Abstract
Background
An increased resting heart rate (RHR) has long been associated with unhealthy life. Nevertheless, it remains uncertain whether time-varying measurements of RHR are predictive of mortality in older persons.
Design
The purpose of this study was to assess the relationship between repeated measurements of RHR and risk of death from all causes among older adults.
Methods
We evaluated repeat measurements of resting heart rate among 5691 men and women (aged 65 years or older) enrolled in the Cardiovascular Health Study. RHR was measured annually for six consecutive years by validated electrocardiogram. All-cause mortality was confirmed by a study-wide Mortality Review Committee using reviews of obituaries, death certificates and hospital records, interviews with attending physicians, and next-of-kin.
Results
Of the study cohort, 974 (17.1%) participants died. Each 10 beat/min increment in RHR increased the risk of death by 33% (adjusted hazard ratio, 95% confidence interval (CI) = 1.33, 1.26–1.40). Similar results were observed (adjusted hazard ratio, 95% CI = 2.21, 1.88–2.59) when comparing the upper-most quartile of RHR (mean = 81 beats/ min) with the lowest (mean = 53 beats/min). Compared with participants whose RHR was consistently ≤65 beats/min during the study period, the risk of death increased monotonically for each 10 beat/min (consistent) increment in RHR, with adjusted hazard ratios (95% CI) ranging from 1.30 (1.23–1.37) for 75 beats/min to 4.78 (3.49–6.52) for 125 beats/ min.
Conclusions
Elevations in the RHR over the course of six years are associated with an increased risk of all-cause mortality among older adults.
Keywords: Time-varying, resting heart rate, all-cause mortality, older
Introduction
Given the substantial burden of adverse health conditions associated with older age, identifying modifiable risk factors for these outcomes is crucial for prolonging the capacity to live independently and function well during the later years of life.1 With advancing age, however, some conventional risk factors implicated in a number of health-related disorders tend to lose their predictive ability.2
A wealth of epidemiological data suggests that a high resting heart rate (RHR) is an important predictor of all-cause mortality.3–6 Hence, highlighting the relative importance of RHR as a distinct marker of health status may provide a straightforward and non-invasive means for risk stratification in the elderly, for whom prevention strategies could be implemented. Prior studies, however, have evaluated RHR at only a single point in time. Therefore, little is known about the deleterious effects of changes in RHR over time, especially in older persons.
In the current investigation, we sought to examine the relationship between repeated measurements of RHR and the risk of death from all-causes in a large cohort of older persons. Specifically, we hypothesized that sustained elevations in RHR over time would be strongly and independently associated with all-cause mortality.
Methods
Study population
In this investigation, the Cardiovascular Health Study (CHS) limited access files were used. The CHS is a population-based longitudinal investigation, designed to determine risk factors for coronary heart disease (CHD), stroke, and other cardiovascular diseases (CVDs) in persons aged 65 years and older.7 The initial sample comprised 5201 men and women enrolled from four US communities: Pittsburgh, Pennsylvania; Forsyth County, North Carolina; Washington County, Maryland; and Sacramento County, California between May 1989–June 1990. An additional cohort of 687, predominantly African Americans, was recruited to the study between November 1992–June 1993. All of the participants were identified from the Medicare eligibility lists of the Health Care Financing Administration. Inclusion criteria required all individuals to be 65 years or older, non-institutionalized, expected to reside in the area for the next three years, capable of providing written informed consent, and did not require the assistance of a proxy respondent. Those who were wheelchair-bound or receiving hospice treatment, radiation therapy or chemotherapy for cancer were excluded. Written informed consent was obtained from each study participant. Each of the institutional review committees of the four study sites as well as the coordinating center at the University of Washington in Seattle approved the study.
Participant evaluation
A more detailed summary of the CHS objectives and procedures as well as sample size and power calculations have been described elsewhere.7 In brief, the baseline evaluation included a home interview and a clinic-based examination. Components of the initial evaluation were repeated annually for six consecutive years. During the home interview, participants were asked whether they had ever smoked in their lifetime. Potential answers were “yes” or “no”. Participants’ level of physical activity was calculated according to a previously validated measure of exercise intensity, which has been described in detail.8 Briefly, four categories of exercise intensity were employed, whereby study members who engaged in one or more of six high-intensity activities (i.e. swimming, hiking, aerobics, tennis, jogging, racquetball) or walking for exercise at a brisk (>4 mph/6.4 kph) pace were classified as having participated in high-intensity exercise. Conversely, those who responded to not participating in any leisure-time activities were categorized as being sedentary. Educational attainment was used as a measure of socio-economic status.
