Abstract
The induction of functional memory CTLs is a major goal of vaccination against intracellular pathogens. IL-12 is critical for the generation of memory CTLs, and inhibition of mTOR by rapamycin can effectively enhance the memory CTL response. Yet, the role of IL-12 in mTOR’s regulation of memory CTL is unknown. Here, we hypothesized that the immunostimulatory effects of mTOR on memory CTLs requires IL-12 signaling. Our results revealed that rapamycin increased the generation of memory CTLs in vaccinia virus infection, and this enhancement was dependent upon the IL-12 signal. Furthermore, IL-12 receptor deficiency diminished the secondary expansion of rapamycin-regulated memory, and resultant secondary memory CTLs were abolished. Rapamycin enhanced IL-12 signaling by up regulating IL-12 receptor β2 expression and STAT4 phosphorylation in CTLs during early infection. In addition, rapamycin continually suppressed T-bet expression in both WT and IL-12 receptor knockout CTLs. These results indicate an essential role for IL-12 in the regulation of memory CTLs by mTOR, and highlight the importance of considering the interplay between cytokines and adjuvants during vaccine design.
Keywords: cytotoxicity T cells, rapamycin, mTOR, cytokines, IL-12, memory CTLs
Introduction
Enhancement of memory CTLs holds promise for vaccination against chronic viral infections, such as HIV. The generation of functional memory CTLs requires inflammatory cytokines along with antigen and co-stimulation 1–5. Among the cytokines, IL-12 and type I interferon have been identified as the major components for providing the third signal to induce fully functional memory CTLs 3, 6–8. The memory CTL response is compromised when CTLs, through receptor deficiencies, are unresponsive to these third-signal cytokines, as has been demonstrated in vaccinia virus and Listeria monocytogenes infections 9. IL-12, in conjunction with antigen and co-stimulation, is capable of programming memory CTLs in vitro 9–11, further supporting the pivotal role of IL-12 in memory CTL induction. IL-12 has been used in preclinical studies, yielding promising results. IL-12 enhances Th1 and CTL responses when co-administered with antigens in gene transfer 12, induces functional memory CTLs when co-administered subcutaneously with peptide 13, 14, and suppresses tumor growth 15–17. Therefore, IL-12 is a critical stimulator of memory CTLs.
mTOR is a conserved signaling integrator for many environmental components such as amino acids and growth factors 18. Interestingly, mTOR was recently found to be a critical regulator of immune functions 19, such as immune homeostasis 18, activation 20, 21, differentiation 22, 23 metabolism, and migration 24, 25. Inhibiting mTOR via rapamycin enhances memory CTLs during LCMV 26 and Listeria Monocytogenes infections 27. Although rapamycin directly interacts with IL-12 in vitro to regulate the balance of T-bet/Eomes expression 11, it is unclear whether rapamycin’s immunomodulatory effects require inflammatory cytokines during infection in animals.
Using adoptive transfer and receptor deficiency in a mouse model, we show that rapamycin substantially increased the quantity of functional and protective memory CTLs during vaccinia virus infection. This rapamycin-induced regulation requires IL-12, as absence of the IL-12 signal reduced the memory CTL response. Additionally, rapamycin directly enhanced IL-12 signaling by up regulating STAT4 phosphorylation, and consistently inhibited T-bet expression in both WT and IL-12 receptor deficient CTLs in infected animals. More importantly, secondary memory CTLs were abolished when the IL-12 signal was absent. Taken together, these data indicate that IL-12 is essential for rapamycin regulation. Therefore, specific inflammatory cytokines may be necessary when rapamycin is used as an adjuvant.
Results
Rapamycin enhances memory CTLs during vaccinia virus infection
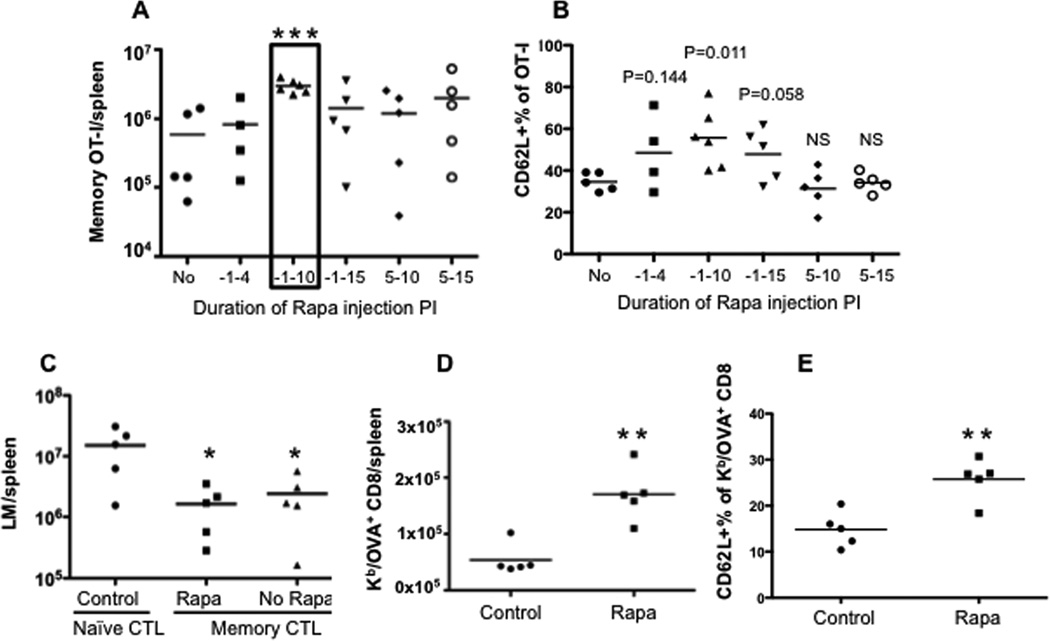
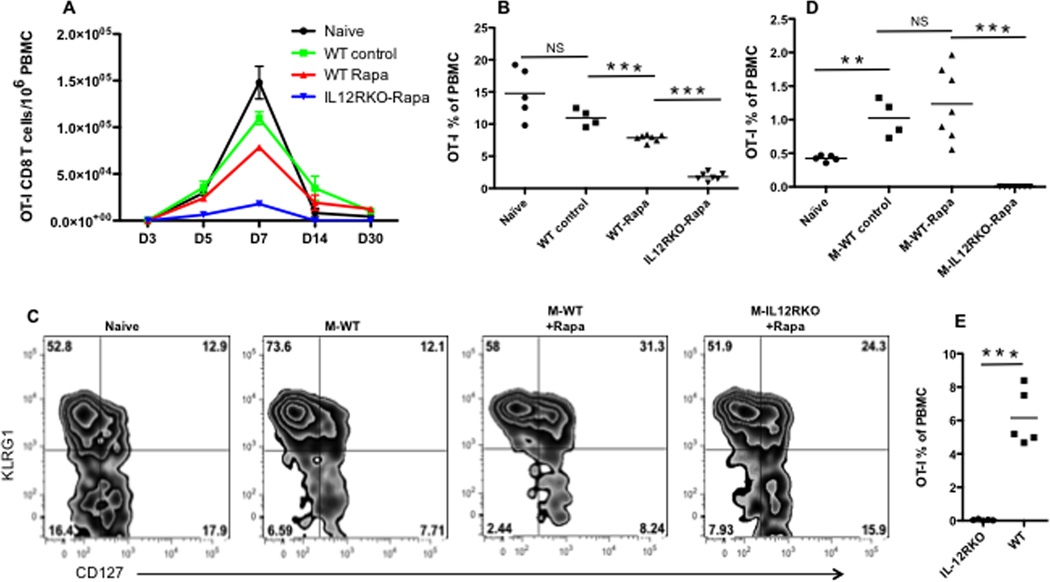
Administration of rapamycin to mice can promote memory CTLs in both LCMV 26 and Listeria Monocytogenes infections (LM) 27. We sought to understand if rapamycin had similar effects on memory differentiation in vaccinia virus (VV) infection. Purified naïve OT-I CD8 T cells were transferred into naïve B6 mice, and the recipients were infected with recombinant VV containing a chicken ovalbumin peptide (VV-OVA) 28. We previously found that high doses of rapamycin have a better regulatory function on IL-12-driven memory CTL programming in vitro than do low doses 10. In addition, high doses of rapamycin can accelerate the transition of effectors to memory CTLs in LCMV infection 26. We speculated that daily administration of high doses of rapamycin early in infection would be immunostimulatory, as this period corresponds to memory CTL programming by IL-12 in vitro10. A high dose of rapamycin was injected daily i.p. during different time windows based on a pilot experiment revealing no difference between D10 and D30 for daily administration (Suppl Fig.1). Memory OT-I cells were examined at D30 post-infection (PI). Consistent with the report by Araki et al. 26, inhibition of mTOR by rapamycin significantly enhanced memory CTLs during VV infection by 4-fold when administered from D-1 to D10 PI (Fig.1A and Suppl Fig.2). The first injection window (D-1 to D4 PI) was not sufficient for rapamycin regulation, and continuous administration of rapamycin after D10 was not beneficial (Fig.1A). Thus, we used D-1 to D10 PI as the standard time window for rapamycin injection for the rest of this project, unless otherwise indicated. The immunostimulatory effect of rapamycin was not a consequence of VV infection delay by rapamycin, as VV was not detectable in tissues (spleen, LN, peritoneal cavity) 5 days post infection in both rapamycin-treated and untreated mice (data not shown). In LCMV infection, low doses of rapamycin applied during the expansion phase increased the frequency of memory CTLs, whereas high doses applied during the contraction phase accelerated memory differentiation 26. Our data showed that administration of high dose rapamycin during the early infection increased memory CTLs. The high dose did not change the kinetics of CTLs response, but delayed both the expansion and contraction phases. The memory CTLs stabilized at a time (D30) comparable to the no rapamycin controls, consistent with an accelerated memory differentiation driven by high dose rapamycin 26. Similar to LCMV infection 26, rapamycin up regulated CD62L expression in memory CTLs (Fig.1B). In addition, bulk splenocytes containing an equal number of memory OT-Is (105) were transferred into naïve B6 mice. They were challenged the next day with recombinant Listeria Monocytogenes containing chicken ovalbumin (LM-OVA) i.v. as we previously reported 9, 29. Memory OT-I cells generated with and without rapamycin achieved similar protection (Fig.1C). To further confirm the effects of rapamycin on the endogenous memory CTL response to VV-OVA infection, we infected naïve B6 mice (no transfer) with VV-OVA with and without rapamycin treatment. Kb/OVA tetramer was used to detect endogenous OVA-specific CD8 T cells 28. We confirmed that rapamycin promoted endogenous memory CTLs similar to memory OT-I cells (Fig.1D). CD62L was up regulated in the rapamycin-treated endogenous memory Kb/OVA positive CTLs (Fig.1E). These data from both the transgenic system and the endogenous CTL response suggest that rapamycin increases the quantity of memory CTLs in response to VV infection and promotes a more central memory phenotype.
Figure 1. Rapamycin enhances memory CTLs during vaccinia virus infection.
Purified naïve OT-I cells were transferred into naïve B6 recipients, which were infected with VV-OVA the next day. Rapamycin was injected daily at 600ug/kg through i.p at the time windows indicated in (A). A. Memory OT-I cells in spleens 30 days post-infection (PI). B. CD62L expression in memory OT-I cells from (A); C. Splenocytes containing 105 memory OT-I cells were transferred into naïve B6, which were challenged with LM-OVA the next day. Bacteria were cultured and counted three days after LM-OVA challenge in spleens. D. Endogenous KbOVA+ memory CD8 cells in VV-OVA infected mice (without transfer of OT-I). Naïve B6 mice (without transfer) were infected with VV-OVA, which were treated with or without rapamycin. E. CD62L expression in KbOVA+ memory CD8 cells from D. Rapamycin injection occurred daily from D-1 to D10 post-infection in D–E. Student’s t test was performed comparing each of the groups with no rapamycin controls (A, B, D and E) or with naïve CTL transferred controls (C). *, P < 0.05; **, P < 0.01; ***, P < 0.001, which will be the same in the rest of this study. The data are representative of three independent experiments with similar results.
IL-12 increases CTL expansion following rapamycin treatment
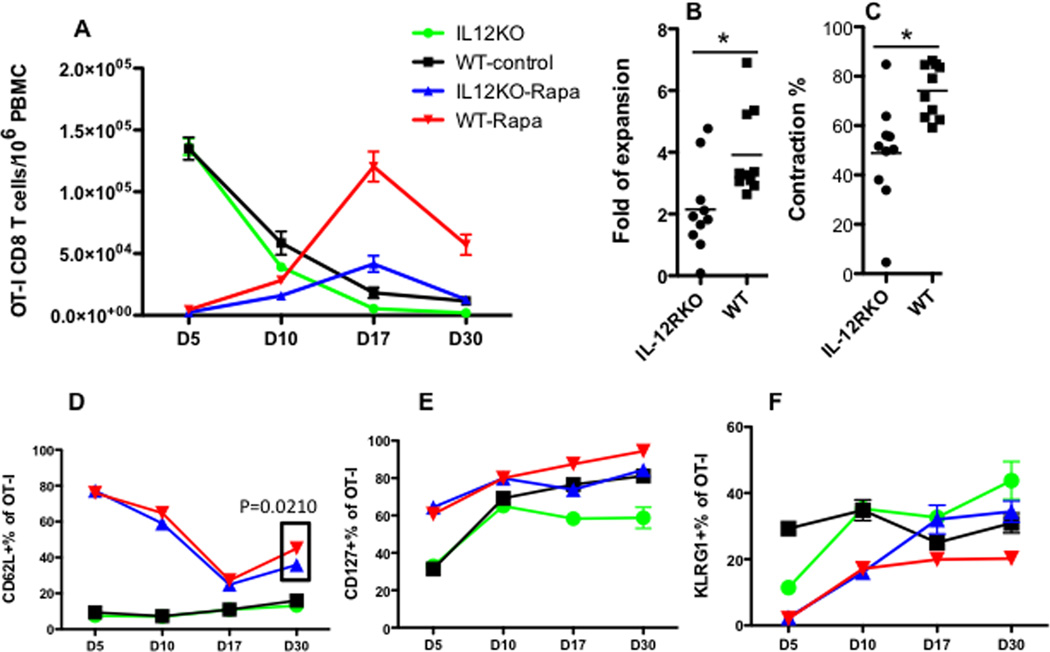
To understand if IL-12 signaling was required for rapamycin’s regulation of memory CTL formation, OT-I cells of wild type (WT) or IL-12 receptor deficient (IL-12RKO) mice 9 (Suppl Fig.3) were transferred into naïve B6 recipients, which were infected with VV-OVA the next day. The recipient mice received daily rapamycin injections from D-1 to D10 PI as illustrated in Fig.1. Compared to untreated controls, effector CTL expansion in rapamycin-treated WT and IL-12RKO groups was reduced by more than 10 times at the peak of expansion (D5) (Fig.2A). This is consistent with the report that a high dose of rapamycin inhibits expansion of effectors in LCMV infection 26. However, CTLS significantly expanded between D5 and D10 in the rapamycin-treated WT and IL-12RKO groups (Fig.2A), and this expansion accelerated upon withdrawal of rapamycin until day 17. Notably, WT OT-Is expanded almost 2 times more than IL-12RKO OT-Is (Fig.2B), and supports the critical role of IL-12 in CTL expansion after rapamycin treatment. Interestingly, we noticed similar inhibition of rapamycin on CTL expansion in vitro, but observed accelerated CTL expansion following transfer into recipients 10. After D17, the CTL population contracted, and a fraction of expanded cells became memory CTLs at D30, remaining stable thereafter (Fig.2A and data not shown). WT OT-Is contracted more than IL-12RKO OT-Is, based on lower expansion of IL-12RKO (Fig.2C). Therefore, IL-12 is critical for optimal CTL expansion and memory formation after rapamycin treatment.
Figure 2. IL-12 increases CTL expansion after rapamycin treatment.
OT-I cells were purified from wild type (WT) or IL-12RKO OT-I mice, which were transferred into naïve B6 mice at 105/mouse through tail vein. Recipients were infected with VV-OVA the next day. Daily rapamycin injection occurred from D-1 to D10 post-infection. A. Comparison of OT-I percentage of PBMCs in blood in different groups. Data were expressed as mean plus SEM of 6–10 mice for each group. B. Comparison of expansion of OT-I after rapamycin withdrawal. Data were calculated by dividing the OT-I percentage at D17 by that at D10 (the last day for rapamycin injection). C. Comparison of contraction of OT-Is after rapamycin withdrawal. Data were calculated by dividing the OT-I percentage at D30 by that at D17. D–F: Comparison of expression of CD62L, CD127 and KLRG1 in OT-I cells in blood samples from (A). Data are representative of three experiments with similar results. Two-way ANOVA was performed in A, D, E and F. Student’s t test was performed in B, C and part of D as the square indicates.
