7 | 19.4 |
|
| ||||||
| Comorbidity Measures† | ||||||
| CCI: Treated, mean | 1.7 | 1.7 | 1.9 | 2.0 | 2.1 | 1.9 |
| CCI: Untreated, mean | 2.1 | 2.2 | 2.3 | 2.5 | 2.6 | 2.4 |
| Number of distinct medications per capita: Treated, mean | 11.5 | 11.3 | 11.3 | 11.5 | 11.5 | 11.4 |
| Number of distrinct medications per capita: Untreated, mean | 8.7 | 9.0 | 8.5 | 9.2 | 9.3 | 9.0 |
Abbreviations: HCV, Hepatitis C virus; CCI, Charlson Comorbidity Index
Treated: received ribavirin + PEG-interferon or interferon alfacon in a given year; Untreated: did not receive ribavirin + peg-interferon or interferon alfacon in a given year
The top 10 therapeutic medication categories used in this chronic HCV cohort included (1) analgesics/antipyretics and opiate agonists, (2) antidepressants, (3) antivirals, (4) gastrointestinal drugs, (5) benzodiazepines, (6) beta-blockers, (7) ACE-inhibitors, (8) anxiolytic/sedative hypnotics, (9) calcium channel blockers, and (10) interferons. The top 40 medications used by HCV patients from 2006-2010 are displayed in Table 3, with the top 200 listed in Appendix B. The most common medication with DDI potential used by HCV patients was acetaminophen/hydrocodone, which was filled 367,166 times among the 53,461 patients with HCV who had prescription insurance benefits between 2006 and 2010. Examination of the top 40 medications for DDI potential with boceprevir or telaprevir in the Liverpool resource showed that 17 were classified as “no clinically significant interaction expected” (42.5%), 21 contained at least one component that was classified as “potential interaction” (52.5%, 5 of which indicated an unlikely pharmacokinetic interaction and/or a drug-disease interaction only), zero medications were classified as “should not be coadministered” (0%), and 2 medications (5.0%) were not listed in the resource.
Table 3. Drug interaction potential of the most utilized medications in U.S. patients with HCV by total number of prescriptions filled.
| Medication | Number of claims | Boceprevir* | Telaprevir* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Interaction | CI | NL | None | Interaction | CI | NL | ||
| 1. Acetaminophen/hydrocodone bitartrate | 367,166 | X§ | X§ | ||||||
| 2. Ribavirin | 176,842 | X | X | ||||||
| 3. Zolpidem tartrate | 169,811 | X | X | ||||||
| 4. Levothyroxine sodium | 155,926 | X | X | ||||||
| 5. Alprazolam | 146,362 | X | X | ||||||
| 6. Lisinopril† | 123,616 | X | X | ||||||
| 7. Peginterferon alfa-2a | 120,191 | X | X | ||||||
| 8. Oxycodone | 118,753 | X | X | ||||||
| 9. Furosemide | 100,413 | X | X | ||||||
| 10. Amlodipine besylate | 93,776 | X | X | ||||||
| 11. Omeprazole | 87,895 | X | X | ||||||
| 12. Acetaminophen/oxycodone | 81,332 | X§ | X§ | ||||||
| 13. Esomeprazole magnesium | 80,526 | X§ | X | ||||||
| 14. Metformin HCl† | 78,229 | X | X | ||||||
| 15. Escitalopram oxalate | 74,171 | X | X | ||||||
| 16. Spironolactone | 72,277 | X | X | ||||||
| 17. Hydrochlorothiazide | 71,311 | X | X | ||||||
| 18. Bupropion HCl | 66,680 | X | X | ||||||
| 19. Tramadol HCl | 65,803 | X | X | ||||||
| 20. Pantoprazole sodium | 62,622 | X | X | ||||||
| 21. Metoprolol succinate† | 61,663 | X | X | ||||||
| 22. Lorazepam | 59,950 | X | X | ||||||
| 23. Azithromycin | 59,784 | X | X | ||||||
| 24. Atenolol | 59,054 | X | X | ||||||
| 25. Peginterferon alfa-2b | 58,846 | X | X | ||||||
| 26. Sertraline | 58,004 | X | X | ||||||
| 27. Clonazepam | 57,336 | X | X | ||||||
| 28. Citalopram hydrobromide† | 55,375 | X | X | ||||||
| 29. Gabapentin | 53,583 | X | X | ||||||
| 30. Trazodone | 53,564 | X | X | ||||||
| 31. Prednisone | 53,292 | X | X | ||||||
| 32. Tacrolimus | 50,389 | X | X | ||||||
| 33. Amoxicillin | 49,285 | X | X | ||||||
| 34. Cyclobenzaprine | 48,168 | X | X | ||||||
| 35. Diazepam | 48,097 | X | X | ||||||
| 36. Potassium chloride | 47,627 | X | X | ||||||
| 37. Sulfamethoxazole/trimethoprim | 46,555 | X§ | X§ | ||||||
| 38. Venlafaxine | 46,376 | X | X | ||||||
| 39. Metoprolol tartrate† | 45,848 | X | X | ||||||
| 40. Carisoprodol | 45,149 | X | X | ||||||
Abbreviations: HCV, Hepatitis C Virus; CI, Contraindicated; NL, Not listed
Interaction potential based on Liverpool Drug Interaction charts[21] with None: “No clinically significant interaction expected”; Interaction: “Potential interaction – may require close monitoring, alteration of drug dosage or timing of administration; Contraindicated: “These drugs should not be coadministered”
Chart indicates drug-disease interaction only with hepatic insufficiency/cirrhosis and/or pharmacokinetic interaction unlikely
Interaction has not been assessed and has been predicted based on metabolic profiles of the drugs
Of 225 drugs listed in the Liverpool resource, 143 were categorized as “potential interaction” or “should not be coadministered” with boceprevir or telaprevir. Of these, 109 were selected for analysis if they had FDA approval during the study period and were not classified as a drug-disease interaction. The medication with the highest exposure was zolpidem, which was used by 14.1% of the subcohort. Medications demonstrating increasing utilization over the study period included pravastatin, tenofovir, buprenorphine, simvastatin, amlodipine, tacrolimus, and prednisone; decreasing utilization was observed for escitalopram and venlafaxine. Simvastatin, which is contraindicated with telaprevir and boceprevir, showed a notable increase in utilization between 2006 and 2010.
Of the most utilized medications by prescription claims or exposure, a subset of 22 highly utilized medications was examined further. The Liverpool resource recommendations are summarized in Table 4. For telaprevir, 13 (59.1%) were listed in the prescribing information, 16 (72.7%) carried a clear recommendation for DDI management, 5 (22.7%) carried a recommendation to avoid coadministration, and 5 (22.7%) were investigated formally in a DDI study. For boceprevir, 9 (40.9%) were listed in the prescribing information, 15 (68.2%) carried a clear recommendation for DDI management, 3 (13.6%) carried a recommendation to avoid coadministration, and 5 (22.7%) were investigated formally in a DDI study. These 22 medications with drug interaction potential are listed by exposure in Table 5. On average, 62.1% of chronic HCV patients were exposed to any of these 22 medications.
Table 4. Liverpool resource recommendations for top medications reported to have interaction potential with boceprevir and telaprevir[21].
| Medication | Classification* | Studied | Clear recommendation | Listed in prescribing information | |||||
|---|---|---|---|---|---|---|---|---|---|
| Boceprevir | Telaprevir | ||||||||
|
| |||||||||
| N | I | C | N | I | C | BOC/TVR | BOC/TVR | BOC/TVR | |
| zolpidem | X | X | No / Yes | No / Yes | No / Yes | ||||
| alprazolam | X | X | Yes / Yes | Yes / Yes | Yes / Yes | ||||
| amlodipine | X | X | No / Yes | Yes / Yes | Yes / Yes | ||||
| prednisone | X | XC | Yes / No | YesPI / Yes | No / Yes | ||||
| tramadol | X | X | No / No | No / No | No / No | ||||
| codeine | X | X | No / No | Yes / Yes | No / No | ||||
| fluticasone | XC | XC | No / No | Yes / Yes | Yes / Yes | ||||
| methylprednisolone | X | XC | No / No | Yes / Yes | No / Yes | ||||
| escitalopram | X | X | Yes / Yes | Yes / Yes | No / Yes | ||||
| trazodone | X | X | No / No | Yes / Yes | Yes / Yes | ||||
| clindamycin (systemic) | X | X | No / No | No / No | No / No | ||||
| diazepam | X | X | No / No | No / No | No / No | ||||
| sertraline | X | X | No / No | No / No | No / No | ||||
| lansoprazole | X | X | No / No | No / No | No / No | ||||
| clonazepam | X | X | No / No | No / No | No / No | ||||
| sildenafil (Viagra only) | X | X | No / No | Yes / Yes | Yes / Yes | ||||
| fluconazole | X | X | No / No | No / No | No / No | ||||
| simvastatin | X | X | No / No | Yes / Yes | Yes / Yes | ||||
| venlafaxine | X | X | No / No | No / No | No / No | ||||
| salmeterol | XC | XC | No / No | Yes / Yes | Yes / Yes | ||||
| clarithromycin | X | X | Yes / No | Yes / Yes | Yes / Yes | ||||
| tacrolimus | X | X | Yes / Yes | Yes / Yes | Yes / Yes | ||||
Abbreviations: N, None; I, Potential interaction; C, Contraindicated; BOC, Boceprevir; TVR, Telaprevir; PI, prescribing information
Interaction potential based on Liverpool Drug Interaction charts with None: “No clinically significant interaction expected”; Potential interaction: “Potential interaction – may require close monitoring, alteration of drug dosage or timing of administration; Contraindicated: “These drugs should not be coadministered”
Bold indicates drug also listed in Table 3
Although drug is classified as potential interaction, recommendation is to avoid coadministration
Table 5. Exposure to drugs reported to have DDI potential with telaprevir or boceprevir in a U.S. Commercial Claims Database, 2006-2010.
| 2006 | 2007 | 2008 | 2009 | 2010 | Weighted Average | |
|---|---|---|---|---|---|---|
| Beneficiaries with pharmacy benefit, n* | (53,461 beneficiaries, 79,185 cross-sections) | |||||
| 11,424 | 11,517 | 19,379 | 18,882 | 17,983 | 79,185 | |
|
| ||||||
| Medication | Proportion exposed, % | |||||
|
| ||||||
| zolpidem | 15.0 | 14.4 | 13.6 | 14.1 | 14.0 | 14.1 |
| alprazolam | 9.7 | 10.6 | 9.2 | 10.0 | 9.9 | 9.8 |
| amlodipine | 7.5 | 7.7 | 8.8 | 10.3 | 10.9 | 9.3 |
| prednisone | 8.5 | 8.7 | 8.8 | 9.7 | 10.0 | 9.2 |
| tramadol | 7.8 | 8.7 | 8.2 | 9.7 | 10.0 | 9.0 |
| codeine | 8.0 | 7.9 | 9.0 | 9.6 | 9.1 | 8.9 |
| fluticasone | 8.0 | 8.1 | 7.9 | 9.3 | 10.2 | 8.8 |
| methylprednisolone | 6.3 | 6.0 | 6.3 | 7.0 | 7.1 | 6.6 |
| escitalopram | 7.9 | 7.4 | 6.2 | 6.0 | 5.1 | 6.4 |
| trazodone | 5.2 | 5.0 | 4.8 | 5.5 | 6.0 | 5.3 |
| clindamycin (systemic) | 5.0 | 5.1 | 4.9 | 5.4 | 5.3 | 5.2 |
| diazepam | 4.9 | 5.0 | 5.0 | 5.4 | 5.1 | 5.1 |
| sertraline | 5.3 | 5.2 | 4.9 | 5.1 | 4.9 | 5.1 |
| lansoprazole | 5.8 | 5.0 | 4.1 | 3.7 | 3.8 | 4.3 |
| clonazepam | 3.9 | 4.1 | 3.9 | 4.3 | 4.4 | 4.1 |
| sildenafil (Viagra only) | 4.1 | 3.3 | 4.1 | 4.3 | 3.7 | 3.9 |
| fluconazole | 3.8 | 4.0 | 3.7 | 4.0 | 4.2 | 3.9 |
| simvastatin | 2.0 | 3.1 | 3.6 | 4.7 | 4.7 | 3.8 |
| venlafaxine | 4.5 | 4.3 | 3.4 | 3.1 | 2.4 | 3.4 |
| salmeterol | 3.5 | 3.3 | 3.0 | 3.2 | 3.0 | 3.2 |
| clarithromycin | 3.8 | 3.3 | 3.2 | 2.8 | 2.8 | 3.1 |
| tacrolimus | 2.5 | 2.7 | 2.9 | 3.3 | 3.5 | 3.0 |
|
| ||||||
| Any medication above | 60.9 | 61.1 | 59.5 | 63.9 | 64.3 | 62.1 |
Abbreviations: DDI, Drug-drug interaction
defined using a subcohort of chronic HCV patients with prescription drug benefits and filling at least one prescription per year
A complete listing of percentages using specific queried medications can be found in Appendix C, including rates of utilization for the medications with DDI potential.
Discussion
Medication utilization has been historically inferred from clinical knowledge or extracted from limited clinical trial data. To our knowledge, this is the first published systematic investigation of modern day clinical medication utilization in a large, real-world database of patients with chronic HCV. Previous studies have been smaller or examined potential interactions within a strictly controlled setting. A 2011 analysis examined the use of certain co-medications with boceprevir triple therapy in three major late phase studies; however, patients with certain comorbidities were excluded, limiting the study's generalizability. [10, 24-26]. Recently, Maasoumy et al. investigated DDI risk during initiation of HCV protease inhibitor therapy in 115 consecutively treated patients seen at a German tertiary referral center. They reported that 38% of 116 outpatient medications used in their cohort had DDI potential or unknown DDI risk and that 49% of patients were exposed to at least one drug with DDI potential during treatment [16]. The four most frequently encountered medication classes in the German cohort included beta-blockers, proton pump inhibitors (PPIs), thyroid hormones, and dihydropyridine calcium channel blockers (CCBs) (e.g., amlodipine), and only 4% of encountered drugs were strictly contraindicated [16]. In contrast, in our large U.S. cohort of chronic HCV subjects (not restricted by treatment), the four most frequently used drug categories included analgesics/antipyretics and opiate agonists, antidepressants, antivirals, and gastrointestinal drugs including PPIs. In our study, 57.5% of the top 40 outpatient medications had DDI potential or unknown DDI risk, and 62% of patients were exposed to at least one of the twenty-two most highly utilized medications with DDI potential. Medications with DDI potential used by greater than 9% of patients included zolpidem, alprazolam, amlodipine, and prednisone. Based on both studies, the DDI risk appears to be substantial among chronic HCV patients.