Assessment of biological covariates
For each evaluation, measured weight (kg) and standing height (cm) were used to calculate body mass index (BMI) using the formula: weight (kg)/height (m2). RHR was obtained with the participant resting for five minutes in the supine position by 12-lead electrocardiogram (ECG) using the MAC PC-DT electrocardiographic recorder (Marquette Electronics Inc., Milwaukee, Wisconsin, USA). This method consisted of recording 10-second data from leads I, II, and V1–V6, simultaneously, with a sampling rate of 250 measurements per second. Following five minutes of rest in the seated position, the 30-second RHR was also determined using the radial pulse.
Samples of venous blood were collected early during each annual visit, following 12-hour fast. Fasting plasma lipid analyses were performed on an Olympus Demand system (Olympus Corp., Lake Success, New York, USA), which involved the collection of high-density lipoprotein (HDL) cholesterol, and triglycerides. Low-density lipoprotein (LDL) cholesterol was determined according to the equation by Friedewald and colleagues. Interleukin-6 (IL-6) and C-reactive protein (CRP) were measured in stored plasma samples by enzyme-linked immunosorbent assay (ELISA; High-sensitivity Quantine kit, R & D Systems, Minneapolis, Minnesota, USA), with an interassay coefficient of variation of 7% and 9%, respectively.
Classification of co-morbidities
Hypertension was defined as systolic blood pressure ≥ 140 mm Hg, or diastolic blood pressure ≥90 mm Hg, or a previous history of hypertension, or self-reported use of anti-hypertensive medication. Type 2 diabetes mellitus was defined as a fasting glucose concentration ≥126 mg/dl (≥7.0 mmol/l), or two hour post-load glucose ≥200 mg/dl (≥11.1 mmol/l), or self-reported use of oral hypoglycemic medication, according to the American Diabetes Association. Atrial fibrillation was self-reported, or otherwise determined by 12-lead resting ECG. CHD was defined by the occurrence of angina pectoris, myocardial infarction (MI), coronary angioplasty, or coronary artery bypass graft surgery.12 Congestive heart failure (CHF) was based on physician diagnosis, supplemented by information from diagnostic and surgical procedures, when available. Family history of CVD was noted when a sibling had suffered either a fatal or non-fatal heart attack. Data regarding prescription medication was collected directly from prescription bottles, while the use of non-prescribed drugs was established by questionnaire.
Ascertainment of death
Cause-specific deaths were unavailable in the limited access files and therefore the primary outcome in this study was all-cause mortality. Study participants were contacted semi-annually and a study-wide Mortality Review Committee confirmed any fatal events from reviews of obituaries, death certificates and hospital records, interviews with attending physicians, next-of-kin, and witnesses. Contacts and proxies for those missing at follow-up were also available for interview. Therefore, ascertainment of mortality status during follow-up was 100% complete.
Statistical methods
Of the 5888 subjects enrolled, 197 were omitted due to RHR values that were dangerously low (≤35 beats/min) or high (≥125 beats/min), occurring at least once, and required clinical attention; hence, the analytic sample comprised 5691 individuals. Baseline characteristics of the study cohort were summarized according to survival status (all-cause mortality), reporting mean and standard error (or median and interquartile range (IQR) for variables that were non-normally distributed), or counts and proportions. The decedents and non-decedents were compared using t-tests for normally distributed continuous data, the Mann-Whitney U test when non-normally distributed, and the chi-squared test for categorical or binary data.
Because RHR can vary over time, we updated RHR at each annual observation, thus it is a time-varying risk factor. This approach provides better accuracy for assessing the relationship between RHR and all-cause mortality, compared with using only a single measurement that is fixed at baseline. Moreover, since we evaluated RHR as a time-varying risk factor, the most immediate value is consistently used for prognostic estimation, thereby enhancing the clinical relevance of this study. Further details of this method have been described elsewhere. For our prospective analyses, we carefully selected all covariates before entering them into our Cox regression models.
