Abstract
Necrotic and apoptotic neuronal cell death can be found in pneumococcal meningitis. We investigated the role of Bcl-2 as an antiapoptotic gene product in pneumococcal meningitis using Bcl-2 knockout (Bcl-2−/−) mice. By using a model of pneumococcal meningitis induced by intracerebral infection, Bcl-2-deficient mice and control littermates were assessed by clinical score and a tight rope test at 0, 12, 24, 32, and 36 h after infection. Then mice were sacrificed, the bacterial titers in blood, spleen, and cerebellar homogenates were determined, and the brain and spleen were evaluated histologically. The Bcl-2-deficient mice developed more severe clinical illness, and there were significant differences in the clinical score at 24, 32, and 36 h and in the tight rope test at 12 and 32 h. The bacterial titers in the blood were greater in Bcl-2-deficient mice than in the controls (7.46 ± 1.93 log CFU/ml versus 5.16 ± 0.96 log CFU/ml [mean ± standard deviation]; P < 0.01). Neuronal damage was most prominent in the hippocampal formation, but there were no significant differences between groups. In situ tailing revealed only a few apoptotic neurons in the brain. In the spleen, however, there were significantly more apoptotic leukocytes in Bcl-2-deficient mice than in controls (5,148 ± 3,406 leukocytes/mm2 versus 1,070 ± 395 leukocytes/mm2; P < 0.005). Bcl-2 appears to counteract sepsis-induced apoptosis of splenic lymphocytes, thereby enhancing clearance of bacteria from the blood.
Streptococcus pneumoniae is one of the most important gram-positive bacterial species causing disease in humans, and the diseases that it causes include pneumonia, otitis media, and meningitis. In adults it is the most common cause of meningitis (7, 39). In spite of the continuing development of new antibiotics, the mortality rate for S. pneumoniae meningitis remains high (7). The overall mortality rate for bacterial meningitis has not changed over the last few decades in developed countries (7, 46, 39). Survivors of meningitis often suffer from severe neurological sequelae (3, 11). The pathological substrate for these long-term problems is neocortical and hippocampal neuronal damage, as observed at autopsy, by hippocampal magnetic resonance imaging volumetry, and in animal models (8, 10, 25, 33, 47, 44). Neuronal apoptosis and necrosis are caused by direct toxicity of bacterial compounds and the inflammatory reaction of the host (6, 21, 38, 40, 43, 47). They are mediated by free radicals, excitatory amino acids, and caspases (4, 5, 22, 25, 26, 34, 43).
The Bcl-2 protein family consists of pro- and antiapoptotic members that can homo- and heterodimerize with each other (23, 27, 35). An excess of proapoptotic dimers leads to cell death, whereas an excess of antiapoptotic molecules prevents cell death (23, 27). Bcl-2 itself is antiapoptotic. Bcl-2 knockout mice have a shortened life span and develop gray hair, polycystic kidneys, and premature lymphocyte apoptosis after initially normal lymphocyte development (19, 29, 31, 42). Also, apoptosis of neurons appears to depend on Bcl-2 family member concentrations. The absence of Bcl-2 results in degeneration of sympathetic, sensory, and motoneurons in the early postnatal period (29). Furthermore, increased susceptibility of aged rabbits to hippocampal apoptosis after aluminum treatment has been correlated with a short-lived early Bcl-2-response and a late intense Bax response, as opposed to the small Bax response and a maintained intense Bcl-2 response in young animals to this stimulus protecting against apoptosis (37). Moreover, Bcl-2 overexpression enhances survival of hippocampal neurons after oxidative stress, glutamate exposure, and hypoglycemia in rats (24). Neurological scores and infarct volume are increased in Bcl-2-deficient mice after 1 h of occlusion of the middle cerebral artery (13). Hypoxia of rat neocortical neuronal cultures leads to Bcl-2 expression and apoptosis that is aggravated when Bcl-2 is antagonized (1).
Therefore, we investigated the effect of an absence of Bcl-2 on the inflammatory changes, clinical course, and neuronal damage in experimental murine pneumococcal meningitis by using Bcl-2 knockout mice.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 2000.)
MATERIALS AND METHODS
Bacteria.
A strain of S. pneumoniae serotype 3 (isolated from a patient with meningitis; a gift from M. G. Täuber, Department of Clinical Microbiology, University of Bern, Bern, Switzerland) was used. Bacteria were grown in tryptic soy broth for 18 h, harvested by centrifugation, washed and resuspended in 0.9% NaCl, divided into aliquots, and stored frozen at −70°C. Frozen aliquots were used for the experiments, and the concentration was adjusted to the required concentration, 104 CFU/25 μl of inoculum. The inoculum size was confirmed by plating 10-fold dilutions on blood agar.
Mice.
Eleven Bcl-2-deficient mice and 13 control littermates with a C57BL/6 background (10 heterozygous and 3 homozygous) that were 40 days old and were kindly provided by B. Holtmann (Department of Neurology, University of Würzburg) were bred as described previously (29). Phenotypically, the Bcl-2-deficient mice had a slightly prolonged life span and exhibited less severe polycystic kidneys than other Bcl-2-deficient mice described previously (31, 42). The Bcl-2-deficient mice weighted 13.5 ± 3.5 g, and the controls weighed 20.0 ± 1.9 g (P = 0.0002). For analysis of Bcl-2 expression in spleens of infected or uninfected mice, 10 6-week-old C57BL/6 mice (Charles River Laboratories, Sulzfeld, Germany) were used. The mice were kept in the Animal Care Facility of the University Hospital of Göttingen at 25°C (room temperature), and water and food were provided ad libitum. The experiments were approved by the Animal Care Committee of the Medical Faculty of the University of Göttingen and by the District Government of Braunschweig, Lower Saxony.
Experimental meningitis.
