Abstract
Objective
To evaluate the impact of implementing an enteral nutrition (EN) algorithm on achieving optimal EN delivery in the Pediatric Intensive Care Unit (PICU).
Design
Prospective pre/post implementation audit of EN practices.
Setting
One 29-bed medical/surgical PICU in a free standing, university affiliated children’s hospital.
Patients
Consecutive patients admitted to the PICU over two 4-week periods pre and post implementation, with a stay of > 24 hours who received EN.
Interventions
Based on the results of our previous study, we developed and systematically implemented a stepwise, evidence and consensus-based algorithm for initiating, advancing and maintaining EN in critically ill children. Three months after implementation, we prospectively recorded clinical characteristics, nutrient delivery, EN interruptions, parenteral nutrition (PN) use, and ability to reach energy goal in eligible children over a 4-week period. Clinical and nutritional variables were compared between the pre and post-intervention cohorts. Time to achieving energy goal was analyzed using Kaplan Meier statistical analysis.
Measurements and Main Results
Eighty patients were eligible for this study and were compared to a cohort of 80 patients in the pre-implementation audit. There were no significant differences in median age, gender, need for mechanical ventilation, time to initiating EN, or use of post-pyloric feeding between the 2 cohorts. We recorded a significant decrease in the number of avoidable episodes of EN interruption (3 vs. 51, p0.0001) and the incidence and duration of PN dependence in patients with avoidable EN interruptions in the post-intervention cohort. Median time to reach energy goal decreased from 4 days to 1 (p<0.0001), with a higher proportion of patients reaching this goal (99% vs. 61%, p = 0.01).
Conclusions
The implementation of an EN algorithm significantly improved EN delivery and decreased reliance on PN in critically ill children. Energy intake goal was reached earlier in a higher proportion of patients.
Keywords: Enteral nutrition, guideline, algorithm, critical illness, children, nutrient delivery
INTRODUCTION
Enteral nutrition (EN) is the preferred mode of nutrient intake in critically ill children with a functioning gut1. In a recent international study of nutrient intake in mechanically ventilated patients, we reported a significantly lower odds of 60-day mortality in patients with greater adequacy of enteral nutrient delivery2. However, despite the preference for EN, there are a number of avoidable barriers in the PICU that impede optimal nutrient delivery at the bedside 3. Delay in initiating and advancing EN can result in failure to reach energy and protein delivery goals 4. In a previous study of nutrient delivery in our PICU, enteral feeds were started early after admission in a majority of patients, but subsequently interrupted for a variety of reasons5. Many of the interruptions were related to the absence of a clear and uniform definition of feeding intolerance, prolonged nutrient deprivation surrounding routine procedures, and mechanical problems with feeding tubes.
There is currently no widely accepted or uniform approach to feeding the critically ill child. The lack of a strong evidence base for bedside nutrition practices in the PICU often results in heterogeneity of nutrition delivery. Algorithms may help optimize nutrient delivery and allow identification of best practices that are associated with desirable outcomes. A small number of institutions have reported significant improvement in nutrient delivery in their PICU after implementing nutrition guidelines6–8. Stepwise advancement of EN has been shown to significantly decrease the time required to achieve goals in this population8. Encouraged by the experience of these centers, we developed a comprehensive goal-directed EN delivery guideline based on local practice deficiencies identified in our pre-implementation audit, a systematic literature search and multidisciplinary consensus. The objective of our current study was to evaluate the impact of implementing this algorithm on achieving optimal EN delivery in the PICU. We hypothesized that implementation of a uniform nutrition guideline would decrease avoidable EN interruptions, increase likelihood of reaching energy delivery goals early via the enteral route and decrease unnecessary reliance on PN in the PICU.