Our study also demonstrates increasingly high comorbidity burden in patients with chronic HCV, based on rates of specific comorbidities as well as Charlson Comorbidity Index. A small cross-sectional retrospective study investigating comorbidities associated with HCV from 1998 to 2007 found that HIV/AIDS, renal disease, diabetes, and obesity were more prevalent in patients with HCV compared with the U.S. population [27]. Higher comorbidity rates may reflect the selective age distribution of the HCV population, but there is also some recent evidence that comorbidity rates may be higher in the HCV population even after adjusting for age [17]. In our study, the potential increasing comorbidity rates may be related to the increasing average age of the underlying HCV population and have implications for increasing polypharmacy and complexity of DDI assessment.
As a result, it is important that practitioners be familiar with current DDI management recommendations. However, as observed with approximately one-third of the drugs identified in this study, many potential DDIs have not been studied formally and thus clear and actionable DDI recommendations often are absent. For those DDIs that do provide actionable recommendations, DDI management depends upon the nature of the interaction. The most well characterized DDIs with telaprevir and boceprevir are those involving the inhibition or induction of oxidative metabolism by CYP3A. For simvastatin (the most highly utilized statin in this population, Appendix B), reasonable intervention options exist and include employing a “statin holiday” by removing medication for the duration of treatment, or stabilizing a patient on an alternative statin with lower interaction potential prior to therapy [28]. For other drugs such as salmeterol, fluticasone, or other inhaled corticosteroids, alternatives such as formoterol or beclomethasone may be employed [5]. Comparatively, use of systemic corticosteroids may be unavoidable in some settings such as maintenance immunosuppression in the transplant setting, and providers must weigh the risk of reduced efficacy of telaprevir and boceprevir due to the possible induction of CYP3A [5, 29, 30]. Management of depression-like symptoms during treatment with peginterferon-based therapies has been shown to affect treatment outcomes and often involves the use of antidepressants such as selective serotonin reuptake inhibitors (SSRIs). [31] SSRIs are generally metabolized by multiple CYP450 enzymes with CYP3A providing only partial contribution, and therefore, the risk of a clinically significant CYP3A-mediated DDI with DAAs is low [5, 14]. In addition, a recent review of data from major clinical trials by Sockalingam et al investigating the neuropsychiatric adverse effects of DAAs as well as DDIs between DAAs and psychiatric medications concluded that DAAs have minimal neuropsychiatric risk [32]. Opioids and other analgesics also are metabolized by multiple pathways including CYP3A, but they are often self-titrated by patients for pain control, complicating the risk of DDIs [33, 34]. In addition, they often are used in combination with acetaminophen, which may represent the greatest risk to patients because providers may not recognize the daily acetaminophen dose from all sources [35].
Less characterized DDIs with boceprevir and telaprevir are those involving hepatic transport proteins [36]. Boceprevir appears to be an inhibitor of organic anion transporting polypeptide 1B (OATP1B) while telaprevir is an inhibitor of OATP1B1 and OATP1B3, organic cation transporter 1 (OCT1), and multidrug and toxin extrusion 1 (MATE1); these transporters are involved in the active uptake of drugs in the liver [36, 37]. Inhibition of these transporters may result in higher systemic exposures but reduced hepatic exposure and efficacy of drugs targeting the liver (e.g., statins, metformin, valsartan) [38-40]. Boceprevir also appears to be a substrate of breast cancer resistance protein (BCRP), a hepatic transporter involved in the active biliary elimination of drugs from the liver [37]. Drugs that are substrates/inhibitors of BCRP (e.g., eltrombopag, cyclosporine) may potentially inhibit the biliary elimination of boceprevir resulting in higher hepatic exposure [41, 42]. Alternatively, boceprevir could increase the hepatotoxic potential of other co-administered BCRP substrates (e.g., methotrexate, rosuvastatin, lapatinib) [43-45]. Additional examination of the potential interactions involving elimination pathways mediated by hepatic transport proteins is needed.
While many drug interactions are predicted from a theoretical understanding of the drug's clinical pharmacology, most are never investigated in DDI studies. Accordingly, at the time of this study, many drugs with theoretical DDI potential through CYP3A had not been formally investigated. While it is impractical and cost-prohibitive to study every possible drug interaction in a formal DDI study, it is also imprudent to risk treatment failure or toxicity without a sound understanding of the DDI potential in a real-world setting. Theoretical prediction is limited by our scientific understanding, which is particularly evident with transporter interactions where the science is in its infancy. In order to mitigate risk, computer simulation with tools such as SimCyp should be considered prior to drug approval to complement formal in vivo studies and establish a better framework for reliable DDI prediction [46, 47]. However, much of the DDI knowledge will still be derived from post-marketing studies and expert opinion, as demonstrated by the majority of interacting medications in this study that were listed in the Liverpool resource but not in the prescribing information. Here, methods such as DDI registries and pharmacovigilance algorithms may complement in vivo studies for detection of DDIs quickly and efficiently [21, 48]. As DDI information is discovered, the prescribing information will remain a reliable source of information about formal DDI studies that were performed by the pharmaceutical company, but resources that are comprehensive, regularly updated, and maintained by clinical experts, such as the Liverpool database, may provide a more optimal model for access to actionable DDI information [21]. The sensitivity and specificity of disease-specific resources such as the Liverpool database also should be compared with other widely-used, validated interaction checking software such as ePocrates [49].
This study has several limitations. ICD-9 definitions, though applied from validated studies, may differ from other definitions such as the Clinical Classification Software from the U.S. Agency for Healthcare Research and Quality; this may partially account for slight differences in comorbidity rates observed in other reports [17, 27]. Medication use was explored in the chronic HCV cohort and not limited to treated patients, so conclusions should be drawn in this context. In addition, only outpatient medication claims were evaluated, so utilization rates may not appropriately estimate medications with use limited to hospital settings. Over-the-counter medications could not be evaluated in this database. This study also was restricted to insured HCV subjects in the U.S. and may not be fully representative of the typical U.S. HCV population [50] or populations in other parts of the world where treatment patterns and comorbidities may differ. However, this study is significant because it examined a wide range of potential prescription medications available both in the U.S. and worldwide for their interaction potential with boceprevir and telepravir, and this information was integrated with the actual exposure to these medications in a real-world setting.
While the focus of this study was to inform DDI assessment for currently available agents, these findings also may have strong implications for other candidates in development. Two new DAAs, simeprevir and sofosbuvir, have been recently approved and others are in Phase 3 evaluation for HCV treatment [51, 52]. An all-oral regimen utilizing a protease inhibitor boosted with ritonavir (ABT-450/r), combined with an NS5A inhibitor and a non-nucleoside polymerase, will likely be licensed by the end of 2014. Moreover, faldaprevir and daclatasvir, which are currently undergoing approval in both the U.S. and Europe, have been shown to be CYP3A substrates and require dose-reductions when used in combination with a ritonavir-boosted anti-retroviral [3, 53-55]. Thus, the information learned here from examining the first two prototypical CYP3A DAAs will remain highly relevant and applicable [56]. As knowledge increases regarding the mechanism(s) and role(s) of metabolism and transport for various drugs, particularly in the setting of liver disease, the information provided here will become highly relevant.
Acknowledgments
Funding sources and disclosures: This research was unfunded. CLM has been supported by a UNC/GlaxoSmithKline Pharmacokinetics/Pharmacodynamics fellowship and is currently employed by Janssen Research & Development, LLC; she has no relevant financial relationships with any company related to this research. Dr. Brouwer is supported, in part, by funding from the National Institutes of Health through award number R01GM41935 from the National Institute of General Medical Sciences. Dr. Fried is funded in part by NIH Mid-Career Mentoring Award K24 DK066144. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Fried serves as ad hoc consultant and receives research grant support from Merck, Vertex, Gilead, AbbVie, BMS, and Janssen.
The University of North Carolina at Chapel Hill Cecil G. Sheps Center provided the access to Truven MarketScan data for this research.
Appendix A. ICD-9 Code Definitions for Selected Comorbidities
| Disease State | ICD-9 Codes |
|---|---|
| Advanced liver disease (includes any of the following: ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, portal hypertension, esophageal varices, hepatorenal syndrome, hepatocellular carcinoma) | 070.44, 070.71, 456.0, 456.1, 456.2x, 572.2, 572.3, 572.4, 572.8, 789.59, 567.23, 155.x |
| Alcohol abuse or dependence | 303.xx, 305.0x, 291.xx |
| Alcoholic liver disease (includes alcoholic fatty liver, acute alcoholic hepatitis, alcoholic cirrhosis of liver, alcoholic liver damage unspecified) | 571.0-, 571.1-, 571.2-, 571.3- |
| Bipolar disorders | 296.0x, 296.1x, 296.4x-296.7x, 296.80, 296.89 |
| Compensated cirrhosis (alcoholic, nonalcoholic, biliary) | 571.2-, 571.5-, 571.6- |
| COPD/asthma | 491.xx-493.xx, 496.x |
| Depression | 296.2x, 296.3x, 311, 309.1, 300.4 |
| Diabetes | 250.xx |
| Drug abuse or dependence (non-tobacco) | 292.xx, 304.xx, 305.2x-305.9x |
| Hepatocellular carcinoma (malignant neoplasm of liver and intrahepatic bile ducts) | 155.x |
| HIV/AIDS, asymptomatic HIV infection, HIV-2 | 042.xx, V08, 079.53 |
| Hypertension | 401.xx, 402.xx, 403.xx, 404.xx, 405.xx |
| Lipid metabolism disorders | 272.xx |
| Liver transplant | V42.7, 50.5x; 996.82, CPT codes 47135, 47136 |
| Non-alcoholic fatty liver disease | 571.8 |
| Overweight and obesity | 278.0x |
| Rheumatologic disease | 710.xx, 714.xx, 725.xx |
| Viral hepatitis B with or without hepatic coma, or carrier | 070.2x, 070.3x, V02.61 |
Abbreviations: ICD-9, International classification of disease, 9th edition; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; CPT: Current Procedural Terminology
Appendix B. Drug Interaction Potential of Top 200 Drugs in Chronic HCV Cohort by Prescriptions Filled
| Medication | Boceprevir | Telaprevir |
|---|---|---|
| 1. Acetaminophen/Hydrocodone BitartrateA |
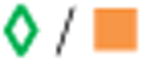
|
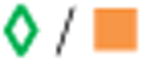
|
| 2. Ribavirin |

