Abstract
Aim
The natural omega-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA), has recently been credited for possessing anticancer properties. Herein, we investigate the cytotoxic actions of DHA-loaded low-density lipoprotein (LDL) nanoparticles in normal and liver cancer cells.
Materials & methods
LDL-DHA nanoparticles were prepared and subjected to extensive biophysical characterization. The therapeutic utility of LDL-DHA nanoparticles was evaluated in normal and malignant murine hepatocyte cell lines, TIB-73 and TIB-75, respectively.
Results & discussion
The engineered LDL-DHA nanoparticles possessed enhanced physical and oxidative stabilities over native LDL and free DHA. Dose–response studies showed that therapeutic doses of LDL-DHA nanoparticles that completely killed TIB-75 were innocuous to TIB-73. The selective induction of lipid peroxidation and reactive oxygen species in the cancer cells was shown to play a central role in LDL-DHA nanoparticle-mediated cytotoxicity.
Conclusion
In summary, these findings indicate that LDL-DHA nanoparticles show great promise as a selective anticancer agent against hepatocellular carcinoma.
Keywords: docosahexaenoic acid, liver cancer, low-density lipoprotein, low-density lipoprotein receptor, nanoparticle, omega-3 fatty acids
In recent years, the dietary benefits of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) have not only been heralded for improving cardiovascular health, but also for preventing cancer. Numerous population ecological studies have shown that a high per capita consumption of cold water fish (a high source of ω-3 PUFAs) correlates with decreased risk of cancer [1–3]. Animal studies also support this inverse association, as they have shown that high dietary intake of ω-3 PUFAs can markedly impede experimental carcinogenesis [4–6]. Collectively, these epidemiologic and preclinical studies indicate that high dietary intake of ω-3 PUFAs can antagonize the initiation and early progression of cancer in situ.
It is less clear what role or benefit ω-3 PUFAs may have in the treatment of pre-existing tumors. To date, many papers have demonstrated the dose-dependent cytotoxicity of ω-3 PUFAs towards various cancer cells in culture [7–9]. However, the local doses of ω-3 PUFAs used to elicit these anticancer effects in culture are difficult, if not impossible, to achieve through dietary consumption [10]. This probably explains why the few studies that attempted to treat established tumors through dietary consumption of ω-3 PUFAs reported inconsistent results with only modest effects [11–13]. Direct intravascular administration of ω-3 PUFAs is not a feasible option due to their poor aqueous solubility and propensity to form emboli [14,15]. Even for the cell culture experiments mentioned above, organic solvents such as ethanol or dimethyl sulfoxide were required to solubilize the ω-3 PUFAs in cell culture media. Alternatively, albumin can be used as a physiological transporter for ω-3 PUFAs; however, there are some concerns that albumin may protect cancer cells against ω-3 PUFA-induced cytotoxicity [16,17]. Fish oil-based emulsions used for parenteral nutrition (e.g., Omegaven®; Fresenius Kabi AG, Bad Homburg, Germany) can transport large amounts of ω-3 PUFAs in the plasma. However, their large heterogenous size (diameters >200 nm), rapid clearance by the mononuclear phagocyte system and poor sensitivity to lipoprotein lipase limit their efficacy to deliver ω-3 PUFAs to tumors [18–20].
Over the last decade, there have been a plethora of studies reporting on the utility of lipoprotein-based nanoparticles to deliver diagnostic and/or therapeutic agents to cancer [21–25]. These carriers are attractive vehicles for oncology applications due to their nanoscale size, fine particle size distribution, high payload-carrying capacity and ability to avoid mononuclear phagocyte system surveillance [26]. Furthermore, cancer cells have a natural proclivity to actively take up lipoproteins to acquire lipids needed for their rapid membrane turnover [27–29]. The lipoprotein platform is also a particularly fitting vehicle for ω-3 PUFAs, as these carriers naturally function to transport lipids in the plasma [26]. In the present study, the ω-3 PUFA docosahexaenoic acid (DHA) was uniformly incorporated into low-density lipoprotein (LDL) nanoparticles (LDL-DHA). The physicochemical and stability properties of this novel nanoparticle were evaluated; in addition, the therapeutic efficacy and the cytotoxic selectivity of the LDL-DHA nanoparticles were investigated in both normal and malignant murine livers cells. The present study shows that LDL-DHA nanoparticles are stable nanoparticle constructs that are able to selectively kill liver cancer cells without harming normal liver cells. Collectively, these findings suggest that LDL-DHA nanoparticles may prove to be a promising and efficacious nanotherapy against hepatocellular carcinoma (HCC).
Materials & methods
Low-density lipoprotein
Human LDL was isolated from apheresis plasma of patients with familial hypercholesterolemia using sequential density gradient ultracentrifugation as described previously by Lund-Katz et al. [30].
Preparation of LDL-DHA nanoparticles
Incorporation of DHA (Nu-chek Prep Inc., MN, USA) into LDL was performed by the reconstitution (core-loading) method [31]. Briefly, lyophilized LDL was subjected to organic extraction with heptane. Following the extraction, DHA was added to the LDL residue and the sample was allowed to sit at 4°C for 90 min. Thereafter, heptane was removed by evaporation and the dried residue was resuspended in 10 mM tricine buffer (10mM tricine; Sigma-Aldrich, MO USA; pH 8.4). After an overnight incubation at 4°C, LDL samples were clarified by low-speed centrifugation and stored under N2 atmosphere at 4°C. Throughout these studies, various LDL particles were used as controls. These included native LDL, as an overall control vehicle, LDL reconstituted with oleic acid (LDL-OA) or LDL reconstituted with oleic acid triglyceride (triolein) (LDL-TO).
Preparation of human serum albumin associated with DHA
Human serum albumin (HSA; 5% w/v) was dissolved in 1 ml of 75 mM KCl solution and pH was adjusted to 7.4. DHA in ethanol (0.125% w/v; final concentration) was added to HSA solution, vortexed briefly and incubated at 37°C for 1 h. Samples were then filtered through a 0.2-μm syringe filter and stored under N2 atmosphere at 2–8°C until further use.
Nanoparticle characterization
Numerous assays were performed to extensively characterize the LDL nanoparticles. These included: structure and composition determination, percent recovery and loading, mean particle size, polydispersity index (PDI), zeta-potential, turbidity measurements, apoprotein secondary structure, agarose gel electrophoresis, and physical and oxidative stability measurements. Full details on these characterization methods are described in the Supplementary Material (see online at: www.futuremedicine.com/doi/suppl/10.2217/NNM.13.187).
Cell culture
The normal mouse hepatocyte cell line TIB-73 (BNL CL.2) and its malignant counterpart TIB-75 (BNL 1ME A.7R.1) were obtained from American Type Culture Collection (VA, USA) and cultured in DMEM supplemented with 10% fetal bovine serum. Cells were grown at 37°C in a humidified in an atmosphere of 5% CO2 incubator.
Immunoblot
Whole-cell lysates were separated on a 10% sodium dodecyl sulfate polyacrylamide gel and transferred to nitrocellulose. Membranes were then probed with anti-LDL receptor (LDLR) antibody kindly provided by Joachim Herz (University of Texas Southwestern Medical Center at Dallas, USA).
Binding & internalization of LDL & LDL-DHA nanoparticles
LDL uniformly labeled with 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate dye (DiI; LDL-DiI) and LDL uniformly loaded and labeled with DHA and DiI dye were prepared according to the method of Pitas et al. [32]. For all of the binding and uptake studies, the cells were incubated with serum-free DMEM media overnight prior to the start of experiments.
For the binding assays, cells were incubated with LDL-DiI/LDL uniformly loaded and labeled with DHA (10 μg/ml) in serum-free DMEM culture medium for 2 h at 4°C. Since receptors are not internalized at 4°C, only binding of the ligand to the cell surface receptors is measured. After washing with phosphate-buffered saline, 1 ml of isopropanol was added to each well and the plates were rocked for 15 min in the dark. The isopropanol extract of DiI was transferred to a tube and centrifuged for 15 min at 3000 rpm. Thereafter, the DiI fluorescence signal was determined using a spectrofluorometer (excitation at 520 nm and an emission scan from 530 to 630 nm). Cells were dissolved with cell lysis buffer (1 g/l sodium dodecyl sulfate in 0.1 M NaOH) for protein determination. The calculated bound LDL-DiI in μg/ml was normalized to cellular protein in mg/ml. Parallel experiments were performed at 37°C to measure total association of LDL-DiI/LDL uniformly loaded and labeled with DHA (bound and internalized) with the cells. The amount of internalized LDL particle was calculated by subtracting the 4°C binding values from the measure of total association at 37°C.
Cell toxicity assay (MTS)
Each cell type was seeded in 96-well plates (2 × 103 cells/well). After 72 h of culture, the cells received various concentrations of the LDL-DHA nanoparticles ranging from 0 to 100 μM (DHA). For controls (LDL-OA/TO), dosing went as high as 200 μM. At the end of the 72-h treatment period, the cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay (MTS; Promega, WI, USA) as recommended by the manufacturer. In brief, 100 μl MTS solution was added to each well and cells were incubated at 37°C for 4 h followed by absorbance readings at 450 nm. The relative cell viability is expressed as a percentage of the nontreated controls.
LDL competition
TIB-73 and TIB-75 cells were incubated with 60 μM LDL-DHA nanoparticles with and without excess native LDL (tenfold) for 4 h, after which both compounds were removed and fresh media without LDL-DHA nanoparticles or native LDL was added for an additional 68 h of incubation (total 72-h incubation). Cells without treatment, with LDL-TO and with native LDL alone were used as controls. After the 72-h incubation period, MTS analyses were performed.
Coculture experiment
Cocultures of TIB-73 and TIB-75 were established and exposed to various LDL nanoparticle treatments. Imaging was performed before and after the treatment period. Full details of the culturing methods performed in this experiment are described in the Supplementary Material.
