Abstract
Excessive worry is associated with a range of psychological disorders. While previous studies have examined genes associated with a range of different anxiety phenotypes, none have explored genes specifically associated with the general tendency to worry. The present study tested associations between trait worry and functional polymorphisms of three candidate genes: the serotonin transporter-linked polymorphic region (5-HTTLPR) of the SLC6A4 gene, the Val66Met region of the brain-derived neurotrophic factor (BDNF) gene, and the Val158Met region of the catechol-O-methyltransferase (COMT) gene. A heterogeneous sample of adult participants (n = 173) completed the Penn State Worry Questionnaire (PSWQ) and provided DNA samples for genotyping. Results revealed a significant interaction between 5-HTTLPR and BDNF genotypes predicting levels of worry. Specifically, there was a significant positive association between 5-HTTLPR short alleles and PSWQ scores, but only in BDNF met allele carriers. COMT genotype was not significantly associated levels of worry, nor did COMT interact with 5-HTTLPR or BDNF genotypes to predict PSWQ scores. These findings provide preliminary evidence about the putative genetic etiology of worrying. Key limitations of the present study and corresponding directions for future research on this topic are discussed.
Keywords: worry, genetic association, 5-HTTLPR, BDNF, COMT
Worry is a type of repetitive thinking focused on undesirable outcomes that could happen in the future (Berenbaum, 2010). Worrying can serve adaptive functions (see Tallis & Eysenck, 1994), but it is also considered a form of anxiety (see Barlow, 1991) and can cause significant distress and impairment. In fact, excessive worry across life domains is the cardinal feature of Generalized Anxiety Disorder (GAD; APA, 2013). But elevated levels of worry are also associated with most other anxiety disorders (e.g., Posttraumatic Stress Disorder, Holeva et al., 2001; Social Phobia; Obsessive-Compulsive Disorder, Abramowitz & Foa, 1998), as well as a number of other forms of psychopathology (e.g., depression, Kertz et al., 2011; eating disorders, Sassaroli et al., 2005; psychosis, Morrison & Wells, 2007). As a result, excessive worry has been characterized as a transdiagnostic problem (e.g., Berenbaum, 2010; Kertz et al., 2012).
A great deal of research has focused on proximal cognitive, emotional, and behavioral processes that contribute to worrying (for a review, see Berenbaum, 2010), but very little work has explored distal antecedents (e.g., genes) that may play a role the etiology of these processes. Nevertheless, there is evidence that worrying is influenced by genetics. For example, behavioral genetic research suggests that genes explain 32% of the variance in GAD (e.g., Hettema et al., 2001), and trait anxiety (which is related to, but distinguishable from, trait worry; see Davey et al., 1992) appears to be even more heritable, at least in children and adolescents (e.g., 45% according to Legrand et al., 1999). Yet while numerous studies have used molecular methods to investigate specific genes associated with a variety of other anxiety phenotypes, no published research has examined genes associated with the tendency to worry. Given that excessive worry is associated with a range of psychological disorders, findings linking specific genes with worrying should have broad clinical implications.
To this end, the goal of the present study was to examine genetic correlates of trait worry. Based upon a review of relevant literatures, we identified three candidate genes:
Serotonin transporter gene – The short allele of the serotonin transporter-linked polymorphic region (5-HTTLPR) of the SLC6A4 gene is associated with elevated levels of trait anxiety (see Schinka et al., 2004) as well as risk factors for anxiety disorders (e.g., attentional biases; Beevers et al., 2007). Further, this allele is associated with Major Depressive Episodes (e.g., Caspi et al., 2003), and research suggests Major Depressive Disorder and GAD may involve entirely shared genetic risk (see Gorwood, 2004).
Brain derived neurotrophic factor (BDNF) gene – The met allele of the Val66Met polymorphism of the BDNF gene is associated with elevated levels of harm avoidance (e.g., Montag et al., 2010). Further, this allele is associated with rumination (e.g., Beevers et al., 2009), which is also a negative form of repetitive thinking that some argue involves the same underlying processes as worry (e.g., Watkins et al., 2005).
