Abstract
Purpose
Parastomal hernia (PH) is a frequent complication from stoma formation after radical cystectomy (RC). We sought to determine the prevalence and risk factors for developing PH following RC.
Material and Methods
Retrospective study of 433 consecutive patients who underwent open RC and ileal conduit between 2006-2010. Postoperative cross-sectional imaging studies performed for routine oncologic follow-up (n=1736) were evaluated for PH, defined as radiographic evidence of protrusion of abdominal contents through the abdominal wall defect created by forming the stoma. Univariable and multivariable Cox regression analyses were used to determine clinical and surgical factors associated with PH.
Results
Complete data were available for 386 patients with radiographic PH occurring in 136. The risk of developing a PH was 27% (95% CI 22-33%) and 48% (95% CI 42-55%) at 1 and 2 years. Clinical diagnosis of PH was documented in 93 patients and 37 were symptomatic. Of 16 patients with clinical PH referred for repair, 8 had surgery. On multivariable analysis, female gender (HR=2.25, 95%CI 1.58-3.21; p<0.0001), higher BMI (HR=1.08 per unit increase 95%CI 1.05-1.12; p<0.0001), and lower preoperative albumin (HR=0.43 per g/dl, 95%CI 0.25-0.75; p=0.003) were significantly associated with PH.
Conclusions
The overall risk of radiographic evidence of PH approached 50% at 2 years. Female gender, higher BMI, and lower preoperative albumin were most associated with developing PH. Identifying those at greatest risk may allow for prospective surgical maneuvers at the time of initial surgery, such as placement of prophylactic mesh in selected patients, to prevent the occurrence of PH.
Keywords: parastomal hernia, risk factors, cystectomy
INTRODUCTION
Ileal conduit remains one of the most commonly used diversions after radical cystectomy (RC). Despite over 60 years of experience, stomal complications remain a substantial problem with a reported incidence of up to 60%.1 Parastomal hernia (PH), defined as an “incisional hernia related to an abdominal wall stoma”, is one of the most frequent complications following stoma formation and has a negative impact on quality of life after RC.2-4 Its incidence varies widely depending on the definition used, the length of follow-up, and whether the diagnosis is made clinically or radiographically.4 Up to 30% of patients with PH require surgical intervention, most commonly due to discomfort, poor fit of the ostomy appliance, or rarely due to obstruction, bowel perforation, or strangulation.2, 5, 6 There is paucity of data about the natural history and risk factors associated with the development of PH after RC and ileal conduit. Most data are adapted from studies in patients undergoing colostomy and ileostomy, in whom issues such as obesity, malnutrition, increasing age, history of radiation exposure, and increased intra-abdominal pressure from chronic coughing, constipation, or ascites, have been cited as potential risk factors.2, 7-10 Technical factors, such as the type of stoma created, the size and location of the abdominal wall defect through which the stoma is formed, and preoperative marking of the stoma site by a wound-ostomy nurse may also impact the risk of PH formation. 11-14
The aim of this study was to determine the prevalence and risk factors for developing a PH following RC and conduit diversion for bladder cancer.
MATERIALS AND METHODS
Patients
This was a retrospective, IRB-approved study of consecutive patients who underwent open RC and ileal conduit at Memorial Sloan-Kettering Cancer Center between January 2006 and October 2010. Patient records were reviewed for documentation of PH on clinical exam, symptoms attributable to the PH, clinical management, referral for PH repair, dates of PH surgery and its indication, and outcome of the repair. The patient characteristics of interest included age, gender, body mass index (BMI) measured at RC, diabetes, smoking history, chronic obstructive pulmonary disease (COPD), estimated blood loss, preoperative albumin, history of prior abdominal surgery and hernia repair, preoperative radiation therapy, neoadjuvant chemotherapy, and stoma type (end-stoma vs. Turnbull loop conduit). All Turnbull loop stomas were performed by a single surgeon (BHB).