Cell death assay
A total of 72 h following LDL nanoparticle treatments (40 μM; as described above) cells were stained with the Promokine (Heidelberg, Germany) Apoptotic/Necrotic Cells Detection Kit according to the manufacturer’s protocols. The annexin V-fluorescein isothiocyanate and propidium iodide (PI) double staining method was used to provide readouts of apoptotic and necrotic cells, respectively. Cells were analyzed by fluorescent microscope (NIKON Eclipse E600 microscope; Nikon, TX, USA) and flow cytometric analysis (FACScan™ flow cytometer; Becton Dickson, CA, USA).
Lipid peroxidation
The total amount of lipid peroxidation products formed in the cells was determined using the thiobarbituric acid method [33].
Cellular reactive oxygen species
Cellular reactive oxygen species (ROS) content was measured by incubating cells with 15 μM 5-(and-6)-carboxy-2′,7′-dichloro-dihydrofluorescein diacetate (DCF) for 1 h at 37°C. After incubation, cells were washed with phosphate-buffered saline, and intracellular ROS accumulation was quantified with fluorescence spectrophotometry (Hitachi F-7000 Fluorescence Spectrophotometer with excitation at 485 nm and an emission scan from 500 to 560 nm; Hitachi, CA, USA) or observed and photographed using a fluorescence microscope (NIKON Eclipse E600 microscope) operating with a green filter set.
Statistical evaluation
The results were expressed as mean ± standard error. Analysis of variance with Tukey’s multiple comparison post hoc testing was used for evaluation of differences between groups. Differences with a p-value of <0.05 were deemed significant.
Results
Nanoparticle characterization
Replacement of the cholesteryl ester/triacylglycerol core of plasma LDL with DHA, as described by the reconstitution method, yields LDL particles that are uniformly loaded with DHA (Figure 1). Compositional analysis (Table 1) shows that, on average, for each LDL-DHA nanoparticle (given one copy of apoB-100 per LDL), 390 phospholipid molecules make up its amphipathic monolayer shell while 1453 molecules of DHA are incorporated into this structure. LDL-DHA nanoparticles do not contain any free unesterified cholesterol, as seen in the surface of native LDL. Free cholesterol, along with the core neutral lipids, is extracted from the LDL particle during the reconstitution process [31]. The efficiency of the reconstitution reaction was evaluated and was found to produce the LDL-DHA nanoparticles with good yield. The percentage protein and DHA recovery from the initial starting materials were 46 and 13%, respectively. In addition, the average lipid- (DHA) to-protein mass ratio was calculated to be 0.82. These recovery values are in keeping with those cited in Krieger et al. for cholesteryl linoleate (the principle cargo of plasma LDL) [31].
Figure 1. Docosahexaenoic acid-loaded low-density lipoprotein nanoparticle.
(A) Schematic drawing of LDL-DHA nanoparticle. (B) Transmission electron microscopy images of (B,i) native LDL and (B,ii) LDL-DHA nanoparticles.
DHA: Docosahexaenoic acid; LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein.
Table 1.
Composition and physicochemical properties of native low-density lipoprotein and low-density lipoprotein nanoparticles.
| Parameter | Native LDL | LDL-TO | LDL-OA | LDL-DHA |
|---|---|---|---|---|
| ApoB-100 (number of molecules per LDL particle) | 1 | 1 | 1 | 1 |
| Phospholipid (number of molecules per LDL particle) | 734 ± 114 | 862 ± 173 | 373 ± 79.7 | 386 ± 113 |
| Cholesterol (number of molecules per LDL particle)† | 2958 ± 378 | Not detectable | Not detectable | Not detectable |
| Lipid cargo (number of molecules per LDL particle) | ‡ | 344 ± 26.6 | 1401 ± 371 | 1453 ± 92 |
| Diameter (nm) | 18.2 ± 0.3 | 20.3 ± 0.7 | 20.0 ± 0.9 | 18.3 ± 0.5 |
| Surface charge (mV) | −8.3 ± 0.7 | −15.0 ± 2.5 | −26.7 ± 6.1 | −21.9 ± 3.3 |
Total cholesterol includes cholesteryl esters and free cholesterol. Literature values indicate that LDL typically carries 1300–1600 cholesteryl esters, and 500–600 free cholesterol molecules [43,79].
LDL also carries approximately 170 triglyceride molecules. DHA typically makes up only 1% of the total fatty acid composition of LDL [80].
The data represent the mean ± the standard error.
LDL: Low-density lipoprotein; LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein; LDL-OA: Low-density lipoprotein reconstituted with oleic acid; LDL-TO: Low-density lipoprotein reconstituted with oleic acid triglyceride.
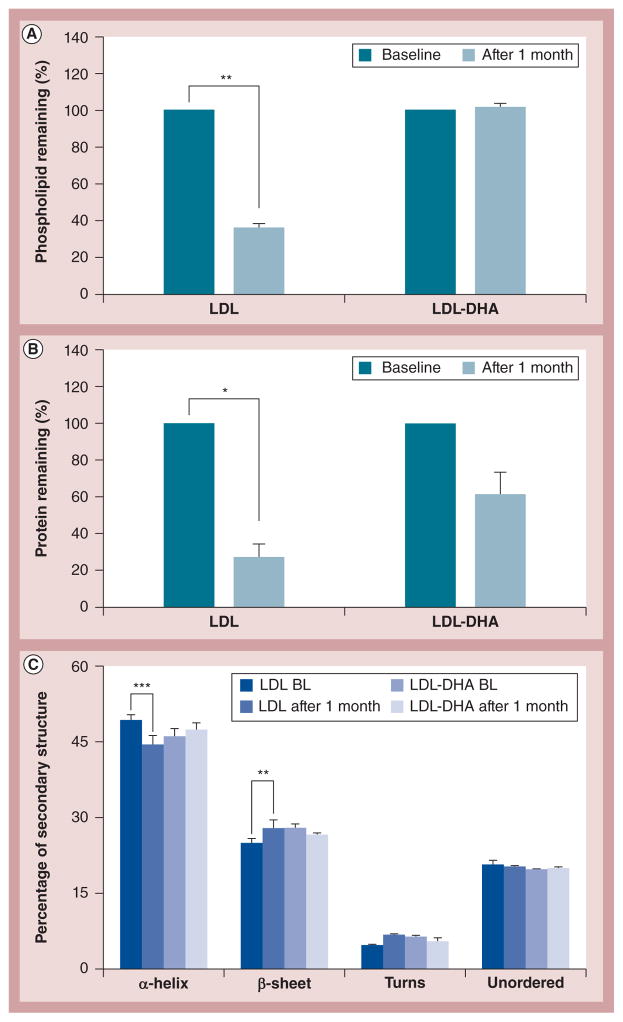
The LDL-DHA nanoparticle retains much of the structure and morphological features of native LDL. Transmisssion electron microscopy micrographs (Figure 1B) and DLS analyses reveal that LDL-DHA nanoparticles consist of a fairly uniform population of quasispherical-shaped particles with an average particle diameter of 18.3 ± 0.53 nm (PDI: 0.26). The other reconstituted LDL nanoparticles, LDL-OA and LDL-TO, also displayed similar size and morphology. The conformational structure of LDL-DHA nanoparticle apoB-100 was also monitored by circular dichroism spectrophotometry (Figure 2C). The spectral secondary structure readings of apoB-100 in LDL-DHA nanoparticles indicate that it contains 46% α-helix, 28% β-sheet, 6% β-turns and 20% unordered structure. The protein secondary structure of apoB-100 from native LDL had a similar conformation (49% α-helix, 25% β-sheet, 5% β-turns and 21% unordered structure). The apoB-100 from LDL-OA and LDL-TO also contained a similar secondary conformation.
Figure 2. Composition characterization and stability.
All of the samples were stored at ambient conditions and were subjected to composition analysis at baseline and at 1 month. (A) Phospholipid content, (B) protein content, and (C) protein secondary structure analysis using circular dichroism. *, ** and *** represent a significant difference from the corresponding group at p < 0.05, p < 0.01 and p < 0.001, respectively. The error bars represent the standard error of the mean.
BL: Baseline; LDL: Low-density lipoprotein; LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein; LDL-DHA-BL: Docosahexaenoic acid-loaded low-density lipoprotein baseline.
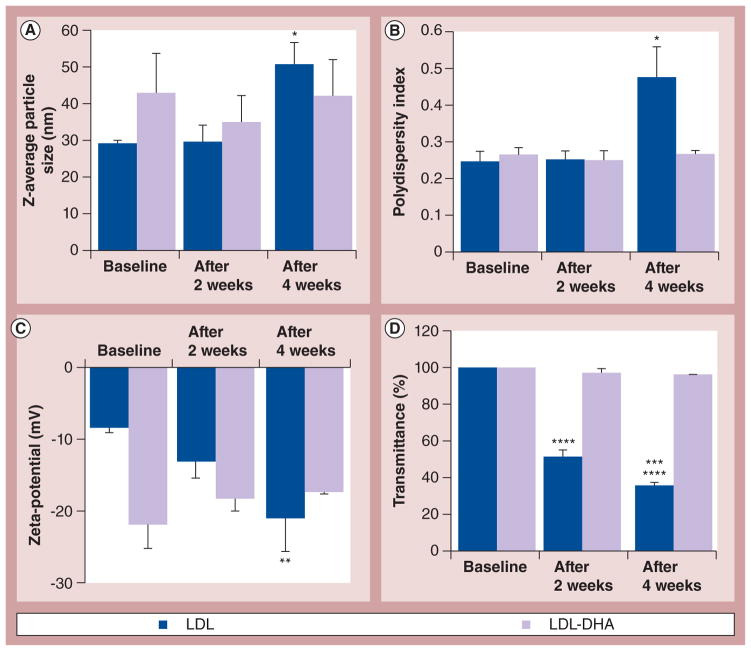
The zeta-potential measure of surface charge for LDL-DHA nanoparticles differed considerably from native LDL (−22 and −8 mV, respectively; Figure 3C). These findings were confirmed by agarose gel electrophoresis as LDL-DHA nanoparticles showed a marked increase in its electrophoretic mobility (Supplementary Figure S1). Interestingly, LDL-OA also displayed a strong electronegative surface charge and electrophoretic mobility while LDL-TO possessed values similar to native LDL.