|

|
| 3. Zolpidem Tartrate |

|

|
| 4. Levothyroxine Sodium |

|

|
| 5. Alprazolam |

|

|
| 6. Lisinopril |

|

|
| 7. Peginterferon Alfa-2a |

|

|
| 8. Oxycodone HCl |

|

|
| 9. Furosemide |

|

|
| 10. Amlodipine Besylate |

|

|
| 11. Omeprazole |

|

|
| 12.Acetaminophen/Oxycodone HClA |
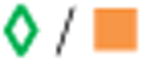
|
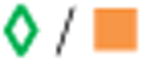
|
| 13. Esomeprazole Magnesium |

|

|
| 14. Metformin HCl |

|

|
| 15. Escitalopram Oxalate |

|

|
| 16. Spironolactone |

|

|
| 17. Hydrochlorothiazide |

|

|
| 18. Bupropion HCl |

|

|
| 19. Tramadol HCl |

|

|
| 20. Pantoprazole Sodium |

|

|
| 21. Metoprolol Succinate |

|

|
| 22. Lorazepam |

|

|
| 23. Azithromycin |

|

|
| 24. Atenolol |

|

|
| 25. Peginterferon Alfa-2b |

|

|
| 26. Sertraline HCl |

|

|
| 27. Clonazepam |

|

|
| 28. Citalopram Hydrobromide |

|

|
| 29. Gabapentin |

|

|
| 30. Trazodone HCl |

|

|
| 31. Prednisone |

|

|
| 32. Tacrolimus |

|

|
| 33. Amoxicillin |

|

|
| 34. Cyclobenzaprine HCl | NL | NL |
| 35. Diazepam |

|

|
| 36. Potassium Chloride |

|

|
| 37. Sulfamethoxazole/Trimethoprim |

|

|
| 38. Venlafaxine HCl |

|

|
| 39. Metoprolol Tartrate |

|

|
| 40. Carisoprodol | NL | NL |
| 41. Fluoxetine HCl |

|

|
| 42. Propranolol HCl |

|

|
| 43. Morphine Sulfate |

|

|
| 44. Lactulose |

|

|
| 45. Insulin Glargine, Recombinant | NL | NL |
| 46. Lansoprazole |

|

|
| 47. Acetaminophen/Propoxyphene NapsylateA |

|

|
| 48. Hydrochlorothiazide/Lisinopril |

|

|
| 49. Ciprofloxacin HCl |

|

|
| 50. Sildenafil CitrateB |
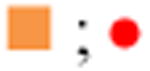
|
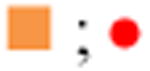
|
| 51. Albuterol SulfateA |