Surgical Technique
Patients were evaluated preoperatively by a wound-ostomy certified nurse to mark the stoma location. Conduits were isolated from the ileum by standard techniques and the decision to perform an end-stoma or Turnbull loop conduit was determined by surgeon preference. After re-establishing bowel continuity and closing the mesenteric defect to prevent internal hernias, a circular segment of skin and subcutaneous fat at the pre-designated stoma site was excised. A cruciate incision was made in the anterior rectus fascia and the fibers of the rectus muscles were separately longitudinally to allow the passage of two fingers through a second incision in the posterior rectus fascia. The ileum was passed through the abdominal wall defect and maturation of the stoma was completed. The decision to place supporting sutures at the level of fascia was made according to surgeon preference.
Definition and classification of parastomal hernia
All postoperative cross-sectional imaging scans (computed tomography [CT] or magnetic resonance imaging [MRI]) obtained for routine oncologic follow-up were reviewed for the presence of a PH by a radiologist blinded to all clinical data. PH was defined as radiographic evidence of the protrusion of abdominal contents through the abdominal wall defect created by forming the stoma. When present, the grade of PH was recorded using a previously published classification system 15 as follows: (1) Type 1 PH: hernia sac that contains the prolapsed bowel loop forming the stoma; (2) Type 2: contains abdominal fat or omentum herniating through the abdominal wall defect created by the stoma and (3) Type 3: contains herniated loops of bowel other than that forming the stoma. (Images 1-3)
Statistical Analyses
Univariable Cox regression was used to determine associations between PH development and patient characteristics. Patients who did not develop a PH were censored at the last time in which they were hernia-free. Additional models for BMI and preoperative albumin were created using restricted cubic splines with knots at the tertiles to account for non-linearity. A multivariable Cox model was created, which included all significant patient characteristics from univariable analysis to assess the association of each when combined in a single model. Additionally, we tested for any effects of differential schedules of follow-up imaging exams. Because patients received a different number of imaging exams over different time periods, separate linear regression models for each patient characteristic were created to ensure that these were not associated with the number of imaging exams a patient received. The outcome for these models was the number of imaging exams a patient received within 2 years of surgery. Patients with less than 9-month radiographic follow-up by cross-sectional imaging and those with incomplete clinical data were excluded from analysis. Statistical analyses were performed using Stata 12.0 (StataCorp, College Station, TX).
RESULTS
Patient Characteristics
The records of 433 consecutive patients undergoing open RC and ileal conduit were reviewed. Radiographic and clinical follow-up was evaluable for 386 patients. A summary of patient characteristics is shown in Table 1.
Table 1.
Patient characteristics. All values are median (IQR) or frequency (%)
| n=386 | |
|---|---|
| Patient Age | 74 (68, 79) |
| Gender | |
| Male | 262 (68%) |
| Female | 124 (32%) |
| BMI | 27.7 (24.5, 31.5) |
| Preoperative Albumin (g/dL) | 4.3 (4.1, 4.5) |
| Estimated Blood Loss (cc) | 725 (500, 1200) |
| Smoker | 305 (79%) |
| Diabetes | 77 (20%) |
| Chronic Obstructive Pulmonary Disease | 26 (7%) |
| Prior Abdominal Surgery | 213 (55%) |
| Prior Hernia Repair | 73 (19%) |
| Preoperative Radiation Therapy | 26 (7%) |
| Neoadjuvant Chemotherapy | 110 (28%) |
| Turnbull Stoma | 109 (28%) |
| Histology | |
| TCC | 311 (81%) |
| Other | 75 (19%) |
| Nodal Status | |
| pN0 | 290 (75%) |
| pN+ | 80 (21%) |
| pNX | 16 (4%) |
| Stage | |
| pT0 | 58 (15%) |
| pTa, pTis, pT1 | 117 (30%) |
| pT2 | 63 (16%) |
| pT3 | 129 (33%) |
| pT4 | 17 (4%) |
| pTX | 2 (1%) |
| Grade | |
| Low | 6 (1.6%) |
| Intermediate | 4 (1.0%) |
| High | 317 (82%) |
| Not graded | 59 (15%) |
Natural history of parastomal hernias
A median of 3 follow-up cross-sectional imaging examinations per patient were individually reviewed (range 1-17) for a total of 1736 imaging examinations in the 386 patients. Radiographic evidence of PH was identified in 137 of 386 patients, with a median follow-up of one year for patients who did not develop a PH. Type 1, 2, and 3 PH developed in 5 (4%), 90 (66%), and 41 (30%) patients, respectively. Progression to a higher type of hernia (e.g. from Type 1 to Type 3) over the course of follow-up occurred in 34 patients. Four of 5 initial Type 1 hernias progressed to Type 3, while 30 of 90 initial Type 2 hernias progressed to Type 3. Figure 1 shows the Kaplan-Meier curve for PH free survival and at 1-year it was 73% (95% CI 67%, 78%).