Figure 3. Physicochemical properties and stability.
All of the samples were stored at ambient conditions and were subjected to physicochemical analysis at baseline, 2 weeks and 4 weeks. (A) Z-average particle size (*p < 0.05), (B) polydispersity index (*p < 0.05, LDL baseline or LDL after 2 weeks vs LDL after 4 weeks), (C) zeta-potential (**p < 0.01, LDL baseline or LDL after 2 weeks vs LDL after 4 weeks), and (D) turbidity (****p < 0.0001, LDL baseline vs LDL after 2 weeks or LDL after 4 weeks; ***p < 0.001, LDL after 2 weeks vs LDL after 4 weeks). The error bars represent the standard error of the mean.
LDL: Low-density lipoprotein; LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein.
Stability experiments
The physicochemical stability of LDL-DHA nanoparticles and native LDL was monitored over a 1-month period under ambient conditions (room temperature and air). LDL-DHA nanoparticles showed notable stability across all of the physicochemical parameters: the phospholipid and DHA content remained unaltered and showed no signs of leakage (Figures 2A & 4A), the Z-average particle size, PDI, turbidity and zeta-potential were similarly unchanged (Figure 3), and the composition of the apoB-100 secondary structure was preserved (Figure 2C). The apoB-100 protein content did decrease on average by 37% in the LDL-DHA nanoparticle samples; however, this change was not deemed significant. Conversely, native LDL particles displayed pronounced degradative changes over this period. The content of apoB-100 protein decreased by 75% and its secondary structure composition was significantly altered (Figures 2B & 2C). Similarly, the phospholipid content in native LDL also decreased by 63% (Figure 2A). Significant flocculation of the native LDL occurred during this time as the Z-average particle size and PDI markedly increased, with a corresponding decrease in turbidity (Figures 3A, 3B & 3D). The zeta-potential of the native LDL also steadily decreased over the 4 weeks (Figure 3C).
Figure 4. Oxidative stability.
(A) HPLC determination of DHA content in LDL nanoparticles and DHA in heptane. (B) Peroxide indices of LDL, DHA in heptane and LDL-DHA nanoparticles. Samples from (A) and (B) were stored at ambient conditions and were subjected to HPLC or peroxide analysis at baseline, 7, 14, 21 and 28 days. (C) Peroxide indices of LDL, DHA in heptane and LDL-DHA nanoparticles over a 4-day period (baseline, 1 day and 4 days) at 40°C. *, **, ***, **** represents a significant difference from the corresponding LDL-DHA group at p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively. (D) Peroxide indices of LDL, DHA in heptane and LDL-DHA nanoparticles at baseline and after a 5-h incubation at 55°C. *** represents a significant difference from the corresponding baseline control group at p < 0.001. The error bars represent the standard error of the mean.
DHA: Docosahexaenoic acid; LDL: Low-density lipoprotein; LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein.
Next, the oxidative stability of LDL-DHA nanoparticles, DHA in heptane and native LDL was measured under ambient and elevated stress conditions (Figure 4). For the samples maintained at ambient room temperature and air for 1 month, the concentration of DHA in heptane decreased by 43%, accompanied by a sharp increase in the peroxide index (12 mEq/kg lipid). Over this time period, native LDL also showed a sharp rise in its peroxide index (14 mEq/kg lipid). Under the same conditions, LDL-DHA nanoparticles experienced little peroxidation (3 mEq/kg lipid increase; Figure 4B). These findings agree with the stable content of DHA in LDL over this time period (Figure 4A). The stability of LDL-DHA nanoparticles was then further tested under conditions of enhanced stress. Each sample was kept under air at 40°C for 4 days. During this stress test period, native LDL and DHA in heptane experienced a drastic rise in peroxide indices (>13.0 mEq/kg lipid). Once again, the peroxidation process was significantly blunted in LDL-DHA nanoparticles, where peroxide levels only reached 5.5 mEq/kg lipid (Figure 4C). In the final stress test, samples were incubated under air at 55°C for 5 h. Even under these extreme conditions, the oxidative stability of LDL-DHA nanoparticles was maintained. By contrast, native LDL and DHA in heptane succumbed to significant degradative peroxidation (Figure 4D).
Biological activity of LDL-DHA nanoparticles
The mouse liver cell lines TIB-73 and TIB-75 displayed markedly different morphological features and patterns of growth (Supplementary Figure S2). The TIB-73 cells demonstrated more epithelial characteristics, as they grew as a monolayer in a ‘cobblestone’ pattern, similar to mature hepatocytes. The TIB-75 cells, on the other hand, displayed more mesenchymal features. These cells grew in a more chaotic manner, often forming multiple layers of cells.
Next, we examined how avidly TIB-73 and TIB-75 cells bind and take up LDL particles. Western blot analysis showed that TIB-73 cells expressed slightly higher levels of LDLR protein than TIB-75 cells (Figure 5A). The binding assay showed that both TIB-73 and TIB-75 cells avidly bound and internalized LDL to a similar degree (Figure 5B). Complementary microscopy experiments showed that the uptake of LDL-DiI was specific (through LDLR), as excess LDL was able to reduce the uptake of the fluorescent LDL (Figure 5D). The binding and uptake of LDL-DHA nanoparticles was similar to that of native LDL for the TIB-73 cells. Competition experiments similarly showed that LDL-DHA nanoparticle uptake was inhibited by excess native LDL (Supplementary Figure S3). The capacity of TIB-75 cells to take up LDL-DHA nanoparticles, however, was less than that for native LDL (Figure 5C). Nevertheless, excess native LDL was still able to reduce the uptake of LDL-DHA nanoparticles in TIB-75 cells, indicating the involvement of LDLR in its internalization (Supplementary Figure S3).
Figure 5. Biological activity of docosahexaenoic acid-loaded low-density lipoprotein.
(A) Representative western blot of LDLR expression in TIB-73 and TIB-75 cells with β-actin expression as a protein loading control. (B & C) The binding and uptake of (B) 10 μg/ml LDL-DiI or (C) LDL-DHA-DiI in TIB-73 and TIB-75 cells following a 2-h incubation at 4°C (external binding) or 37°C (total binding and uptake). Internalization of LDL-DiI or LDL-DHA-DiI is calculated from the difference between the 37 and 4°C experiments. Values are expressed as μg of LDL-DiI or LDL-DHA-DiI per mg of cell protein. Values represent the mean of four independent experiments where each condition was performed in duplicate. (D) Fluorescent images of TIB-73 and TIB-75 cells treated with 10 μg/ml LDL-DiI or 10 μg/ml LDL-DHA-DiI and excess native LDL (500 μg/ml) for 2 h at 37°C. The error bars represent the standard error of the mean. LDL: Low-density lipoprotein; LDL-DHA-DiI: Low-density lipoprotein uniformly loaded and labeled with docosahexaenoic acid and 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate dye; LDL-DiI: Low-density lipoprotein uniformly labeled with 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate dye; LDLR: Low-density lipoprotein receptor.
Cytotoxicity experiments
The sensitivity of the liver cell lines to escalating concentrations of LDL-DHA nanoparticles was evaluated by the MTS assay (Figure 6). LDL-DHA nanoparticles were rapidly cytotoxic to the TIB-75 cells. The viability of these cells quickly declined with the initial doses of LDL-DHA nanoparticles (10–40 μM) and complete ablation of the TIB-75 cells was achieved at 60 μM LDL-DHA nanoparticle treatment. Conversely, LDL-DHA nanoparticles appeared to be innocuous to the TIB-73 cells over much of the dose–response curve. In fact, the viability of the treated TIB-73 cells exceeded that of the baseline controls up to 70 μM LDL-DHA nanoparticles. It was not until the LDL-DHA nanoparticles reached concentrations exceeding 80 μM that the TIB-73 cells experienced cytotoxic effects.
Figure 6. Docosahexaenoic acid-loaded low-density lipoprotein anticancer activity.
TIB-73 and TIB-75 cells were treated for 72 h with increasing concentrations of (A) vehicle control LDL-TO, (B) LDL-DHA or (C) TIB-75 cells treated with human serum albumin associated with DHA or LDL-DHA. Following treatment, cell viability was determined by MTS assay where cell viability is normalized to the untreated cell controls. (D) Cocultures of TIB-75 and TIB-73 cells were treated with 60 μM LDL-DHA or LDL-TO for 72 h. After treatment, the cells were imaged at 10× magnification on an inverted bright field microscope and at 0.5× magnification on a dissecting microscope. The error bars represent the standard error of the mean. The triolein dose is equivalent to three oleic fatty acid chains (e.g., 50 μM oleic acid triglyceride ≈ 150 μM oleic acid).
DHA: Docosahexaenoic acid; LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein; LDL-TO: Low-density lipoprotein reconstituted with oleic acid triglyceride.
The specificity of LDL-DHA nanoparticle toxicity towards malignant TIB-75 cells was confirmed with corresponding treatments of LDL-TO and LDL-OA (Figure 6A & Supplementary Figure S4). MTS readings indicated that neither LDL-TO nor LDL-OA elicited significant cytotoxicity towards TIB-73 or TIB-75 cells over an equivalent dose range compared with LDL-DHA nanoparticles.