METHODS
Algorithm/guideline development
A stepwise, evidence-based algorithm for initiating, advancing and maintaining EN in critically ill children was developed using a multidisciplinary consensus approach. A full description of the process is beyond the scope of this manuscript and will be described separately. In brief, representatives from critical care nursing, critical care medicine, respiratory therapy, nutrition, gastroenterology, surgery and pharmacy, were among the key stakeholders that participated in the algorithm development process. Working groups were assigned eight major areas of inquiry; i) nutritional assessment and screening, ii) indications and contraindications to EN, iii) advancement strategies for EN, iv) risk factors and strategies to prevent aspiration of gastric contents, v) definition of EN intolerance and management strategies, vi) role of EN adjuncts such as antacids and pro-motility agents, vii) bowel management strategies to prevent and treat constipation, and viii) recommendations for fasting time around procedures.. The designated groups examined the literature using a systematic approach for searching, grading and reporting the evidence on each area, and made recommendations based on the available evidence. These recommendations were translated into stepwise decision points in the EN algorithm.
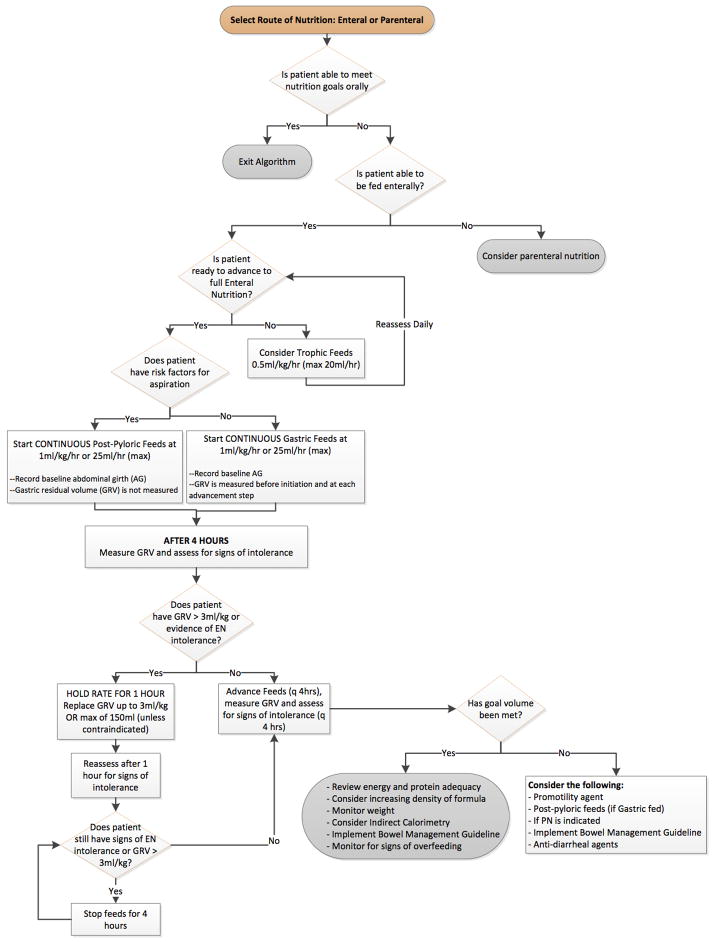
The final guideline was drafted in the form of a stepwise algorithm, and included, a) nutritional assessment and establishing nutrient intake goals, b) selection of the mode of nutrition (EN vs. PN) and selecting route of EN (gastric vs. post pyloric), c) initiation of EN, and d) maintenance of EN. Figure 1 shows a condensed/simplified version of our full EN algorithm. (The criteria for ‘feeding intolerance’ and ‘risk of aspiration’ are available as supplementary files.) The institutional nutritional advisory committee approved the guideline. An initial pre-test administered to physicians and nurses allowed identification of areas of knowledge deficits and need for education. A computerized learning module, one-on-one education and weekly nutrition rounds performed by members of the guideline development committee were included in the educational phase prior to dissemination of the guidelines via paper and electronic format to all personnel. We also incorporated reminders to review the nutrition algorithm and EN goals during daily bedside rounds.
Figure 1.
Stepwise algorithm for initiating and advancing enteral nutrition in the pediatric intensive care unit.