Catechol-O-methyltransferase (COMT) gene – The met allele of the Val158Met polymorphism of the COMT gene is associated with elevated levels of generalized anxiety (Olsson et al., 2007) as well as processes that play a role in the development of anxiety disorders (e.g., failure to extinguish conditioned fear; Londsdorf et al., 2009).
Though these genes have been linked to a number of different anxiety and worry-related phenotypes, previous studies have yielded some inconsistent findings (e.g., Frustaci et al., 2008; Samochowiec et al., 2004; Sen et al., 2004; Wray et al., 2008). There are several possible reasons for these inconsistencies, which our study may be able to address. First, in light of evidence that worry is transdiagnostic, focusing on specific diagnoses (e.g., GAD) may be overly narrow and could lead to false negatives (i.e., control participants with excessive worry). Similarly, given that worry is dimensional in nature (see Ruscio, Borkovec, & Ruscio, 2001), approaches that use arbitrary cut-offs to characterize individuals with high or low levels of worry may ignore meaningful variance and reduce statistical power. Furthermore, unlike prior work, we used a measure with very strong psychometric properties that can differentiate worry from general distress, depression, and other forms of anxiety (see Behar et al, 2003, Brown et al., 1992, and Nitschke et al., 2001). Finally, there is emerging evidence that these candidate genes may function interactively. For example, Olsson and colleagues (2007) found that participants homozygous for both the COMT met allele and the 5-HTTLPR short allele had the highest levels of generalized anxiety, compared to other genotype combinations for these two polymorphisms. Further, Clasen and colleagues (2011) found that 5-HTTLPR and BDNF genotypes interactively predict rumination in the context of life stress, while Kaufman and colleagues (2006) reported an interaction between these two polymorphisms predicting depression in children with a history of maltreatment. Such findings are bolstered by emerging evidence of biological epistasis between these candidate genes in brain regions implicated in disorders associated with excessive worry (e.g., amygdala, anterior cingulate cortex; see Pezawas et al., 2008 and Etkin et al., 2010). Thus, in addition to testing associations between trait worry and these three polymorphisms separately, we also examined interactions between them. We hypothesized that risk alleles of each of these candidate genes would be associated with worrying, either additively or interactively.
Methods
Participants
A total of 173 individuals (68% female; mean age = 25.3, SD = 9.7, range = 17–69) participated in the study (note: this sample is independent from those used by Wells et al., 2010 and Clasen et al., 2011). These individuals were recruited from the Austin community via introductory psychology classes at the University of Texas and advertisements placed online, on campus, and at various locations around the city (e.g., community centers, libraries, coffee shops). Those recruited from psychology classes (47% of the sample) received course credit in exchange for their participation. All other participants were paid for their time. Overall, 67% identified as Caucasian, 14% as Hispanic, 12% as Asian American, 3% as African American, and 2% as “other” (approximately 3% did not report their race/ethnicity).
Materials
Trait worry was measured using the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990). The PSWQ is composed of 16 items (e.g., “My worries overwhelm me”), and participants rate how typical each statement is of them on a scale from 1 (“not at all typical”) to 5 (“very typical”). Thus, scores can range from 16 to 80. Past research suggests that the PSWQ has excellent test-retest reliability (r = .92 over 8–10 weeks; Meyer et al., 1990), as well as good convergent and discriminant validity (with other self-report measures and clinical ratings of related constructs; see Brown et al., 1992, Meyer et al., 1990, and Nitschke et al., 2001). Internal consistency of the PSWQ (measured using Cronbach’s alpha) in the present sample was .96.
The 5-HTTLPR, which maps to17q11.1–17q12, contains a 44 bp insertion/deletion in the 5′ regulatory region of the gene (Heils et al., 1996). This polymorphism in the promoter appears to be associated with variations in transcriptional activity: the long variant (528 bp) has approximately three times the basal activity of the shorter promoter (484 bp) with the deletion (Lesch et al., 1996). The assay is a modification of the method of Lesch and colleagues (1996). The primer sequences are forward, 5′-GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled), and reverse, 5′-GAGGGACTGAGCTGGACAACCAC-3′. These primer sequences yield products of 484 or 528 bp. The polymorphic fragments were separated using an ABI Prism 3130xl DNA sequencer. Allele sizes were scored independently by two investigators and inconsistencies were reviewed and rerun when necessary.