Since albumin functions as one of the major transporters of free fatty acid in the plasma, we decided to evaluate the efficacy of HSA associated with DHA (HSA-DHA) to kill TIB-75 cells (Figure 6C). HSA-DHA failed to induce any toxicity in TIB-75 cells over an extended dose range up to 200 μM. Rather, the viability of TIB-75 was higher than that of untreated controls for most of the treatment doses of HSA-DHA. Interestingly, HSA-DHA treatment consistently compromised the viability of TIB-73 cells to 70% of that of the untreated controls over the entire dose range (Supplementary Figure S5).
To further validate the MTS dose–response findings, cocultures of TIB-73 and TIB-75 were grown in 60-mm culture dishes (Figure 6D). Over the duration of these experiments, each cell type displayed its typical phenotypes. As expected, treating the cocultured cells with LDL-TO for 72 h did not hinder the proliferation of either cell type; both cell types seemed to thrive under these conditions. In a similar manner, cocultures of TIB-73 and TIB-75 were treated with LDL-DHA nanoparticles (60 μM). At the end of the treatment period, TIB-73 cells did not experience any toxicity and continued to grow in their ‘cobblestone’ manner, filling much of their half of the culture dish. The TIB-75 cells had a much different fate; by the end of the 3-day incubation period, they were completely killed. Only cell debris remained in the hemisphere initially occupied by TIB-75. Overall, these findings were consistent with the MTS dose–response studies and clearly demonstrated the selective cytotoxicity of LDL-DHA nanoparticles towards the malignant TIB-75 cells.
LDL competition
LDL competition studies showed that excess native LDL was able to fully block the LDL-DHA nanoparticle-mediated toxicity in TIB-75 cells (Figure 7). The protective effects of native LDL against LDL-DHA nanoparticles could not be assessed in TIB-73 cells at this dose; however, at higher doses of LDL-DHA nanoparticles, the excess native LDL proved protective for TIB-73 cells (data not shown). These findings, combined with the earlier fluorescence experiments, collectively indicate that LDL and LDL-DHA nanoparticles are internalized into cells by a similar uptake mechanism, which is mainly governed by LDLR.
Figure 7. The effect of excess native low-density lipoprotein on the efficiency of docosahexaenoic acid-loaded low-density lipoprotein cell killing.
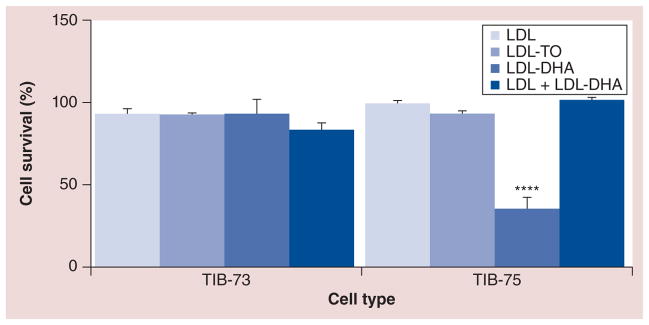
LDL-DHA nanoparticles (60 μM) was added to TIB-73 and 75 cells with and without excess native LDL (tenfold) for 4 h, after which both compounds were removed and fresh media without LDL-DHA or native LDL was added for an additional 68 h of incubation (total 72-h incubation). Cells without treatment, with LDL-TO and with native LDL alone were used as controls. After the 72-h incubation period, MTS analyses were performed. The error bars represent the standard error of the mean.
**** represents a significant difference from the remaining groups at p < 0.0001.
LDL: Low-density lipoprotein; LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein; LDL-TO: Low-density lipoprotein reconstituted with oleic acid triglyceride.
Mode of cell death
To elucidate the specific cell death pathways elicited by LDL-DHA nanoparticle treatment, annexin V-fluorescein isothiocyanate and PI staining was performed in conjunction with flow cytometry and fluorescence microscopy (Figure 8). FACS analysis showed that neither LDL-TO nor LDL-DHA nanoparticle treatments (60 μM) induced greater rates of apoptosis or necrosis in TIB-73 cells compared with normal untreated controls (~5–6%). This was also confirmed with fluorescence microscopy as the LDL-TO- and LDL-DHA nanoparticle-treated TIB-73 images looked identical to the untreated TIB-73 group. For the TIB-75 cells, FACS analysis showed that LDL-DHA nanoparticle treatment markedly activated both cell death pathways. TIB-75 cells showed significantly higher amounts of late apoptosis/early necrosis and late necrosis compared with all of the other groups. This was also vividly demonstrated in the fluorescence microscopy images as LDL-DHA nanoparticle-treated TIB-75 cells showed intense individual and coregistered staining of annexin V and PI, which was absent from the images of the other groups.
Figure 8. Mode of cell death.
(A–F) Fluorescence microscopy of TIB-73 and TIB-75 cells following a 72-h treatment with LDL nanoparticles (40 μM). The mode of cell death was assessed by staining the cells with annexin V-fluorescein isothiocyanate to detect apoptosis (green) and propidium iodide to detect necrosis (red). Concurrent apoptosis and necrosis coregistered as yellow fluorescence. The treatment groups are as follows: (A) TIB-73 untreated; (B) TIB-73 LDL-TO treated; (C) TIB-73 LDL-DHA nanoparticle treated; (D) TIB-75 untreated; (E) TIB-75 LDL-TO treated; and (F) TIB-75 LDL-DHA nanoparticle treated. (G) Quantitative analysis of the same treatment groups was performed by FACS analysis using two-color flow cytometric measurements. Quadrant gating was used to determine the percentage of cells in different stages of apoptosis and necrosis. Results are expressed as percentage of total cell population. The error bars represent the standard error of the mean.
** represents a significant difference from the corresponding control groups at p < 0.01.
LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein; LDL-TO: Low-density lipoprotein reconstituted with oleic acid triglyceride.
Mechanism of selective cytotoxicity
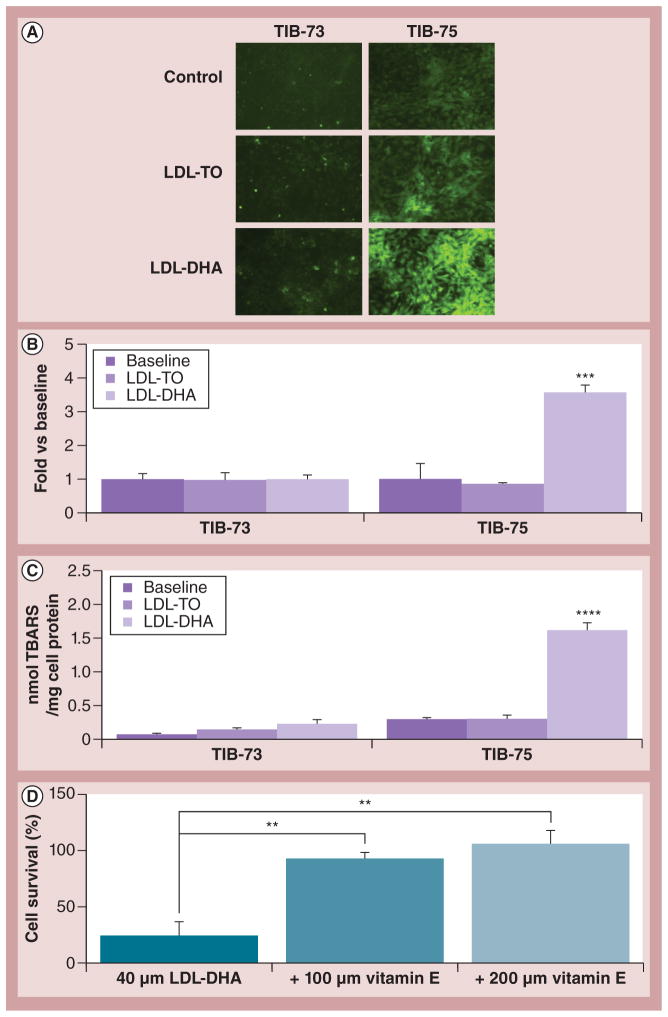
To investigate the mechanism of LDL-DHA nanoparticle selective toxicity, lipid peroxidation and the production of ROS were examined in the cells before and following treatment (Figure 9). Prior to LDL-DHA nanoparticle treatment, paired comparisons of untreated TIB-73 and TIB-75 were performed (Supplementary Figure S6). TIB-75 cells were found to have higher levels of oxidative stress as indicated by higher DCF fluorescence (ROS) and thiobarbituric acid reactive species (lipid peroxidation) readings. Thereafter, LDL-DHA nanoparticle treatment was shown to selectively induce even greater amounts of lipid peroxidation and ROS in TIB-75 cells (Figures 9A–9C). Thiobarbituric acid reactive species and DCF-reactive species were significantly elevated three- to four-fold in the LDL-DHA nanoparticle-treated TIB-75 cells. Neither biomarker was elevated in the TIB-73 cells following LDL-DHA nanoparticle treatment. An additional experiment later showed that coincubation with the antioxidant vitamin E was able to effectively abrogate the cytotoxicity of LDL-DHA nanoparticles in TIB-75 cells (Figure 9D). Collectively, these findings support the central role of lipid peroxidation and ROS induction in the selective killing of malignant TIB-75 cells by LDL-DHA nanoparticles.
Figure 9. Mechanism of selective cytotoxicity.
(A) Microscopy of 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate fluorescence in TIB-73 and TIB-75 cells at baseline and 24 h following treatment with 60 μM LDL-TO or LDL-DHA nanoparticles (20× magnification). (B) Quantification of 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate fluorescence in TIB-73 and TIB-75 cells by fluorometer (excitation 485 nm and emission 526 nm). Values represent the fold difference in 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate fluorescence per mg of cell protein normalized to the untreated cells (n = 3). (C) TBARS analysis of TIB-73 and TIB-75 cells at baseline and 24 h following treatment with 40 μM LDL-TO or LDL-DHA nanoparticles. TBARS values were normalized to cell protein (n = 5). ***, **** represents a significant difference from the remaining groups at p < 0.001 and p < 0.0001, respectively. (D) Cell viability assay of TIB-75 cells following 72 h of treatment with 40 μM LDL-DHA nanoparticles with or without 100 or 200 μM vitamin E (n = 4). ** represents a significant difference from the corresponding groups at p < 0.01. The error bars represent the standard error of the mean.