Figure 1.
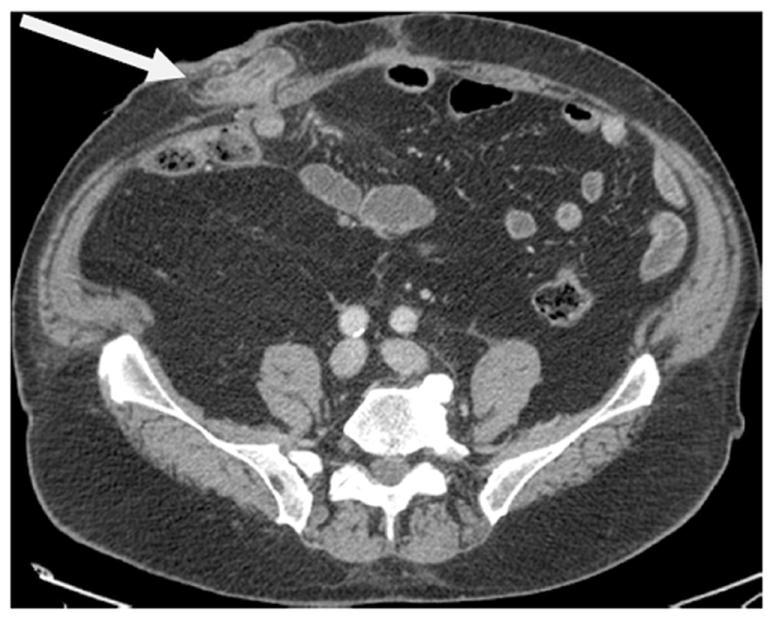
Type 1 parastomal hernia
Clinically evident PH were documented in 93 of 137 patients with radiographic evidence of a PH. Of the 93 patients with a clinically evident PH, 32 (34%) were Type 2 PH and 61 (66%) were Type 3. There were no clinically evident Type 1 PH documented. Symptoms related to the PH, such as pain, discomfort, bowel incarceration, appliance difficulties, or leakage attributable to the PH were present in 37 of 93 patients (40%). Three patients (3%) were asymptomatic and 53 (57%) had no documentation regarding symptoms related to the PH (Table 2). For patients with a clinically evident PH, an abdominal hernia belt or binder was prescribed for 75 patients (81%) and 16 (17%) were referred to general surgery for possible repair. Only 8 patients (9%) underwent surgical repair. Two PH repairs were performed emergently for bowel incarceration and ultimately 3 of 8 repairs developed a recurrent PH. Nearly half of the patients (n=45) with clinically evident hernias had locally recurrent or metastatic disease and none with advanced disease underwent surgery or consultation for hernia repair. There were no patients with clinically detected but radiographically occult PH.
Table 2.