We evaluated the non-parametric Spearman’s rank-order correlations for all covariates. When a correlation coefficient was greater than 0.33, denoting high collin-earity, we chose a single factor based on clinical judgment and the strength of association with the mortality outcome. Next, we modeled associations for RHR measured continuously (10 beat/min increments) and categorically (fourth versus first RHR quartile) over the course of the study period with all-cause mortality, using multivariable Cox regression, reporting hazard ratios (HRs) with 95% confidence intervals (CIs). To select the remaining candidate factors for inclusion in the multivariable analysis, we used a forward selection procedure as advocated by Hosmer and Lemeshow. Specifically, this procedure involved selecting the remaining candidate factors with p-values <0.25 in bivariate analysis, and including them in a forward selection model. In this iterative procedure, the covariate with the lowest p-value was sequentially added to the multivariable model, until the covariate with the lowest p value was eventually ≥0.05. Lastly, an interaction between RHR and sex was evaluated, but did not achieve statistical significance; thus, the reduced model without the interaction term was used. With the exception of age (65–69, 70–74, 75–79, ≥80 years old), sex, race (Caucasian versus non-Caucasian), exercise (sedentary, low, average, high intensity), and education attained (high school or less, high school graduate or technical college, college graduate), all other measures collected during annual assessments were entered as time-varying covariates. Although the amount of missing data was small, ranging from n = 3 (0.03%) for HDL cholesterol to n = 398 (7.0%) for IL-6, we imputed values for missing covariates using the last-observation-carried-forward method to maintain complete information for the multivariable analysis.
We also assessed the association between RHR and all-cause mortality according to 10 beat/min categories ranging from 36–65 beats/min (reference group), to 125 beats/min (highest group). We additionally tested the association between RHR and all-cause mortality stratifying by categories of age, as well as by pre-specified co-morbidities at baseline, adjusting for those covariates that were retained during the forward selection procedures.
Lastly, we performed an additional set of analyses to test the robustness of our findings. First, since ECG may not be routinely obtained in clinical practice, we repeated the analyses using RHR as determined by radial pulse. The level of agreement between RHR as measured by ECG and radial pulse was high, with intraclass correlation coefficient = 0.68 (p < 0.001). Second, we performed a complete-case analysis, including the 4181 (73.4%) participants who had no missing data on any variable at baseline. Third, as cardiovascular medications (i.e. beta blockers) may lower the RHR, we excluded those who reported the use of such agents (n = 1678) at baseline. Results were considered statistically significant at a two-tailed p-value of <0.05. Statistical calculations were computed using STATA version 11.2 (StataCorp LP, College Station, Texas, USA) and SAS version 9.3 (SAS Inc., North Carolina, USA).
Results
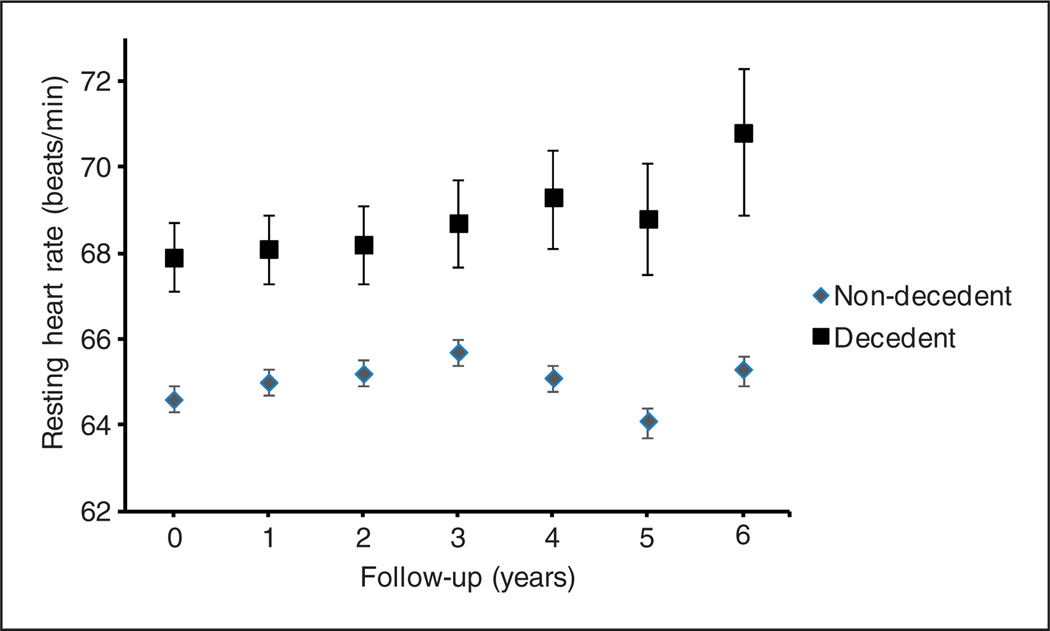
Over the course of this study (duration 7.9 years), 974 (17.1%) participants died (median length-of-survival 6.9 years). Baseline characteristics of the sample are presented in Table 1. Most notably, compared with the non-decedents, the decedents were older, were more likely to be male, and have a history of smoking, were more physically inactive, and were less-well educated. IL-6 and CRP were higher among the decedents, whereas BMI, LDL, and HDL cholesterol were lower compared with the non-decedents. Most other measures including co-morbidities and cardiovascular medications were present relatively more often among the decedents. At each time point, RHR was found to be consistently higher among the decedents as compared with the non-decedents (Figure 1).