Rapamycin treatment postponed the down regulation of CD62L until D10 (Fig.2D), which is consistent with its effects during in vitro stimulation 10. The continued expansion of OT-Is upon the withdrawal of rapamycin led to a quick down regulation of CD62L, although expression of CD62L remained higher than in their untreated counterparts (Fig.2D). CD62L was upregulated in rapamycin-regulated memory CTLs regardless of the presence or absence of IL-12 at D30 after the viral infection (p<0.001 Two-way ANOVA). However, there was a significant difference between WT and IL-12RKO OT-I cells treated with rapamycin—WT OT-Is with rapamycin had slightly but significantly (p=0.021 t test) higher expression of CD62L than IL-12RKO treated with rapamycin. This suggests that IL-12 may partially contribute to the development of a more central memory phenotype (Fig.2D). Furthermore, IL-7 receptor α (CD127) expression was upregulated by rapamycin in both groups (p<0.001 Two-way ANOVA), and WT OT-I cells expressed higher levels than IL-12RKO at D17 and D30 (Fig.2E). In addition, KLRG1 expression was downregulated by rapamycin (p<0.001 Two-way ANOVA), but the absence of the IL-12 signal led to differential expression levels (p<0.001 Two-way ANOVA) (Fig.2F). These data suggest that rapamycin favors a central memory CTL phenotype (CD62Lhi/CD127hi/KLRG1lo), and the IL-12 signal may contribute to this phenotype.
Rapamycin enhances memory CTLs in tissues
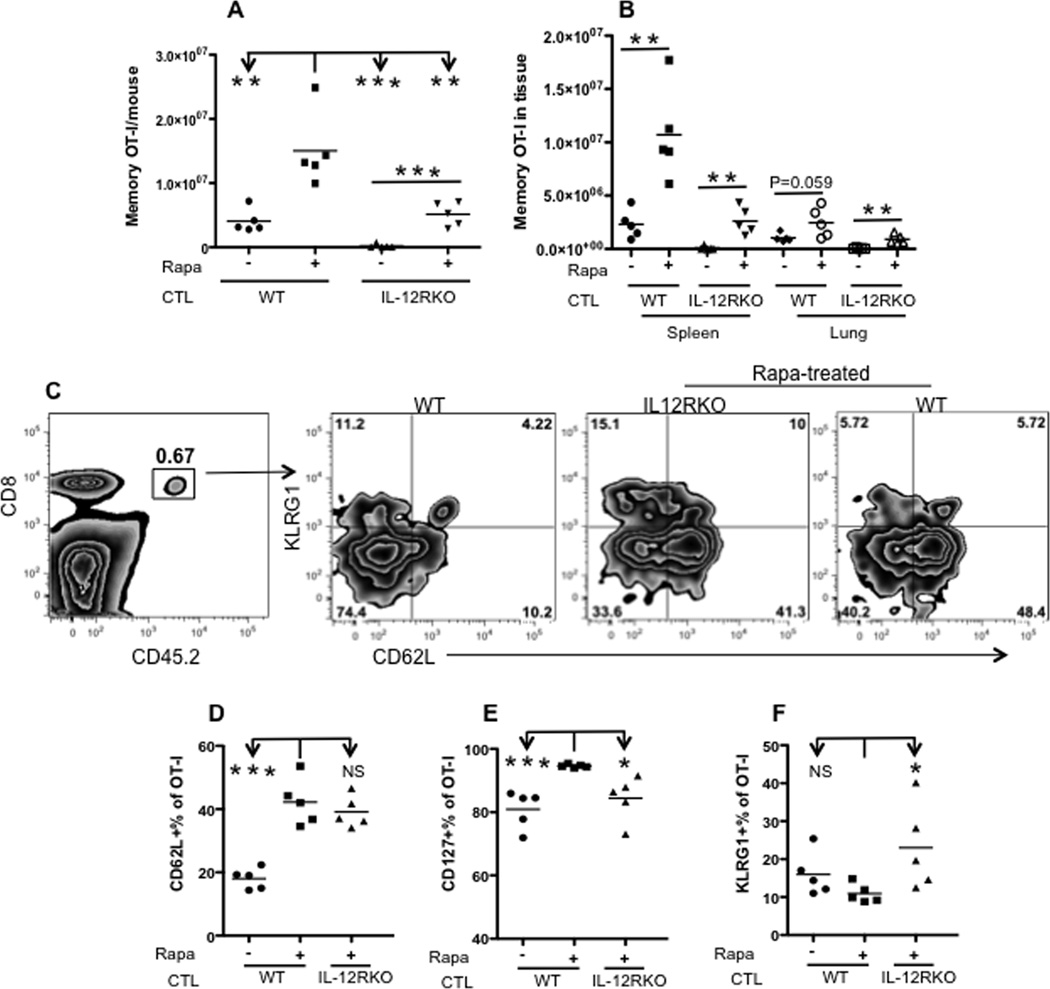
We sought to determine whether our observations regarding memory CTLs in blood also applied to CTLs in tissues. Memory mice, 40 days post VV-OVA infection and 30 days post rapamycin administration, were analyzed. Single cells were isolated from peripheral lymph nodes, spleen, bone marrow (two sets of femur) and lung. Similar to CTLs from the blood, rapamycin treatment significantly increased WT and IL-12RKO OT-Is in tissues compared to corresponding controls (Fig.3A). Yet, achieving optimal CTL memory requires IL-12: the IL-12 signal (WT) enhanced the rapamycin-treated memory 3-fold compared to IL-12 deficiency (rapamycin-treated IL-12RKO) (Fig.3A).
Figure 3. Rapamycin enhances memory CTLs in tissues.
Memory OT-I cells were analyzed in memory mice (similar to those in Fig. 2A) 40 days after VV-OVA infection. A. Comparison of total memory OT-I cells from peripheral lymph nodes, spleen, lung and two sets of femur from each mouse. B. Tissue distribution of memory OT-I cells in spleen and lung. Data were calculated by dividing the number of memory OT-I in one tissue by the number in all examined tissues. C. Representative expression of CD62L/CD127/KLRG1 and corresponding statistics (Student’s t test) (D–F) of memory OT-I cells in spleens from (A). The experiment was repeated three times and similar results were obtained.
To investigate whether rapamycin altered migration of memory CTLS, we analyzed the tissue distribution of memory OT-Is. Although rapamycin treatment increased the number of memory OT-Is in tissues in both WT and IL-12RKO (Fig.3B), rapamycin-regulated memory OT-Is tended to remain in the spleen (p=0.057) compared to CTLs not treated with rapamycin (Suppl Fig.4A). This trend disappeared in IL-12RKO OT-Is (p=0.578) which were retained in the spleen at similar percentages regardless of the exposure to rapamycin (Suppl Fig.4A and B). In contrast, memory CTLs in the lung were significantly reduced (by about 10%) after rapamycin treatment in both WT and IL-12RKO OT-I groups (Suppl Fig.4A), consistent with the observation of enhanced central memory phenotype due to rapamycin. The memory OT-Is in spleens from rapamycin-treated mice exhibited increased expression of CD62L when compared to WT controls (Fig.3C–D). Similar to blood samples (Fig.2E and F), rapamycin-treated WT memory CTLs in spleens had slightly but significantly higher expression of CD127, but lower expression of KLRG1 compared to their IL-12RKO counterparts (Fig.3E–F). These observations were similarly reflected in memory OT-Is from most tissues (some differences were not significant), although expression levels varied among tissues in the same animals (Suppl fig.4C–E). For example, memory CTLs in the lung had the lowest CD62L expression but the highest KLRG1 expression, which is consistent with an effector memory phenotype (Suppl fig.4C–E). These results suggest a general trend: rapamycin promotes a central memory phenotype of CTLs in tissues and in the periphery.