Audit of nutritional outcomes following implementation of EN algorithm
The impact of this intervention was examined by an audit of bedside nutrition practices over a 4-week period that mimicked the pre-intervention audit 5 in patients who received EN with a length of stay > 24 hours in the Medical/Surgical PICU at Boston Children’s Hospital. After obtaining approval from the institutional review board, we prospectively recorded nutrient delivery, actual amount and route of nutrient intake, interruptions to EN, PN usage and adequacy of energy delivery in this cohort. Clinical characteristics, details of enteral nutrient delivery, EN interruptions, PN use, and the ability and time to reach energy goal via the enteral route, were compared between the pre- and post-implementation cohorts. A dedicated nutrition team defined individual daily energy and volume goals for each patient. Standard energy equations predicted (either the Schofield or WHO) were used to determine basal energy requirement. Clinical status was taken into account to determine the need for any additional “stress-factors” as appropriate. For subjects over the age of 18 years, the WHO equation was used when an accurate height or length was not available. The Schofield equation was preferred when both weight and height were reliably obtained. Nutrient delivery goals were reassessed regularly by the nutrition team during the course of illness and adjusted as necessary.
Data recording and analysis
Primary outcomes for the study included; a) total and avoidable interruptions to EN (number and duration of each episode), b) time to initiate EN after PICU admission, c) time to reach prescribed energy goal, and d) PN use in patients with EN interruption. All episodes of interruption to EN delivery were examined, and avoidable episodes were identified a priori by consensus among the multidisciplinary group of investigators. Nurses completed the nutrition audit twice daily at the end of each 12-hour shift. These documents were examined daily by nursing investigators to allow for capture of any missing data. The accuracy of each nutrition audit was crosschecked with the existing electronic medical record (EMR). Clinical data such as duration of mechanical ventilation support, and length of PICU stay were abstracted retrospectively from patient charts following completion of enrollment.
Patient characteristics were described using frequency tables for categorical variables and using measures of central tendency with spread for non-categorical variables. Variables that were reasonably normally distributed were described using mean and standard deviation (SD), whereas those displaying a high degree of skew were characterized by their median and interquartile range (IQR). Comparisons in patient characteristics were made between the cohorts before and after implementation of the nutrition algorithm. Tests of significance for 2-group comparisons included Fisher exact test for categorical variables, and Student t-test and the Mann-Whitney rank sum test for normal and skewed distributions, respectively. Kaplan Meier curves were generated for the 2 cohorts to compare the proportion of patients achieving energy delivery goal during the PICU course, censored to 12 days. The logrank sum test and hazards ratio were used to test the significance of difference between these cohorts.
RESULTS
A total of 150 patients were admitted to the PICU during this audit. Eighty consecutive patients, who received EN and had a PICU length of stay of more than 24 hours, were eligible for the study. These patients were compared to a cohort of 80 patients (from 118 consecutive admissions) enrolled in the pre-implementation phase of the study. Details of the clinical and nutritional characteristics of the pre-implementation cohort have been previously described 5. Table 1 and 2 describe the baseline characteristics and nutrition variables of eligible patients in the pre- and post-intervention cohorts. The post-implementation cohort had a lower number of children less than 1 year of age and a higher proportion of surgical patients, particularly those with esophageal atresia and otolaryngology procedures. These differences were not statistically significant, however. There was a significantly higher number of children with respiratory illnesses (p < 0.005) in the pre-intervention cohort. There were no significant differences in median age, gender, need for mechanical ventilation, and length of PICU stay between the 2 cohorts.
TABLE 1.