The Val66Met polymorphism (rs6265) of the BDNF gene was genotyped using Taqman assay C___11592758_10 and the Val158Met polymorphism (rs4680) of the COMT gene was genotyped using Taqman assay C___25746809_50 (Applied Biosystems, Foster City, CA). Both were performed using an ABI 7300 real-time PCR system.
Procedure
Each participant provided informed consent, then completed questionnaires and provided DNA for genotyping. Genomic DNA was isolated from buccal cells using a modification of published methods (e.g., Freeman et al., 1997; Lench et al., 1998). First, the cheeks and gums were rubbed for 20 seconds with three sterile, cotton-tipped wooden swabs. The swabs were then placed in a 50-ml capped polypropylene tube containing lysis buffer (500 μl of 1 M Tris-HCL; 200 mM disodium ethylene diaminetetracetic acid [EDTA], pH 8.0; 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride). Next, participants rinsed out their mouth vigorously with 10 ml of distilled water for 20 seconds, and the expelled rinse was added to the tube. The tubes were stored at 4° C until the DNA was extracted using alcohol precipitation.
Statistical Analyses
Linear associations between risk alleles for our three candidate genes and scores from the PSWQ were examined using (separate) hierarchal regression analyses. Specifically, we created dummy coded variables for each gene, with individuals who were homozygous for the non-risk allele coded as −1, individuals who were heterozygous coded as 0, and individuals who were homozygous for the risk allele coded as 1. These variables were then entered as predictors of PSWQ scores in the regression analyses. To test for gene-by-gene interactions, variables for the two genes of interest were entered in the first step, then a product score of these two variables were entered in the second step. The three-way interaction between our candidate genes was not examined due to lack of statistical power. Because the age range of our sample was large, along with evidence that genetic influences can vary across development (see Kendler et al., 2008) and that mean levels of worry are lower in late adulthood (e.g., Basevitz et al., 2008), age was entered as a covariate in the first step of all the analyses. Similarly, because our sample was racially heterogeneous and allele frequencies can vary across racial/ethnic groups (see Hutchison et al., 2004), dummy-coded variables for each (self-reported) racial group were also entered as covariates. Importantly, the effects were consistent with those reported below when: a) these two covariates were not included in the analyses; b) only data from those participants who identified as Caucasian were included in the analyses (see Table A in the Supplementary Materials for these results); or c) the analyses were conducted separately for males and females, or for those who received course credit for participating and those who received payment.
Results
Two samples failed genotyping for COMT and 12 failed genotyping for BDNF – data from these participants were excluded from analyses involving these specific genes. Genotype frequencies for the three candidate genes are shown in Table 1. None of these distributions differed from Hardy-Weinberg equilibrium (ps > .30), nor did they differ significantly amongst Caucasian and non-Caucasian participants (ps > .13). PSWQ scores were normally distributed in the sample (M = 48.4, SD = 16.8) and the range of these scores was quite large (17–80; possible scores range from 16 to 80). Nevertheless, elevated levels of worry were not rare − 47 participants had PSWQ scores of 62 or greater (which represents a proposed cutoff for identifying individuals with pathological worry in unselected samples; Behar et al., 2003).
Table 1.
Genotype frequencies for the sample as a whole and broken down by self-reported race/ethnicity (Caucasian vs. non-Caucasian), along with chi-square values from statistical tests for Hardy-Weinberg equilibrium (for the total sample) and between-group differences.