Memory CTLs derived from rapamycin treatments in the absence of the IL-12 signal are functional
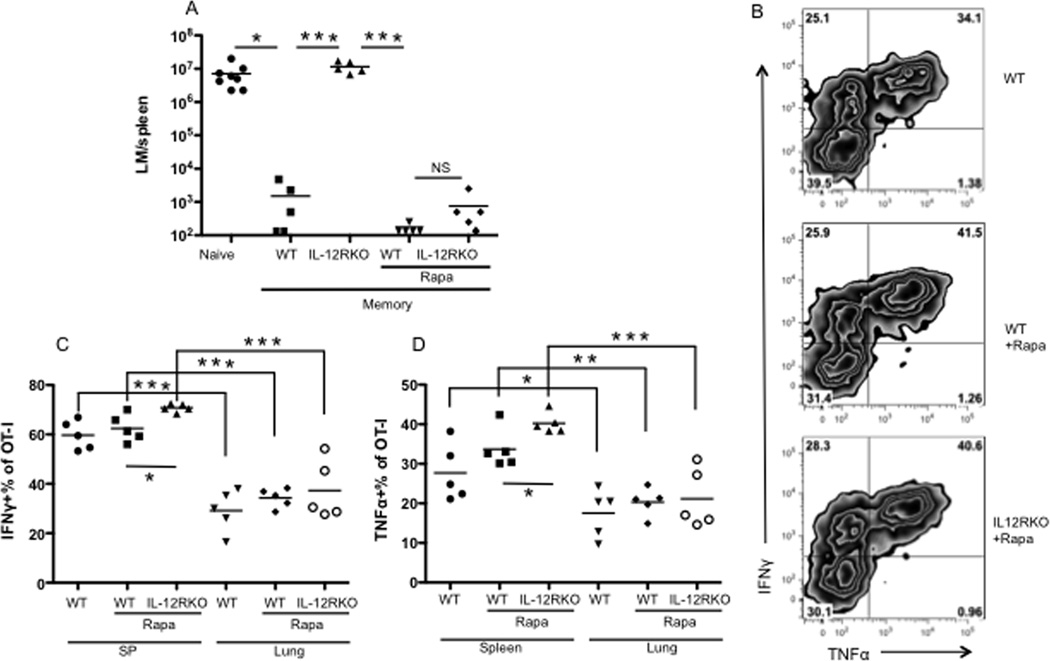
Quantitative measurements of memory CTLs do not necessarily reflect functionality, as demonstrated by exhausted CTLs in chronic LCMV infection 30–32. To test if the CTLs in this study were functional, memory mice were challenged with LM-OVA 9, 10. The memory mice which had originally received IL-12RKO OT-Is were not protected against LM-OVA challenge, as is consistent with our previous report 9 (Fig.4A). Notably, treatment with rapamycin rescued functions of IL-12RKO CTLs and enabled them to respond to challenge, reaching levels of protection similar to WT with or without rapamycin treatments (Fig.4A). Endogenous Kb/OVA CD8 T cells were undetectable (data not shown), suggesting that memory IL-12RKO OT-Is were responsible for the enhanced memory protection in IL-12RKO OT-I transfer mice. IFNγ and TNFα have been closely associated with memory CTL function, and these rapamycin-regulated memory IL-12RKO CTLs had slightly but significantly higher production of both molecules compared to WT controls (Fig.4B–D). Notably, there were significant differences in IFNγ and TNFα production by memory CTLs from different tissues within the same individual: CTLs in lungs produced the lowest amount of IFNγ and TNFα, whereas CTLs in spleens, lymph nodes, and bone marrow produced more of these cytokines (Fig.4C–D and data not shown). These data suggest that the rapamycin-regulated memory CTLs are functional and protective, even in the absence of IL-12.
Figure 4. Rapamycin-regulated memory CTLs are functional in the absence of the IL-12 signal.
A. Memory mice (similar to those in Fig. 2A) were challenged with LM-OVA, and bacterium was recovered from the spleen 3 days after challenge. B–D. Resting memory OT-I cells in different tissues were examined for production of IFNγ and TNFα. Representative cytokine expression in spleen (B) and comparison between spleen and lung (C–D). These are representative of three independent experiments with similar results.
IL-12 is required for secondary expansion of memory CTLs regulated by rapamycin
A functional memory response is characterized by rapid expansion and quick control of reinfection upon pathogen re-challenge 33, 34. To test secondary expansion ability, an equal number (105) of memory OT-Is from each treatment group was transferred into naïve recipients, which were then challenged with LM-OVA. OT-Is became detectable at D5, peaked at D7, and contracted thereafter (Fig.5A). IL-12RKO OT-Is had the smallest expansion at D7, which was significantly lower than the other groups (Fig.5B). Furthermore, this group (IL-12RKO) contracted the most, becoming almost undetectable at D14 post-challenge (Fig.5A). Interestingly, rapamycin-regulated WT memory OT-Is were significantly lower than WT memory controls at D7 (Fig.5B), but both achieved a similar level of secondary memory (D30 after re-challenge Fig.5A). Additionally, the absence of IL-12 signaling in the primary response caused weaker activation of memory CTLs, as demonstrated by a lower KLRG1 expression and reduced down-regulation of CD62L at D7 (Fig.5C and Suppl Fig.5A–C) and D5 (data not shown). The extent of expansion was predictive of the resultant secondary memory: secondary memory CTLs were undetectable in the IL-12RKO+rapamycin group (Fig.5D). Secondary memory from either WT memory or WT+rapamycin memory CTLs was higher than in naïve controls (Fig.5D). To confirm the absence of memory CTLs, memory mice in IL-12RKO+rapamycin group and WT +rapamycin group were challenged with VV-OVA at D60 post LM-OVA infection. There was no detectable expansion of OT-I at D5 in IL-12RKO+rapamycin group, whereas a huge expansion was detected in WT (Fig.5E). Collectively, lack of the IL-12 signal causes defective secondary expansion and abolishes secondary memory formation.
Figure 5. IL-12 is required for secondary expansion of memory CTLs regulated by rapamycin.
Naïve mice having received naïve or IL-12RKO OT-I cells were split into two groups: rapamycin-treated and untreated control. These mice were then infected with VV-OVA. Splenocytes containing 105 memory OT-I cells from each of the treatments were transferred into naïve B6 mice, which were challenged the next day with LM-OVA. Memory IL-12RKO OT-Is without rapamycin were at or below detectable level, so were excluded in transfer. OT-I populations were tracked in the blood at various time points. A. Kinetics of OT-I populations. Data are expressed as mean plus SEM of 4–7 mice. Comparison of OT-I percentage of PBMCs at D7 (B) or D30 (D) after LM-OVA challenge. C. Comparison of expression of KLRG1/CD127/CD62L in OT-Is at D7 after LM-OVA challenge. E. Mice that have received rapamycin-treated first memory OT-Is (IL-12RKO and WT), were infected with LM-OVA as did in A. These memory mice were challenged again with VV-OVA 60 days after LM-OVA infection, and CTL expansion was examined day D5. The results are representative of two separate experiments with similar results. Student’s t test was performed in B D and E.