LDL-DHA: Docosahexaenoic acid-loaded low-density lipoprotein; LDL-TO: Low-density lipoprotein reconstituted with oleic acid triglyceride; TBARS: Thiobarbituric acid reactive species.
Discussion
Characterization of LDL-DHA nanoparticles
The concept of utilizing LDL as a drug-delivery vehicle was first proposed over three decades ago [28,34,35]. In these studies, plasma LDL was described as a potential transport platform on which anticancer agents could be incorporated and specifically delivered to cancer cells. Over the years, many groups have attempted to formulate various chemotherapeutics (e.g., doxorubicin, vincristine and paclitaxel, among others) with LDL [36–38]. However, these LDL–drug complexes suffered from poor stability, altered apoB-100 integrity and low drug loading capacity [37–41]. In the present study, an alternate approach was taken towards LDL-mediated cancer therapy; instead of trying to incorporate and transport conventional chemotherapeutics in LDL, the natural lipid, DHA, was selected as the bioactive cargo. Being a natural lipid, DHA readily incorporates into LDL through the reconstitution process; over 1400 DHA molecules are accommodated into the core shell structure of LDL. This is exceptionally good loading considering that LDLs typically carry 1500 cholesteryl esters. Previous investigators have tried to ‘enrich’ plasma LDL with PUFA by feeding African Green Monkeys a diet high in ω-3 fatty acids for 3–5 years [42]. Subsequent purification of their plasma yielded LDL containing up to 6% DHA fatty acids. The considerable time and cost associated with this procedure, combined with the relatively small increases of DHA enrichment, severely limits the practicality of this dietary supplementation approach. The LDL reconstitution method [31] offers an efficient and facile approach to incorporating DHA into LDL particles.
The LDL-DHA nanoparticles formed from this procedure retain nearly all of the physicochemical properties and biological activities of their native counterparts. Particle size, morphology and apoB-100 protein conformation were all comparable with that seen in native LDL. The only LDL-DHA nanoparticle parameters that differed significantly from native LDL were the particles’ concentration of phospholipids and surface charge properties (zeta-potential).
The amphipathic phospholipid/protein outer layer of native LDL serves to thermodynamically stabilize the hydrophobic core of the particle. Approximately 700 phospholipid molecules make up this monolayer shell, which is highly dynamic and fluid, in spite of the presence of free cholesterol in the slightly deeper interfacial layer [43]. Similarly, the globular domains of apoB-100 flexibly surround the LDL particle in a ‘belt-like’ manner, interspersing the phospholipid layer and changing its configuration as needed [44,45]. The outer shell of LDL-DHA nanoparticles, as well as LDL-OA, contained significantly fewer phospholipids than that of native LDL. In spite of this deficit, the dynamic and flexible phospholipid/protein shell of these reconstituted nanoparticles was able to maintain the stability of its core and the entire lipoprotein structure. In fact, as will be discussed later, the overall stability of the LDL-DHA nanoparticles was observed to be superior to that of native LDL.
LDL-DHA and LDL-OA nanoparticles also had significantly greater negative surface charge compared with LDL-TO and native LDL particles. These findings were determined by zeta-potential measurements and confirmed by agarose gel electrophoresis. Typically, such negatively charged surface properties are associated with oxidative degradation of LDL. However, the intact apoB-100 conformation, low peroxide values and avid/intact LDLR binding affinity of these nano-particles argue against this view. Given that only LDL particles reconstituted with free fatty acids demonstrated this property, perhaps the anionic species of the unesterified fatty acids, potentially situated in the interfacial layer, may be contributing to this negative surface charge [46,47]. Studies are ongoing in our laboratory to elucidate these findings.
Enhanced nanoparticle stability
The pronounced electronegative surface charge of LDL-DHA nanoparticles probably contributes to the enhanced colloidal stability of this particle over its native counterpart [47]. In general, higher zeta-potential values, regardless of their positive or negative charge, induce stronger electrostatic repulsions that resist flocculation and promote greater stability of particles [48]. The results from our study were also consistent with this principle. Over the 1-month study period under ambient temperature and air, LDL-DHA nanoparticles maintained zeta-potential values between −22 and −17 mV. Corresponding values for Z-average particle size, polydispersity and turbidity of the LDL-DHA nanoparticles remained unchanged, indicating good colloidal stability. Conversely, native LDL, which had a much weaker initial surface charge (−8 mV), underwent significant aggregation, as seen by the increased Z-average particle size and polydispersity and the sharp drop in its turbidity readings. At the end of the 4-week period, the zeta-potential value for native LDL dropped to −21 mV; these changes were the result of oxidative degradation. The corresponding levels of apoB-100 protein, secondary structure conformation and peroxide values for native LDL confirm these oxidative changes. These findings are in agreement with others who have shown that the human LDL will undergo significant degradative changes even when stored under mild conditions [49,50].
Interestingly, LDL-DHA nanoparticles were also resistant to oxidative degradation. Reconstituted DHA displayed remarkable retention and stability in the LDL particle, as there was less than 10% loss over a 4-week period at room temperature. Conversely, free DHA in organic solvents rapidly degrades under these conditions (Figure 4A). The DHA molecules probably assume a tightly packed conformation in the confines of the LDL particle, similar to that of DHA micelles or emulsions in aqueous solutions. Several reports have shown that, in this conformation, DHA is more resistant to oxidative attack [51,52]. In addition, the phospholipid protein shell of LDL may shield the DHA from its surrounding aqueous environment and limit the accessibility of oxidizing agents [53,54]. Indeed, the phospholipid protein shell presents a barrier to solubilized O2, as its diffusion is ten-times faster in aqueous media than in the lipid milieu of LDL [55]. Although O2 tends to partition into lipids over water, the ordered phospholipid protein shell, along with the tightly packed DHA in the LDL particle, probably constrains the dissolution of O2 into the particle [55]. Surprisingly, it can also be said that the incorporation of DHA into the LDL platform imparted enhanced oxidative stability to the overall LDL nanoparticle structure as well. The mechanism of this protection is unknown, but other studies corroborate these findings in demonstrating decreased oxidative susceptibility among LDLs with high DHA content [56,57].
The stability of the LDL-DHA nanoparticles was maintained even when confronted with enhanced oxidative stress. Stress test conditions involving moderate temperature elevations for prolonged periods (4 days at 40°C) or pronounced temperature elevations for shorter durations (5 h at 55°C) both induced significant peroxide production in free DHA and native LDL, indicating marked auto-oxidation. These findings were expected as elevated temperatures significantly increase the permeability of O2, the kinetics of lipid auto-oxidation and the overall denaturation of LDL [58]. However, under these same conditions, the integrity of the LDL-DHA nanoparticles was retained. It stands to reason that the protective mechanism described above also shielded the LDL-DHA nanoparticles from extensive oxidative deterioration during the stress tests.
Biological activity of LDL-DHA nanoparticles
The biological activity of the LDL-DHA nanoparticles was evaluated in the paired normal and malignant murine liver cell lines TIB-73 and TIB-75, respectively. TIB-73 is an immortalized hepatocyte cell line derived from the liver of BALB/c mice, which is neither tumorigenic when injected into mice nor capable of anchorage-independent growth [59]. TIB-73 cells show typical normal hepatocyte morphology and produce liver-specific proteins aldolase B, α-fetoprotein, ferritin and γ-glutamyltransferase, as well as the less specific markers aspartate aminotransferase and alanine aminotransferase [60]. TIB-75 is a HCC cell line derived from TIB-73 by transformation with methylcholanthrene epoxide [59]. TIB-75 is tumorigenic when injected into mice [59] and expresses high levels of HGF, epithelial–mesenchymal transition traits, COX-2 and Akt activity [61]. Comparative studies of these two cell lines offer an excellent opportunity for identifying cancer-specific pathways and sensitivities. Distinct differences were noted in their morphology in cell culture. The TIB-73 cell line displayed typical cobblestone epithelial growth patterns. Conversely, TIB-75 demonstrated more chaotic mesenchymal morphology consistent with a malignant phenotype [61]. Only a modest difference in LDLR expression was seen between the two cell lines, with TIB-73 presenting slightly more protein. In most accounts, malignant transformation is commonly associated with the overexpression of the LDLR protein [62,63]. Although the TIB-75 cell line displayed slightly less LDLR than its normal counterpart, this deficit did not hinder the ability of the TIB-75 cells to bind or internalize LDL (Figure 5). This compensation in the TIB-75 cells may be the result of faster binding and uptake kinetics of LDLR for LDL; alternatively, the TIB-75 cells may recruit other cell-surface receptors and transporters (e.g., lipoprotein-binding site and LDLR-related protein 6, among others) [64,65] to help engage and internalize LDL.
The TIB-73 cells bound and internalized LDL-DHA nanoparticles in a similar LDLR-dependent manner as native LDL. These results further confirm that the apoB-100 conformation and LDLR binding properties were retained in the reconstituted LDL-DHA nanoparticle. Conversely, for the TIB-75 cells, the uptake of LDL-DHA nanoparticles was less than that for native LDL. Although the mechanism of uptake for LDL-DHA nanoparticles seems to occur mainly through LDLR, the compensatory mechanisms described above for native LDL uptake do not seem to operate for LDL-DHA nanoparticle uptake. Perhaps the more electronegatively charged LDL-DHA nanoparticles may be experiencing unique hindrances binding/interacting at the TIB-75 cell surface. Clearly, further studies are needed to fully characterize the binding/uptake kinetics of LDL particles in TIB-75 cells.