| Polymorphism | Sample Group | Homozygous, non-risk allele | Heterozygous | Homozygous, risk allele | χ2 | p |
|---|---|---|---|---|---|---|
| 5-HTTLPR | Total sample | 49 | 90 | 44 | .04 | .84 |
| Caucasian | 35 | 58 | 25 | 3.39 | .18 | |
| Non-caucasian | 10 | 26 | 17 | |||
|
| ||||||
| BDNF | Total sample | 91 | 71 | 9 | 1.05 | .31 |
| Caucasian | 65 | 43 | 4 | 3.98 | .14 | |
| Non-caucasian | 20 | 23 | 4 | |||
|
| ||||||
| COMT | Total sample | 52 | 96 | 33 | .95 | .33 |
| Caucasian | 24 | 63 | 30 | 3.73 | .16 | |
| Non-caucasian | 6 | 26 | 20 | |||
Notes. 5-HTTLPR risk allele = short; BDNF risk allele = met; COMT risk allele = met. Tests of race/ethnicity group differences were conducted through cross-tabulation (Pearson chi-square) analyses. Hardy-Weinberg equilibrium analyses test whether the observed distribution of genotypes within a sample deviates significantly the expected distribution, based upon the observed distribution of alleles within the sample. In all of these analyses, the null hypothesis is no deviation/difference.
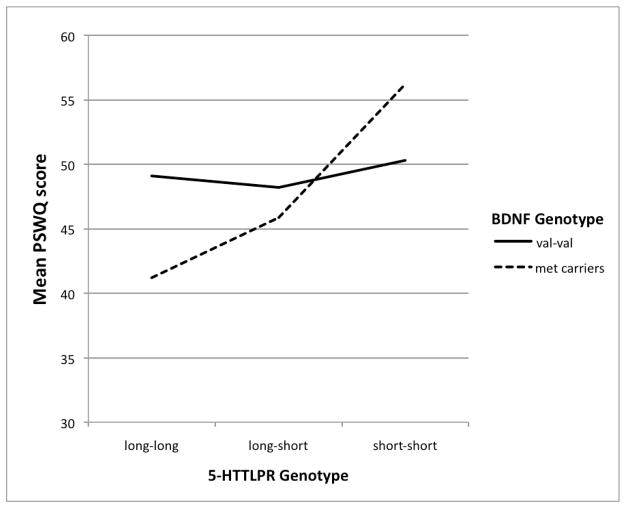
Regression analyses revealed a significant association between 5-HTTLPR genotype and PSWQ scores, β = .17, ΔR2 = .027, p = .03. Specifically, number of short alleles was positively associated with levels of worry. While there was no direct association between BDNF genotype and PSWQ scores, β = −.02, ΔR2= .000, p = .85, there was a significant interaction between BDNF and 5-HTTLPR genotypes predicting PSWQ scores, β = .22, ΔR2 = .027, p = .04 (see Table 2, top). This interaction is portrayed graphically in Figure 1. Follow-up analyses revealed a significant positive association between number of 5-HTTLPR short alleles and levels of worry in BDNF met allele carriers (r = .31, p < .01), but not in val/val homozygotes (r = .02, p = .84). Importantly, these correlations were significantly different from one another, Z = 1.83, p < .05. COMT genotype was not significantly associated with PSWQ scores, β = −.01, ΔR2 = .000, p = .87, nor did COMT interact with 5-HTTLPR or BDNF genotypes to predict worry (ps > .5; see Table 2, middle and bottom).
Table 2.
Results from the hierarchal regression analyses examining 2-way interactions between 5-HTTLPR and BDNF (top), 5-HTTLPR and COMT (middle) and COMT and BDNF (bottom).