DEMOGRAPHICS OF PATIENTS RECEIVING ENTERAL NUTRITION AND WITH LENGTH OF STAY > 24 HOURS: PRE- AND POST-INTERVENTION COHORTS
| Pre-intervention cohort (N=80) | Post-intervention cohort (N=80) | P value | |
|---|---|---|---|
|
| |||
| Age at PICU admission (years); Median (IQR) | 6.5 (1.5, 15) | 7.4 (2.2, 12.9) | P = 0.7 |
|
| |||
| Age <1 year, N (%) | 14 (17) | 11 (14) | P = 0.5 |
|
| |||
| Female gender, N (%) | 41 (51) | 41 (51) | P = 1.0 |
|
| |||
| Patients admitted to the surgical service, N (%) | 47 (50) | 50 (62) | P = 0.74 |
|
| |||
| PICU length of stay, median (IQR) days | 3.0 (1.0, 9.0) | 3.0 (1.7, 8.0) | P = 0.93 |
|
| |||
| Mechanically ventilated, N (%) | 36 (45) | 37 (46) | P = 0.9 |
|
| |||
| Admitted to Medical Service N (%) | 33 (41) | 30 (38) | |
| Diagnostic categories | |||
| Respiratory Illness | 15 | 9 | |
| Neurologic/Seizures | 8 | 8 | |
| Shock | 2 | 4 | |
| Oncology/Stem Cell Transplant | 3 | 5 | |
| Other | 5 | 4 | |
| Admitted to Surgical Service N (%) | 47 (59) | 50 (62) | |
| Diagnostic categories | |||
| Neurosurgery | 17 | 11 | |
| General Surgery | 14 | 11 | |
| Long Gap Esophag Atresia | 0 | 3 | |
| Trauma | 2 | 3 | |
| Plastic Surgery | 7 | 6 | |
| Orthopedic Surgery | 4 | 4 | |
| Otolaryngology Surgery | 3 | 10 | |
| Other | 0 | 2 | |
PICU = pediatric intensive care unit, IQR = interquartile range
TABLE 2.
ENTERAL NUTRITION (EN) DELIVERY IN CRITICALLY ILL CHILDREN: COMPARISON OF PRE- AND POST-INTERVENTION COHORTS
| Variable: | 2008 N = 80 |
2011 N = 80 |
P value |
|---|---|---|---|
| Days from PICU admission to starting EN Median (IQR) | 1.0 (0.0, 3.5) | 1.0 (2.0, 3.5) | P = 0.08 |
| Energy goal reached during PICU course*, n (%) | 49 (61) | 79 (99) | P= 0.01* |
| Median (IQR) time to reach energy intake goal, in days | 4 (1, 8) | 1 (1, 3) | P<0.01* |
| Post-pyloric feeding (n, %) | 16 (20) | 15 (19) | P = 0.9 |
| Number of total EN interruptions (n) | 88 | 83 | - |
| Patients with EN interruption (n, %) | 24 (30) | 28 (34) | P = 0.6 |
| Interruptions to EN (Average/patient), mean (SD) median (IQR) | 3.7 (+/− 3.1) | 2.8 (+/− 2.3) | P = 0.28 |
| Avoidable interruptions to EN (n, % of all EN interruptions) | 51 (58) | 3 (3.7) | P < 0.0001* |
| Patients on EN + PN during PICU course, n (%) | 13 (16) | 10 (12.5) | P = 0.65 |
| PN days, median (IQR) | 6 (3.0, 10.0) | 7.5 (3.7, 14.8) | P = 0.55 |
EN = enteral nutrition, PN = parenteral nutrition, IQR = interquertile range
There were no significant differences in time to initiating EN (median of 1 day) or the use of post-pyloric feeding route (19% vs. 20%) between the groups. Total duration of EN interruption during the study period decreased from 1483 hours to 796 hours after the intervention. We recorded a significant decrease in the number of avoidable episodes of EN interruption (3 vs. 51, p<0.0001) and the incidence and duration of PN dependence in patients with avoidable EN interruptions in the post-intervention cohort. Time to reach energy goal was significantly decreased (1 vs. 4 days, p<0.05), with a higher incidence of patients reaching this goal (99% vs. 61%, p = 0.01). Figure 2 shows the Kaplan Meier curves illustrating the proportion of patients reaching energy goal over time in the 2 cohorts. The cumulative proportion of patients reaching energy goal by sequential day on the PICU after admission was significantly different between the 2 cohorts, p<0.0001; hazard ratio (CI) of 0.29 (0.16 to 0.52). The post-intervention cohort not only had a higher proportion of patients that achieved energy goal enterally, but this goal was achieved earlier after admission.