|

|
| 52. Fluticasone Propionate |

|

|
| 53. Ibuprofen |

|

|
| 54. Estradiol | NL | NL |
| 55. Simvastatin |

|

|
| 56. Promethazine HCl |

|

|
| 57. Paroxetine HCl |

|

|
| 58. Temazepam |

|

|
| 59. Amlodipine Besylate/Benazepril HCl |
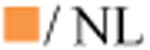
|
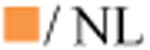
|
| 60. Nadolol | NL | NL |
| 61. Levofloxacin |

|

|
| 62. Duloxetine HCl |

|

|
| 63. CephalexinA |

|

|
| 64. Hydrochlorothiazide/Triamterene |
|
|
| 65. Epoetin Alfa |

|

|
| 66. Amoxicillin/Clavulanate Potassium |

|

|
| 67. Glipizide | NL | NL |
| 68. Fexofenadine HCl |

|

|
| 69. Tamsulosin HCl |

|

|
| 70. Methadone HCl |

|

|
| 71. Fentanyl |

|

|
| 72. Atorvastatin Calcium |

|

|
| 73. Pregabalin |

|

|
| 74. Valsartan |

|

|
| 75. Quetiapine Fumarate |

|

|
| 76. Fluticasone Propionate/Salmeterol Xinafoate |
|
|
| 77. Clopidogrel Hydrogen Sulfate |

|

|
| 78. Triamcinolone Acetonide | NL | NL |
| 79. Amitriptyline HCl |

|

|
| 80. Hydrocodone Bitartrate/Ibuprofen |

|

|
| 81. Valacyclovir HCl | NL | NL |
| 82. Hydrochlorothiazide/Valsartan |

|

|
| 83. Warfarin Sodium |

|

|
| 84. Eszopiclone | NL | NL |
| 85. Methylprednisolone |

|

|
| 86. UrsodiolA |

|

|
| 87. Diltiazem HCl |

|

|
| 88. Mycophenolate Mofetil |

|

|
| 89. Conjugated Estrogens | NL | NL |
| 90. Acetaminophen/Codeine PhosphateA |
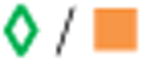
|
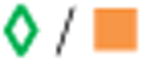
|
| 91. Hydromorphone HCl |

|

|
| 92. Hydroxyzine HCl |

|

|
| 93. Carvedilol |

|

|
| 94. TadalafilB |
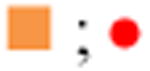
|
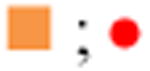
|
| 95. Montelukast Sodium |

|

|
| 96. Nifedipine |

|

|
| 97. Acyclovir | NL | NL |
| 98. Naproxen |

|

|
| 99. Doxycycline Hyclate |

|

|
| 100. Meloxicam | NL | NL |
| 101. Alendronate SodiumA 101 |

|

|
| 102. Mometasone Furoate |

|

|
| 103. Clonidine HCl | NL | NL |
| 104. Folic Acid |

|

|
| 105. Ezetimibe |

|

|
| 106. Ramipril |

|

|
| 107. Celecoxib |

|

|
| 108. Fluconazole |

|

|
| 109. Buprenorphine HCl/Naloxone HCl |
|
|
| 110. Glimepiride |

|

|
| 111. Lamotrigine |

|

|
| 112. Pioglitazone HCl |

|

|
| 113. Allopurinol |

|

|
| 114. Losartan Potassium |

|

|
| 115. Metronidazole |

|

|
| 116. Rabeprazole Sodium | NL | NL |
| 117. Rifaximin |

|

|
| 118. Verapamil HCl |

|

|
| 119. Amphetamine Salt Combination |

|

|
| 120. Clindamycin HCl |

|

|
| 121. Filgrastim |

|

|
| 122. Ergocalciferol | NL | NL |
| 123. Glyburide | NL | NL |
| 124. Fenofibrate |

|

|
| 125. Mirtazapine |

|

|
| 126. Enalapril Maleate |

|

|
| 127. Hydrochlorothiazide/Olmesartan Medoxomil |

|

|
| 128. Clobetasol Propionate | NL | NL |
| 129. Olmesartan Medoxomil |

|

|
| 130. Insulin Aspart, Recombinant | NL | NL |
| 131. Benazepril HCl | NL | NL |
| 132. Ranitidine HCl |

|

|
| 133. AlbuterolA |

|

|
| 134. Insulin Lispro, Recombinant | NL | NL |
| 135. Emtricitabine/Tenofovir Disoproxil Fumarate |

|
|
| 136. Vardenafil HCl |

|

|
| 137. Topiramate |

|

|
| 138. Metoclopramide HCl |

|

|
| 139. Tizanidine HCl | NL | NL |
| 140. Rosuvastatin Calcium |

|

|
| 141. Sumatriptan Succinate | NL | NL |
| 142. Varenicline |

|

|
| 143. Hydrochlorothiazide/Losartan Potassium |

|

|
| 144. Interferon Alfacon-1 | NL | NL |
| 145. Pravastatin Sodium |

|

|
| 146. Codeine Phosphate/Guaifenesin |
|
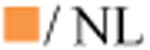
|
| 147. Diclofenac Sodium |

|

|
| 148. Chlorpheniramine Polistirex/Hydrocodone Polistirex |

|

|
| 149. Moxifloxacin HCl |

|

|
| 150. Modafinil | NL | NL |
| 151. Penicillin V Potassium |

|

|
| 152. Tiotropium Bromide | NL | NL |
| 153. Testosterone | NL | NL |
| 154. Codeine Phosphate/Promethazine HCl |

|

|
| 155. Ritonavir |

|

|
| 156. Methocarbamol | NL | NL |
| 157. Sitagliptin Phosphate |

|

|
| 158. Ondansetron HCl |

|

|
| 159. Nystatin | NL | NL |
| 160. Hydroxychloroquine Sulfate |

|

|
| 161. Acetaminophen/Butalbital/CaffeineA |
|
|
| 162. Benzonatate | NL | NL |
| 163. Methylphenidate HCl |

|

|
| 164. Albuterol Sulfate/Ipratropium BromideA |

|

|
| 165. Buspirone HCl | NL | NL |
| 166. Ezetimibe/Simvastatin |

|

|
| 167. Doxazosin Mesylate |

|

|
| 168. Conjugated Estrogens/ Medroxyprogesterone Acetate | NL / NL | NL / NL |
| 169. Quinapril HCl |

|

|
| 170. Cetirizine HCl | NL | NL |
| 171. Irbesartan |

|

|
| 172. Ropinirole HCl | NL | NL |
| 173. Insulin Human Isophane (NPH) | NL | NL |
| 174. Lithium Carbonate |

|

|
| 175. Metaxalone | NL | NL |
| 176. Clarithromycin |

|

|
| 177. Risedronate Sodium | NL | NL |
| 178. Aripiprazole |

|

|
| 179. Efavirenz/Emtricitabine/Tenofovir Disoproxil Fumarate |
|
|
| 180. Ibandronate Sodium |

|

|
| 181. Lidocaine |

|

|
| 182. Betamethasone Dipropionate/ Clotrimazole | NL / NL | NL / NL |
| 183. Omega-3-Acid Ethyl EstersA |

|

|
| 184. CyclosporineA |

|

|
| 185. Atazanavir Sulfate |

|

|
| 186. Glyburide/Metformin HCl |
|
|
| 187. Cyclosporine, ModifiedA |

|

|
| 188. Mupirocin | NL | NL |
| 189. Colchicine |

|

|
| 190. Niacin | NL | NL |
| 191. Hydrochlorothiazide/Irbesartan |

|

|
| 192. Famotidine | NL | NL |
| 193. Atropine Sulfate/Diphenoxylate HCl | NL / NL | NL / NL |
| 194. Fluocinonide | NL | NL |
| 195. Terazosin HCl | NL | NL |
| 196. Etanercept |