Mice were anesthetized with 100 mg of ketamin per kg and 10 mg of xylazine per kg. Then eight Bcl-2-deficient mice and nine control mice (three Bcl-2+/+ mice and six Bcl-2+/− mice) were infected by injection of 104 CFU in 25 μl of 0.9% saline into the right forebrain with a 27-gauge needle at a depth of 2 mm from the surface of the skull; this depth ensured that the bevel of the needle was partially placed in the subarachnoid space, which allowed a significant part of the inoculum to spread into the subarachnoid space (10). Twenty-five microliters of 0.9% saline was injected into uninfected controls (three Bcl-2−/− mice and four Bcl-2+/− mice) in the same way. After recovery from anesthesia the mice were clinically normal without apparent deficits. The mice were monitored by using clinical scores, a tight rope test, and weight before infection and 12, 24, 32, and 36 h after infection. The clinical scores were determined as follows: 0, healthy; 1, slightly lethargic; 2, moderately lethargic but able to walk; 3, severely lethargic and unable to walk; 4, dead (10, 44). At 36 h after infection the mice were sacrificed by decapitation, and blood was collected. Then the brain and spleen were removed from each mouse. Bacterial counts were determined for blood, spleen, and cerebellar homogenates. A part of the spleen and the brain were fixed in 4% paraformaldehyde and embedded in paraffin for hematoxylin-eosin staining and in situ tailing (47). For analysis of Bcl-2 expression in the spleens of wild-type mice, C57BL/6 mice were infected with 104 CFU in 25 μl of 0.9% saline (n = 5), or 25 μl of saline was injected (n = 5). After 36 h the mice were killed by cervical dislocation and perfused after cardiac puncture with 10 ml of a 0.9% saline solution. Then the spleen was removed from each mouse and homogenized in 10 μl of phosphate-buffered saline per μg (wet weight). Homogenates were immediately snap frozen in liquid nitrogen.
Tight rope test.
An adapted string test, originally described by Miquel and Blasco (30), was used to evaluate motor performance. Mice were taken by the tail and placed with their front paws in the middle of a 60-cm-long tight rope about 60 cm above the floor. A box with a padded floor was placed beneath the rope to prevent falling mice from being injured. Healthy mice placed on the rope tried to reach one end of the rope, usually by using their hind paws and tail for climbing. The time until the end of the rope was reached was determined. Based on whether the animal reached the end and the time required, a tight rope test performance score was given. Mice that reached one end in less than 7 s were given a score of 1. An additional point was added for every additional 6 s that was needed. Mice that hung on the rope for 60 s but did not reach one end were given a score of 11. Mice that fell before that time were given a point in addition to the 11 points for every 6 s before 60 s that they fell. Mice that fell after less than 6 s received the highest score, 20. Therefore, a low score indicated good performance in the tight rope test (44).
Histology.
One-micrometer coronary sections of the brain were stained with hematoxylin and eosin and examined by light microscopy. Brain sections were scored semiquantitatively for inflammation and neuronal damage. Neuronal damage was assessed in four regions: hippocampus, dentate gyrus, basal ganglia, and neocortex. The percentage of damaged neurons was estimated and was expressed as a score, as follows: 0, no damaged cells; 1, ≤10% of the neuronal cells damaged; 2, 11 to 30% of the neuronal cells damaged; and 3, >30% of the neuronal cells damaged. Meningeal inflammation was assessed as follows. By using a 40-fold magnification field three meningeal, the two temporobasal, and the interhemispheral regions and the third ventricle were examined to determine the number of leukocytes. A score was given based on the number of leukocytes present, as follows: 0, no leukocytes; 1, 1 to 10 leukocytes; 2, 11 to 50 leukocytes; 3, more than 50 leukocytes. All regional scores were added. Thus, the maximum score possible was 21 (10).
In situ tailing.
To assess apoptotic cell death, in situ tailing was performed with brain and spleen sections as described previously (47). Deparaffinized and hydrated 5-μm sections of the spleen and 1-μm coronal sections of the brain were treated with 50 μg of proteinase K (Sigma, Deisenhofen, Germany) per ml for 15 min at 37°C in a reaction mixture containing 10 μl of 5× tailing buffer, 1 μl of digoxigenin DNA labeling mixture, 2 μl of cobalt chloride, 12.5 U of terminal transferase, and the amount of distilled water necessary to bring the volume to 50 μl. After washing, a solution of alkaline phosphatase-labeled anti-digoxigenin antibody in 10% fetal calf serum (1:250) was placed on the sections for 60 min at 37°C. The color reaction was developed with 4-nitroblue tetrazolium chloride-5-bromo-4-chloro-3-indolylphosphate (BCIP). The sections were counterstained with nuclear fast red-aluminum hydroxide (all reagents were obtained from Boehringer, Mannheim, Germany) (47).
Quantification of lymphocyte apoptosis in the spleen.
The area of three follicles per section was measured for sections stained with hematoxylin and eosin by using a microscope (Olympus BX51; Olympus, Hamburg, Germany) equipped with a digital camera (SIS Color View) attached to a computer with software for image analysis (analySIS [Special ′SIS Docu]; Soft Imaging System; www.soft-imaging.net), and the numbers of apoptotic leukocytes per square millimeter in the consecutive sections stained by in situ tailing were determined for the follicles used. The number of apototic cells per square millimeter was calculated.
Quantification of meningeal PML apoptosis.
For brain sections stained by in situ tailing the numbers of apoptotic polymorphonuclear leukocytes (PML) were determined by assessing 300 PML per animal by using a microscope equipped with an ocular grid.
All investigators involved in the scoring and assessment of histological sections were blinded with respect to allocation of the samples.
Immunohistochemistry.