Rapamycin enhances IL-12 signaling in early infection and consistently inhibits T-bet expression
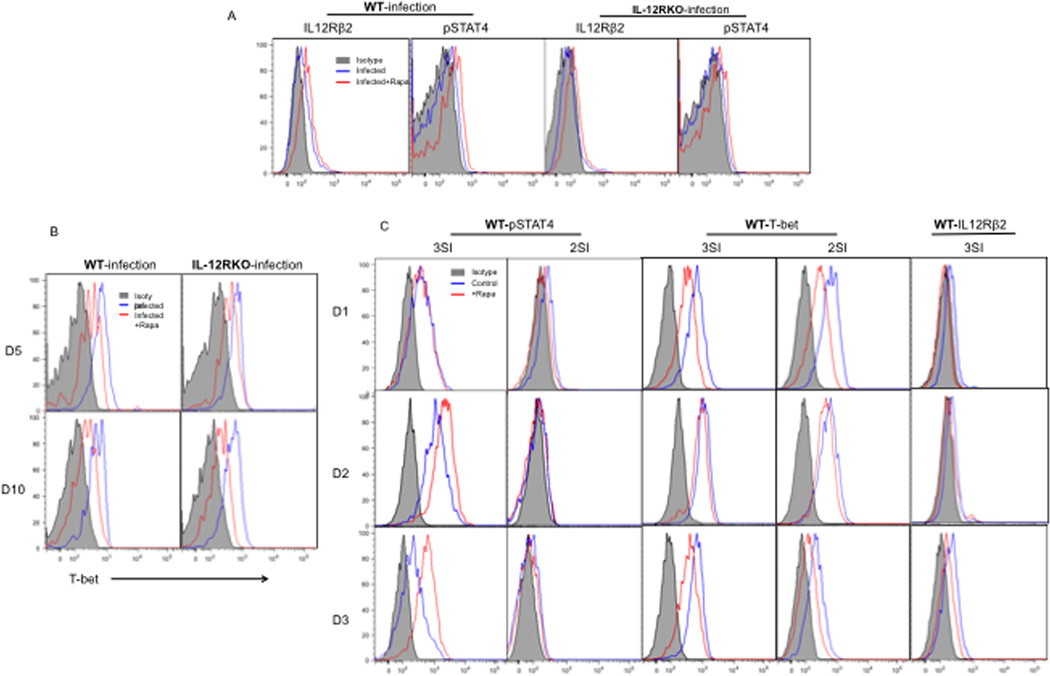
Rapamycins enhancement of memory CTL formation may be due to direct interactions with IL-12 signaling within CTLs, or result indirectly from interactions with other cells. To address this question, naïve WT and IL-12RKO OT-I cells were transferred into recipient B6 mice, which were infected with VV the next day. Rapamycin was administered as in Fig.2. OT-Is were analyzed for IL-12 signaling and other pathways at different time points post infection. IL-12 receptors are composed of two subunits, β1 (shared with IL-23) and β2 (binding p35 of IL-12, so is unique to IL-12) 35–37. Our IL-12RKO OT-Is are deficient in the β1 subunit. β1 and β2 are differentially expressed in immune cells38. In naïve CD4 cells, β1 is expressed but β2 is absent 39. The expression of β2 is induced by IFN-γ, but inhibited by IL-4 during activation 39. In CD8 T cells, both β1 and β2 can be regulated by cytokine stimulation (IL-12 or type I IFN), but the speed and magnitude of up-regulation is different between the two subunits. The transcriptional expression of β2 was upregulated earlier and with greater magnitude than was β1 (under what treatment? IFN or IL-12 or both?) 40. Administration of rapamycin increased IL-12R β2 expression in both WT and IL-12RKO OT-Is during early infection (days 3–5), but not β1 (Fig.6A and data not shown). Type I IFN receptor subunit 1 was not affected by rapamycin (Suppl.6A). This indicates that IL-12R β2 is up regulated by rapamycin. In addition to the receptor expression, rapamycin up regulated the phosphorylation of STAT4 in both WT and IL-12RKO OT-I cells, but not the expression of JAK2 on the protein level (Fig.6A). This suggests that rapamycin directly enhances STAT4 activation during early infection through the IL-12 signaling pathway and/or other cytokines9, 41–43. T-bet is a transcription factor responsible for CTL effector function 44, 45. Rapamycin regulates IL-12-driven memory programming by inhibiting T-bet, and promoting Eomes expression 11. Consistent with this, administration of rapamycin suppressed T-bet expression in both WT and IL-12RKO CTLs at days 5 and 10 post infection (Fig.6B and data not shown), but Eomes expression was not affected (Suppl.6B). Thus, rapamycin’s suppression of CTL effector function may contribute to the enhanced memory in both WT and IL-12RKO OT-Is. Interestingly, mTOR phosphorylation was not altered by rapamycin at days 5 and 10 post infection, indicating that rapamycin may work through pathways other than mTOR (Suppl.6C). Therefore, our findings suggest that rapamycin can both directly augment IL-12 signaling during early infection and suppress CTL effector function.
Figure 6. Rapamycin enhances IL-12 signaling in early infection and consistently inhibits T-bet expression.
Naïve WT or IL-12RKO OT-I cells were transferred into recipient B6 mice, which were infected with VV-OVA the next day. High doses of rapamycin were administered daily between D-1 and D10 post VV-OVA infection. OT-I cells in spleens were examined at days 5 (A) and 10 after infection (B). The results are representative of 5 mice per group, and similar data were obtained in two separate experiments. C. Sorted WT OT-I cells were stimulated with 3SI (antigen+B7+IL-12) or 2SI (antigen+B7) in the presence or absence of rapamycin as we previously reported 10. Programmed CTLs were examined at day 3 post-stimulation. The T-bet was examined on effector CTLs generated in vivo (B) and in vitro (C). These are representatives of two independent experiments with similar results.
To confirm the direct effects of rapamycin on IL-12 signaling observed in animals, sorted naïve OT-I cells were cultured in the presence (3SI) or absence (2SI) of IL-12 in addition to antigen and B7 stimulation 10, 46. Indeed, rapamycin directly enhanced and extended STAT4 phosphorylation when IL-12 was present (Fig.6C). Consistent with data in VV infection (Fig.6A), rapamycin directly inhibited T-bet expression independent of IL-12 (Fig.6C) as previously reported 11. In contrast to in vivo, IL-12Rβ2 was inhibited by rapamycin in both 2SI and 3SI stimulation (data not shown). Therefore, rapamycin can directly enhance IL-12 signaling but this does not necessarily occur through direct regulation of IL-12 receptors.
Long-term administration of rapamycin at low doses is equally effective as high doses
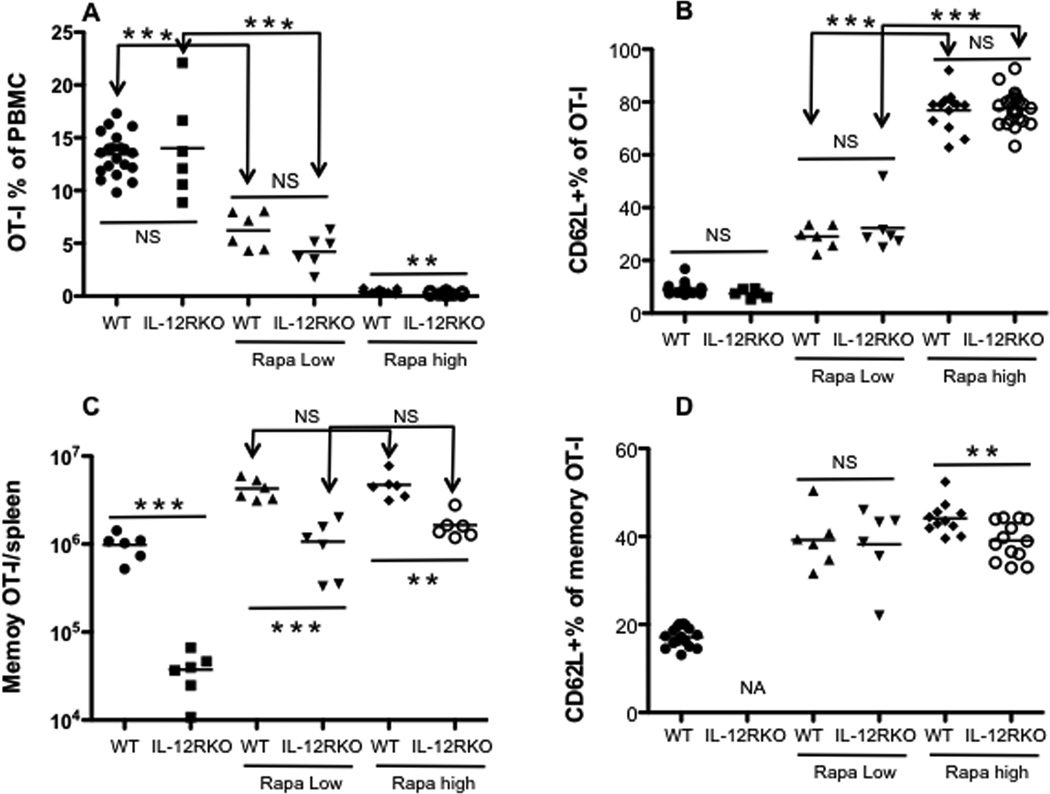
Long-term administration of low doses of rapamycin enhances memory CTLs in LCMV infection 26. To test if the same is true in VV infection, naïve OT-I cells were transferred into B6 mice, which were infected with VV-OVA. Rapamycin was administered at either low doses from −1 to 30 days post-infection, or high doses from −1 to 10 PI. Indeed, high doses of rapamycin (-1 to 10 PI) dramatically suppressed CTL expansion in both WT and IL-12RKOs (Fig.7A). Yet, low doses of rapamycin inhibited CTL expansion in both WT and IL-12RKO CTLs, albeit more in IL-12RKOs (Fig.7A). This suggests that inhibition of CTL expansion by rapamycin is dose-dependent, and the IL-12 signal may lessen this inhibition, at least partially.