Figure 2.
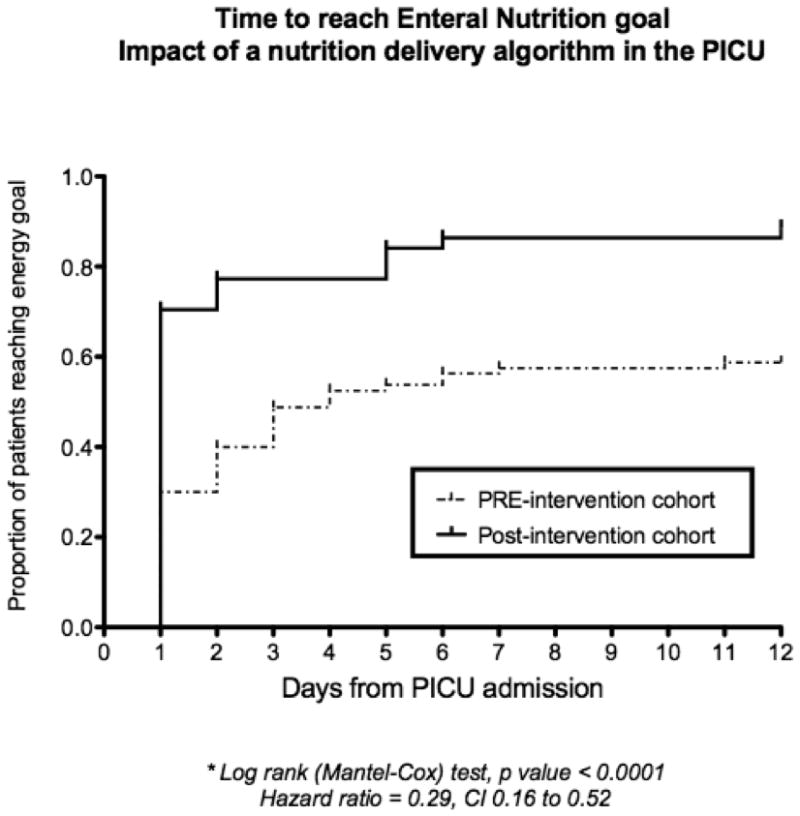
Kaplan Meier plot showing the proportion of patients in the PICU achieving energy goal in relation to days since admission.
In a subgroup analysis, there were no significant differences in the likelihood of reaching goal (p = ) or of number of patients with EN interruptions (p = ) between patients under the age of 1 year and older. In mechanically ventilated children, energy goal was reached in 86% compared to 100% of those not mechanically ventilated (p=0.6). The number of patients with EN interruptions was higher (57% vs. 16%) in the mechanically ventilated group (p = 0.02).
Using standard per patient hospital charges for PN, costs increased from a median (IQR) of 1151 (822.5, 1974) to 1590 (689, 2640) in the post-implementation cohort. However, majority of PN use was in patients with esophageal atresia (N=3, 54 PN days) in the post intervention period. The PN use (days) and charges (U.S. dollars) in patients with avoidable EN interruptions were reduced from a median of 8 days and $1316 to 1 day and $164 after algorithm implementation.
DISCUSSION
Despite early initiation of EN in the PICU, a variety of barriers impede maintenance of feeding during the course of critical illness 3,5,9. Energy and protein deficits accrued due to suboptimal nutrient delivery are difficult to overcome and persist throughout the illness course4,10. Failure to meet energy and protein goals has been associated with poor clinical outcomes from critical illness in adult and pediatric populations11–13. Early EN delivery may have important associations with desirable outcomes in critically ill children13. In our current study, we report a significant improvement in the likelihood and time to achieve nutrition goals following implementation of a stepwise EN guideline. The duration of EN deprivation and PN usage as a consequence of avoidable EN interruptions was dramatically reduced after this intervention. The time to reach energy intake goal decreased from 4 days to 1.0 days and the proportion of patients that reached goal EN during their PICU course increased from 61% to 99%. The PN use in the post-implementation cohort was increased overall, reflecting the higher proportion of surgical and in particular esophageal atresia patients, who were dependent on long-term PN. However, PN use secondary to avoidable EN interruptions was significantly lower with cost savings from lower PN charges. Mechanically ventilated children remain at a higher risk of EN interruptions. Thus, our current study has demonstrated improvement in EN delivery and decrease in the incidence of avoidable PN reliance in the PICU population following implementation of an EN algorithm. The impact of this intervention on clinical outcomes such as length of stay, acquired infections and nutritional status on discharge needs to be shown in a larger multicenter study.