The following antibodies were used for immunohistochemical analysis of the spleen: monoclonal mouse anti-human Bcl-2 (clone 124; dilution, 1:25; DAKO, Glostrup, Denmark), monoclonal mouse anti-human CD8 (clone C8/144B; dilution, 1:100; DakoCytomation, Glostrup, Denmark), and rat anti-human CD3 (clone CD3-12; dilution, 1:200; Serotec, Düsseldorf, Germany). Deparaffinized and hydrated 1-μm sections (for double staining with CD3 and CD8 after in situ tailing) were pretreated by microwaving them five times for 3 min in 10 mM citric acid buffer (pH 6.0). Positive and negative control sections were included for each individual antibody. Staining after the primary antibodies were omitted revealed no immunoreactivity. The following secondary antibodies were used: for Bcl-2 staining and double staining of in situ tailed sections with CD8, a biotinylated sheep anti-mouse immunoglobulin antibody (RPN1001; Amersham Biosciences, Freiburg, Germany); and for double staining of in situ tailed sections with CD3, a biotinylated goat anti-rat immunoglobulin (RPN1005; Amersham Biosciences). The color reaction was developed by using a peroxidase-conjugated avidin-biotin complex (Vectastain ABC kit; Vector, Peterborough, United Kingdom) with diaminobenzidine (Roche, Basle, Switzerland) as a chromogen (brown) for Bcl-2 and new fuchsin (red) for CD8 and CD3. Slices were counterstained with hemalaun.
Bcl-2 Western blotting.
For protein extraction murine spleen tissue was homogenized, mixed with an equal volume of reducing sample buffer, and boiled for 5 min. After quantitation of total protein by the bicinchoninic acid assay (Pierce, Rockford, Ill.) 50 μg of each specimen was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The blot was blocked and then incubated with anti-Bcl-2 mouse monoclonal immunoglobulin G1 (sc-7382; Santa Cruz Biotechnology, Heidelberg, Germany) and peroxidase-conjugated goat anti-mouse immunoglobulin G (Jackson Immunoresearch Laboratories, West Grove, Pa.). The reaction was developed with the ECL enhanced chemiluminescence Western blotting detection reagents, and the emitted light was detected and quantified by using a chemiluminescence camera (Fluor S MAX Multiimager; Bio-Rad Laboratories, Hemel Hempstead, United Kingdom).
Statistics.
Data were expressed as means ± standard deviations if they were normally distributed. Groups were compared by using a t test for independent samples. When there was not a normal distribution, the median and the 25th and 75th percentiles were used, unless indicated otherwise. Then groups were compared by using the Mann-Whitney U test. A P value of <0.05 was considered significant.
RESULTS
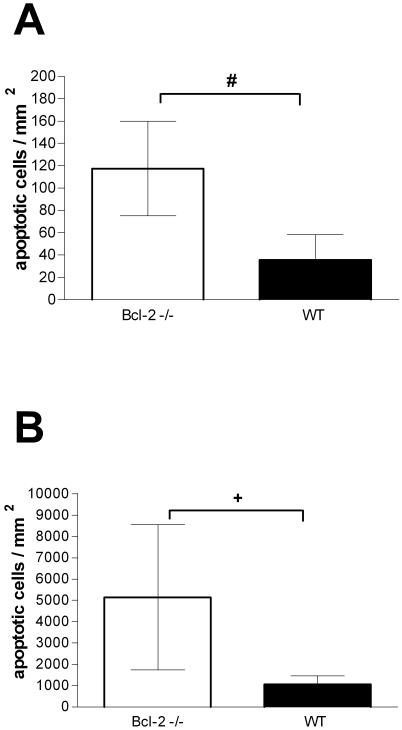
At the beginning of the experiment all mice were healthy. Bcl-2-deficient mice and controls into which saline was injected intracerebrally did not develop signs of clinical disease within 36 h. Also, the performance in the tight rope test was not altered, and the results for the two groups were not significantly different. At zero time the median score in the tight rope test for the Bcl-2−/− mice was 3 and the minimum and maximum scores were 2 and 4, respectively, whereas for the Bcl-2+/− mice the median score was 2 and the minimum and maximum scores were 2 and 3, respectively (P = 0.4). At 36 h the median score for the Bcl-2−/− mice was 2 and the minimum and maximum scores were 2 and 4, respectively, whereas for the Bcl-2+/− mice the median score was 2 and the minimum and maximum scores were 2 and 2, respectively (P = 0.4). These mice did not show any neuronal damage other than that at the site of injection. The spleens of these mice contained few apoptotic lymphocytes, but the number of these cells was significantly greater in Bcl-2-deficient mice than in the controls. The spleens of the Bcl-2−/− mice contained 118 ± 42 apoptotic cells/mm2, whereas the spleens of the control littermates contained 36 ± 23 apoptotic cells/mm2 (P = 0.02) (Fig. 1A and 2E and F).
FIG. 1.
Numbers of apoptotic lymphocytes in spleen follicles in Bcl-2-deficient mice (open bars) and controls (wild type [WT]) (solid bars). (A) Results 36 h after intracerebral injection of 25 μl of saline (n = 3 and n = 4 for the Bcl-2-deficient mice and controls, respectively). (B) Results 36 h after intracerebral injection of 104 CFU of S. pneumoniae in 25 μl (n = 8 and n = 9 for the Bcl-2-deficient mice and controls, respectively). Note the difference in the scales of the ordinate axes. The bars indicate means, and the error bars indicate standard deviations. A number sign indicates that the P value is 0.02, and a plus sign indicates that the P value is <0.005 (as determined by a t test).
FIG. 2.