Table 1.
Baseline uncorrelated characteristics according to decedent status
| Non-decedent (n = 4717) |
Decedent (n = 974) |
p valuea | |
|---|---|---|---|
| Age in years, n (%) | |||
| 65–69 | 2,183 (46.3) | 249 (25.1) | <0.001 |
| 70–74 | 1591 (33.7) | 287(30.0) | |
| 75–79 | 743 (15.6) | 279 (28.6) | |
| >80 | 200 (4.4) | 159 (16.3) | |
| Male, n (%) | 1853 (39.3) | 552 (56.7) | <0.001 |
| Caucasian, n (%) | 4004 (85.0) | 803 (82.4) | 0.06 |
| Ever smoker, n (%) | 2460 (52.2) | 579 (59.5) | <0.001 |
| Exercise intensity, n (%) | |||
| Sedentary | 362 (7.7) | 148 (15.2) | <0.001 |
| Low | 2201 (46.7) | 504 (51.9) | |
| Average | 1690 (35.8) | 268 (27.6) | |
| High | 462 (9.8) | 52 (5.3) | |
| Education level, n (%) | |||
| Some high school or less | 1310 (27.8) | 365 (37.5) | <0.001 |
| High school graduate or technical college | 2413 (51.2) | 437 (45.0) | |
| College graduate or professional | 994 (21.0) | 172 (17.5) | |
| Body mass index, (kg/m2) | 26.7 ±0.07 | 26.2 ±0.15 | 0.001 |
| Lipids, mg/dl | |||
| LDL cholesterol | 130.7 ±0.59 | 125.8 ± 1.42 | 0.002 |
| HDL cholesterol | 54.7 ±0.26 | 51.4 ±0.57 | <0.001 |
| Triglycerides | 120.0 (92.0–164.0) | 122.5 (91.0–166.5) | 0.53 |
| Interleukin–6, pg/ml | 1.59 (1.11–2.40) | 2.27 (1.56–3.43) | <0.001 |
| C–reactive protein, mg/l | 1.83 (0.93–3.25) | 2.33 (1.17–4.92) | <0.001 |
| Co-morbidities, n (%) | |||
| Hypertension | 2019 (42.8) | 518 (53.2) | <0.001 |
| Type 2 diabetes mellitus | 1340 (28.4) | 403 (41.2) | <0.001 |
| Atrial fibrillation | 97 (2.1) | 39 (4.0) | <0.001 |
| Coronary heart disease | 802 (17.0) | 302 (31.0) | <0.001 |
| Congestive heart failure | 144 (3.1) | 108 (11.1) | <0.001 |
| Family history of cardiovascular disease | 1375 (31.8) | 297 (34.0) | 0.26 |
| Cardiovascular medication use, n (%) | |||
| Beta-blocker | 595 (12.6) | 107 (11.0) | 0.12 |
| ACE-inhibitor | 286 (6.1) | 106 (10.9) | <0.001 |
| Aspirin | 144 (3.1) | 43 (4.4) | 0.03 |
| Calcium channel blocker | 583 (12.4) | 174 (17.9) | <0.001 |
ACE: angiotensin converting enzyme; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Continuous data are presented as mean ± standard error (SE) or median (interquartile range). Categorical data are shown as number and percentage values.
t-test and χ2 test were used.