|

|
| 197. Nortriptyline HCl |

|

|
| 198. Azathioprine |

|

|
| 199. Chlorhexidine Gluconate | NL | NL |
| 200. PEG Electrolyte Lavage Solution | NL | NL |
Abbreviations: HCV, Hepatitis C Virus; NL, Not listed;
Names differ between MarketScan database and hep-druginteractions.org as follows, respectively: acetaminophen listed as paracetamol, propoxyphene listed as dextropropoxyphene, albuterol listed as salbutamol, cephalexin listed as cefalexin, ursodiol listed as ursodeoxycholic acid, alendronate listed as alendronic acid, Omega-3-Acid Ethyl Esters listed as fish oils, cyclosporine listed as ciclosporin
Drugs list different recommendation for different indication/usage
*Hep-druginteractions.org last accessed 9/11/2013
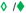 No clinically significant interaction expected
No clinically significant interaction expected
 Potential interaction – may require close monitoring, alteration of drug dosage or timing of administration
Potential interaction – may require close monitoring, alteration of drug dosage or timing of administration
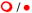 These drugs should not be coadministered
These drugs should not be coadministered
Empty symbols indicate the interaction has not been assessed and has been predicted based on the metabolic profiles of the drugs.
Appendix C. Comprehensive List of Queried Drugs with Population Exposures from 2006-2010, by Medication Class
| 2006 | 2007 | 2008 | 2009 | 2010 | Weighted Average | |
|---|---|---|---|---|---|---|
| (53,461 beneficiaries, 79,185 cross-sections) | ||||||
|
| ||||||
| Enrollees with Pharmacy Benefit, n | 11,424 | 11,517 | 19,379 | 18,882 | 17,983 | 79,185 |
|
| ||||||
| Medication, % | ||||||
|
| ||||||
| HEPATITIS C RELATED | ||||||
|
| ||||||
| Treatment | ||||||
|
| ||||||
| ribavirin | 27.6 | 24.3 | 21.1 | 20.1 | 16.9 | 21.3 |
| peginterferon alfa-2a | 16.2 | 14.2 | 13.3 | 14.0 | 12.5 | 13.8 |
| peginterferon alfa-2b | 10.5 | 9.3 | 6.9 | 5.2 | 4.0 | 6.7 |
| interferon alfacon-1 | 2.2 | 1.8 | 1.2 | 1.1 | 0.9 | 1.3 |
|
| ||||||
| Side Effect Management | ||||||
|
| ||||||
| epoetin alfa | 5.6 | 4.5 | 3.5 | 3.0 | 2.5 | 3.6 |
| filgrastim | 2.8 | 2.6 | 2.2 | 2.1 | 1.7 | 2.2 |
| darbepoetin alfa | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 |
|
| ||||||
| Transplant | ||||||
|
| ||||||
| tacrolimus | 2.5 | 2.7 | 2.9 | 3.3 | 3.5 | 3.0 |
| mycophenolate | 1.6 | 1.7 | 1.9 | 2.2 | 2.3 | 2.0 |
| cyclosporine | 1.2 | 1.1 | 1.1 | 1.2 | 1.4 | 1.2 |
| sirolimus | 0.5 | 0.4 | 0.4 | 0.5 | 0.5 | 0.5 |
| azathioprine | 0.6 | 0.5 | 0.5 | 0.6 | 0.5 | 0.5 |
|
| ||||||
| NERVOUS SYSTEM | ||||||
|
| ||||||
| Antidepressants | ||||||
|
| ||||||
| escitalopram | 7.9 | 7.4 | 6.2 | 6.0 | 5.1 | 6.4 |
| bupropion | 6.8 | 6.0 | 5.2 | 5.7 | 5.4 | 5.7 |
| citalopram | 4.2 | 4.7 | 4.8 | 6.1 | 6.4 | 5.4 |
| trazodone | 5.2 | 5.0 | 4.8 | 5.5 | 6.0 | 5.3 |
| sertraline | 5.3 | 5.2 | 4.9 | 5.1 | 4.9 | 5.1 |
| fluoxetine | 3.7 | 3.5 | 3.4 | 3.7 | 3.4 | 3.5 |
| venlafaxine | 4.5 | 4.3 | 3.4 | 3.1 | 2.4 | 3.4 |
| paroxetine | 3.7 | 3.3 | 2.9 | 2.8 | 2.4 | 3.0 |
| duloxetine | 2.6 | 3.0 | 2.8 | 2.8 | 2.5 | 2.7 |
| amitriptyline | 2.7 | 2.7 | 2.3 | 2.5 | 2.3 | 2.5 |
| mirtazapine | 1.4 | 1.5 | 1.3 | 1.4 | 1.5 | 1.4 |
| nortriptyline | 0.8 | 0.8 | 0.8 | 1.0 | 1.0 | 0.9 |
| doxepin | 0.7 | 0.7 | 0.6 | 0.8 | 0.7 | 0.7 |
| desipramine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
|
| ||||||
| Antipsychotics / Neuroleptics | ||||||
|
| ||||||
| quetiapine | 1.9 | 2.2 | 2.1 | 2.2 | 2.2 | 2.1 |
| prochlorperazine | 1.8 | 1.9 | 1.9 | 1.7 | 1.8 | 1.8 |
| aripiprazole | 0.6 | 0.5 | 0.7 | 1.1 | 1.1 | 0.8 |
| lithium carbonate | 0.6 | 0.6 | 0.5 | 0.6 | 0.7 | 0.6 |
| risperidone | 0.6 | 0.6 | 0.6 | 0.6 | 0.7 | 0.6 |
| olanzapine | 0.9 | 0.6 | 0.5 | 0.6 | 0.5 | 0.6 |
| clozapine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| pimozide | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| Anxiolytics / Sedatives / Hypnotics | ||||||
|
| ||||||
| zolpidem | 15.0 | 14.4 | 13.6 | 14.1 | 14.0 | 14.1 |
| alprazolam | 9.7 | 10.6 | 9.2 | 10.0 | 9.9 | 9.8 |
| lorazepam | 5.5 | 5.4 | 5.4 | 5.9 | 6.1 | 5.7 |
| diazepam | 4.9 | 5.0 | 5.0 | 5.4 | 5.1 | 5.1 |
| temazepam | 3.2 | 3.6 | 2.9 | 3.2 | 3.1 | 3.2 |
| eszopiclone | 3.8 | 3.5 | 2.4 | 1.9 | 1.9 | 2.5 |
| buspirone | 1.0 | 1.0 | 0.9 | 1.0 | 1.0 | 1.0 |
| triazolam | 0.4 | 0.5 | 0.4 | 0.5 | 0.4 | 0.5 |
| midazolam (iv) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| midazolam (po) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| Opioid Dependence | ||||||
|
| ||||||
| methadone | 1.4 | 1.2 | 1.2 | 1.3 | 1.4 | 1.3 |
| buprenorphine | 0.6 | 0.7 | 1.0 | 1.3 | 1.5 | 1.1 |
|
| ||||||
| Pain | ||||||
|
| ||||||
| hydrocodone | 33.7 | 35.0 | 32.0 | 35.5 | 34.9 | 34.2 |
| oxycodone | 14.6 | 14.9 | 15.1 | 16.5 | 17.7 | 15.9 |
| tramadol | 7.8 | 8.7 | 8.2 | 9.7 | 10.0 | 9.0 |
| codeine | 8.0 | 7.9 | 9.0 | 9.6 | 9.1 | 8.9 |
| gabapentin | 4.3 | 4.8 | 4.9 | 5.7 | 6.6 | 5.4 |
| morphine sulfate | 1.8 | 2.2 | 2.1 | 2.6 | 2.6 | 2.3 |
| hydromorphone | 1.5 | 1.8 | 2.1 | 2.6 | 2.5 | 2.2 |
| fentanyl | 1.8 | 1.8 | 1.8 | 1.7 | 1.6 | 1.7 |
| meperidine | 1.5 | 1.1 | 1.0 | 0.7 | 0.7 | 0.9 |
| oxymorphone | 0.0 | 0.3 | 0.4 | 0.4 | 0.4 | 0.3 |
|
| ||||||
| Muscle Relaxants | ||||||
|
| ||||||
| cyclobenzaprine | 7.8 | 7.6 | 7.5 | 8.6 | 8.5 | 8.1 |
| carisoprodol | 3.2 | 3.5 | 3.0 | 3.2 | 3.0 | 3.2 |
| methocarbamol | 1.5 | 1.8 | 1.6 | 1.7 | 2.1 | 1.8 |
| metaxalone | 2.1 | 2.2 | 1.7 | 1.3 | 1.1 | 1.6 |
| tizanidine | 1.3 | 1.4 | 1.2 | 1.2 | 1.3 | 1.3 |
|
| ||||||
| Migraine | ||||||
|
| ||||||
| sumatriptan | 1.3 | 1.2 | 1.2 | 1.2 | 1.5 | 1.3 |
| ergotamine | 0.2 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 |
| dihydroergotamine | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| methylergonovine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| Anticonvulsants | ||||||
|
| ||||||
| clonazepam | 3.9 | 4.1 | 3.9 | 4.3 | 4.4 | 4.1 |
| lamotrigine | 1.0 | 1.0 | 1.1 | 1.2 | 1.4 | 1.2 |
| topiramate | 1.2 | 1.2 | 1.0 | 1.1 | 1.2 | 1.1 |
| levetiracetam | 0.3 | 0.4 | 0.5 | 0.7 | 0.7 | 0.5 |
| phenytoin | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| carbamazepine | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 |
| phenobarbital | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 |
|
| ||||||
| Miscellaneous | ||||||
|
| ||||||
| pregabalin | 2.0 | 2.8 | 2.8 | 2.6 | 2.1 | 2.5 |
| varenicline | 0.8 | 4.1 | 2.9 | 2.5 | 1.9 | 2.4 |
| modafinil | 1.2 | 1.2 | 1.1 | 1.0 | 0.8 | 1.0 |
| amphetamine salt | 0.9 | 0.8 | 0.8 | 0.9 | 1.0 | 0.9 |
| methylphenidate | 0.8 | 0.8 | 0.6 | 0.7 | 0.9 | 0.7 |
| ropinirole | 0.7 | 0.8 | 0.5 | 0.7 | 0.8 | 0.7 |
|
| ||||||
| METABOLIC | ||||||
|
| ||||||
| Hypertension / Cardiovascular | ||||||
|
| ||||||
| hydrochlorothiazide | 14.4 | 15.6 | 15.0 | 16.0 | 16.3 | 15.5 |
| lisinopril | 8.6 | 10.9 | 11.4 | 13.1 | 14.5 | 12.0 |
| furosemide | 8.1 | 9.1 | 8.9 | 9.8 | 10.8 | 9.5 |
| spironolactone | 6.5 | 6.4 | 6.3 | 7.2 | 8.1 | 7.0 |
| valsartan | 3.4 | 3.4 | 3.8 | 3.7 | 4.0 | 3.7 |
| benazepril | 3.1 | 3.3 | 2.9 | 3.0 | 2.7 | 3.0 |