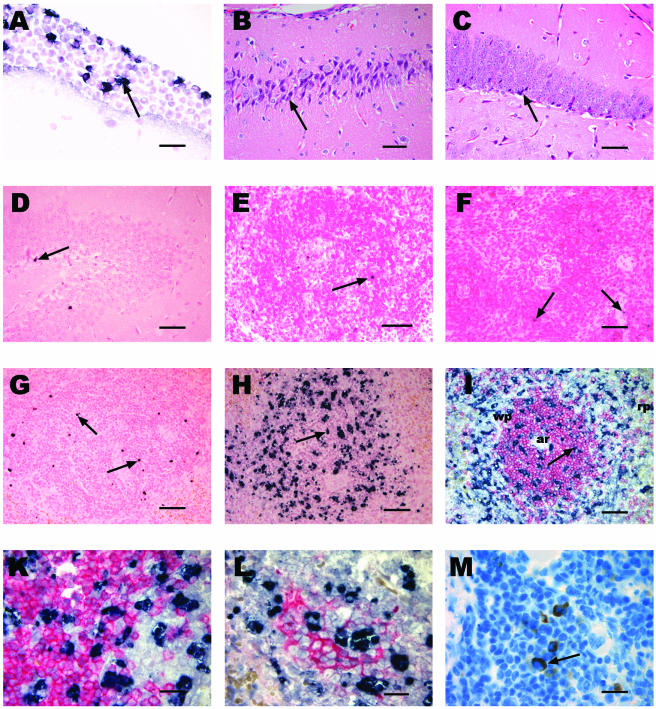
Histology and immunohistochemistry of the brain and spleen in experimental S. pneumoniae meningitis in Bcl-2-deficient mice and controls. (A) In situ tailing of the brain showing meningeal inflammation in a Bcl-2-deficient mouse with apoptotic PML (arrow). Bar, 20 μm. (B and C) Hematoxylin and eosin staining showing necrotic neuronal damage (arrow) in the CA3 region of the hippocampus of a Bcl-2-deficient mouse (B) and in the dentate gyrus of a Bcl-2-deficient mouse (C). (D) In situ tailing of the brain showing an apoptotic neuron (arrow) in the dentate gyrus of a Bcl-2-deficient mouse. (E and F) In situ tailing of the spleen showing few apoptotic lymphocytes (arrow) in a heterozygous control mouse (E) and in a Bcl-2-deficient mouse (F) into which saline was injected. (G and H) In situ tailing of the spleens of a wild-type mouse (G) with few apoptotic lymphocytes (arrow) and a Bcl-2-deficient mouse (H) showing a markedly increased number of apoptotic lymphocytes (arrow) 36 h after intracerebral infection with S. pneumoniae. Bars in panels B to H, 50 μm. (I) Double staining of spleen slices with in situ tailing (black) and CD3 (red) showing apoptotic lymphocytes (arrow) mainly in the white pulp (wp) within and outside T-cell regions. Note the arteriole (ar) in the center of the T-cell region located in the periarteriolar lymphatic sheath. The red pulp (rp) is visible at the edge and does not contain as many apoptotic cells. Bar, 50 μm. (K) Higher magnification showing that there are no cells that show clear double staining with in situ tailing (black) and CD3 (red) due to the shrunken cytoplasm of apoptotic cells. (L) Double staining with in situ tailing (black) and CD8 (red) showing apoptotic lymphocytes within a cytotoxic T-cell cluster and also outside this area. (M) Bcl-2 staining (arrow) in splenic lymphocytes. Bars in panels K to M, 20 μm.
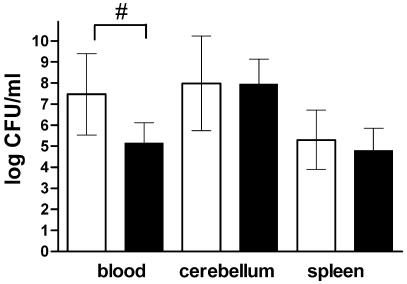
Bcl-2-deficient mice infected intracerebrally with pneumococci developed lethargy and other clinical signs of meningitis earlier than control mice developed such signs (Table 1). This was also reflected by the worse performance of the Bcl-2−/− mice in the tight rope test (Table 1). The bacterial titers in the blood were significantly higher in Bcl-2-deficient mice at 36 h than in the control littermates; the values for the Bcl-2−/− and control mice were 7.46 ± 1.93 and 5.16 ± 0.96 log CFU/ml, respectively (P = 0.0074) (Fig. 3). The bacterial titers in cerebellar and splenic homogenates were not significantly different; the values for the cerebellum for the Bcl-2−/− mice and the control littermates were 7.98 ± 2.25 and 7.95 ± 1.19 log CFU/ml, respectively (P = 0.97), and the values for the spleen were 5.30 ± 1.41 and 4.80 ± 1.06 log CFU/ml, respectively (P = 0.43). Severe secondary neuronal damage (other than the damage at the site of injection, where moderate direct injury occurred) was found predominantly in the hippocampal formation in hematoxylin-and eosin-stained brain sections (Fig. 2B and C). There were no significant differences when Bcl-2-deficient mice were compared with the control littermates for each individual region of the brain examined (data not shown). Also, the difference in the overall damage scores did not reach statistical significance; the overall neuronal damage scores (sums of scores for the four regions examined) for the Bcl-2−/− mice and the control littermates were 6.5 (25th and 75th percentiles, respectively, 5.0 and 8.0) and 5.0 (25th and 75th percentiles, respectively, 4.3 and 6.5), respectively (P = 0.20). In situ tailing revealed that the neuronal damage was mainly necrotic. Only a few animals had one or two apoptotic neurons per section as determined by in situ tailing (Fig. 2D).
TABLE 1.
Clinical scores and tight rope test results during the course of meningitis in Bcl-2-deficient mice and wild-type littermates
| Time (h)a | Clinical score
|
Tight rope test
|
|
||||
|---|---|---|---|---|---|---|---|
| Median (25th percentile, 75th percentile)
|
Pb
|
Mean (25th percentile, 75th percentile)
|
Pb
|
||||
| Bcl-2−/− mice (n = 8) | Controls (n = 9) | Bcl-2−/− mice (n = 8) | Controls (n = 9) | ||||
| 0 | 0 (0, 0) | 0 (0, 0) | NS | 2.5 (2, 6) | 2 (1, 3) | NS | |
| 12 | 0 (0, 0.5) | 0 (0, 0) | NS | 9.5 (3.5, 11) | 3 (2, 3) | 0.046 | |
| 24 | 0 (0, 1.5) | 0 (0, 0) | 0.046 | 11 (4.5, 13) | 3 (2, 6) | 0.059 | |
| 32 | 2 (1.5, 2.5) | 1 (0, 1) | 0.0025 | 17 (12.5, 20) | 11 (11, 11) | 0.046 | |
| 36 | 3 (2.5, 3) | 1 (1, 2) | 0.0055 | 18 (16.5, 20) | 14 (11, 17) | NS | |
Time after infection. The data for time zero are preinfection data.