Symptoms attributable to PH in patients with clinically evident PH (n=93)
| Appliance Issues (poor fit, leakage) | 15 (16%) |
| Self-reported bother due to PH | 10 (10%) |
| Pain and discomfort associated with PH | 8 (9%) |
| Incarceration of PH | 5 (5%) |
| Asymptomatic | 3 (3%) |
| No documentation of symptoms | 53 (57%) |
Association between patient characteristics and development of parastomal hernias
Table 3 displays the association between patient characteristics and time to development of PH. On univariable analysis, female gender (HR 1.85, 95% CI 1.31, 2.62; p= 0.0005), BMI (HR 1.07 per unit increase in BMI, 95% CI 1.04, 1.11; p< 0.0001), and preoperative albumin (HR 0.54 per g/dl, 95% CI 0.31, 0.94; p= 0.029) were statistically significantly associated with the development of a PH. These same patient characteristics remained significant in multivariable analysis: female gender (HR 2.25, 95% CI 1.58, 3.21; p<0.0001), BMI (HR 1.08, 95% CI 1.05, 1.12; p<0.0001), and preoperative albumin (HR 0.43, 95% CI 0.25, 0.75; p<0.003)
Table 3.
Univariable Cox proportional hazards regression models for the outcome of time to first hernia development.
| HR (95% CI) | ||
|---|---|---|
| Female | 1.85 (1.31, 2.62) | p-value |
| BMI | 1.07 (1.04, 1.11) | 0.0005 |
| Preoperative Albumin (g/dL) | 0.54 (0.31, 0.94) | <0.0001 |
| Age | 0.99 (0.97, 1.00) | 0.029 |
| Diabetes | 1.07 (0.71, 1.63) | 0.12 |
| Smoker | 0.89 (0.59, 1.35) | 0.7 |
| Chronic Obstructive Pulmonary Disorder | 1.34 (0.70, 2.56) | 0.6 |
| Estimate Blood Loss (per 100 cc) | 1.01 (0.98, 1.03) | 0.4 |
| Prior Abdominal Surgery | 1.10 (0.78, 1.55) | 0.6 |
| Prior Hernia Repair | 1.17 (0.79, 1.75) | 0.4 |
| Preoperative Radiation Therapy | 0.59 (0.26, 1.35) | 0.6 |
| Neoadjuvant Chemotherapy | 0.92 (0.63, 1.35) | 0.2 |
| Turnbull Stoma | 1.10 (0.76, 1.57) | 0.7 |
| Pathological Stage | 0.6 | |
| pT0 | Ref. | 0.7 |
| pTa, pTis, pT1 | 0.98 (0.61, 1.57) | Ref. |
| pT2 | 1.10 (0.63, 1.91) | - |
| pT3 | 0.86 (0.51, 1.45) | - |
| pT4 | 0.62 (0.19, 2.04) | - |
| pTX | 2.17 (0.51, 9.21) | - |
DISCUSSION
In this study, we describe the prevalence and risk factors associated with the development of PH in patients who underwent RC with ileal conduit diversion. Most existing literature on PH centers on their repair with relatively few reports examining the risk factors associated with their development, especially after RC. Identifying those at risk for PH is clinically relevant as many patients require surgical correction due to symptoms such as pain or discomfort, issues related to the appliance, or occasionally bowel obstruction, perforation, or strangulation. 16 Some surgeons have advocated prevention as the best strategy and three prospective, randomized trials of prophylactic mesh placed at the time of stoma formation demonstrated significant reduction in the rates of PH by more than 50% without significant associated morbidity, such as wound or mesh infections, erosion, or obstruction. 17, 18,19, 20 This approach has been successfully adopted in the colorectal surgery community without adverse sequelae and has become standard in some institutions. 21
In our study, the risk of developing a PH by 2 years after surgery was 48%, higher than rates reported in the literature which range from 2.3% to 33%. 2, 8,10, 12, 22 The higher rate of PH in our cohort likely relates to the use of radiographic diagnosis. Most studies use clinical definitions for PH, based upon the finding of protrusion in the vicinity of the stoma on physical exam, but methods for detection vary greatly, i.e., supine vs upright, with or without Valsalva maneuvers, self-reported vs documented by the examining surgeon. 23 Radiographic definitions of PH have the advantage of being morphologic, reproducible, less impacted by physical factors such as body habitus, and allow objective documentation of the size of hernias and changes over time. Radiographic diagnosis is typically more sensitive, generally associated with higher detection rates, and may lead to a greater identification of clinically insignificant hernias. 