Figure 1.
Mean resting heart rate at each year of study follow-up according to all-cause mortality. Error bars represent 95% confidence limits.
As shown in Table 2, in multivariable analyses, each 10 beat/min increment in RHR was associated with a 33% greater risk of all-cause mortality. Additionally, study members belonging to the highest quartile of RHR (mean=81 beats/min) had more than a twofold increase in their adjusted risk of dying, compared with those in the lowest quartile (mean RHR=53 beats/min).
Table 2.
Adjusted hazard ratios for the association between resting heart rate and all-cause mortality
| Adjusted hazard ratio (with 95% CIs)a |
||
|---|---|---|
| Per 10 beat/min increment |
Q4 (81 beats/min) versus Q1 (53 beats/min) |
|
| Resting heart rate | 1.33 (1.26–1.40) | 2.21 (1.88–2.59) |
| Age in years | ||
| 65–69 | 1.00 (reference) | 1.00 (reference) |
| 70–74 | 1.32 (1.10–1.58) | 1.32 (1.10–1.58) |
| 75–79 | 2.41 (2.00–2.91) | 2.39 (1.98–3.88) |
| >80 | 4.55 (3.61–5.72) | 4.47 (3.54–5.61) |
| Male sex | 2.13 (1.84–2.46) | 2.12 (1.83–2.46) |
| Ever smoke | 1.33 (1.15–1.54) | 1.34 (1.16–1.55) |
| Exercise intensity | ||
| Sedentary | 1.00 (reference) | 1.00 (reference) |
| Low | 0.67 (0.56–0.80) | 0.66 (0.56–0.79) |
| Average | 0.47 (0.39–0.58) | 0.47 (0.38–0.57) |
| High | 0.39 (0.28–0.56) | 0.39 (0.27–0.55) |
| Education level | ||
| Some high school or less | 1.00 (reference) | 1.00 (reference) |
| High school graduate or technical college | 0.96 (0.83–1.12) | 0.98 (0.83–1.16) |
| College graduate or professional | 0.76 (0.62–0.92) | 0.74 (0.59–0.92) |
| Body mass index | 0.94 (0.92–0.95) | 0.94 (0.92–0.95) |
| Type 2 diabetes mellitus | 1.54 (1.33–1.79) | 1.59 (1.37–1.83) |
| C–reactive protein | 1.01 (1.00–1.02) | 1.02 (1.01–1.02) |
| Interleukin–6 | 1.08 (1.06–1.10) | 1.08 (1.06–1.10) |
| Coronary heart disease | 1.53 (1.31–1.79) | 1.52 (1.31–1.77) |
| Congestive heart failure | 1.67 (1.32–2.10) | 1.67 (1.32–2.11) |
| ACE–inhibitors | 1.55 (1.30–1.86) | 1.57 (1.31–1.87) |
| Aspirin | 1.37 (1.05–1.79) | 1.36 (1.05–1.78) |
ACE: angiotensin converting enzyme; CI: confidence interval; Q: quartile.
Multivariable models also included: race, hypertension, high-density lipoprotein and low-density lipoprotein cholesterol, atrial fibrillation, beta blocker, and calcium channel blocker therapy.