P value as determined by the Mann-Whitney U test. NS, not significant.
FIG. 3.
Bacterial titers in blood, cerebellar, and spleen homogenates of Bcl-2-deficient mice (open bars) (n = 8) and controls (solid bars) (n = 9) 36 h after intracerebral infection with 104 CFU of S. pneumoniae in 25 μl. The bars indicate means, and the error bars indicate standard deviations. A number sign indicates that the P value is <0.01 (as determined by a t test).
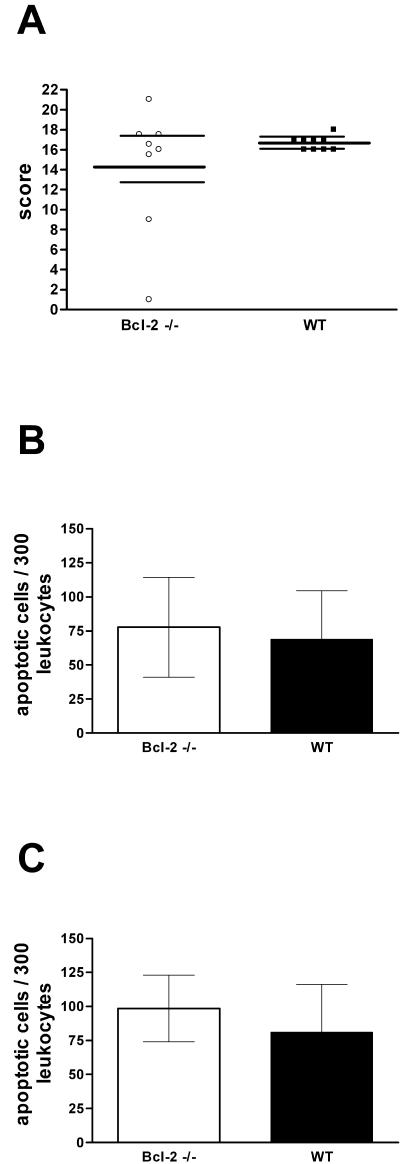
Both groups developed histologically confirmed meningitis with high inflammation scores; the scores for the Bcl-2−/− mice and the control littermates were 16.2 (25th and 75th percentiles, respectively, 12.2 and 17.5) and 17 (25th and 75th percentiles, respectively, 16.0 and 17.0), respectively (P = 0.67) (Fig. 2A and 4A). Sections stained by in situ tailing showed that high proportions of the PML at the site of infection and in meningeal infiltrates were apoptotic. There were no significant differences between the groups, however; for the meningeal infiltrate the values for the Bcl-2−/− mice and the control littermates were 78 ± 37 and 69 ± 36 apoptotic cells/300 PML, respectively (P = 0.64) (Fig. 4B), and for the intraparenchymal infiltrate the values were 98 ± 24 and 81 ± 35 apoptotic cells/300 PML, respectively (P = 0.39) (Fig. 4C).
FIG. 4.
Meningeal inflammation in experimental S. pneumoniae meningitis 36 h after infection. Mice were infected intracerebrally with 104 CFU of S. pneumoniae in 25 μl of saline. ○ and open bars, Bcl-2-deficient mice (n = 8); ▪ and solid bars, controls (wild type [WT]) (n = 9). (A) Meningeal inflammation score (median and 25th and 75th percentiles). (B) Apoptotic PML in the meningeal infiltrate. (C) Apoptotic PML in cerebral infiltrate. The bars indicate means, and the error bars indicate standard deviations. No statistically significant differences were observed.
In the spleen 36 h after infection marked apoptosis of follicular lymphocytes was observed by in situ tailing. The number of apoptotic lymphocytes was higher in Bcl-2-deficient mice; the values for Bcl-2−/− mice and the control littermates were 5,148 ± 3,406 and 1,070 ± 395 apoptotic lymphocytes/mm2, respectively (P = 0.0018) (Fig. 1B and 2G and H). Most apoptotic lymphocytes were located in the white pulp of the spleen. Double staining with CD3 and CD8 showed that cells were positive for in situ tailing in regions with T lymphocytes and cytotoxic T lymphocytes and also outside T-cell regions (Fig. 2I, K, and L). However, it was difficult to morphologically identify double-stained lymphocytes in these areas since the cytoplasm of apoptotic lymphocytes was shrunken, which impeded staining for lymphocyte markers (Fig. 2K and L).
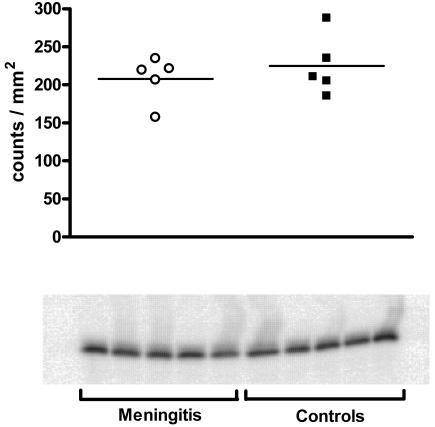
In wild-type mice we observed expression of Bcl-2 in the spleen by Western blotting. However, expression of Bcl-2 did not increase in mice infected intracerebrally with pneumococci compared to mice into which saline was injected (Fig. 5). Bcl-2-positive cells were found in spleen follicles by immunocytochemistry (Fig. 2M).
FIG. 5.
Bcl-2 expression in C57BL/6 wild-type mice 36 h after intracerebral infection with pneumococci (n = 5) or injection of normal saline (n = 5) as determined by Western blotting. ○, mice with pneumococcal meningitis (n = 5); ▪, uninfected controls (n = 5).