24 The length of follow-up probably impacts the rates of PH with the lowest published PH rates seen in studies with the shortest follow-up. 8 The median follow-up for patients who did not develop a PH was 12 months. The overall clinical PH rate in our cohort was 24% (93 of 386 patients), which is consistent with the clinically detectable PH rates reported elsewhere. 2, 8,10,12,22 After initial detection, progression to a higher grade of hernia over the course of follow-up occurred in 25% of patients. Eighty percent of patients with Type 1 and 30% of patients with Type 2 PH eventually progressed to Type 3 hernias over the period of follow-up. The high rate of progression of Type 1 and Type 2 PH suggests the relevance of radiographic identification. Standardized radiographic classification systems for reporting PH are relatively new and though the experience with them is limited to a few prospective reports, they appear to have good concordance between identifying clinically symptomatic and radiographically evident hernias. Type 1 hernias are the least common and based upon the finding of redundant, prolapsed conduit above the fascia separate from the stomal opening at the skin surface. In these studies, radiographic Type 3 hernias were universally identified on physical exam while Grade 2 hernias had a concordance rate of 60-80%, suggesting radiographic identification of PH can be a relevant means for reporting incidence rates, especially in the retrospective setting. 11, 15
We found female gender, BMI, and preoperative albumin levels were significantly associated with the development of PH. There was no statistically significant association between PH and age, diabetes, smoking history, COPD, estimated blood loss, prior abdominal surgery, prior hernia repair, preoperative radiation therapy, neoadjuvant chemotherapy, and stoma type. Due to very few patients (n = 10) receiving adjuvant or salvage chemotherapy prior to developing a PH, we were not able to evaluate their association. Our findings are concordant with others that have found increasing rates of PH after cystectomy and colorectal resections in patients with elevated BMI.2,7-9,10 While malnutrition has been reported previously as a risk factor for PH development 24, this is the first report to our knowledge to identify a quantifiable variable, preoperative albumin level, that is significantly associated with PH development.. Like other investigators analyzing factors associated with hernia formation, we found female gender was significantly associated with PH development. 25, 26 Similar to Klein and colleagues, we did not find an association between the type of stoma (end- versus Turnbull loop stoma) and the risk of developing a PH after ileal conduit. 27 Since all Turnbull stomas in this series were performed by a single surgeon (BHB), this analysis may be impacted by surgeon technique rather than by the type of diversion performed and is a limitation in this study.
Though 40% of patients were symptomatic, only 17% of those with clinically evident hernias were referred for surgical consultation and 9% underwent repair. The low rates of referral may be reflective of several factors including the significant morbidity associated with repair, the high rate of metastatic or advanced disease in our population (48%), and high recurrence rates associated with PH repair. This may account for why 80% were managed conservatively with abdominal binders/hernia belts.
Our study is limited by its retrospective nature. Symptoms were only recorded when there was explicit documentation that the hernia was felt to be responsible. This strict clinical reporting requirement may have underestimated the symptoms associated with PH in our cohort and explain why 57% did not have any documentation of symptoms related to the PH.