Anticancer activity
The findings of the present study clearly demonstrate that LDL-DHA nanoparticles preferentially kill cancer cells. Therapeutic concentrations of LDL-DHA nanoparticles that are lethal to monocultures of malignant TIB-75 prove to be innocuous to TIB-73. Furthermore, the coculture experiments vividly show the ability of LDL-DHA nanoparticles to preferentially ablate cancer cells in the presence of normal cells. The normal TIB-73 counterparts do not experience any collateral injury during these experiments and continue to grow. Similarly, in the body, malignant cells grow next to normal tissues, and thus the findings of these experiments suggest that LDL-DHA nanoparticles may be equally discriminating in an in vivo setting. Such pronounced therapeutic selectivity is rarely seen and is highly desired in oncology research. The anticancer actions of PUFAs have been reported since the early 1980s [66,67]. In these studies, Dippenaar and colleagues proposed that the viability of cancer cells was dependent upon PUFA deficiency; they went on to show that supplementation with PUFA (particularly γ-linolenic acid) significantly inhibited the growth of a variety of malignant cells [66]. Since these studies, researchers have relied on organic solvents to solubilize these lipids for biological investigation. Until the present study, adequate physiological delivery of PUFA, apart from oral intake, remained elusive. While albumin serves as a major colloidal carrier in plasma, its limited fatty acid-carrying capacity, indiscriminant distribution and ambiguous intracellular delivery potentially detract from its utility as an anticancer drug-delivery vehicle. Our studies show that when DHA is associated with HSA, it is ineffective at killing TIB-75 cells up to doses of 200 μM. These results are corroborated by the finding of others who report that albumin-mediated delivery of ω-3 PUFA only weakly inhibits proliferation or the induction of apoptosis in cancer cells [42]. Further studies by Kanno et al. concluded that albumin is able to modulate the actions of DHA and thereby protect cancer cells from DHA-mediated injury [16]. The LDL nanoparticle, on the other hand, has proven to be very effective at delivering and mediating the cytotoxic actions of DHA towards cancer cells. Following internalization of the LDL-DHA nanoparticle complex, the liver cancer cells experience pronounced cytotoxic reactions that activate both apoptotic and necrotic processes. This mixed pattern of cell death indicates that several ante mortem pathways are probably triggered by LDL-DHA nanoparticle treatment. In this event, an intense activation of death processes along multiple fronts overwhelms the homeostatic systems in the TIB-75 cells, resulting in rapid and severe cell kill. It should be noted that the anticancer efficacy of LDL-DHA nanoparticles is not dependent upon the overexpression of LDLR on cancer cells, as is seen with other receptor-mediated nanotherapies. The fact that TIB-75 expresses less LDLR and takes up equal or slightly less LDL-DHA nanoparticles than TIB-73 suggests that the selective cytotoxicity of this therapy is mainly governed by DHA.
To date, several mechanisms have been proposed to explain the anticancer actions of DHA, one of which is the general cytotoxic effects of fatty acids. The dual polar–nonpolar nature of nonesterified fatty acids allows these molecules to behave like detergents [68,69]. Ionized fatty acid micelles are able to solubilize membrane lipids and proteins and disrupt the integrity of cell membranes [68]. This indiscriminant toxicity was not evident with equimolar treatments of LDL-OA or LDL-TO, and thus it can be reasoned that the cytotoxic properties of DHA cannot be explained by this general ‘detergent’ or lipotoxic effect.
Lipid peroxidation and oxidative stress are commonly cited as pathways of DHA-induced cytotoxicity due to the high oxidative susceptibility from the many bisallylic hydrogens present in this fatty acid molecule. The findings of the present study also support this premise. In addition to succumbing to catastrophic cytotoxicity, LDL-DHA nanoparticle-treated TIB-75 cells experienced a drastic increase in the intracellular levels of lipid peroxides and ROS. During the lipid peroxidation process, the long unsaturated hydrocarbon chain of DHA is first degraded to various lipid hydroperoxides and finally to a variety of alkoxyl and peroxyl radicals [70]. High levels of alkoxyl aldehyde end products (e.g., malondialdehyde), as determined from their chromogen adducts with thiobarbituric acid reactive species, attest to escalated activities of the lipid peroxidation pathways [71]. Similarly, the pronounced DCF fluorescence detected in TIB-75 cells indicate that the ROS production in these cells far exceeds their endogenous antioxidative defenses [72]. The products of both pathways, highly reactive aldehydes and ROS species, are both known to activate both apoptosis and necrosis processes [73–76]. The lipid peroxidation and ROS-mediated cell death of TIB-75 was later validated with studies involving antioxidant supplementation. Coincubation with vitamin E, a known suppressor of lipid peroxidation and ROS production, was able to effectively protect the TIB-75 cells from LDL-DHA nanoparticle-induced cell death. The exact molecular mechanisms, whereby LDL-DHA nanoparticle lipid peroxidation and ROS processes lead to cell death, remains to be elucidated. Until this is resolved, the involvement of other highly cited DHA targets such as the Wnt/β-catenin pathway [8], PI3K/p38 MAPK pathways [77] and activation of caspase cascades should not be discounted, as they may prove to be secondary or downstream effectors in LDL-DHA nanoparticle-induced cytotoxicity.
Concomitant analysis of the TIB-73 cells following LDL-DHA nanoparticle treatment revealed that they neither experienced cell kill nor elevated lipid peroxidation or ROS production. The TIB-73 cells were able to fully tolerate the LDL-DHA nanoparticle treatments at doses that were lethal to their malignant counterparts. It should be noted that the lack of toxicity experienced by the TIB-73 cells was not the result of avoidance or reduced intracellular uptake; in fact, the TIB-73 cells internalized equal amounts or slightly more LDL-DHA nanoparticles (nonsignificant) than the TIB-75 cells (Figure 5). These findings suggest that the TIB-73 cells were able to metabolize and process the LDL-DHA nanoparticles in a benign nontoxic manner. One possible explanation may be that cancer cells (unlike normal cells) tend to operate at higher basal levels of oxidative stress (Supplementary Figure S6) [78]. Under these conditions, LDL-DHA nanoparticle-induced lipid peroxidation and ROS production would preferentially injure the cancer cells over the normal cells. Additional studies are ongoing in our laboratory to fully elucidate the factors that sensitize and protect malignant and normal cells, respectively, to LDL-DHA nanoparticle-mediated toxicity.
Conclusion
In conclusion, our study indicates that the LDL nanocarrier is an effective and appropriate transporter for DHA in the current murine system. Incorporation of DHA into the LDL particle imparts unexpected physicochemical properties that enhance the physical and oxidative stabilities of this complex over native LDL and free DHA. Finally, the LDL-DHA nanoparticle was shown to be preferentially cytotoxic to malignant murine liver cells. Normal murine liver cells did not experience any harm from the LDL-DHA nanoparticle treatment at therapeutic doses that were lethal to HCC cells. Preliminary studies in our laboratory have shown that the therapeutic efficacy of LDL-DHA nanoparticles is not limited to mouse cells; both rat and human cancer cells experience similar effects. LDL-DHA nanoparticles show great promise as an anticancer agent against such a challenging malignancy as HCC; further preclinical and clinical testing is warranted.
Future perspective
To date, ω-3 PUFAs, such as DHA, mainly serve as adjuvants for palliative or nutritional support in cancer care. While the anticancer cytotoxic properties of ω-3 PUFAs have been known for some time, the methods used for their delivery, which include organic solvents or albumin, are either impractical for in vivo applications or lack efficacy. The present study showcases the LDL nanoparticle delivery system, which enables DHA to elicit selective, lethal anticancer effects. This therapeutic strategy opens the possibility that DHA may be deliverable to tumors at sufficient concentrations to induce tumoricidal effects. As such, LDL-DHA nanoparticles may be able to serve as an active anticancer agent. Although the LDL-DHA nanoparticle may be an attractive biologic therapeutic, several caveats should be addressed. First, LDL-DHA nanoparticles are a blood-based product, thus concerns of infectious agents are warranted. Like all other blood products, the plasma-derived LDL must be subjected to intense screening and purification to meet regulatory standards. The source of plasma may be from healthy volunteers, in which case it would be an allogeneic-based therapy (equivalent to a blood transfusion, which is routinely performed in most medical centers); alternatively plasma/LDL may be drawn from the same patient to provide an isogeneic treatment. The latter approach would be important when dealing with a shortage of compatible donors. While these procedures would require a coordinated effort by the clinical staff, precedence for the use of plasma-derived colloidal carriers, such as LDL, is set by ABRAXANE® (Celgene Corp., NJ, USA), one of the most successful anticancer nanoparticle therapies to date. The second major issue facing LDL-DHA nanoparticle therapy is the naturally high concentrations of circulating LDL in the blood (~120 mg/dl). This would probably dilute and inhibit the actions of LDL-DHA nanoparticles. In addition, systemic injections of LDL-DHA nanoparticles would result in the rapid sequestration of these nanoparticles by high LDLR-expressing tissues, such as the liver and adrenal glands. To overcome these limitations a local regional approach would be required where LDL-DHA nanoparticles would be administered into a compartment or vessel in direct proximity to the tumor. This would ensure that efficacious concentrations of LDL-DHA nanoparticles would still reach the tumor and eliminate competition from normal tissues. In the case of HCC, hepatic artery infusions can be performed as HCCs are mainly vascularized by the hepatic artery. The surrounding normal liver receives most of its blood supply from the portal vein. Preclinical testing of local regional LDL-DHA nanoparticle treatments are currently being investigated our laboratory.
Supplementary Material
Executive summary.
Docosahexaenoic acid is an ideal cargo for low-density lipoprotein-mediated transport
Low-density lipoprotein (LDL) is a principle carrier of cholesterol and fat in the body.
Docosahexaenoic acid (DHA) is a natural polyunsaturated fatty acid that has unique chemical and biological properties.
LDL nanoparticles have the capacity to transport over 1400 DHA fatty acid molecules each.