The benefits of a protocolized or guideline driven nutrition delivery strategy have been described in previous studies 14. Adult centers that utilize an enteral feeding protocol have been shown to start feeds earlier and have a higher percentage of patients reach goal feeds 15. In their single center effort to optimize nutrient delivery, Petrillo-Albarano et al emphasized the importance of a comprehensive protocol that addresses common barriers to reaching and maintaining goal EN 7. By including recommendations for the management of intolerance and constipation, they were able to decrease the time to goal feeds from a median of 32 hours to 14 hours. Similarly, Meyer et al implemented a detailed nutrition protocol that improved EN delivery in their PICU to 70% of goal estimated energy requirement (EER) from 15% prior to protocol introduction 6. Briassoulis et al demonstrated the effectiveness of a protocolized approach to achieving gastric feedings in critically ill children 8. Early initiation of full strength gastric feeds and stepwise increase over the first 5 days after admission, helped early achievement of nutrient delivery targets in their study. Our current study reinforces the role of uniform guidelines in improving bedside nutrient delivery during critical illness. In particular, we have shown significant increase in the proportion of patients reaching goal (Figure 2) and the time to reach goal after admission was decreased. Hence, protocolized advancement of EN in eligible patients may allow early EN goals to be achieved. The successful application of such a uniform guideline requires that the intervention is developed by consensus with key stakeholders and that local barriers and knowledge gaps are addressed.
Previous studies have looked at common barriers to maintaining goal EN in an ICU. Rogers et al reported that fluid restriction was a major factor limiting nutrient intake in pediatric cardiac patients 3. Fasting prior to procedures, failure to reinstitute timely nutrient intake after procedures, and mechanical issues with feeding tubes have all been identified as other major contributing factors to EN interruptions 3,5,6,16. These interruptions to EN may result in a nutrient deprivation for a high proportion of the illness course. EN intolerance remains the most commonly reported barrier to optimizing EN intake in the PICU 3,5,6,17. However, a unifying definition of EN intolerance does not exist. As a result, a variety of clinical signs and symptoms have been used to determine EN intolerance in critically ill children 17. Gastric residual volume (GRV) is routinely measured in the intensive care environment despite lack of evidence to support it as a useful marker of EN intolerance 18,19. There is no consensus on the threshold of GRV that defines EN intolerance, and the practice of isolated GRV measurements to guide EN advancement is questionable 20,21. A majority of the episodes of EN interruption at our center were due to heterogeneous practice around fasting times for procedures, lack of a uniform definition and inconsistency in managing EN intolerance, varied EN advancement strategies, and failure to prioritize nutritional support during daily rounds 5. Hence, our algorithm focused on a uniform definition and approach to EN intolerance and made clear recommendations for the rate of advancing EN in the PICU. The algorithm also included guidelines for selecting the gastric versus post-pyloric route, and the use of adjuncts including a detailed stepwise protocol for preventing and managing constipation.