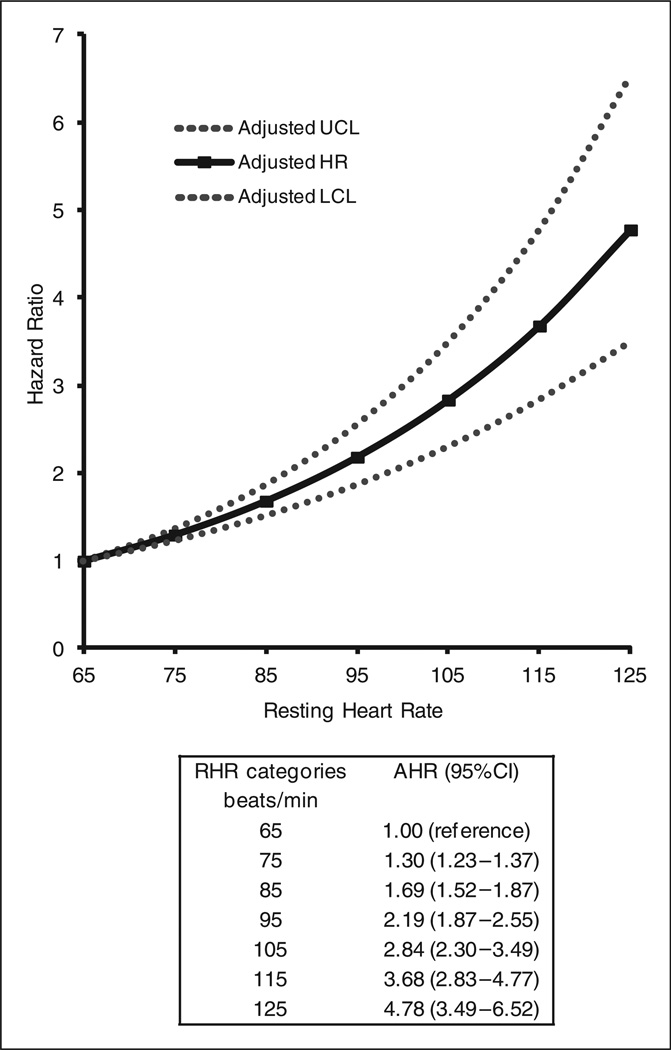
Figure 2 describes the multivariable association between increasing 10 beats/min categories of RHR and the risk of dying from all-causes. Relative to the reference group, each category of RHR was associated with all-cause mortality. Over the course of the study period, the adjusted risk increased monotonically according to each 10 beat/min consistent increment in RHR, with adjusted HRs (95% CI) ranging from 1.30 (1.23–1.37) for 75 beats/min to 4.78 (3.49–6.52) for 125 beats/min.
Figure 2.
Multivariable associations between time-varying resting heart rate (10 beat/min categories) and risk of dying from all-causes. Adjusted model included covariates retained in mul-tivariable analyses as described in Table 2.
AHR: adjusted hazard ratio; HR: hazard ratio; LCL: lower confidence limit; RHR: resting heart rate; UCL: upper confidence limit.
As shown in Table 3 (Supplementary Material), elevations in the RHR were associated with a higher risk of death across three of the five co-morbidity subgroups, with the strongest association observed for hypertension. Also, the association between RHR and all-cause mortality was observed for each of the four age groups (Figure 3, Supplementary Material).
The results for all-cause mortality did not change appreciably when RHR (per 10 beat/min increments) was assessed by radial pulse instead of ECG (HR, 95% CI=1.29, 1.21–1.36; p<0.001), when only complete cases were analyzed (HR, 95% CI= 1.34, 1.27–1.42; p < 0.001), when participants who reported the use of cardiovascular medications at baseline were omitted (HR, 95% CI=1.33, 1.24–1.43; p<0.001), or when we stratified according to beta-blocker treatment (HR, 95% CI=1.35, 1.11–1.65; p=0.003).
Discussion
Over the course of the study period, we found elevations in the RHR to be strongly related to the risk of death from any-cause in older persons. Our observations support, as well as extend, previous reports that have found an association between a fast RHR and all-cause mortality when using only a single measure.3–5
Few prior studies evaluated the longitudinal changes in RHR with the risk of adverse health outcomes. Jouven et al.16 highlighted the prognostic implications of elevations in the RHR with risk of death from all-causes among apparently healthy middle-aged men. Following adjustment, an increment of more than 3 beats/min from the reference category (64–70 beats/ min) after five years increased the adjusted hazard for mortality by approximately 20%.16 Likewise, Nauman and colleagues17 demonstrated that elevations in the RHR measured almost 10 years apart strongly influenced the risk of death from all-causes among middle-aged adults without known CVD. In that study, subjects whose RHR was less than 70 beats/ min at baseline but greater than 85 beats/min at the second measurement had an adjusted risk of 1.5 (95% CI, 1.2–1.9) for all-cause mortality. However, a potential pitfall of these studies is the limited number of available RHR measurements, suggesting it may have been challenging to formally account for annual changes in RHR over the entire course of these study periods. The repeated measurements of RHR collected at one-year intervals in this study afforded a unique opportunity to enhance our understanding about the promising relationship between RHR and mortality, by utilizing as much RHR data as possible.