DISCUSSION
Bcl-2 knockout mice initially show normal lymphocyte development, but there is premature lymphocyte death (19, 29, 31, 42). In immunocompetent individuals lymphocyte death can be accelerated by stimuli, such as sepsis (14, 18). In our study Bcl-2-deficient mice developed clinically more-severe pneumococcal meningitis despite similar cerebellar bacterial titers, meningeal inflammation scores, and neuronal damage in the hippocampal formation, yet the bacterial titers in the blood were higher. This coincided with markedly enhanced lymphocyte apoptosis in the spleens of Bcl-2-deficient mice, which exceeded by far that in control mice (the means were 5,148 and 1,070 lymphocytes/mm2, respectively). Bcl-2-deficient mice which received saline contained few apoptotic lymphocytes, yet the lymphocyte apoptosis was higher in uninfected Bcl-2-deficient mice than in the healthy wild-type littermates (the means were 118 and 36 lymphocytes/mm2, respectively) (Fig. 1A and 2E and F). Lymphocyte apoptosis in the spleen appeared to affect all types of lymphocytes and, probably, also dendritic cells, although we were not able to show double staining for various lymphocyte markers with conventional immunohistochemistry, since cells that had undergone apoptotic cell death had lost the rim of cytoplasm.
Bcl-2−/− mice were less able to clear bacteria from the blood, which led to more severe sepsis. Also, in patients dying from sepsis and multiorgan dysfunction there is marked lymphocyte apoptosis in the spleen affecting B cells and CD4-positive T cells (14, 18). Hotchkiss et al. concluded that this acquired immunodeficiency may be partially responsible for the high mortality rate for sepsis. Accordingly, it was suggested that inhibition of lymphocyte apoptosis could be a new treatment option to improve survival in sepsis (35). The role of lymphocytes in sepsis is not completely clear. Certainly, B lymphocytes proliferate in bacterial infections and produce antibodies that may be important for opsonization of bacteria to improve their phagocytosis. Apoptotic lymphocytes are taken up by phagocytes themselves. When there is overwhelming death of lymphocytes, as occurs in Bcl-2-deficient mice during sepsis, the dying lymphocytes may divert the capacity of the phagocytic system, interfering with the phagocytosis of bacteria (42). Furthermore, macrophages react in an anti-inflammatory manner when they ingest apoptotic lymphocytes, impairing the host response to infection (18).
We demonstrated that there was increased pneumococcal sepsis-induced lymphocyte apoptosis in the spleens of Bcl-2-deficient mice by in situ tailing. In pigs, bacterial endotoxins that cause septic shock and multiorgan failure induce apoptosis in the liver and spleen that correlates with a decrease in the Bcl-2 protein level (12). In our study, after intracerebral challenge with pneumococci wild-type mice did not show any change in the Bcl-2 protein concentration in the spleen, as estimated by Western blotting. Therefore, the presence of normal amounts of Bcl-2 may be sufficient to prevent the overwhelming lymphocyte apoptosis in gram-positive infections seen in Bcl-2-deficient mice. However, overexpression of Bcl-2 in transgenic mice led to a decrease in apoptosis of spleno- and thymocytes and to improved survival in sepsis (15, 16). Also, murine splenic T cells overexpressing Bcl-2 showed reduced apoptosis in vitro after stimulation with myelin oligodendrocyte glycoprotein (36). In conclusion, Bcl-2 is involved in prevention of infection-induced apoptosis of splenocytes and thymocytes. Bcl-2 probably acts as an inhibitor of activation of caspases involved in the mitochondrial pathway (41). Accordingly, caspase inhibitors were able to prevent lymphocyte apotosis in the cecal ligation and puncture model of polymicrobial sepsis (17). In meningitis treated with antibiotics, PML in the subarachnoid space preferentially die by apoptosis and are removed by macrophages (32). Meningeal inflammation and PML apoptosis in the subarachnoid space were not influenced by the lack of Bcl-2 in our experiments, suggesting that Bcl-2 plays only a minor role in regulation of the cellular immune response to bacteria in the central nervous system. The meningeal infiltrate in pneumococcal meningitis consists mainly of PML. The leukocyte subgroups affected by apoptosis and regulated by Bcl-2 in sepsis are, however, B lymphocytes and CD4+ T helper cells (18). In vitro, PML underwent mainly necrosis when they were exposed to live pneumococci, but they exhibited features of apoptosis when they were exposed to heat-inactivated pneumococci (48). Bcl-2 may, therefore, be of minor importance in the regulation of PML death in the subarachnoid space in untreated meningitis.
In focal ischemia, neuronal apoptosis was markedly increased in Bcl-2 knockout mice (13). Accordingly, overexpression of Bcl-2 in transgenic mice protected the mice from ischemia-induced neuronal apoptosis (28). Furthermore, intravenous administration of a TAT-Bcl-X(L) fusion protein, containing Bcl-X(L) (another antiapoptotic member of the Bcl-2 family), which is able to cross the blood-brain barrier, can reduce neuronal damage in the brain in a murine stroke model (20). In bacterial meningitis Bcl-2 was expressed in apoptotic hippocampal neurons (9). Bacterial meningitis leads to oxidative stress, which has been shown to trigger neuronal necrosis and apoptosis in the central nervous system (4, 9, 25). Therefore, we also expected that neuronal damage would be increased in Bcl-2 knockout mice. However, in Bcl-2 knockout mice with pneumococcal meningitis neuronal damage was not significantly enhanced. Apoptosis, however, accounted only for a small part of the neuronal damage in this model (10). Neuronal damage in meningitis can be a consequence of several alternative pathogenetic mechanisms (34). Infection-induced neuronal apoptosis could in part be caused by tumor necrosis factor alpha-induced caspase activation, which many researchers believe is not influenced by Bcl-2 (2, 17, 43, 45). The role of Bcl-2 in neuronal damage during bacterial meningitis needs to be investigated further by using a model of central nervous system infection with more pronounced apoptotic neuronal damage.
In conclusion, this study provided evidence concerning the role of Bcl-2 in preventing infection-induced apoptosis of lymphocytes in the spleen. A Bcl-2 deficiency leads to a more fulminant clinical course of pneumococcal meningitis after intracerebral infection, but it does not influence meningeal inflammation, PML apoptosis within the central nervous system, and necrotic neuronal damage. Future research could be targeted at direct application or stimulation of the synthesis of antiapoptotic members of the Bcl-2 protein family to prevent lymphocyte apoptosis in bacterial infections and thereby improve survival.