Our study has a number of limitations. We have reported a single center’s experience with EN delivery. While our patient population may be comparable to other like sized PICU’s there are likely unique features that may make some of our observations not generalizable. At the time of the post-intervention audit the unit census included long gap esophageal atresia patients undergoing esophageal growth induction, who were dependent on PN. This could have decreased some of the impact of the intervention in our study by increasing the percentage of PN use and lowering percentage of EN days. In addition, we observed some differences in patient case type including fewer patients with respiratory illness and more otolaryngology surgery cases during the post-intervention period. However, the otolaryngology cases included complex laryngotracheal reconstructions that require longer PICU stay and may be challenging to achieve EN goals due to airway concerns. Such seasonal and longitudinal changes in the case mix of a PICU are not avoidable, and may have influenced the impact of our intervention. Our multidisciplinary PICU does not include infants with congenital heart disease following repair. This patient population presents unique challenges to nutrient delivery. A positive impact of a uniform algorithm on nutrient delivery in this group was demonstrated by Braudis et al from the cardiac intensive care unit at our institution 22. In our study only 54% of the total cohort received at least some EN. The remaining 46% were either in the PICU for < 24 hours or had a documented absolute or relative contraindication to EN. We do not routinely measure resting energy expenditure in all patients nor are all patients weighed on admission so energy and volume goals were estimated in a majority of the patients in our cohort. The post implementation audit of EN nutrition practices took place 3 months after the new EN algorithm was introduced. Clinicians had received recent education about the algorithm and there was an increased awareness of EN on rounds. Repeat audits of EN practices will need to be performed to measure long-term adherence to the guidelines and its continued impact on nutrition delivery in the future. A multicenter trial may be indicated to examine the true impact of utilizing a protocolized approach to EN delivery on clinical outcomes in the PICU population. Finally the evidence for best nutrition practices in critically ill children remains scant, and guides to feeding this population will need to evolve as future studies continue to illuminate this area and instruct ways to achieve optmail nutrition delivery in this vulnerable cohort.
CONCLUSIONS
The implementation of a step-wise EN algorithm significantly improved EN delivery and decreased the reliance on PN in critically ill children. The guideline, developed by a multidisciplinary group of experts and key stakeholders was based on available evidence, institutional barriers and knowledge gaps. Energy intake goal was reached earlier and in a higher proportion of patients. The implementation of the algorithm also minimized avoidable interruptions to EN. The impact of optimizing EN delivery on clinical outcomes in the PICU population needs to be evaluated.
Supplementary Material
Acknowledgments
FUNDING: Partially supported by a grant from the Program for Patient Safety and Quality 2009 funding cycle at Boston Children’s Hospital.
References
- 1.Mehta NM, Compher C. AspeN Clinical guidelines: nutrition support of the critically ill child. Jpen. 2009;33:260–76. doi: 10.1177/0148607109333114. [DOI] [PubMed] [Google Scholar]
- 2.Mehta NM, Bechard LJ, Cahill N, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study*. Critical care medicine. 2012;40:2204–11. doi: 10.1097/CCM.0b013e31824e18a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers EJ, Gilbertson HR, Heine RG, Henning R. Barriers to adequate nutrition in critically ill children. Nutrition. 2003;19:865–8. doi: 10.1016/s0899-9007(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 4.Hulst JM, Joosten KF, Tibboel D, van Goudoever JB. Causes and consequences of inadequate substrate supply to pediatric ICU patients. Curr Opin Clin Nutr Metab Care. 2006;9:297–303. doi: 10.1097/01.mco.0000222115.91783.71. [DOI] [PubMed] [Google Scholar]