| Variable | F | (Δ)R2 | Beta | SE | t | P-value |
|---|---|---|---|---|---|---|
| Step 1 | 1.20 | .046 | .31 | |||
| (Constant) | 4.20 | .77 | 5.46 | .00 | ||
| Age | −.02 | .01 | −2.18 | .03 | ||
| Caucasian | −.70 | .76 | −.92 | .36 | ||
| African American | −.97 | .97 | −1.00 | .32 | ||
| Asian | −.54 | .79 | −.69 | .49 | ||
| Hispanic | −.68 | .78 | −.87 | .39 | ||
| Native American | −.78 | 1.30 | −.60 | .55 | ||
|
| ||||||
| Step 2 | 1.71 | .022 | .18 | |||
| 5-HTTLPR | .23 | .13 | 1.84 | .07 | ||
| BDNF | −.03 | .15 | −.17 | .87 | ||
|
| ||||||
| Step 3 | 4.40 | .027 | .04 | |||
| 5-HTTLPR x BDNF | .43 | .21 | 2.10 | .04 | ||
|
| ||||||
| Step 2 | 1.89 | .023 | .15 | |||
| 5-HTTLPR | .23 | .12 | 1.94 | .05 | ||
| COMT | −.03 | .12 | −.21 | .83 | ||
|
| ||||||
| Step 3 | .26 | .002 | .61 | |||
| 5-HTTLPR x COMT | .09 | .17 | .51 | .61 | ||
|
| ||||||
| Step 2 | .02 | .000 | .98 | |||
| BDNF | −.03 | .15 | −.19 | .85 | ||
| COMT | −.01 | .13 | −.06 | .96 | ||
|
| ||||||
| Step 3 | .44 | .003 | .51 | |||
| BDNF x COMT | .15 | .23 | .66 | .51 | ||
Notes. Betas presented above are unstandardized, and p-values are two-tailed. Since each regression analysis was intended to test a distinct hypothesis, these analyses were conducted separately to preserve degrees of freedom. However, the results from Step 1 are presented only once given that all of the predictors in this step were the same across all three of these analyses.
Figure 1.
Mean PSWQ scores as a function of 5-HTTLPR and BDNF genotypes. Scores from the BDNF val-met and met-met groups are collapsed (into a “met carrier” group), both for ease of interpretation and in line with the results from the follow-up (correlational) analyses described in the text. On that note, we also conducted follow-up simple slope analyses, which revealed that the slope of the association between 5-HTTLPR short alleles and worry approached statistical significance at one BDNF met allele (t = 1.81, p = .07), and reached significance at a point just beyond this (1.05 met alleles). For the specific numbers of participants in our sample with each genotype combination, please see Table B in the Supplementary Materials.
Discussion
This is the first published study to identify specific genes associated with the tendency to worry. Specifically, we found that 5-HTTLPR genotype was significantly associated with trait worry scores, with short alleles predicting higher levels of worry. Importantly, this relationship was moderated by BDNF genotype, such that the association was only present in BDNF met allele carriers. Whereas other studies have consistently linked 5-HTTLPR genotypes with trait anxiety (see Schinka et al., 2004), our results demonstrate the importance of also considering BDNF genotypes when examining the tendency to worry. These findings are consistent with previous evidence of an epistatic relationship between 5-HTTLPR and BDNF genotypes at the neural level (e.g., connectivity and interactions between amygdala and anterior cingulate; see Pezawas et al., 2008 and Outhred et al., 2012), as well as predicting other traits associated with emotional distress (e.g., stress-induced rumination, Clasen et al., 2011; cognitive reactivity, Wells et al., 2010). More generally, the results from this study provide preliminary evidence about the putative genetic etiology of trait worry, and illustrate the potential utility of examining transdiagnostic phenotypes and gene-by-gene interactions in genetic research.
Our confidence in the conclusions that can be drawn from these findings is dampened by some key limitations of the present study, which in turn highlight important directions for future research on this topic. First and foremost, the size of the sample used in this study was relatively small (particularly by the standards of genetic association research), thus limiting our statistical power to detect effects. Sensitivity power analyses (conducted using G*Power 3.1; Faul et al., 2007) revealed that we had at least 80% power to detect (R2) effects of approximately 0.045, supporting the argument that some of the null findings from this study (e.g., the lack of an association between worry and COMT genotype) could reflect Type II errors. Further, our sample was relatively heterogeneous (e.g., in terms of age and race/ethnicity). While this is a common issue in research on individual differences and can even be considered a methodological strength in some respects (e.g., regarding generalizability), the limitations of such heterogeneity are more salient in genetic research given evidence that these sorts of demographic variables can confound results (see Hutchison et al., 2004 and Kendler et al., 2008). Finally, the scope of the present study was somewhat limited, in that we only examined three candidate genes and did not measure potential environmental influences on levels of worry. Though our methodological approach was influenced by the constraints on statistical power outlined above, the fact that we did not examine other factors that could moderate some of the relationships we tested (e.g., childhood maltreatment, social support; see Kaufman et al., 2006) could also explain some of our null findings. Along these lines, it is important to note the observed effects were small, both in an absolute sense and relative to best estimates regarding the heritability of trait worry (e.g., Hettema et al., 2001; Legrand et al., 1999) - while this is typically the case in candidate gene research, it highlights the fact that other genetic and environmental factors clearly play a role in the etiology of trait worry as well. Despite these limitations, the present study represents an important first step in exploring the genetic underpinnings of worrying, an important trandiagnostic dimension of psychopathology.