Despite the low rates of surgical repair in our cohort, PH represent a clinically significant problem. Surgical repair of PH has been associated with significant recurrence rates depending on the approach. Local repair using native tissues have high recurrence rates ranging from 46-100% and relocation of the stoma to another quadrant of the abdomen still requires closure of the original defect, placing both sites at risk for hernias. 28 Varying techniques of mesh placement for PH repair have all demonstrated roughly equal improvements over local tissue repairs with recurrence rates of approximately 10%; however, with complication rates of 65% in some series, PH repairs are often performed only when compelling indications dictate. 28 The high prevalence of PH, the negatively associated quality of life issues, morbidity of surgical repair, and high recurrences rates have prompted surgeons to attempt to prevent their appearance from the time of stoma formation. Level 1 evidence from prospective, randomized trials have demonstrated significantly reduced rates of PH when mesh is placed prophylactically. 17,18,20 Identifying those at greatest risk may allow for prospective, risk-adapted surgical maneuvers at the time of RC to prevent the occurrence of PH.
CONCLUSION
Cross-sectional imaging performed for routine oncologic follow-up after RC allows objective measurement of the time to first hernia and provides an understanding of the natural history of PH after RC. While 40% with clinically evident hernias were symptomatic, only 9% of patients underwent repair. There was a low likelihood of requiring emergent surgery for PH. The overall risk of radiographic evidence of PH approached 50% at 2 years and female gender, higher BMI, and lower preoperative albumin were most associated with developing PH.
Figure 2.
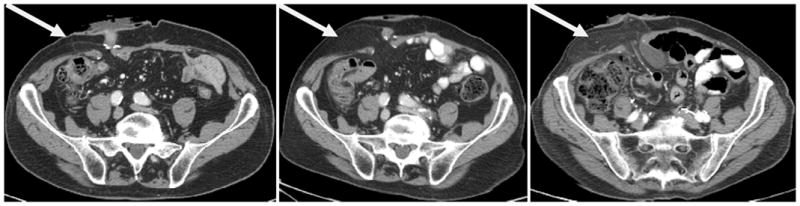
Type 2 parastomal hernia demonstrating progressive fat herniation over 30 months of follow up
Figure 3.
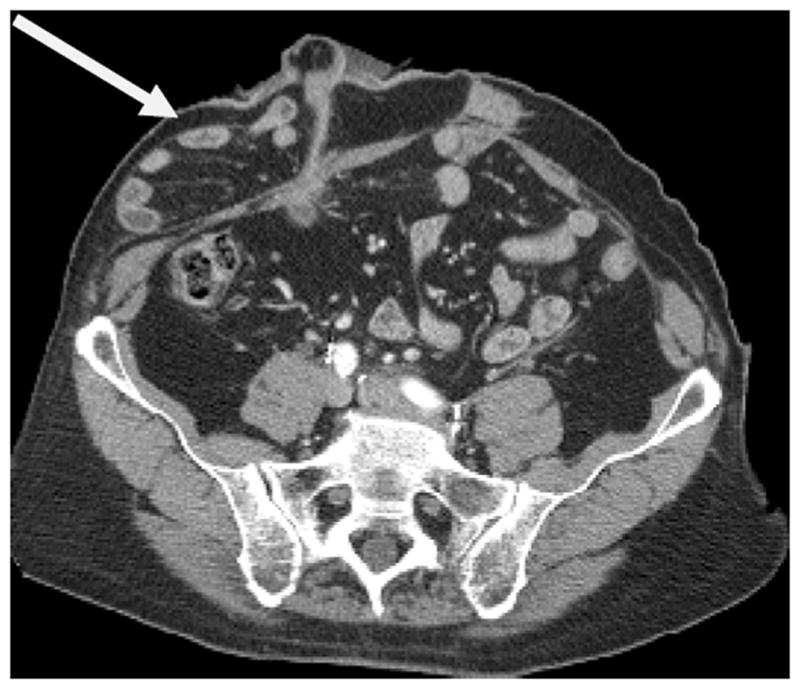
Type 3 parastomal hernia
Figure 4.