DHA-loaded LDL nanoparticles have enhanced material properties
DHA-loaded LDL (LDL-DHA) nanoparticles possess a moderate electronegative surface charge.
LDL-DHA nanoparticles resist degradative changes (protein loss, flocculation) with long-term storage at ambient conditions.
The integrity of DHA is preserved in the LDL nanoparticle even with prolonged air exposure or elevated temperatures.
The incorporation of DHA into LDL helps protect the LDL particle from oxidative deterioration.
LDL-DHA nanoparticles elicit selective anticancer effects
LDL-DHA nanoparticles are avidly taken up in normal and malignant cells.
LDL-DHA nanoparticles are cytotoxic to malignant cells and is able to kill murine liver cancer cells.
Therapeutic doses of LDL-DHA nanoparticles do not injure normal murine liver cells.
Elevations in lipid peroxidation and reactive oxygen species contribute to LDL-DHA nanoparticle-induced preferential killing of liver cancer cells.
Acknowledgments
The authors would like to thank J Herz for kindly providing the anti-low-density lipoprotein receptor antibody and P Thomas for access to his circular dichroism spectrometer. In addition, we would like to thank SY Kim for her technical experimental assistance.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported in part by the American Gastroenterological Association Research Foundation Scholar Award (IR Corbin), the Southwestern Small Animal Imaging Research Program (SW-SAIRP; NCI U24 CA126608) and the University of Texas Southwestern Medical Center President’s Research Council Award (IR Corbin). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74(1):159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasazuki S, Inoue M, Iwasaki M, et al. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer. 2011;129(7):1718–1729. doi: 10.1002/ijc.25802. [DOI] [PubMed] [Google Scholar]
- 3.Sawada N, Inoue M, Iwasaki M, et al. Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology. 2012;142(7):1468–1475. doi: 10.1053/j.gastro.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Berquin IM, Min Y, Wu R, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117(7):1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto S, Senzaki H, Kiyozuka Y, et al. Effects of fatty acids on liver metastasis of ACL-15 rat colon cancer cells. Nutr Cancer. 1998;31(2):143–150. doi: 10.1080/01635589809514694. [DOI] [PubMed] [Google Scholar]
- 6.Braden LM, Carroll KK. Dietary polyunsaturated fat in relation to mammary carcinogenesis in rats. Lipids. 1986;21(4):285–288. doi: 10.1007/BF02536414. [DOI] [PubMed] [Google Scholar]
- 7.Kang KS, Wang P, Yamabe N, Fukui M, Jay T, Zhu BT. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE. 2010;5(4):e10296. doi: 10.1371/journal.pone.0010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim K, Han C, Dai Y, Shen M, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8(11):3046–3055. doi: 10.1158/1535-7163.MCT-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindskog M, Gleissman H, Ponthan F, Castro J, Kogner P, Johnsen JI. Neuroblastoma cell death in response to docosahexaenoic acid: sensitization to chemotherapy and arsenic-induced oxidative stress. Int J Cancer. 2006;118(10):2584–2593. doi: 10.1002/ijc.21555. [DOI] [PubMed] [Google Scholar]
- 10.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J Lipid Res. 1998;39(2):286–292. [PubMed] [Google Scholar]
- 11.Gleissman H, Segerstrom L, Hamberg M, et al. Omega-3 fatty acid supplementation delays the progression of neuroblastoma in vivo. Int J Cancer. 2011;128(7):1703–1711. doi: 10.1002/ijc.25473. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi M, Minami M, Yagasaki R, et al. Chemoprevention of DMBA-induced mammary carcinogenesis in rats by low-dose EPA and DHA. Br J Cancer. 1997;75(3):348–353. doi: 10.1038/bjc.1997.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swamy MV, Citineni B, Patlolla JM, Mohammed A, Zhang Y, Rao CV. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr Cancer. 2008;60(Suppl 1):81–89. doi: 10.1080/01635580802416703. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Reilly CS. Fat embolism. Contin Educ Anaesth Crit Care Pain. 2007;7(5):148–151. [Google Scholar]
- 15.Namani T, Ishikawa T, Morigaki K, Walde P. Vesicles from docosahexaenoic acid. Colloids Surf B Biointerfaces. 2007;54(1):118–123. doi: 10.1016/j.colsurfb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Kanno S, Kurauchi K, Tomizawa A, Yomogida S, Ishikawa M. Albumin modulates docosahexaenoic acid-induced cytotoxicity in human hepatocellular carcinoma cell lines. Toxicol Lett. 2011;200(3):154–161. doi: 10.1016/j.toxlet.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 18.Gura KM, Duggan CP, Collier SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118(1):e197–e201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 19.Lutz O, Meraihi Z, Mura JL, Frey A, Riess GH, Bach AC. Fat emulsion particle size: influence on the clearance rate and the tissue lipolytic activity. Am J Clin Nutr. 1989;50(6):1370–1381. doi: 10.1093/ajcn/50.6.1370. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira FL, Rumsey SC, Schlotzer E, Hansen I, Carpentier YA, Deckelbaum RJ. Triglyceride hydrolysis of soy oil vs fish oil emulsions. J Parenter Enteral Nutr. 1997;21(4):224–229. doi: 10.1177/0148607197021004224. [DOI] [PubMed] [Google Scholar]
- 21.Marotta DE, Cao WG, Wileyto EP, et al. Evaluation of bacteriochlorophyll-reconstituted low-density lipoprotein nanoparticles for photodynamic therapy efficacy in vivo. Nanomedicine (Lond) 2011;6(3):475–487. doi: 10.2217/nnm.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahzad MM, Mangala LS, Han HD, et al. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13(4):309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P, Hatziieremia S, Elliott MA, et al. Uptake of synthetic low density lipoprotein by leukemic stem cells – a potential stem cell targeted drug delivery strategy. J Control Release. 2010;148(3):380–387. doi: 10.1016/j.jconrel.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Corbin IR, Ng KK, Ding L, Jurisicova A, Zheng G. Near-infrared fluorescent imaging of metastatic ovarian cancer using folate receptor-targeted high-density lipoprotein nanocarriers. Nanomedicine (Lond) 2013;8(6):875–890. doi: 10.2217/nnm.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng KK, Lovell JF, Zheng G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res. 2011;44(10):1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotto AM, Jr, Pownall HJ, Havel RJ. Introduction to the plasma lipoproteins. Methods Enzymol. 1986;128:3–41. doi: 10.1016/0076-6879(86)28061-1. [DOI] [PubMed] [Google Scholar]
- 27.Favre G. Targeting of tumor cells by low density lipoproteins: principle and use of ellipticin derivatives. C R Seances Soc Biol Fil. 1992;186(1–2):73–87. [PubMed] [Google Scholar]
- 28.Gal D, Ohashi M, MacDonald PC, Buchsbaum HJ, Simpson ER. Low-density lipoprotein as a potential vehicle for chemotherapeutic agents and radionucleotides in the management of gynecologic neoplasms. Am J Obstet Gynecol. 1981;139(8):877–885. doi: 10.1016/0002-9378(81)90952-2. [DOI] [PubMed] [Google Scholar]
- 29.Ho YK, Smith RG, Brown MS, Goldstein JL. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood. 1978;52(6):1099–1114. [PubMed] [Google Scholar]
- 30.Lund-Katz S, Laplaud PM, Phillips MC, Chapman MJ. Apolipoprotein B-100 conformation and particle surface charge in human LDL subspecies: implication for LDL receptor interaction. Biochemistry. 1998;37(37):12867–12874. doi: 10.1021/bi980828m. [DOI] [PubMed] [Google Scholar]
- 31.Krieger M, McPhaul MJ, Goldstein JL, Brown MS. Replacement of neutral lipids of low density lipoprotein with esters of long chain unsaturated fatty acids. J Biol Chem. 1979;254(10):3845–3853. [PubMed] [Google Scholar]
- 32.Pitas RE, Innerarity TL, Weinstein JN, Mahley RW. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981;1(3):177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- 33.Erdahl WL, Krebsbach RJ, Pfeiffer DR. A comparison of phospholipid degradation by oxidation and hydrolysis during the mitochondrial permeability transition. Arch Biochem Biophys. 1991;285(2):252–260. doi: 10.1016/0003-9861(91)90357-o. [DOI] [PubMed] [Google Scholar]
- 34.Counsell RE, Pohland RC. Lipoproteins as potential site-specific delivery systems for diagnostic and therapeutic agents. J Med Chem. 1982;25(10):1115–1120. doi: 10.1021/jm00352a001. [DOI] [PubMed] [Google Scholar]
- 35.Krieger M, Brown MS, Faust JR, Goldstein JL. Replacement of endogenous cholesteryl esters of low density lipoprotein with exogenous cholesteryl linoleate. Reconstitution of a biologically active lipoprotein particle. J Biol Chem. 1978;253(12):4093–4101. [PubMed] [Google Scholar]
- 36.Firestone RA. Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells. Bioconjug Chem. 1994;5(2):105–113. doi: 10.1021/bc00026a002. [DOI] [PubMed] [Google Scholar]
- 37.Kader A, Davis PJ, Kara M, Liu H. Drug targeting using low density lipoprotein (LDL): physicochemical factors affecting drug loading into LDL particles. J Control Release. 1998;55(2–3):231–243. doi: 10.1016/s0168-3659(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 38.Masquelier M, Vitols S, Palsson M, Mars U, Larsson BS, Peterson CO. Low density lipoprotein as a carrier of cytostatics in cancer chemotherapy: study of stability of drug–carrier complexes in blood. J Drug Target. 2000;8(3):155–164. doi: 10.3109/10611860008996861. [DOI] [PubMed] [Google Scholar]