Several lines of investigation support the notion that a faster RHR amplifies the risk of adverse health conditions by compromising the balance between myocardial oxygen demand and supply. A higher RHR shortens the fraction of cardiac cycle occupied by diastole, which in turn diminishes coronary blood supply, encouraging greater myocardial oxygen consumption and oxidative stress.18 These functional alterations are accompanied by heightened inflammatory activity, which plays a pivotal role in disturbing endothelial function by restricting the bioavailability of nitric oxide.19 These changes expose the endothelium to a number of deleterious risk factors that provoke impaired vascular tone as well as structural wall dysre-gulation.20 In the light of this, an elevated RHR may represent a modifiable risk factor that contributes to mortality in later life. Slowing the RHR may therefore forestall the risk associated with all-cause mortality.
Indeed, a number of trials have demonstrated a beneficial effect of reducing the RHR pharmacologically.21,22 Albeit, this method of intervention is unlikely to be recommended to the general population, as no trial has yet evaluated the efficacy of pharmacological treatment for lowering the RHR in apparently healthy individuals.23 It is possible, however, that the positive effects that accompany some lifestyle modifications may be mediated, in part, by reducing the RHR.24 For instance, physical training may exert beneficial health effects by augmenting parasympathetic outflow while diminishing sympathetic overactivity in the human heart.25 Moreover, slowing the RHR through physical activity could improve a host of cardiovascular risk factors, including obesity, type 2 diabetes mellitus, hypertension, and increased inflammatory activity,26–29 and in turn, reduce the burden of CVD. Nonetheless, further examination of the potential role of exercise training towards slowing the RHR is warranted.
Our analyses focused on all-cause mortality, as cause-specific deaths were unavailable in the limited access dataset. Thus, we were unable to determine the relationship between RHR and CVD mortality. Older adults are frequently accompanied by multiple co-morbidities, and it is rare for a single condition of health status to be the sole predictor of adverse outcomes.30 We reviewed prior reports from the CHS to guide our selection of previously known predictors that may have influenced the relationship between RHR and mortality. Nonetheless, we cannot exclude the possibility that unmeasured factors may have given rise to confounding. Despite the large sample size, the number of participants belonging to increasing 10 beats/min subgroups as well as for some of the specific co-morbidity groups was relatively small, leading to estimates with wide confidence intervals. Although we conducted a number of sensitivity checks, we were unable to identify (and omit) persons who had a pacemaker in the limited access dataset. Nevertheless, important strengths of the current study include its large sample size, repeat measurements of RHR, control for an extensive collection of clinically meaningful cov-ariates known to influence RHR, complete ascertainment of mortality, and an analytic strategy that included a series of sensitivity analyses, each of which yielded comparable results. For example, since ECG may not be as readily accessible in some clinics, we further evaluated the association between RHR assessed by radial pulse and all-cause mortality. The results were similar, suggesting that the radial pulse may also prove useful when assessing the risk related with all-cause mortality.
In this study, we found elevations in the RHR to be associated with a greater risk of all-cause mortality among older adults. These observations confirm prior reports of increased mortality among those who presented with a high RHR, and demonstrate a novel relationship between RHR determined annually over the course of this study and all-cause mortality. As an easily measurable and modifiable clinical parameter, the RHR constitutes a significant threat towards unhealthy life in the burgeoning population of older persons.
Supplementary Material
Acknowledgements
The authors would like to thank all study members who participated in the CHS. They are also grateful to the CHS team and coordinating centers involved in subject recruitment, sample, and handling of data. The contents of this article are solely the responsibility of the authors and do not necessarily represent the opinions or official views of the NIA, National Center for Advanced Translational Sciences (NCATS), National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI), or the CHS.
Funding
This study was conducted at the Yale Claude D Pepper Older Americans Independence Center (P30AG21342). Thomas Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging (NIA).
Footnotes
Conflicts of interest
None declared.
Supplementary Material
The online data supplements are available at http://cpr.sagepub.com.