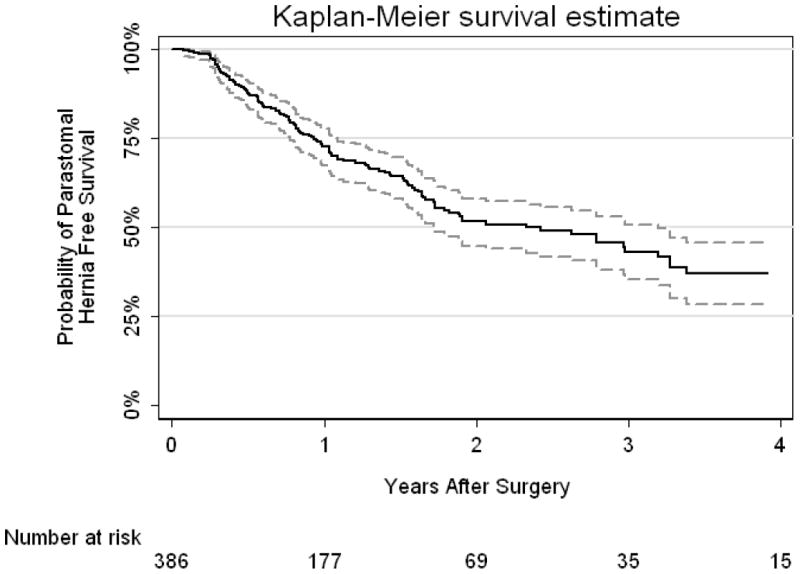
Kaplan-Meier curve for parastomal hernia free survival with 95% CI interval.
Abbreviations
- PH
parastomal hernia
- RC
radical cystectomy
- CI
confidence interval
- HR
hazards ratio
- IQR
interquartile range
- BMI
body mass index
- CT
computed tomography
- MRI
magnetic resonance imaging
- COPD
chronic obstructive pulmonary disease
References
- 1.Farnham SB, Cookson MS. Surgical complications of urinary diversion. World J Urol. 2004;22:157. doi: 10.1007/s00345-004-0429-5. [DOI] [PubMed] [Google Scholar]
- 2.Kouba E, Sands M, Lentz A, et al. Incidence and risk factors of stomal complications in patients undergoing cystectomy with ileal conduit urinary diversion for bladder cancer. J Urol. 2007;178:950. doi: 10.1016/j.juro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Pearl RK. Parastomal hernias. World J Surg. 1989;13:569. doi: 10.1007/BF01658872. [DOI] [PubMed] [Google Scholar]
- 4.Israelsson LA. Parastomal hernias. Surg Clin North Am. 2008;88:113. doi: 10.1016/j.suc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Goligher JC, Lloyd-Davies OV, Robertson CT. Small-gut obstructions following combined excision of the rectum with special reference to strangulation round the colostomy. Br J Surg. 1951;38:467. doi: 10.1002/bjs.18003815208. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson AM, Collins JP. Strangulated para-ileostomy hernia. Aust N Z J Surg. 1977;47:86. doi: 10.1111/j.1445-2197.1977.tb03941.x. [DOI] [PubMed] [Google Scholar]
- 7.De Raet J, Delvaux G, Haentjens P, et al. Waist circumference is an independent risk factor for the development of parastomal hernia after permanent colostomy. Dis Colon Rectum. 2008;51:1806. doi: 10.1007/s10350-008-9366-5. [DOI] [PubMed] [Google Scholar]
- 8.Parmar KL, Zammit M, Smith A, et al. A prospective audit of early stoma complications in colorectal cancer treatment throughout the Greater Manchester and Cheshire colorectal cancer network. Colorectal Dis. 2011;13:935. doi: 10.1111/j.1463-1318.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 9.Arumugam PJ, Bevan L, Macdonald L, et al. A prospective audit of stomas--analysis of risk factors and complications and their management. Colorectal Dis. 2003;5:49. doi: 10.1046/j.1463-1318.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 10.Nastro P, Knowles CH, McGrath A, et al. Complications of intestinal stomas. Br J Surg. 2010;97:1885. doi: 10.1002/bjs.7259. [DOI] [PubMed] [Google Scholar]
- 11.Seo SH, Kim HJ, Oh SY, et al. Computed tomography classification for parastomal hernia. J Korean Surg Soc. 2011;81:111. doi: 10.4174/jkss.2011.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilgrim CH, McIntyre R, Bailey M. Prospective audit of parastomal hernia: prevalence and associated comorbidities. Dis Colon Rectum. 2010;53:71. doi: 10.1007/DCR.0b013e3181bdee8c. [DOI] [PubMed] [Google Scholar]
- 13.Emmott D, Noble MJ, Mebust WK. A comparison of end versus loop stomas for ileal conduit urinary diversion. J Urol. 1985;133:588. doi: 10.1016/s0022-5347(17)49099-9. [DOI] [PubMed] [Google Scholar]