- 5.Mehta NM, McAleer D, Hamilton S, et al. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. Jpen. 2010;34:38–45. doi: 10.1177/0148607109348065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer R, Harrison S, Sargent S, Ramnarayan P, Habibi P, Labadarios D. The impact of enteral feeding protocols on nutritional support in critically ill children. J Hum Nutr Diet. 2009;22:428–36. doi: 10.1111/j.1365-277X.2009.00994.x. [DOI] [PubMed] [Google Scholar]
- 7.Petrillo-Albarano T, Pettignano R, Asfaw M, Easley K. Use of a feeding protocol to improve nutritional support through early, aggressive, enteral nutrition in the pediatric intensive care unit. Pediatr Crit Care Med. 2006;7:340–4. doi: 10.1097/01.PCC.0000225371.10446.8F. [DOI] [PubMed] [Google Scholar]
- 8.Briassoulis GC, Zavras NJ, Hatzis MT. Effectiveness and safety of a protocol for promotion of early intragastric feeding in critically ill children. Pediatr Crit Care Med. 2001;2:113–21. doi: 10.1097/00130478-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 9.de Neef M, Geukers VG, Dral A, Lindeboom R, Sauerwein HP, Bos AP. Nutritional goals, prescription and delivery in a pediatric intensive care unit. Clinical nutrition (Edinburgh, Scotland) 2008;27:65–71. doi: 10.1016/j.clnu.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Hulst J, Joosten K, Zimmermann L, et al. Malnutrition in critically ill children: from admission to 6 months after discharge. Clinical nutrition (Edinburgh, Scotland) 2004;23:223–32. doi: 10.1016/S0261-5614(03)00130-4. [DOI] [PubMed] [Google Scholar]
- 11.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. Jpen. 2003;27:355–73. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 12.Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan C. Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med. 2011 doi: 10.1097/PCC.0b013e3181fe279c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikhailov TA, Kuhn EM, Manzi J, et al. Early Enteral Nutrition Is Associated With Lower Mortality in Critically Ill Children. JPEN Journal of parenteral and enteral nutrition. 2014 doi: 10.1177/0148607113517903. [DOI] [PubMed] [Google Scholar]
- 14.Kattelmann KK, Hise M, Russell M, Charney P, Stokes M, Compher C. Preliminary evidence for a medical nutrition therapy protocol: enteral feedings for critically ill patients. J Am Diet Assoc. 2006;106:1226–41. doi: 10.1016/j.jada.2006.05.320. [DOI] [PubMed] [Google Scholar]
- 15.Heyland DK, Cahill NE, Dhaliwal R, Sun X, Day AG, McClave SA. Impact of enteral feeding protocols on enteral nutrition delivery: results of a multicenter observational study. JPEN Journal of parenteral and enteral nutrition. 2010;34:675–84. doi: 10.1177/0148607110364843. [DOI] [PubMed] [Google Scholar]
- 16.Keehn A, O’Brien C, Mazurak V, et al. Epidemiology of Interruptions to Nutrition Support in Critically Ill Children in the Pediatric Intensive Care Unit. JPEN Journal of parenteral and enteral nutrition. 2013 doi: 10.1177/0148607113513800. [DOI] [PubMed] [Google Scholar]
- 17.Tume L, Latten L, Darbyshire A. An evaluation of enteral feeding practices in critically ill children. Nurs Crit Care. 15:291–9. doi: 10.1111/j.1478-5153.2010.00420.x. [DOI] [PubMed] [Google Scholar]
- 18.Hurt RT, McClave SA. Gastric residual volumes in critical illness: what do they really mean? Critical care clinics. 2010;26:481–90. viii–ix. doi: 10.1016/j.ccc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Kuppinger DD, Rittler P, Hartl WH, Ruttinger D. Use of gastric residual volume to guide enteral nutrition in critically ill patients: a brief systematic review of clinical studies. Nutrition. 2013;29:1075–9. doi: 10.1016/j.nut.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Horn D, Chaboyer W, Schluter PJ. Gastric residual volumes in critically ill paediatric patients: a comparison of feeding regimens. Australian critical care: official journal of the Confederation of Australian Critical Care Nurses. 2004;17:98–100. 2–3. doi: 10.1016/s1036-7314(04)80011-0. [DOI] [PubMed] [Google Scholar]
- 21.DeLegge MH. Managing gastric residual volumes in the critically ill patient: an update. Current opinion in clinical nutrition and metabolic care. 2011;14:193–6. doi: 10.1097/MCO.0b013e328341ede7. [DOI] [PubMed] [Google Scholar]
- 22.Braudis NJ, Curley MA, Beaupre K, et al. Enteral feeding algorithm for infants with hypoplastic left heart syndrome poststage I palliation. Pediatr Crit Care Med. 2009;10:460–6. doi: 10.1097/PCC.0b013e318198b167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.