Acknowledgments
This project was supported by a grant from the Deutsche Forschungsgemeinshaft (#Na165/4-3) to R.N.
Editor: V. J. DiRita
REFERENCES
- 1.Banasiak, K. J., T. Cronin, and G. G. Haddad. 1999. Bcl-2 prolongs neuronal survival during hypoxia-induced apoptosis. Mol. Brain Res. 72:214-225. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan, I., S. L. Leib, M. Bergeron, L. Chow, and M. G. Täuber. 1997. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J. Infect. Dis. 176:693-697. [DOI] [PubMed] [Google Scholar]
- 3.Bohr, V., O. B. Paulson, and N. Rasmussen. 1984. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch. Neurol. 41:1045-1049. [DOI] [PubMed] [Google Scholar]
- 4.Böttcher, T., J. Gerber, A. Wellmer, A. V. Smirnov, F. Fakhrjanali, E. Mix, J. Pilz, U. K. Zettl, and R. Nau. 2000. Rifampin reduces production of reactive oxygen species of CSF phagocytes and hippocampal neuronal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 181:2095-2098. [DOI] [PubMed] [Google Scholar]
- 5.Braun, J. S., R. Novak, H. K. Herzog, S. M. Bodner, J. L. Cleveland, and E. I. Tuomanen. 1999. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 5:298-302. [DOI] [PubMed] [Google Scholar]
- 6.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand, M. L., S. B. Calderwood, D. J. Weber, S. I. Miller, F. S. Southwick, V. S. Caviness, Jr., and M. N. Swartz. 1993. Acute bacterial meningitis in adults. A review of 493 episodes. N. Engl. J. Med. 328:21-28. [DOI] [PubMed] [Google Scholar]
- 8.Free, S. L., L. M. Li, D. R. Fish, S. D. Shorvon, and J. M. Stevens. 1996. Bilateral hippocampal volume loss in patients with a history of encephalitis or meningitis. Epilepsia 37:400-405. [DOI] [PubMed] [Google Scholar]
- 9.Gerber, J., W. Brück, C. Stadelmann, S. Bunkowski, H. Lassmann, and R. Nau. 2001. Expression of death-related proteins in dentate granule cells in human bacterial meningitis. Brain Pathol. 11:422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber, J., G. Raivich, A. Wellmer, T. Kunst, A. Werner, W. Brück, and R. Nau. 2001. A mouse model of Streptococcus pneumoniae meningitis mimicking human disease. Acta Neuropathol. 101:499-508. [DOI] [PubMed] [Google Scholar]
- 11.Grimwood, K., V. A. Anderson, L. Bond, C. Catroppa, R. L. Hore, E. H. Keir, T. Nolan, and D. M. Roberton. 1995. Adverse outcomes of bacterial meningitis in school-age survivors. Pediatrics 95:646-656. [PubMed] [Google Scholar]
- 12.Haendeler, J., U. K. Meßmer, B. Brüne, E. Neugebauer, and S. Dimmeler. 1996. Endotoxic shock leads to apoptosis in vivo and reduces Bcl-2. Shock 6:405-409. [DOI] [PubMed] [Google Scholar]
- 13.Hata, R., F. Gillardon, T. M. Michaelidis, and K.-A. Hossmann. 1999. Targeted disruption of the bcl-2 gene in mice exacerbates focal ischemic brain injury. Metabol. Brain Dis. 14:117-124. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss, R. S., P. E. Swanson, B. D. Freeman, K. W. Tinsley, J. P. Cobb, G. M. Matuschak, T. G. Buchman, and I. E. Karl. 1999. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27:1230-1251. [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss, R. S., P. E. Swanson, C. M. Knudson, K. C. Chang, J. P. Cobb, D. F. Osborne, K. M. Zollner, T. G. Buchman, S. J. Korsmeyer, and I. E. Karl. 1999. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162:4148-4156. [PubMed] [Google Scholar]
- 16.Hotchkiss, R. S., K. W. Tinsley, P. E. Swanson, K. C. Chang, J. P. Cobb, T. G. Buchman, S. J. Korsmeyer, and I. E. Karl. 1999. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. N. Y. Acad. Sci. 96:14541-14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss, R. S., K. C. Chang, P. E. Swanson, K. W. Tinsley, J. J. Hui, P. Klender, S. Xanthoudakis, S. Roy, C. Black, E. Grimm, R. Aspiotis, Y. Han, D. W. Nicholson, and I. E. Karl. 2000. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1:496-501. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss, R. S., K. W. Tinsley, P. E. Swanson, R. E. Schmieg, J. J. Hui, K. C. Chang, D. F. Osborne, B. D. Freeman, J. P. Cobb, T. G. Buchman, and I. E. Karl. 2001. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166:6952-6963. [DOI] [PubMed] [Google Scholar]
- 19.Kamada, S., A. Shimono, Y. Shinto, T. Tsujimura, T. Takahashi, T. Noda, Y. Kitamura, H. Kondoh, and Y. Tsujimoto. 1995. Bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 55:354-359. [PubMed] [Google Scholar]
- 20.Kilic, E., G. P. Dietz, D. M. Hermann, and M. Bähr. 2002. Intravenous TAT-Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann. Neurol. 52:617-622. [DOI] [PubMed] [Google Scholar]
- 21.Kim, Y. S., and M. G. Täuber. 1996. Neurotoxicity of glia activated by Gram-positive bacterial products depends on nitric oxide production. Infect. Immun. 64:3148-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koedel, U., and H. W. Pfister. 1999. Oxidative stress in bacterial meningitis. Brain Pathol. 9:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korsmeyer, S. J. 1999. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. 59(Suppl.):1693s-1700s. [PubMed] [Google Scholar]
- 24.Lawrence, M. S., D. Y. Ho, G. K. Steinberg, and R. M. Sapolsky. 1996. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J. Neurosci. 16:486-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leib, S. L., Y. S. Kim, L. L. Chow, R. A. Sheldon, and M. G. Täuber. 1996. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J. Clin. Investig. 98:2632-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leib, S. L., Y. S. Kim, D. M. Ferriero, and M. G. Täuber. 1996. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J. Infect. Dis. 173:166-171. [DOI] [PubMed] [Google Scholar]