| lidocaine (IV) | 2.2 | 2.3 | 2.5 | 2.6 | 2.4 | 2.4 |
| triamterene | 2.8 | 2.6 | 2.3 | 2.4 | 2.2 | 2.4 |
| clonidine | 2.1 | 2.3 | 2.0 | 2.1 | 2.3 | 2.1 |
| olmesartan | 1.7 | 2.2 | 2.1 | 2.4 | 2.1 | 2.1 |
| losartan | 1.9 | 2.0 | 1.7 | 2.1 | 2.7 | 2.1 |
| clopidogrel | 1.7 | 2.0 | 1.9 | 2.1 | 1.9 | 1.9 |
| ramipril | 1.7 | 1.4 | 1.2 | 1.1 | 1.0 | 1.2 |
| enalapril | 1.2 | 1.0 | 1.2 | 1.1 | 1.0 | 1.1 |
| irbesartan | 1.3 | 1.0 | 1.3 | 1.1 | 0.8 | 1.1 |
| digoxin (oral) | 0.5 | 0.6 | 0.6 | 0.5 | 0.5 | 0.5 |
| amiodarone | 0.1 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 |
| flecainide | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| propafenone | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| sildenafil (Revatio) | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| bosentan | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| quinidine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| tadalafil (Adcirca) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| Beta Blockers | ||||||
|
| ||||||
| metoprolol | 7.1 | 8.0 | 7.7 | 8.0 | 8.1 | 7.8 |
| atenolol | 4.4 | 4.6 | 4.4 | 4.5 | 4.0 | 4.4 |
| propranolol | 3.1 | 3.5 | 3.3 | 3.7 | 3.8 | 3.5 |
| nadolol | 2.3 | 2.3 | 2.6 | 3.0 | 3.4 | 2.8 |
| carvedilol | 1.0 | 1.5 | 1.8 | 2.2 | 2.3 | 1.8 |
| bisoprolol | 0.5 | 0.5 | 0.4 | 0.4 | 0.4 | 0.5 |
| nebivolol | N/A | N/A | 0.2 | 0.6 | 0.8 | 0.5 |
|
| ||||||
| Calcium Channel Blockers | ||||||
|
| ||||||
| amlodipine | 7.5 | 7.7 | 8.8 | 10.3 | 10.9 | 9.3 |
| diltiazem | 1.8 | 1.5 | 1.3 | 1.4 | 1.6 | 1.5 |
| nifedipine | 1.2 | 1.3 | 1.4 | 1.4 | 1.4 | 1.4 |
| verapamil | 1.5 | 1.3 | 1.1 | 1.1 | 1.0 | 1.2 |
| felodipine | 0.5 | 0.5 | 0.4 | 0.3 | 0.3 | 0.4 |
| nisoldipine | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 0.2 |
| nicardipine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hyperlipidemia | ||||||
| nicardipine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| Hyperlipidemia | ||||||
|
| ||||||
| any statin queried | 5.9 | 7.3 | 7.4 | 9.3 | 9.1 | 8.0 |
| simvastatin | 2.0 | 3.1 | 3.6 | 4.7 | 4.7 | 3.8 |
| atorvastatin | 2.4 | 2.2 | 2.1 | 2.2 | 1.7 | 2.1 |
| rosuvastatin | 0.8 | 0.9 | 0.9 | 1.2 | 1.2 | 1.0 |
| pravastatin | 0.4 | 0.7 | 0.8 | 1.3 | 1.4 | 1.0 |
| lovastatin | 0.7 | 0.8 | 0.5 | 0.5 | 0.4 | 0.5 |
| fluvastatin | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| ezetimibe | 2.7 | 3.0 | 2.2 | 1.6 | 1.2 | 2.0 |
| fenofibrate | 1.4 | 1.2 | 1.2 | 1.3 | 1.2 | 1.3 |
| gemfibrozil | 0.5 | 0.6 | 0.6 | 0.5 | 0.6 | 0.5 |
| Niacin | 0.5 | 0.6 | 0.8 | 0.7 | 0.8 | 0.7 |
| colesevelam | 0.6 | 0.6 | 0.6 | 0.7 | 0.8 | 0.7 |
|
| ||||||
| Diabetes | ||||||
|
| ||||||
| insulin (any type) | 7.3 | 7.1 | 7.2 | 7.7 | 7.8 | 7.4 |
| metformin | 5.6 | 6.2 | 6.6 | 7.2 | 7.5 | 6.7 |
| glipizide | 2.2 | 2.2 | 2.1 | 2.2 | 2.0 | 2.1 |
| glyburide | 1.7 | 1.8 | 1.7 | 1.7 | 1.4 | 1.6 |
| pioglitazone | 1.1 | 1.8 | 1.6 | 1.6 | 1.4 | 1.5 |
| glimepiride | 1.4 | 1.3 | 1.4 | 1.4 | 1.4 | 1.4 |
| sitagliptin | 0.0 | 0.8 | 1.2 | 1.5 | 1.6 | 1.1 |
| rosiglitazone | 1.1 | 1.0 | 0.4 | 0.3 | 0.3 | 0.5 |
|
| ||||||
| INFECTIOUS DISEASE | ||||||
|
| ||||||
| Antibacterials | ||||||
|
| ||||||
| amoxicillin | 20.8 | 20.2 | 19.4 | 20.0 | 21.0 | 20.2 |
| azithromycin | 14.1 | 15.4 | 16.0 | 16.8 | 17.4 | 16.2 |
| ciprofloxacin (systemic) | 9.2 | 10.3 | 10.4 | 11.3 | 12.2 | 10.8 |
| levofloxacin | 11.2 | 11.2 | 9.9 | 8.9 | 7.9 | 9.6 |
| trimethoprim/sulfamethoxazole | 8.0 | 8.8 | 8.7 | 9.8 | 10.3 | 9.2 |
| cephalexin | 10.1 | 9.9 | 8.4 | 8.8 | 9.2 | 9.1 |
| doxycycline | 4.5 | 4.9 | 5.1 | 5.4 | 5.6 | 5.2 |
| clindamycin (systemic) | 5.0 | 5.1 | 4.9 | 5.4 | 5.3 | 5.2 |
| moxifloxacin | 3.5 | 3.8 | 3.6 | 3.2 | 2.9 | 3.4 |
| penicillin v potassium | 3.8 | 3.4 | 3.1 | 3.0 | 2.8 | 3.2 |
| clarithromycin | 3.8 | 3.3 | 3.2 | 2.8 | 2.8 | 3.1 |
| rifaximin | 1.1 | 1.5 | 1.6 | 2.0 | 3.0 | 1.9 |
| erythromycin (systemic) | 1.5 | 1.4 | 1.3 | 1.2 | 1.2 | 1.3 |
| neomycin sulfate (systemic) | 1.0 | 1.0 | 0.8 | 0.7 | 0.7 | 0.8 |
| tetracycline | 0.7 | 0.7 | 0.6 | 0.5 | 0.6 | 0.6 |
| gentamicin | 0.5 | 0.5 | 0.5 | 0.7 | 0.6 | 0.6 |
| ofloxacin | 0.5 | 0.7 | 0.5 | 0.4 | 0.4 | 0.5 |
| rifampin | 0.3 | 0.4 | 0.3 | 0.3 | 0.4 | 0.3 |
|
| ||||||
| Antifungals | ||||||
|
| ||||||
| metronidazole | 4.8 | 5.2 | 5.1 | 5.2 | 5.5 | 5.2 |
| fluconazole | 3.8 | 4.0 | 3.7 | 4.0 | 4.2 | 3.9 |
| nystatin | 2.6 | 2.8 | 2.5 | 2.7 | 3.0 | 2.7 |
| ketoconazole | 1.5 | 1.7 | 1.5 | 1.7 | 1.6 | 1.6 |
| terbinafine | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 |
| itraconazole | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| voriconazole | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 |
| posaconazole | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| Antivirals | ||||||
|
| ||||||
| valacyclovir | 3.0 | 3.1 | 3.2 | 3.2 | 3.3 | 3.2 |
| acyclovir | 2.9 | 2.8 | 2.8 | 3.0 | 3.0 | 2.9 |
|
| ||||||
| HIV | ||||||
|
| ||||||
| tenofovir | 0.7 | 1.0 | 1.5 | 1.9 | 2.2 | 1.6 |
| emtricitabine | 0.5 | 0.9 | 1.3 | 1.6 | 1.8 | 1.3 |
| ritonavir | 0.5 | 0.8 | 1.0 | 1.2 | 1.1 | 1.0 |
| lamivudine | 1.0 | 0.8 | 0.9 | 1.0 | 0.9 | 0.9 |
| efavirenz | 0.6 | 0.5 | 0.8 | 0.9 | 1.0 | 0.8 |
| atazanavir | 0.2 | 0.4 | 0.4 | 0.6 | 0.6 | 0.5 |
| abacavir | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.4 |
| zidovudine | 0.5 | 0.3 | 0.4 | 0.4 | 0.3 | 0.4 |
| lopinavir/ritonavir | 0.2 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 |
| raltegravir | N/A | 0.0 | 0.1 | 0.3 | 0.4 | 0.2 |
| darunavir | 0.0 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 |
| fosamprenavir | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| nevirapine | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 |
| nelfinavir | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| didanosine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| stavudine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| etravirine | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| saquinavir | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 |
| maraviroc | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| indinavir | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| delavirdine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| tipranavir | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| MEN & WOMEN'S HEALTH | ||||||
|
| ||||||
| Hormone therapy | ||||||
|
| ||||||
| estradiol (excluding EE) | 3.6 | 3.4 | 2.9 | 3.2 | 3.4 | 2.9 |
| conjugated estrogens | 3.1 | 2.8 | 2.3 | 2.3 | 2.0 | 2.4 |
| testosterone | 1.3 | 1.5 | 1.6 | 1.7 | 2.0 | 1.7 |
| ethinyl estradiol (EE) | 1.8 | 1.7 | 1.5 | 1.5 | 1.3 | 1.5 |
| medroxyprogresterone | 1.0 | 1.0 | 1.1 | 1.0 | 1.1 | 1.0 |
| norethindrone | 0.8 | 1.0 | 0.8 | 0.9 | 0.9 | 0.9 |
| progesterone | 0.5 | 0.6 | 0.6 | 0.7 | 0.7 | 0.6 |
| drospirenone | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 |
|
| ||||||
| Erectile dysfunction | ||||||
|
| ||||||
| sildenafil (Viagra) | 4.1 | 3.3 | 4.1 | 4.3 | 3.7 | 3.9 |
| tadalafil (Cialis) | 1.7 | 1.8 | 2.4 | 2.5 | 2.4 | 2.2 |
| vardenafil | 1.3 | 1.4 | 1.4 | 1.6 | 1.4 | 1.5 |
|
| ||||||
| Benign prostatic hyperplasia | ||||||