References
- 1.Katz S, Branch LG, Branson MH, et al. Active life expectancy. New Engl J Med. 1983;309:1218–1224. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 2.Palatini P, Casiglia E, Julius S, et al. High heart rate: A risk factor for cardiovascular death in elderly men. Arch Intern Med. 1999;159:585–592. doi: 10.1001/archinte.159.6.585. [DOI] [PubMed] [Google Scholar]
- 3.O’Hartaigh B, Bosch JA, Pilz S, et al. Influence of resting heart rate on mortality in patients undergoing coronary angiography (from the Ludwigshafen Risk and Cardiovascular Health (LURIC) study) Am J Cardiol. 2012;110:515–520. doi: 10.1016/j.amjcard.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 4.Cooney MT, Vartiainen E, Laatikainen T, et al. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Perk G, Stessman J, Ginsberg G, et al. Sex differences in the effect of heart rate on mortality in the elderly. JAGS. 2003;51:1260–1264. doi: 10.1046/j.1532-5415.2003.51410.x. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MT, Marott JL, Allin KH, et al. Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: The Copenhagen City Heart Study. Eur J Prev Cardiol. 2012;19:102–108. doi: 10.1177/1741826710394274. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 8.Siscovick DS, Fried L, Mittelmark M, et al. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 12.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allison PD. Survival analysis using SAS: A practical guide. 2nd ed. Cary, NC: SAS Institute; 2010. [Google Scholar]
- 14.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 15.Hosmer DWJ, Lemeshow S. Applied logistic regression. New York: John Wiley; 2000. [Google Scholar]
- 16.Jouven X, Empana JP, Escolano S, et al. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. Am J Cardiol. 2009;103:279–283. doi: 10.1016/j.amjcard.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 17.Nauman J, Janszky I, Vatten LJ, et al. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306:2579–2587. doi: 10.1001/jama.2011.1826. [DOI] [PubMed] [Google Scholar]
- 18.Orso F, Baldasseroni S, Maggioni AP. Heart rate in coronary syndromes and heart failure. Prog Cardiovasc Dis. 2009;52:38–45. doi: 10.1016/j.pcad.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Desjardins F, Balligand JL. Nitric oxide-dependent endothelial function and cardiovascular disease. Acta Clin Belg. 2006;61:326–334. doi: 10.1179/acb.2006.052. [DOI] [PubMed] [Google Scholar]
- 20.Park BJ, Lee HR, Shim JY, et al. Association between resting heart rate and arterial stiffness in Korean adults. Arch Cardiovasc Dis. 2010;103:246–252. doi: 10.1016/j.acvd.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 22.Fox K, Ford I, Steg PG, et al. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 23.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: Executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 24.Pittaras AM, Faselis C, Doumas M, et al. Heart rate at rest, exercise capacity, and mortality risk in veterans. Am J Cardiol. 2013;112:1605–1609. doi: 10.1016/j.amjcard.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Carter JB, Banister EW, Blaber AP. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 2003;33:33–46. doi: 10.2165/00007256-200333010-00003. [DOI] [PubMed] [Google Scholar]
- 26.O’Hartaigh B, Bosch JA, Carroll D, et al. Evidence of a synergistic association between heart rate, inflammation, and cardiovascular mortality in patients undergoing coronary angiography. Eur Heart J. 2012;34:932–941. doi: 10.1093/eurheartj/ehs396. [DOI] [PubMed] [Google Scholar]
- 27.Shigetoh Y, Adachi H, Yamagishi S, et al. Higher heart rate may predispose to obesity and diabetes mellitus: 20-Year prospective study in a general population. Am J Hypertens. 2009;22:151–155. doi: 10.1038/ajh.2008.331. [DOI] [PubMed] [Google Scholar]
- 28.Carnethon MR, Yan L, Greenland P, et al. Resting heart rate in middle age and diabetes development in older age. Diabetes Care. 2008;31:335–339. doi: 10.2337/dc07-0874. [DOI] [PubMed] [Google Scholar]
- 29.Palatini P, Dorigatti F, Zaetta V, et al. Heart rate as a predictor of development of sustained hypertension in subjects screened for stage 1 hypertension: The HARVEST Study. J Hypertens. 2006;24:1873–1880. doi: 10.1097/01.hjh.0000242413.96277.5b. [DOI] [PubMed] [Google Scholar]
- 30.Tinetti ME, McAvay GJ, Murphy TE, et al. Contribution of individual diseases to death in older adults with multiple diseases. JAGS. 2012;60:1448–1456. doi: 10.1111/j.1532-5415.2012.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.