- 27.Lincz, L. F. 1998. Deciphering the apoptotic pathway: all roads lead to death. Immunol. Cell. Biol. 76:1-19. [DOI] [PubMed] [Google Scholar]
- 28.Martinou, J.-C., M. Dubois-Dauphin, J. K. Staple, I. Rodriguez, H. Frankowski, M. Missotten, P. Albertini, D. Talabot, S. Catsicas, C. Pietra, and J. Huarte. 1994. Overexpression of Bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13:1017-1030. [DOI] [PubMed] [Google Scholar]
- 29.Michaelidis, T. M., M. Sendtner, J. D. Cooper, M. S. Airaksinen, B. Holtmann, M. Meyer, and H. Thoenen. 1996. Inactivation of Bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal developement. Neuron 17:75-89. [DOI] [PubMed] [Google Scholar]
- 30.Miquel, J., and M. Blasco. 1978. A simple technique for evaluation of vitality loss in aging mice, by testing their muscular coordination and vigor. Exp. Gerontol. 13:389-396. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama, K., K.-I. Nakayama, I. Negishi, K. Kuida, H. Sawa, and D. Y. Loh. 1994. Targeted disruption of bcl-2αβ in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl. Acad. Sci. USA 91:3700-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nau, R., U. Zettl, J. Gerber, F. Trostdorf, U. Michel, T. Böttcher, H. Schmidt, S. Adler, and W. Brück. 1998. Granulocytes in the subarachnoid space of humans and rabbits with bacterial meningitis undergo apoptosis and are eliminated by macrophages. Acta Neuropathol. (Berlin) 96:472-480. [DOI] [PubMed] [Google Scholar]
- 33.Nau, R., A. Soto, and W. Brück. 1999. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J. Neuropathol. Exp. Neurol. 58:265-274. [DOI] [PubMed] [Google Scholar]
- 34.Nau, R., and W. Brück. 2002. Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci. 25:38-45. [DOI] [PubMed] [Google Scholar]
- 35.Oberholzer, C., A. Oberholzer, M. Clare-Salzer, and L. L. Moldawer. 2001. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 15:879-892. [DOI] [PubMed] [Google Scholar]
- 36.Okuda, Y., M. Okuda, and C. C. A. Bernard. 2002. The suppression of T cell apoptosis influences the severity of disease during the chronic phase but not the recovery from acute phase of experimental autoimmune encephalomyelitis in mice. J. Neuroimmunol. 131:115-125. [DOI] [PubMed] [Google Scholar]
- 37.Savory, J., J. K. S. Rao, Y. Huang, P. R. Letada, and M. M. Herman. 1999. Age-related hippocampal changes in Bcl-2:Bax ratio, oxidative stress, redox-active iron and apoptosis associated with aluminium-induced neurodegeneration: increased susceptibility with aging. Neurotoxicology 20:805-818. [PubMed] [Google Scholar]
- 38.Schmidt, H., A. Tlustochowska, K. Stuertz, M. Djukic, J. Gerber, E. Schütz, U. Kuhnt, and R. Nau. 2001. Organotypic hippocampal cultures—a model of brain tissue damage in Streptococcus pneumoniae meningitis. J. Neuroimmunol. 113:30-39. [DOI] [PubMed] [Google Scholar]
- 39.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, and B. A. Perkins. 1997. Active Surveillance Team. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 40.Stringaris, A. K., J. Geisenhainer, F. Bergmann, C. Balshüsemann, U. Lee, G. Zysk, T. J. Mitchell, B. U. Keller, U. Kuhnt, J. Gerber, A. Spreer, M. Bähr, U. Michel, and R. Nau. 2002. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen activated protein kinase. Neurobiol. Dis. 11:355-368. [DOI] [PubMed] [Google Scholar]
- 41.Tinsley, K. W., S. L. Cheng, T. G. Buchman, K. C. Chang, J. J. Hui, P. E. Swanson, I. E. Karl, and R. S. Hotchkiss. 2000. Caspases-2, -3, -6, and -9, but not caspase-1, are activated in sepsis-induced thymocyte apoptosis. Shock 13:1-7. [DOI] [PubMed] [Google Scholar]
- 42.Veis, D. J., C. M. Sorenson, J. R. Shutter, and S. J. Korsmeyer. 1993. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229-240. [DOI] [PubMed] [Google Scholar]
- 43.von Mering, M., A. Wellmer, U. Michel, S. Bunkowski, A. Tlustochowska, W. Brück, U. Kuhnt, and R. Nau. 2001. Transcriptional regulation of caspases in experimental pneumococcal meningitis. Brain Pathol. 11:282-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wellmer, A., C. Noeske, J. Gerber, U. Munzel, and R. Nau. 2000. Spatial memory and learning deficits after experimental pneumococcal meningitis in mice. Neurosci. Lett. 296:137-140. [DOI] [PubMed] [Google Scholar]
- 45.Wellmer, A., J. Gerber, J. Ragheb, G. Zysk, T. Kunst, A. Smirnov, W. Brück, and R. Nau. 2001. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun. 69:6881-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenger, J. D., A. W. Hightower, R. R. Facklam, S. Gaventa, C. V. Broome, and Bacterial Meningitis Study Group. 1990. Bacterial meningitis in the United States. J. Infect. Dis. 162:1316-1323. [DOI] [PubMed] [Google Scholar]
- 47.Zysk, G., W. Brück, J. Gerber, Y. Brück, H. W. Prange, and R. Nau. 1996. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 55:722-728. [DOI] [PubMed] [Google Scholar]
- 48.Zysk, G., L. Bejo, B. K. Schneider-Wald, R. Nau, and H. Heinz. 2000. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin. Exp. Immunol. 122:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]