Supplementary Material
Acknowledgments
This research was supported by grant R01MH076897 from the National Institute of Mental Health and grant R01DA032457 from the National Institute of Drug Abuse awarded to Christopher G. Beevers, as well as 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs awarded to John E. McGeary.
Footnotes
The authors do not have any conflicts of interest to disclose.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- Abramowitz JS, Foa EB. Worries and obsessions in individuals with obsessive–compulsive disorder with and without comorbid generalized anxiety disorder. Behaviour Research and Therapy. 1998;36:695–700. doi: 10.1016/S0005-7967(98)00058-8. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Barlow DH. Disorders of emotion. Psychological Inquiry. 1991;2:58–71. doi: 10.1207/s15327965pli0201_15. [DOI] [Google Scholar]
- Basevitz P, Pushkar D, Chaikelson J, Conway M, Dalton C. Age-related differences in worry and related processes. The International Journal of Aging and Human Development. 2008;66:283–305. doi: 10.2190/AG.66.4.b. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. Journal of Abnormal Psychology. 2007;116:208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, McGeary JE. The BDNF Val66Met polymorphism is associated with rumination in healthy adults. Emotion. 2009;9:579–584. doi: 10.1037/a0016189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar E, Alcaine O, Zuellig AR, Borkovec TD. Screening for generalized anxiety disorder using the Penn State Worry Questionnaire: A receiver operating characteristic analysis. Journal of Behavior Therapy and Experimental Psychiatry. 2003;34:25–43. doi: 10.1016/S0005-7916(03)00004-1. [DOI] [PubMed] [Google Scholar]
- Berenbaum H. An initiation-termination two-phase model of worrying. Clinical Psychology Review. 2010;30:962–975. doi: 10.1016/j.cpr.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Brown TA, Antony MM, Barlow DH. Psychometric properties of the Penn State Worry Questionnaire in a clinical anxiety disorders sample. Behavior Research and Therapy. 1992;30:33–37. doi: 10.1016/0005-7967(92)90093-V. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, McGeary JE, Knopik V, Beevers CG. 5-HTTLPR and BDNF Val66Met polymorphisms moderate effects of stress on rumination. Genes, Brain, and Behavior. 2011;10:740–746. doi: 10.1111/j.1601-183X.2011.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlations and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Graig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavioral Genetics. 1997;27:251–257. doi: 10.1023/A:1025614231190. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Pozzi G, Gianfagna F, Manzoli L, Boccia S. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58:163–170. doi: 10.1159/000182892. [DOI] [PubMed] [Google Scholar]
- Gorwood P. Generalized anxiety disorder and major depressive disorder comorbidity: An example of genetic pleiotropy? European Psychiatry. 2004;19:27–33. doi: 10.1016/j.eurpsy.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of the human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS. A population-based twin study of generalized anxiety disorder in men and women. Journal of Nervous and Mental Disease. 2001;189:413–420. doi: 10.1097/00005053-200107000-00001. [DOI] [PubMed] [Google Scholar]
- Holeva V, Tarrier N, Wells A. Prevalence and predictors of acute stress disorder and PTSD following road traffic accidents: Thought control strategies and social support. Behavior Therapy. 2001;32:65–83. doi: 10.1016/S0005-7894(01)80044-7. [DOI] [Google Scholar]