Figure 7. Long-term administration of low doses of rapamycin enhances memory CTLs to levels comparable to high doses.
Naïve WT or IL-12RKO OT-I cells were transferred into recipient B6 mice, which were infected with VV-OVA the next day. Low doses of rapamycin were administered daily between D-1 and D30 post VV-OVA infection, whereas high doses were administered between D-1 and D10 post-infection. OT-I populations were tracked in blood samples. A and C. Comparison of OT-I percentage of PBMCs at day 5 (A) or memory OT-Is in spleen at day 40 (C) after VV-OVA infection. B and D. Comparison of expression of CD62L in OT-Is in blood samples at D5 and D40 after VV-OVA infection. The results are representative of two separate experiments with similar results. Student’s t test was performed in A–D.
In regards to surface molecules, there was no significant difference in KLRG1 and CD127 expression between both doses (data not shown). However, high expression of CD62L was associated with high doses of rapamycin (Fig.7B), whereas CD62L expression was dampened under low doses of rapamycin in both WT and IL-12RKO. Interestingly, rapamycin’s regulation of CD62L at the expansion stage is not dependent on IL-12 signaling (Fig.7B). Despite the differences in expansion and expression of surface molecules, memory CTLs reached similar levels in both WT and IL-12RKO OT-Is regardless of the dose of rapamycin (Fig.7C). Furthermore, CTLs from both doses tended towards central memory phenotype - CD62L positive, and mostly KLRG1 negative and CD127 positive (Fig.7D and data not shown). These data suggest that the long-term administration of low doses of rapamycin has similar effects on memory CTLs compared to short-term administration of high doses.
Requirement of the IL-12 signal for memory expansion is independent of the rapamycin dosage
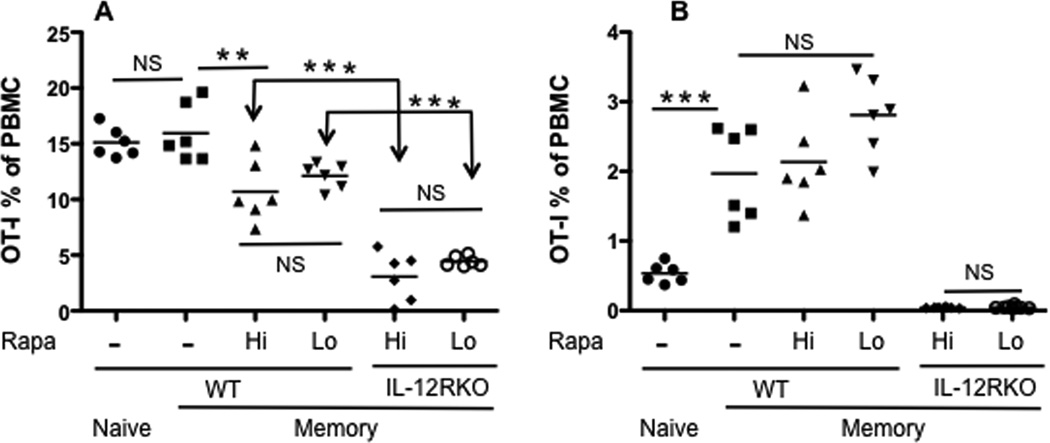
It was possible that the impaired secondary expansion of rapamycin-regulated memory IL-12RKO CTLs was a consequence of high dosage. To address this question, spleen cells containing an equal number of memory OT-Is from each treatment (high and low doses of rapamycin) were transferred into naïve B6 recipients, which were challenged with LM-OVA the next day. At the peak of response (day 7 after re-challenge), rapamycin-regulated IL-12RKO OT-Is were significantly lower than WT regardless of the dosage used during primary activation (Fig.8A), and expansion was only detectable 5 days after re-challenge (data not shown). Consistently, resultant secondary memory CTLs were abolished in IL-12RKO OT-Is derived from both high and low doses of rapamycin (Fig.8B). No phenotypic difference was observed in resultant secondary memory CTLs from low and high dose rapamycin-regulated primary WT memory (data not shown). Therefore, the requirement of IL-12 for secondary memory expansion is independent of the rapamycin dosage.
Figure 8. Requirement of the IL-12 signal for memory expansion is independent of the rapamycin dosage.
Naïve mice, having received naïve or IL-12RKO OT-I cells, were infected with VV-OVA with high or low doses of rapamycin. Splenocytes containing 105 memory OT-I cells from each of the treatments were transferred into naïve B6 mice, which were challenged the next day with LM-OVA. Naïve and WT memory controls (without rapamycin) were included. OT-I populations were tracked in blood samples at different time points. A. Comparison of OT-I percentage of PBMCs in the blood at D7 (A) or at D30 (B) after LM-OVA challenge. The results are representative of two separate experiments with similar results. Student’s t test was performed in A–B. One-way ANOVA was performed in B for comparison of three groups.
Discussion
Inhibiting mTOR by rapamycin effectively enhances memory CTLs in LCMV and Listeria infections 26, 27. Yet, whether the immunostimulatory effects of rapamycin require the presence of inflammatory cytokines is unknown. In this report, we confirmed that rapamycin enhances the formation of functional memory CTLs in vaccinia virus infection, and demonstrated that IL-12 signaling is necessary for achieving the optimal memory CTL response.
Consistent with our previous report 9, IL-12 signal is required for memory formation. Deficiency of the IL-12 signal led to almost undetectable memory, despite similar effector expansion (Fig.2A). When rapamycin was administered to recipients, memory CTLs increased (Fig.2A). However, the presence of IL-12 signaling significantly enhanced the effects of rapamycin by 3–4 fold, and shifted the CTL population to a more central memory phenotype. As IL-12 plays a critical role in the differentiation of Th1 and the establishment of a strong CTL response 47, 48, it is not surprising that this cytokine is required for optimal memory CTL formation following rapamycin treatment. Cessation of rapamycin treatment in primary VV-OVA infection enhanced effector expansion (Fig.2B), and subsequently improved memory CTL formation (Fig.2A). Consistent with a recent report from the Ahmed lab 26, high doses of rapamycin inhibited effector expansion (Fig.2A). However, this strong inhibition did not abolish expansion—CTLs still expanded substantially when high doses of rapamycin were administered (Fig.2A). In addition, these effectors exhibited a period of delayed expansion upon termination of rapamycin treatment, and IL-12 contributed to the strength of this post-rapamycin expansion (Fig.2A). Compared to long-term administration of low doses of rapamycin, high doses yielded a similar effect within a shorter time window (Fig.7C).
Rapamycin promotes a central memory phenotype in a monkey model 26 and can program memory CTLs in short-term culture in vitro in the presence of IL-12 10, 11. In support of these findings, we found that rapamycin drove upregulation of CD62L regardless of the presence or absence of IL-12. However, the lack of the IL-12 signal reduced the expression of CD127 (IL-7 receptor alpha), which suggests decreased responsiveness to IL-7, a critical cytokine for the maintenance and homeostasis of memory CTLs 2, 49–52. Furthermore, the absence of IL-12 signal increased KLRG1 expression, an inhibitory receptor for T cells and a marker for short-lived effectors 53, 54. IL-12 marginally affected CD62L expression, if any (Fig.2D, Fig.3D, Fig.7B and Suppl.4C). These data indicate that memory CTL regulation by rapamycin requires IL-12 to maintain a strong and healthy central-memory CTL phenotype. This quantitative and qualitative regulation by rapamycin was similarly achieved from both high (Fig.2) and low doses (Fig.7). The requirement of IL-12 for the secondary memory response is evident. Rapamycin-regulated memory IL-12RKO CTLs expanded much less than WT CTLs treated with rapamycin. Moreover, there was no detectable secondary memory (Figs.5 and 8). As a common practice in vaccination, boosting with either vectors or adjuvant is used to increase the quantity and quality of memory CTLs 55–58. Our data clearly suggest that enhancing memory CTLs using an mTOR inhibitor, such as rapamycin, requires IL-12 for both optimal primary memory and functional secondary responses. Of course, this does not necessarily exclude the need for other inflammatory cytokines, such as type I interferon, which are critical for the immune response against certain infectious pathogens such as LCMV 34.