|
| ||||||
| tamsulosin | 1.8 | 2.3 | 2.5 | 3.0 | 3.0 | 2.6 |
| doxazosin | 0.6 | 0.6 | 0.7 | 0.7 | 0.7 | 0.7 |
| terazosin | 0.5 | 0.6 | 0.5 | 0.7 | 0.8 | 0.6 |
| alfuzosin | 0.2 | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 |
|
| ||||||
| GASTROINTESTINAL | ||||||
| Reflux | ||||||
|
| ||||||
| omeprazole | 5.2 | 7.2 | 8.3 | 10.8 | 12.4 | 9.2 |
| esomeprazole | 8.4 | 8.4 | 7.1 | 7.1 | 6.1 | 7.2 |
| pantoprazole | 6.1 | 5.3 | 5.4 | 6.2 | 5.3 | 5.6 |
| lansoprazole | 5.8 | 5.0 | 4.1 | 3.7 | 3.8 | 4.3 |
| ranitidine | 1.7 | 1.7 | 1.5 | 1.7 | 1.7 | 1.7 |
| rabeprazole | 2.1 | 1.6 | 1.6 | 1.4 | 0.9 | 1.5 |
| famotidine | 1.0 | 1.2 | 0.9 | 1.3 | 1.2 | 1.1 |
| cimetidine | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 |
|
| ||||||
| Other | ||||||
|
| ||||||
| promethazine | 10.6 | 10.8 | 9.8 | 10.3 | 9.7 | 10.1 |
| lactulose | 4.6 | 5.1 | 5.0 | 5.7 | 6.3 | 5.4 |
| ondansetron | 1.5 | 1.9 | 2.6 | 3.6 | 4.5 | 3.0 |
| metoclopramide | 3.0 | 3.4 | 3.0 | 2.9 | 2.0 | 2.8 |
| ursodiol | 2.0 | 1.8 | 1.8 | 2.1 | 2.2 | 2.0 |
| mesalamine | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 0.6 |
| domperidone | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| cisapride | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
|
| ||||||
| OTHER | ||||||
|
| ||||||
| Asthma / Pulmonary disease | ||||||
|
| ||||||
| albuterol | 8.8 | 8.8 | 8.7 | 9.9 | 10.2 | 9.4 |
| fluticasone (in any product) | 8.0 | 8.1 | 7.9 | 9.3 | 10.2 | 8.8 |
| fluticasone (not in combination) | 5.2 | 5.4 | 5.5 | 6.7 | 7.8 | 6.2 |
| mometasone | 3.8 | 3.7 | 3.5 | 3.2 | 3.2 | 3.4 |
| salmeterol | 3.5 | 3.3 | 3.0 | 3.2 | 3.0 | 3.2 |
| fluticasone proprionate/salmeterol | 3.4 | 3.2 | 2.9 | 3.2 | 2.9 | 3.1 |
| montelukast | 2.1 | 2.1 | 1.9 | 1.9 | 1.8 | 1.9 |
| ipratropium | 1.9 | 1.9 | 1.8 | 2.0 | 1.8 | 1.9 |
| tiotropium | 1.1 | 1.3 | 1.2 | 1.3 | 1.4 | 1.3 |
| budesonide | 1.1 | 1.1 | 1.1 | 1.2 | 1.4 | 1.2 |
|
| ||||||
| Arthritis / Osteoporosis | ||||||
|
| ||||||
| meloxicam | 1.7 | 2.2 | 2.8 | 3.2 | 3.8 | 2.9 |
| celecoxib | 2.5 | 2.4 | 2.1 | 2.1 | 1.7 | 2.1 |
| alendronate | 1.9 | 1.7 | 1.8 | 1.8 | 1.7 | 1.8 |
| hydroxychloroquine sulfate | 0.8 | 0.8 | 0.8 | 0.9 | 1.0 | 0.9 |
| risedronate | 1.0 | 0.8 | 0.8 | 0.7 | 0.5 | 0.8 |
| etanercept | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 | 0.4 |
|
| ||||||
| Gout | ||||||
|
| ||||||
| allopurinol | 1.3 | 1.2 | 1.3 | 1.6 | 1.6 | 1.5 |
| colchicine | 0.9 | 0.9 | 0.8 | 1.0 | 1.1 | 1.0 |
|
| ||||||
| Steroids | ||||||
|
| ||||||
| prednisone | 8.5 | 8.7 | 8.8 | 9.7 | 10.0 | 9.2 |
| methylprednisolone | 6.3 | 6.0 | 6.3 | 7.0 | 7.1 | 6.6 |
| dexamethasone (systemic) | 2.1 | 2.1 | 2.0 | 2.2 | 2.3 | 2.2 |
| prednisolone | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
|
| ||||||
| Other | ||||||
|
| ||||||
| levothyroxine | 8.5 | 8.2 | 8.0 | 9.2 | 9.3 | 8.7 |
| potassium chloride | 5.4 | 5.2 | 5.3 | 5.6 | 5.5 | 5.4 |
| benzonatate | 2.5 | 2.4 | 2.7 | 3.0 | 2.9 | 2.7 |
| diclofenac | 2.0 | 1.9 | 2.5 | 3.0 | 3.5 | 2.7 |
| warfarin | 1.6 | 1.8 | 1.7 | 1.8 | 2.1 | 1.8 |
| atropine | 1.9 | 1.8 | 1.6 | 1.8 | 1.6 | 1.7 |
| tamoxifen | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 |
| ergonovine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Muhlberger N, Schawarzer R, Lettmeier B, Sroczynsk G, Zeuzem S, Siebert U. HCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health. 2009;9:34. doi: 10.1186/1471-2458-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Assocation for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Barritt AS, Fried MW. Maximizing opportunities and avoiding mistakes in triple therapy for hepatitis C virus. Gastroenterology. 2012;142(6):1314–1323. doi: 10.1053/j.gastro.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiser JJ, Burton JR, Anderson PL, Everson GT. Review and management of drug interactions with boceprevir and telaprevir. Hepatology. 2012;55(5):1620–8. doi: 10.1002/hep.25653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss J, Becker JP, Haefeli WE. Telaprevir is a substrate and moderate inhibitor of P-glycoprotein, a strong inductor of ABCG2, but not an activator of PXR in vitro. Int J Antimicrob Agents. 2014;43(2):184–8. doi: 10.1016/j.ijantimicag.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Hulskotte EG, Feng HP, Xuan F, van Zutven MG, Treitel MA, Hughes EA, et al. Pharmacokinetic interactions between the hepatitis C virus protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, darunavir, and lopinavir. Clin Infect Dis. 2013;56(5):718–26. doi: 10.1093/cid/cis968. [DOI] [PubMed] [Google Scholar]
- 8.Garg V, Chandorkar G, Yang Y, Adda N, McNair L, Alves K, et al. The effect of CYP3A inhibitors and inducers on the pharmacokinetics of telaprevir in healthy volunteers. Br J Clin Pharmacol. 2013;75(2):431–9. doi: 10.1111/j.1365-2125.2012.04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond KP, Wolfe P, Burton JR, Jr, Predhomme JA, Ellis CM, Ray ML, et al. Pharmacokinetic interaction between boceprevir and etravirine in HIV/HCV seronegative volunteers. J Acquir Immune Defic Syndr. 2013;62(1):67–73. doi: 10.1097/QAI.0b013e318275da93. [DOI] [PubMed] [Google Scholar]
- 10.Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705–16. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 11.Bifano M, Hwang C, Oosterhuis B, Hartstra J, Grasela D, Tiessen R, et al. Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents: ritonavir-boosted atazanavir, efavirenz and tenofovir. Antivir Ther. 2013;18(7):931–40. doi: 10.3851/IMP2674. [DOI] [PubMed] [Google Scholar]
- 12.Burger D, Back D, Buggisch P, Buti M, Craxi A, Foster G, et al. Clinical management of drug-drug interactions in HCV therapy: challenges and solutions. J Hepatol. 2013;58(4):792–800. doi: 10.1016/j.jhep.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 13.van Heeswijk RP, Beumont M, Kauffman RS, Garg V. Review of drug interactions with telaprevir and antiretrovirals. Antivir Ther. 2013;18(4):553–60. doi: 10.3851/IMP2527. [DOI] [PubMed] [Google Scholar]
- 14.Wilby KJ, Greanya ED, Ford JA, Yoshida EA, Partovi N. A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplant patients. Ann Hepatol. 2012;11(2):179–85. [PubMed] [Google Scholar]
- 15.Back D, Else L. The importance of drug-drug interactions in the DAA era. Dig Liver Dis. 2013;45(Suppl 5):S343–8. doi: 10.1016/j.dld.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Maasoumy B, Port K, Calle Serrano B, Markova AA, Sollik L, Manns MP, et al. The clinical significance of drug-drug interactions in the era of direct-acting anti-viral agents against chronic hepatitis C. Aliment Pharmacol Ther. 2013;38:1365–72. doi: 10.1111/apt.12523. [DOI] [PubMed] [Google Scholar]
- 17.Louie KS, St Laurent S, Forssen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect Dis. 2012;12:86. doi: 10.1186/1471-2334-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Zayadi AR. Hepatitis C comorbidities affecting the course and response to therapy. World J Gastroenterol. 2009;15(40):4993–9. doi: 10.3748/wjg.15.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 20.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 21.University of Liverpool HIV & Hepatitis Pharmacology Group Drug Interaction Charts. [Online, 09 April 2014] Available from: http://www.hep-druginteractions.org/Interactions.aspx.