- Hutchison KE, Stallings M, McGeary JE, Bryan A. Population stratification in the candidate gene study: Fatal threat or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, et al. Brain-derived neurotrophic factor–5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: Evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38:1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertz SJ, Bigda-Peyton JS, Rosmarin DH, Bjorgvinsson T. The importance of worry across diagnostic presentations: Prevalence, severity, and associated symptoms in a partial hospital setting. Journal of Anxiety Disorders. 2012;26:126–133. doi: 10.1016/j.janxdis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Legrand LN, McGue M, Iacono WG. A twin study of state and trait anxiety in childhood and adolescence. Journal of Child Psychology and Psychiatry. 1999;40:953–958. doi: 10.1111/1469-7610.00512. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;1:1356–1358. doi: 10.1016/S0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: Possible implications for gene-environment interaction in anxiety disorder. Psychological Science. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Montag C, Basten U, Stelzel C, Fiebach CJ, Reuter M. The BDNF Val66Met polymorphism and anxiety: Support for animal knock-in studies from a genetic association study in humans. Psychiatry Research. 2010;179:86–90. doi: 10.1016/j.psychres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Morrison AP, Wells A. Relationships between worry, psychotic experiences and emotional distress in patients with schizophrenia spectrum diagnoses and comparisons with anxious and non-patient groups. Behaviour Research and Therapy. 2007;45:1593–1600. doi: 10.1016/j.brat.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25:1–22. doi: 10.1023/A:1026485530405. [DOI] [Google Scholar]
- Olsson CA, Byrnes GB, Anney RJL, Collins V, Hemphill SA, Williamson R, Patton GC. COMT Val158Met and 5HTTLPR functional loci interact to predict persistence of anxiety across adolescence: Results from the Victorian Adolescent Health Cohort Study. Genes, Brain and Behavior. 2007;6:647–652. doi: 10.1111/j.1601-183X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- Outhred T, Das P, Dobson-Stone C, Griffiths K, Felmingham KL, Bryant RA, et al. The functional epistasis of 5-HTTLPR and BDNF Val66Met on emotion processing: A preliminary study. Brain and Behavior. 2012;2:778–788. doi: 10.1002/brb3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, et al. Evidence of biological epistatis between BDNF and SLC6A4 and implications for depression. Molecular Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Borkovec TD, Ruscio J. A taxometric investigation of the latent structure of worry. Journal of Abnormal Psychology. 2001;110:413–422. doi: 10.1037//0021-843X.110.3.413. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Hajduk A, Samochowiec A, Horodnicki J, Stepień G, Grzywacz A, Kucharska-Mazur J. Association studies of MAO-A, COMT, and 5-HTT genes polymorphisms in patients with anxiety disorders of the phobic spectrum. Psychiatry Research. 2004;128:21–26. doi: 10.1016/j.psychres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Sassaroli S, Bertelli S, Decoppi M, Crosina M, Milos G, Ruggiero GM. Worry and eating disorders: A psychopathological association. Eating Behaviors. 2005;6:301–307. doi: 10.1016/j.eatbeh.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Schinka JA, Busch RM, Robichaux-Keene N. A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Molecular Psychiatry. 2004;9:197–202. doi: 10.1038/sj.mp.4001405. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Tallis F, Eysenck MH. Worry: Mechanisms and modulating influences. Behavioural and Cognitive Psychotherapy. 1994;22:37–56. doi: 10.1017/S1352465800011796. [DOI] [Google Scholar]
- Watkins ER, Moulds M, Mackintosh B. Comparisons between rumination and worry in a non-clinical population. Behaviour Research and Therapy. 2005;43:1577–1585. doi: 10.1016/j.brat.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Wells TT, Beevers CG, McGeary JE. Serotonin transporter and BDNF genetic variants interact to predict cognitive reactivity in healthy adults. Journal of Affective Disorders. 2010;126:223–229. doi: 10.1016/j.jad.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, James MR, Dumenil T, Handoko HY, Lind PA, Montgomery GW, Martin NG. Association study of candidate variants of COMT with neuroticism, anxiety and depression. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147:1314–1318. doi: 10.1002/ajmg.b.30744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.