Rapamycin may directly and indirectly regulate IL-12 signaling. IL-12R β2 expression was enhanced by rapamycin, while no change was observed in β1 expression during infection (Fig.6A). This could indicate that rapamycin affects IL-12 function in memory generation through differential regulation of IL-12 receptor subunits. However, both IL-12Rβ1 and β2 were inhibited by rapamycin in CTLs when IL-12 was provided in vitro (Fig.6C). Therefore, the enhanced expression of IL-12Rβ2 by rapamycin during infection may be indirect, possibly occurring through other mechanisms. More importantly, inhibition of mTOR in vitro in the presence of IL-12 leads to enhanced memory programming 10, 11, suggesting that mTOR may affect downstream IL-12 signaling. Although the IL-12 signaling was disrupted in IL-12RKO OT-Is due to β1 deficiency, the STAT4 phosphorylation was similarly upregulated by rapamycin during the early infection (Fig.6A). In addition, rapamycin enhanced STAT4 phosphorylation in CTLs in vitro only in the presence of IL-12 (Fig.6C), suggesting that this may be due to the combined effects of IL-12 and other cytokines, such as type I IFN 41–43, 59 and IL-3, IL-5 and IL-6 60–67. Importantly, these effects were transient and only happened early in the infection, suggesting that the regulatory function of rapamycin for cytokine signaling may be generally short-lived. Rapamycin might also influence other components involved in IL-12 signaling that have not been addressed in this study. A global comparison of transcriptome or protein profiling between rapamycin-treated and control in both WT and IL-12RKO OT-Is is currently underway and will provide more defined answers about the molecular mechanisms underlying rapamycin regulation.
It was recently reported that a third signal is required for secondary expansion of memory CTLs in a pathogen-dependent manner 34. Different pathogens may cause distinct inflammatory milieus, and the induction of memory CTLs depends on unique cytokines, such as type I IFN for LCMV 8 and IL-12 for vaccinia virus and listeria monocytogenes 9,34. The ability of CTLs to undergo secondary expansion requires the presence of pathogen-specific third-signal cytokines during priming 34. Our data further support this discovery by illustrating that rapamycin-regulated memory CTL expansion requires a third signal during priming. We cannot rule out the possibility that IL-12 is required for the secondary expansion of memory CTLs, since in this experimental setting there is a lack of IL-12 signaling in both priming and memory stages. Once available, a conditional knockout model will be more suitable to address this question. Although the requirements for reactivating memory CTLs are still subject to debate, dendritic cells are essential for optimal CTL responses to secondary infections 68, 69. This implies that co-stimulation and/or inflammation is essentially involved in the reactivation of memory CTLs 70. Recently, we reported that boosting with peptide requires adjuvant for memory CTL generation 55, so it seems that cytokines are needed. The immune response to live attenuated pathogens is usually stronger than that against killed vaccines 57, 71. Thus, induction of functional memory CTLs using killed vaccines is very challenging, and often requires effective adjuvants and multiple boosts 13, 14, 57, 71, 72. As shown in this report, the inhibition of mTOR and the provision of the IL-12 signal may provide the stimulation necessary to enhance the immune response against killed pathogens.
In summary, we found that IL-12 is critical for rapamycin regulation of memory CTLs in two aspects: 1) IL-12 enhances the regulatory function of rapamycin quantitatively and qualitatively. 2) The presence of IL-12 during priming is required for secondary expansion of memory CTLs regulated by rapamycin. When an mTOR inhibitor is used as adjuvant to enhance memory CTLs during vaccination, it is important to provide sufficient required inflammatory cytokines, such as IL-12.
Materials and methods
Mice and reagents
OT-I mice and OT-I mice deficient for IL-12 receptor β1 (gifts from Dr. Mescher, University of Minnesota) have a transgenic TCR specific for H-2Kb and OVA257–264 9,10,73. Mice were maintained under specific pathogen-free conditions at the University of Maryland, and these studies have been reviewed and approved by the Institutional Animal Care and Use Committee. C57BL/6 male mice were purchased from the National Cancer Institute. Vaccinia virus preferentially accumulates in ovaries 74, 75, which may cause variation in CTL activation under different treatments. As a result, only male recipient mice were used for vaccinia virus infection. Phospho-Stat4 (Tyr693) (D2E4) and Jak2 (D2E12) were purchased from Cell signaling Technology (Danvers, MA). All the rest directly conjugated fluorescent antibodies were purchased from BD Biosciences, eBioscience or Biolegend. Rapamycin were purchased from EMD (Gibbstown, NJ). Kb/OVA tetramer is a gift from Dr. Jameson from University of Minnesota.
Viruses and bacteria
Recombinant VV-GFP-JAW-OVA (VV-OVAp) expresses the OVA257–264 epitope fused C-terminally to GFP and the transmembrane region of JAW-1 (a gift from Dr. Jameson, University of Minnesota) 28, 76. Viral titers were determined by plaque assays using 143B cells, and mice were infected i.p. with 5 × 106 PFUs. Recombinant Listeria monocytogenes (a gift from Dr. Jameson, University of Minnesota) expressing full-length secreted ovalbumin (LM-OVA) was used for inoculation at either 104 CFU/mouse (for secondary expansion of memory CTLs) or 5 × 105 CFU/mouse (for memory CTL protection) via i.v. Mouse spleens were harvested 3 days after LM-OVA challenge, and LM-OVA was cultured using TSB plates for comparison of protection as in our previous reports 9, 10.
Administration of rapamycin
Mice were injected daily with rapamycin (EMD Gibbstown, NJ) through i.p. during a treatment period. Two different treatment periods were used: 1) high dose (600 µg/kg) administration during VV-OVA infection (day −1 prior to day 10 post-infection), or as indicated; 2) low dose (75 µg/kg) administration during VV-OVA infection (day −1 prior to infection to day 30 post-infection), as previously reported 26. Control mice received shame treatment.
Naive T cell purification
This was performed as previously reported 9, 10. Briefly, inguinal, axillary, brachial, cervical, and mesenteric lymph nodes (LNs) were harvested from WT OT-I or IL-12RKO OT-I mice, pooled, and disrupted to obtain a single cell suspension. Cells were incubated with FITC-labeled antibodies specific for CD4, B220, I-Ab, and CD44. Anti-FITC magnetic MicroBeads (Miltenyi Biotech, Auburn CA) were then added and the suspension passed through separation columns attached to a MACS magnet. Cells that did not bind were collected with a purity >95% CD8+ cells and <0.5% CD44hi cells.
Adoptive transfer and flow cytometric analysis
This is the same as we previously reported 9, 10. Purified OT-I cells were adoptively transferred into normal C57BL/6NCr mice by i.v. (tail vein) injection at 105 cells/mouse and OT-I cells were identified as CD8+CD45.2+ cells. Blood samples were drawn at indicated times, and the analysis of memory CTLs was based on samples from blood and/or tissues. Single cell suspensions were prepared, viable cell counts were performed (trypan blue) and the percentage of OT-I cells in the sample was determined by flow cytometry. Background for determining OT-I cell numbers was determined by identical staining of cells from normal C57BL/6 mice (no adoptive transfer). Analysis was done using a FACSCalibur™ flow cytometer and CELLQuest™ software (BD Biosciences) to determine the percentage and total OT-I cells in the samples. Flowjo software (Tree Star Inc. Ashland, OR) was used for data analysis.
Tissue harvest and digestion
Mice were euthanized by CO2 and peripheral lymph nodes and spleens were directly picked up and homogenized using 15 ml glass grinders. Lungs were perfused using 1× PBS at about 30 ml per mouse, cut into small pieces (1 mm3), homogenized with a 10 ml pipette and resuspended in 4 ml Collagenase D (Roche, Indianapolis, IN). For complete digestion, lung tissues were kept in a water bath (37 °C) for 25 min. Digestion was stopped by the addition of 0.1 M EDTA, and digested tissues were homogenized using glass grinders. Bone marrow was harvested by flushing cut bones with 1× PBS.
Intracellular cytokine staining after in vitro stimulation
Single cell suspension from adoptively transferred mice was incubated at 2 × 106 cells/ml in RP-10 with 0.2 µM OVA257–264 peptide and 1 µl Brefeldin A (Biolegend) for 3.5 hrs at 37°C 9, 10. Cells were fixed in fixing buffer (Biolegend) for 15 min at 4°C, permeablized in Saponin-containing Perm/Wash buffer (Biolegend) for another 15 min at 4°C, and stained with PE-conjugated antibody to IFNγ or APC-conjugated antibody to TNFα for 30 min at 4°C. Cells were then washed once with Perm/Wash buffer, and once with PBS containing 2% FBS.