- 22.Merck & Co., Inc. Highlights of prescribing information: Victrelis. Whitehouse Station, NJ: 2011. [Online, 09 April 2014] Available from: http://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf. [Google Scholar]
- 23.Vertex Pharmaceuticals. Highlights of prescribing information: Incivek. Cambridge, MA: 2011. [Online,09 April 2014]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201917lbl.pdf. [Google Scholar]
- 24.Poordad F, McCone J, Jr, Macon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacon BR, Gorden SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poordad F, Lawitz E, Gordon S, Bouliere M, Vierling JM, Poynard T, et al. American Society of Liver Diseases. San Francisco: 2011. Concomitant Medication use (drug interactions) in Patients with Hepatitis C Genotype 1 Treated with Boceprevir Combination Therapy. [Google Scholar]
- 27.Basseri B, Yamini D, Chee G, Enayati PD, Tran T, Poordad F. Comorbidities associated with the increasing burden of hepatitis C infection. Liver Int. 2010;30(7):1012–8. doi: 10.1111/j.1478-3231.2010.02235.x. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78(6):393–403. doi: 10.3949/ccjm.78a.10073. [DOI] [PubMed] [Google Scholar]
- 29.Anglicheau D, Flamant M, Schlageter MH, Martinez F, Cassinat B, Beaune P, et al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18(11):2409–14. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- 30.Konishi H, Sumi M, Shibata N, Takada K, Minouchi T, Yamaji A. Influence of intravenous methylprednisolone pulse treatment on the disposition of ciclosporin and hepatic CYP3A activity in rats. J Pharm Pharmacol. 2004;56(4):477–83. doi: 10.1211/0022357023114. [DOI] [PubMed] [Google Scholar]
- 31.Leutscher PD, Lagging M, Buhi MR, Pederson C, Norkrans G, Langeland N, et al. Evaluation of depression as a risk factor for treatment failure in chronic hepatitis C. Hepatology. 2010;52(2):430–5. doi: 10.1002/hep.23699. [DOI] [PubMed] [Google Scholar]
- 32.Sockalingam S, Tseng A, Giguere P, Wong D. Psychiatric treatment considerations with direct acting antivirals in hepatitis C. BMC Gastroenterol. 2013;13:86. doi: 10.1186/1471-230X-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613–24. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruce RD, Moody DE, Altice FL, Gourevitch MN, Friedland GH. A review of pharmacological interactions between HIV or hepatitis C virus medications and opioid agonist therapy: implications and management for clinical practice. Expert Rev Clin Pharmacol. 2013;6:249–69. doi: 10.1586/ecp.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guggenheimer J, Moore PA. The therapeutic applications of and risks associated with acetaminophen use: a review and update. J Am Dent Assoc. 2011;142:38–44. doi: 10.14219/jada.archive.2011.0026. [DOI] [PubMed] [Google Scholar]
- 36.Kunze A, Huwyler J, Camenisch G, Gutmann H. Interaction of the antiviral drug telaprevir with renal and hepatic drug transporters. Biochem Pharmacol. 2012;84:1096–102. doi: 10.1016/j.bcp.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 37.Chu X, Cai X, Cui D, Tang C, Ghosal A, Chan G, et al. In vitro assessment of drug-drug interaction potential of boceprevir associated with drug metabolizing enzymes and transporters. Drug Metab Dispos. 2013;41:668–81. doi: 10.1124/dmd.112.049668. [DOI] [PubMed] [Google Scholar]
- 38.Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH. Interaction between polymorphisms in the OCT1 and MATE1 transporter and metformin response. Pharmacogenet Genomics. 2010;20:38–44. doi: 10.1097/FPC.0b013e328333bb11. [DOI] [PubMed] [Google Scholar]
- 39.Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- 40.Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34:1247–54. doi: 10.1124/dmd.105.008938. [DOI] [PubMed] [Google Scholar]
- 41.Qadir M, O'Loughlin KL, Fricke SM, Williamson NA, Greco WR, Minderman H, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–6. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 42.Allred AJ, Bowen CJ, Park JW, Peng B, Williams DD, Wire MB, et al. Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers. Br J Clin Pharmacol. 2011;72:321–9. doi: 10.1111/j.1365-2125.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res. 2003;63:5538–43. [PubMed] [Google Scholar]
- 44.Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34:738–42. doi: 10.1124/dmd.105.007534. [DOI] [PubMed] [Google Scholar]
- 45.Polli JW, Humphreys JE, Harmon KA, Castellino S, O'Mara MJ, Olson KL, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 46.Yeo KR, Jamei M, Rostami-Hodjegan A. Predicting drug-drug interactions: application of physiologically based pharmacokinetic models under a systems biology approach. Expert Rev Clin Pharmacol. 2013;6:143–57. doi: 10.1586/ecp.13.4. [DOI] [PubMed] [Google Scholar]
- 47.Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5:211–23. doi: 10.1517/17425250802691074. [DOI] [PubMed] [Google Scholar]
- 48.Caccia S, Garattini S, Pasina L, Nobili A. Predicting the clinical relevance of drug interactions from pre-approval studies. Drug Saf. 2009;32:1017–39. doi: 10.2165/11316630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Perkins NA, Murphy JE, Malone DC, Armstrong EP. Performance of drug-drug interaction software for personal digital assistants. Ann Pharmacother. 2006;40:850–5. doi: 10.1345/aph.1G603. [DOI] [PubMed] [Google Scholar]
- 50.Stepanova M, Kanwal F, El-Serag HB, Younossi ZM. Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology. 2011;53:737–45. doi: 10.1002/hep.24131. [DOI] [PubMed] [Google Scholar]
- 51.Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: The randomized PILLAR study. Hepatology. 2013;58:1918–29. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Torres M, Lawitz E, Kowdley KV, et al. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naive patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58:663–8. doi: 10.1016/j.jhep.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Li Y, Zhou J, Ramsden D, Taub ME, O'Brien D, Xu J, Busacca CA, et al. Enzyme-transporter interplay in the formation and clearance of abundant metabolites of faldaprevir found in excreta but not in circulation. Drug Metab Dispos. 2014;42(3):384–93. doi: 10.1124/dmd.113.055863. [DOI] [PubMed] [Google Scholar]
- 54.Bertz R. 14thInternational Workshop on Clincial Pharmacology of HIV Therapy. 2013 Session 5. [Google Scholar]
- 55.Sabo J, Kort J, Haschke M, Ballow C, Girlich B, Feifel U, et al. Pharmacokinetic interactions of darunavir/ritonavir, efavirenz, and tenofovir with the hepatitis C virus protease inhibitor faldaprevir in healthy volunteers. 20th CROI. 2013 Oral abstract 35. [Google Scholar]
- 56.Kiser JJ, Flexner C. Direct-acting antiviral agents for hepatitis C virus infection. Annu Rev Pharmacol Toxicol. 2013;53:427–49. doi: 10.1146/annurev-pharmtox-011112-140254. [DOI] [PMC free article] [PubMed] [Google Scholar]