- 14.McGrath A, Porrett T, Heyman B. Parastomal hernia: an exploration of the risk factors and the implications. Br J Nurs. 2006;15:317. doi: 10.12968/bjon.2006.15.6.20679. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Matias J, Serra-Aracil X, Darnell-Martin A, et al. The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis. 2009;11:173. doi: 10.1111/j.1463-1318.2008.01564.x. [DOI] [PubMed] [Google Scholar]
- 16.Marimuthu K, Vijayasekar C, Ghosh D, et al. Prevention of parastomal hernia using preperitoneal mesh: a prospective observational study. Colorectal Dis. 2006;8:672. doi: 10.1111/j.1463-1318.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 17.Janes A, Cengiz Y, Israelsson LA. Randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surg. 2004;91:280. doi: 10.1002/bjs.4417. [DOI] [PubMed] [Google Scholar]
- 18.Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J, et al. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg. 2009;249:583. doi: 10.1097/SLA.0b013e31819ec809. [DOI] [PubMed] [Google Scholar]
- 19.Janes A, Cengiz Y, Israelsson LA. Preventing parastomal hernia with a prosthetic mesh. Arch Surg. 2004;139:1356. doi: 10.1001/archsurg.139.12.1356. [DOI] [PubMed] [Google Scholar]
- 20.Hammond TM, Huang A, Prosser K, et al. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia. 2008;12:475. doi: 10.1007/s10029-008-0383-z. [DOI] [PubMed] [Google Scholar]
- 21.Janes A, Cengiz Y, Israelsson LA. Experiences with a prophylactic mesh in 93 consecutive ostomies. World J Surg. 2010;34:1637. doi: 10.1007/s00268-010-0492-6. [DOI] [PubMed] [Google Scholar]
- 22.Caricato M, Borzomati D, Ausania F, et al. Prognostic factors after surgery for locally recurrent rectal cancer: an overview. Eur J Surg Oncol. 2006;32:126. doi: 10.1016/j.ejso.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Janes A, Weisby L, Israelsson LA. Parastomal hernia: clinical and radiological definitions. Hernia. 2011;15:189. doi: 10.1007/s10029-010-0769-6. [DOI] [PubMed] [Google Scholar]
- 24.Ripoche J, Basurko C, Fabbro-Perray P, et al. Parastomal hernia. A study of the French federation of ostomy patients. J Visc Surg. 2011;148:e435. doi: 10.1016/j.jviscsurg.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Hong SY, Oh SY, Lee JH, et al. Risk factors for parastomal hernia: based on radiological definition. J Korean Surg Soc. 2013;84:43. doi: 10.4174/jkss.2013.84.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song IH, Ha HK, Choi SG, et al. Analysis of risk factors for the development of incisional and parastomal hernias in patients after colorectal surgery. J Korean Soc Coloproctol. 2012;28:299. doi: 10.3393/jksc.2012.28.6.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein EA, Montie JE, Montague DK, et al. Stomal complications of intestinal conduit urinary diversion. Cleve Clin J Med. 1989;56:48. doi: 10.3949/ccjm.56.1.48. [DOI] [PubMed] [Google Scholar]
- 28.Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. Br J Surg. 2003;90:784. doi: 10.1002/bjs.4220. [DOI] [PubMed] [Google Scholar]