Statistical analysis
Data was graphed and analyzed using a two-tailed Student's t test or Two-way ANOVA (GraphPad Prism 5.0 software. La Jolla, CA) 9, 10. Comparisons with a P value of <0.05 were considered significantly different.
Supplementary Material
Acknowledgements
We thank Drs. MF Mescher and SC Jameson from the University of Minnesota for providing reagents. The authors have declared that no competing interests exist. This work was supported by National Institutes of Health Grants R21AI095715A (to X. Z.) and Startup from UMD (to X. Z.).
Footnotes
Conflict of Interest
All other authors declare no financial or commercial conflict of interest.
References
- 1.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 3.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9(9):662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 5.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31(6):859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22(3):333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 8.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202(5):637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182(5):2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Garcia K, Sun Z, Xiao Z. Temporal Regulation of Rapamycin on Memory CTL Programming by IL-12. PLoS ONE. 2011;6(9):e25177. doi: 10.1371/journal.pone.0025177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32(1):67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinushi M, Tahara H. Cytokine gene-mediated immunotherapy: current status and future perspectives. Cancer Sci. 2009;100(8):1389–1396. doi: 10.1111/j.1349-7006.2009.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J Immunol. 1999;163(5):2561–2567. [PubMed] [Google Scholar]
- 14.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168(11):5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 15.Rubinstein MP, Cloud CA, Garrett TE, Moore CJ, Schwartz KM, Johnson CB, et al. Ex Vivo Interleukin-12-Priming During CD8(+) T Cell Activation Dramatically Improves Adoptive T Cell Transfer Antitumor Efficacy in a Lymphodepleted Host. J Am Coll Surg. 2012 doi: 10.1016/j.jamcollsurg.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabel MS, Arora A, Su G, Griffith KA, Mathiowitz E, Reineke JJ, et al. Generation of a tumor-specific systemic response after intratumoral injection of IL-12 and IL-18-loaded polylactic acid microspheres. Journal of immunotherapy (Hagerstown, Md. : 1997) 2007;30(8):808–816. doi: 10.1097/CJI.0b013e318156e6a7. [DOI] [PubMed] [Google Scholar]
- 17.Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF. Cutting Edge: IL-12 and Type I IFN Differentially Program CD8 T Cells for Programmed Death 1 Re-expression Levels and Tumor Control. J Immunol. 2013;191(3):1011–1015. doi: 10.4049/jimmunol.1300652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12(5):325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9(5):324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzman SD, O'Gorman WE, Villarino AV, Gallo E, Friedman RS, Krummel MF, et al. Duration of antigen receptor signaling determines T-cell tolerance or activation. Proc Natl Acad Sci U S A. 2010;107(42):18085–18090. doi: 10.1073/pnas.1010560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183(8):4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33(3):301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11(2):109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. J Exp Med. 2007;204(11):2667–2677. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth K, Garcia K, Sun Z, Tuo W, Xiao Z. Repetitive peptide boosting progressively enhances functional memory CTLs. Biochem Biophys Res Commun. 2012 doi: 10.1016/j.bbrc.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106(21):8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 33.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8(2):107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 34.Keppler SJ, Aichele P. Signal 3 requirement for memory CD8+ T-cell activation is determined by the infectious pathogen. Eur J Immunol. 2011;41(11):3176–3186. doi: 10.1002/eji.201141537. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr., et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181(5):1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong Z, Wen Z, Darnell JE., Jr. Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine & amp; growth factor reviews. 2003;14(5):361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 38.Kindt TJ, Goldsby RA, Osborne BA. Kuby Immunology. Sixth edn. New York: W.H.Freeman and Company; 2007. [Google Scholar]
- 39.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the Interleukin (IL)-12R β2 Subunit Expression in Developing T Helper 1 (Th1) and Th2 Cells. J Exp Med. 1997;185(5):817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183(3):1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gil MP, Ploquin MJ, Watford WT, Lee SH, Kim K, Wang X, et al. Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood. 2012;120(18):3718–3728. doi: 10.1182/blood-2012-05-428672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297(5589):2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, et al. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185(9):4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 46.Sun Z, Smyth K, Garcia K, Mattson E, Li L, Xiao Z. Nicotine Inhibits Memory CTL Programming. PLoS ONE. 2013;8(7):e68183. doi: 10.1371/journal.pone.0068183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Den Broek M, Bachmann MF, Kohler G, Barner M, Escher R, Zinkernagel R, et al. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-gamma and nitric oxide synthetase 2. J Immunol. 2000;164(1):371–378. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 49.Obar JJ, Crist SG, Leung EK, Usherwood EJ. IL-15-independent proliferative renewal of memory CD8+ T cells in latent gammaherpesvirus infection. J Immunol. 2004;173(4):2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- 50.Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 2010;184(1):35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115(5):1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177(7):4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jonjic S. Functional plasticity and robustness are essential characteristics of biological systems: lessons learned from KLRG1-deficient mice. Eur J Immunol. 2010;40(5):1241–2413. doi: 10.1002/eji.201040506. [DOI] [PubMed] [Google Scholar]
- 55.Smyth K, Garcia K, Sun Z, Tuo W, Xiao Z. Repetitive peptide boosting progressively enhances functional memory CTLs. Biochem Biophys Res Commun. 2012;424(3):635–640. doi: 10.1016/j.bbrc.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265(1):125–137. doi: 10.1111/j.1365-2796.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- 57.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends in immunology. 2004;25(2):98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Ramshaw IA, Ramsay AJ. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today. 2000;21(4):163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 59.Brierley MM, Fish EN. Review: IFN-alpha/beta receptor interactions to biologic outcomes: understanding the circuitry. Journal of interferon & amp; cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2002;22(8):835–845. doi: 10.1089/107999002760274845. [DOI] [PubMed] [Google Scholar]
- 60.Bacon CM, McVicar DW, Ortaldo JR, Rees RC, O’Shea JJ, Johnston JA. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J Exp Med. 1995;181(1):399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller M, Briscoe J, Laxton C, Guschin D, Ziemiecki A, Silvennoinen O, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- 62.Laurence A, Pesu M, Silvennoinen O, O’Shea J. JAK Kinases in Health and Disease: An Update. The open rheumatology journal. 2012;6:232–244. doi: 10.2174/1874312901206010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93(3):397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 64.O’Shea JJ. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7(1):1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- 65.Pazdrak K, Stafford S, Alam R. The activation of the Jak-STAT 1 signaling pathway by IL-5 in eosinophils. J Immunol. 1995;155(1):397–402. [PubMed] [Google Scholar]
- 66.Silvennoinen O, Witthuhn BA, Quelle FW, Cleveland JL, Yi T, Ihle JN. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, et al. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 68.Belz GT, Wilson NS, Smith CM, Mount AM, Carbone FR, Heath WR. Bone marrow-derived cells expand memory CD8+ T cells in response to viral infections of the lung and skin. Eur J Immunol. 2006;36(2):327–335. doi: 10.1002/eji.200535432. [DOI] [PubMed] [Google Scholar]
- 69.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22(5):561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, et al. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179(10):6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 71.Woodberry T, Gardner J, Elliott SL, Leyrer S, Purdie DM, Chaplin P, et al. Prime boost vaccination strategies: CD8 T cell numbers, protection, and Th1 bias. J Immunol. 2003;170(5):2599–2604. doi: 10.4049/jimmunol.170.5.2599. [DOI] [PubMed] [Google Scholar]
- 72.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115(3):739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogquist K, Jameson S, Heath W, Howard J, Bevan M, Carbone F. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 74.Karupiah G, Coupar B, Ramshaw I, Boyle D, Blanden R, Andrew M. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunology and cell biology. 1990;68(Pt 5):325–333. doi: 10.1038/icb.1990.44. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Y, Adams YF, Croft M. Preferential replication of vaccinia virus in the ovaries is independent of immune regulation through IL-10 and TGF-beta. Viral immunology. 2011;24(5):387–396. doi: 10.1089/vim.2011.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao Z, Curtsinger JM, Prlic M, Jameson SC, Mescher MF. The CD8 T cell response to vaccinia virus exhibits site-dependent heterogeneity of functional responses. Int Immunol. 2007;19(6):733–743. doi: 10.1093/intimm/dxm039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.