- 39.Masquelier M, Tirzitis G, Peterson CO, et al. Plasma stability and cytotoxicity of lipophilic daunorubicin derivatives incorporated into low density lipoproteins. Eur J Med Chem. 2000;35(4):429–438. doi: 10.1016/s0223-5234(00)00139-2. [DOI] [PubMed] [Google Scholar]
- 40.Lestavel-Delattre S, Martin-Nizard F, Clavey V, et al. Low-density lipoprotein for delivery of an acrylophenone antineoplastic molecule into malignant cells. Cancer Res. 1992;52(13):3629–3635. [PubMed] [Google Scholar]
- 41.Hammel M, Laggner P, Prassl R. Structural characterisation of nucleoside loaded low density lipoprotein as a main criterion for the applicability as drug delivery system. Chem Phys Lipids. 2003;123(2):193–207. doi: 10.1016/s0009-3084(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 42.Edwards IJ, Berquin IM, Sun H, et al. Differential effects of delivery of omega-3 fatty acids to human cancer cells by low-density lipoproteins versus albumin. Clin Cancer Res. 2004;10(24):8275–8283. doi: 10.1158/1078-0432.CCR-04-1357. [DOI] [PubMed] [Google Scholar]
- 43.Hevonoja T, Pentikainen MO, Hyvonen MT, Kovanen PT, Ala-Korpela M. Structure of low density lipoprotein (LDL) particles: basis for understanding molecular changes in modified LDL. Biochim Biophys Acta. 2000;1488(3):189–210. doi: 10.1016/s1388-1981(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Walsh MT, Small DM. Apolipoprotein B is conformationally flexible but anchored at a triolein/water interface: a possible model for lipoprotein surfaces. Proc Natl Acad Sci USA. 2006;103(18):6871–6876. doi: 10.1073/pnas.0602213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Segrest JP, Jones MK, De Loof H, Dashti N. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res. 2001;42(9):1346–1367. [PubMed] [Google Scholar]
- 46.Aggerbeck LP, Kezdy FJ, Scanu AM. Enzymatic probes of lipoprotein structure. Hydrolysis of human serum low density lipoprotein-2 by phospholipase A2. J Biol Chem. 1976;251(12):3823–3830. [PubMed] [Google Scholar]
- 47.Jayaraman S, Gantz DL, Gursky O. Effects of oxidation on the structure and stability of human low-density lipoprotein. Biochemistry. 2007;46(19):5790–5797. doi: 10.1021/bi700225a. [DOI] [PubMed] [Google Scholar]
- 48.Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. Physico–chemical stability of colloidal lipid particles. Biomaterials. 2003;24(23):4283–4300. doi: 10.1016/s0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- 49.Schuh J, Fairclough GF, Jr, Haschemeyer RH. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci USA. 1978;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh S, Lee DM. Conformational studies of lipoprotein B and apolipoprotein B: effects of disulfide reducing agents, sulfhydryl blocking agent, denaturing agents, pH and storage. Biochim Biophys Acta. 1986;876(3):460–468. doi: 10.1016/0005-2760(86)90032-9. [DOI] [PubMed] [Google Scholar]
- 51.Duh P-D, Yen W, Yen G-C. Oxidative stability of polyunsaturated fatty acids and soybean oil in an aqueous solution with emulsifiers. J Amer Oil Chem Soc. 1999;76(2):201–204. [Google Scholar]
- 52.Miyashita K, Nara E, Ota T. Oxidative stability of polyunsaturated fatty acids in an aqueous solution. Biosci Biotechnol Biochem. 1993;57(10):1638–1640. [Google Scholar]
- 53.Khan MA, Shahidi F. Tocopherols and phospholipids enhance the oxidative stability of borage and evening primrose triacylglycerols. J Food Lipids. 2000;7(3):143–150. [Google Scholar]
- 54.Zimet P, Livney YD. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for @w-3 polyunsaturated fatty acids. Food Hydrocol. 2009;23(4):7–7. [Google Scholar]
- 55.Moller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem. 2005;280(10):8850–8854. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 56.de Ruiz GJ, de Mertxe R, del Cerro A, de Fernandez LE, Amiano P, Dorronsoro M. Habitual fish intake is associated with decreased LDL susceptibility to ex vivo oxidation. Lipids. 2002;37(4):333–341. doi: 10.1007/s11745-002-0900-8. [DOI] [PubMed] [Google Scholar]
- 57.Calzada C, Colas R, Guillot N, et al. Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis. 2010;208(2):467–472. doi: 10.1016/j.atherosclerosis.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 58.Schuster B, Prassl R, Nigon F, Chapman MJ, Laggner P. Core lipid structure is a major determinant of the oxidative resistance of low density lipoprotein. Proc Natl Acad Sci USA. 1995;92(7):2509–2513. doi: 10.1073/pnas.92.7.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shangguan D, Meng L, Cao ZC, et al. Identification of liver cancer-specific aptamers using whole live cells. Anal Chem. 2008;80(3):721–728. doi: 10.1021/ac701962v. [DOI] [PubMed] [Google Scholar]
- 60.Martin-Orozco E, Chicano A, Ruiz-Alcaraz AJ, et al. Synthetic oligodeoxynucleotides induce MAP kinases activation in murine TIB-73 hepatocytes. Histol Histopathol. 2010;25(7):831–840. doi: 10.14670/HH-25.831. [DOI] [PubMed] [Google Scholar]
- 61.Ogunwobi OO, Liu C. Hepatocyte growth factor upregulation promotes carcinogenesis and epithelial–mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathways. Clin Exp Metastasis. 2011;28(8):721–731. doi: 10.1007/s10585-011-9404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakagawa T, Ueyama Y, Nozaki S, et al. Marked hypocholesterolemia in a case with adrenal adenoma – enhanced catabolism of low density lipoprotein (LDL) via the LDL receptors of tumor cells. J Clin Endocrinol Metab. 1995;80(1):92–96. doi: 10.1210/jcem.80.1.7829645. [DOI] [PubMed] [Google Scholar]
- 63.Peterson C, Vitols S, Rudling M, Blomgren H, Edsmyr F, Skoog L. Hypocholesterolemia in cancer patients may be caused by elevated LDL receptor activities in malignant cells. Med Oncol Tumor Pharmacother. 1985;2(3):143–147. doi: 10.1007/BF02934541. [DOI] [PubMed] [Google Scholar]
- 64.Brissette L, Falstrault L. Analysis of the binding and association of human intermediate density lipoproteins to HepG2 cells. Biochim Biophys Acta. 1992;1165(1):84–92. doi: 10.1016/0005-2760(92)90079-b. [DOI] [PubMed] [Google Scholar]
- 65.Ye ZJ, Go GW, Singh R, Liu W, Keramati AR, Mani A. LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake. J Biol Chem. 2012;287(2):1335–1344. doi: 10.1074/jbc.M111.295287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dippenaar N, Booyens J, Fabbri D, Engelbrecht P, Katzeff IE. The reversibility of cancer: evidence that malignancy in human hepatoma cells is gamma-linolenic acid deficiency-dependent. S Afr Med J. 1982;62(19):683–685. [PubMed] [Google Scholar]
- 67.Leary WP, Robinson KM, Booyens J, Dippenaar N. Some effects of gamma-linolenic acid on cultured human oesophageal carcinoma cells. S Afr Med J. 1982;62(19):681–683. [PubMed] [Google Scholar]
- 68.Pande SV, Mead JF. Inhibition of enzyme activities by free fatty acids. J Biol Chem. 1968;243(23):6180–6185. [PubMed] [Google Scholar]
- 69.Shaw W. Possible role of lysolecithins and nonesterified fatty acids in the pathogenesis of Reye’s syndrome, sudden infant death syndrome, acute pancreatitis, and diabetic ketoacidosis. Clin Chem. 1985;31(7):1109–1115. [PubMed] [Google Scholar]
- 70.Siddiqui RA, Harvey K, Stillwell W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem Phys Lipids. 2008;153(1):47–56. doi: 10.1016/j.chemphyslip.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 72.Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2′,7′-dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radic Res. 2010;44(6):587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 73.Lopez-Sanchez N, Rodriguez JR, Frade JM. Mitochondrial c-Jun NH2-terminal kinase prevents the accumulation of reactive oxygen species and reduces necrotic damage in neural tumor cells that lack trophic support. Mol Cancer Res. 2007;5(1):47–60. doi: 10.1158/1541-7786.MCR-06-0233. [DOI] [PubMed] [Google Scholar]
- 74.Gardner AM, Xu FH, Fady C, et al. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med. 1997;22(1–2):73–83. doi: 10.1016/s0891-5849(96)00235-3. [DOI] [PubMed] [Google Scholar]
- 75.Vanlangenakker N, Vanden Berghe T, Krysko DV, Festjens N, Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr Mol Med. 2008;8(3):207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 76.de Villiers WJ, Song Z, Nasser MS, Deaciuc IV, McClain CJ. 4-Hydroxynonenal-induced apoptosis in rat hepatic stellate cells: mechanistic approach. J Gastroenterol Hepatol. 2007;22(3):414–422. doi: 10.1111/j.1440-1746.2006.04625.x. [DOI] [PubMed] [Google Scholar]
- 77.Toit-Kohn JL, Louw L, Engelbrecht AM. Docosahexaenoic acid induces apoptosis in colorectal carcinoma cells by modulating the PI3 kinase and p38 MAPK pathways. J Nutr Biochem. 2009;20(2):106–114. doi: 10.1016/j.jnutbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–798. [PubMed] [Google Scholar]
- 79.Shen BW, Scanu AM, Kezdy FJ. Structure of human serum lipoproteins inferred from compositional analysis. Proc Natl Acad Sci USA. 1977;74(3):837–841. doi: 10.1073/pnas.74.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quilliot D, Walters E, Bohme P, et al. Fatty acid abnormalities in chronic pancreatitis: effect of concomitant diabetes mellitus. Eur J Clin Nutr. 2003;57(3):496–503. doi: 10.1038/sj.ejcn.1601556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.