Abstract
Study Objective
National guidelines recommend annual Chlamydia trachomatis and Neisseria gonorrhea screening for sexually active youth at-risk for infection. These infections have serious sequelae in women if untreated, and methods to improve testing are needed. We hypothesized that an electronic method of identifying at-risk youth would significantly increase testing for these sexually transmitted infections during emergency department (ED) visits.
Methods
We developed an audio-enhanced computer-assisted self-interview (ACASI) to obtain sexual histories from ED patients and an embedded decision-tree to create an STI testing recommendation. ED healthcare providers were prompted via the electronic medical record to review the participant answers and testing recommendations, and to offer testing to at-risk youth. Patients 15-21 years old visiting the St. Louis Children's Hospital ED, regardless of complaint, were eligible for participation.
Results
STI testing among all 15-21 year old ED patients increased from 9.3% in the three months prior to the ACASI, to 17.8% during the eight-month period the ACASI was available, and diminished to 12.4% in the three months after ACASI withdrawal (P<0.001). During the ACASI period we approached 51.4% of eligible patients and enrolled 59.8% (800/1337) of those approached. Among ACASI participants, 52.4% (419/800) received a recommendation to receive STI testing. Of these, 52.7% (221/419) received testing in the ED, and 18.1% (40/221) of those tested were positive for chlamydia and/or gonorrhea, 55% of whom (22/40) had chief complaints unrelated to STIs. Most (89%) participants rated the ACASI easy to use.
Conclusions
STI testing in the ED significantly increased during ACASI use and diminished after withdrawal. The ACASI was well accepted by youth and holds promise for enhancing STI testing in the ED.
Introduction
Background
More than 1.5 million people in the United States were diagnosed with the sexually transmitted infections (STIs) Chlamydia trachomatis and Neisseria gonorrhea in 2010, with the highest incidence in the 15-24 age group.1 While many infections are asymptomatic, untreated women may experience severe pain, pelvic inflammatory disease, or infertility. The Centers for Disease Control and Prevention (CDC) recommends yearly chlamydia testing for sexually active women under age 26, men who have sex with men, and men evaluated in high prevalence settings (e.g., adolescent clinics), with three-month retesting after treatment for a positive test.2 Most commercial kits test for both chlamydia and gonorrhea, because of the high rate of co-infection.2 Despite testing recommendations, more than 50% of women do not receive the recommended annual screening.3 St. Louis, Missouri ranks among U.S. cities with the highest incidence of both chlamydia and gonorrhea,1,4 and effective methods to increase testing and treatment for youth are needed.
Importance
Opportunities to screen a large number of youth exist in the pediatric emergency department (ED), but a STI risk assessment is difficult to conduct because of constraints on time and privacy. Previous studies examining STI testing in adult and pediatric EDs have used universal testing rather than risk assessment.5-10 However, the ED workflow does not provide healthcare providers the opportunity to interview every youth privately in order to proactively screen for problems unrelated to their primary complaint. Audio-enhanced Computer-Assisted Self Interviews (ACASIs) have an audio-component to aid comprehension and could facilitate efficient, targeted screening. Targeted screening using an ACASI would focus testing resources on youth who are sexually active or engaging in at-risk behaviors. ACASI's have been used in the pediatric ED to screen for mental health issues,11,12 to obtain a medical history,13,14 and in other settings to obtain accurate information from youth about sensitive topics such as sexual activity.15-18 ACASI's can be used in a variety of medical settings,19 and are of increasing value with the increased use of electronic medical records (EMR).
Goals of This Investigation
We hypothesized that use of an ACASI in a pediatric ED would increase youth STI testing more than staff education alone, and that youth would find the ACASI easy to use and prefer it over other methods of disclosing this sensitive health information.
Materials and Methods
Study Design and Setting
St. Louis Children's Hospital (SLCH) is a tertiary care, freestanding children's hospital in urban St. Louis, MO. The SLCH ED treats approximately 50,000 patients annually, and serves many youth from areas of St. Louis with a high prevalence of STIs.
We conducted a quasi-experimental study using an interrupted time series with four consecutive time periods to evaluate the effect of the ACASI on gonorrhea and chlamydia testing frequency in the SLCH ED. The four time periods were, in order: usual care (historical control) [A], education only [B], ACASI + education [C], and education only again [B] after the ACASI was withdrawn. The institutional review board (IRB) at Washington University School of Medicine approved this study.
ED Medical and Nursing Staff Education
ACASI implementation required improved awareness of STI testing recommendations. In order to isolate the effect of this heightened awareness, we provided educational lectures about youth and STI testing prior to ACASI implementation. The lecture was developed by an experienced specialist in adolescent medicine (KP) and delivered by the first author (FAA). The lecture addressed how to discuss sensitive issues with youth and the importance of STI testing in this group, with a focus on chlamydia and gonorrhea. We encouraged ED staff to use urine-based testing that used nucleic acid amplification methods.20,21 During the ACASI testing period, we provided an overview of the process and of how to use the ACASI information. We gave lectures during regularly available education time slots for faculty, fellows, residents, and staff (e.g., during ED resident conferences, division CME, and nursing conferences). Attendance was encouraged but not mandatory, which is typical at our institution.
ACASI Development and Testing Recommendations
We created a branch-logic questionnaire (Appendix 1) to identify patients who met CDC criteria for testing or who endorsed other symptoms that indicated a need for testing, and software for administering the questionnaire on a laptop computer. Prior to implementing the ACASI in the ED, we conducted 25 cognitive interviews at a local youth wellness facility (median age 18 years, IQR 18-19 years, 80% female) to assess comprehension and ease of using the ACASI. We used their responses to modify the questionnaire. The overall Flesh-Kincaid reading level of the introduction and the questions in the ACASI was 6.9.
Testing recommendations were based on the CDC recommendations,2 and our local disease epidemiology that indicated yearly testing for sexually active men (Appendix 2). A decision tree in the software (Appendix 3) used responses to classify participants into one of four recommendation groups. Group 1 included sexually active participants in need of immediate testing, either because their testing history indicated a need for testing according to CDC recommendations or because they endorsed symptoms we believed indicated possible active infection (genitourinary discharge, dysuria, or pain with intercourse). Group 2 included sexually active participants deemed at elevated risk for infection based on their sexual history, but who already satisfied CDC testing recommendations and did not endorse STI-related symptoms; Group 2 recommendation was for more frequent outpatient testing, but without indication for immediate testing. Group 3 included participants without a history of sexual activity and sexually active participants with sufficient testing and without elevated risk and testing was not recommended. Group 4 included participants who skipped too many questions to create a recommendation. After completion, participants viewed their testing recommendations on screen and could provide phone numbers to receive test results and an email address to receive informational material.
Eligibility Criteria
We offered the ACASI from April 18, 2011 through December 20, 2011. Enrollment was conducted by research assistants (RAs), usually 8:00 AM-midnight, seven days a week. Eligible participants were 15-21 years old seeking care in the SLCH ED. Patients presenting for evaluation of abuse or sexual assault, requiring activation of the trauma system, with level-1 or level-2 triage scores (highest severity), with disabilities that prevented independent computer use, psychiatric chief complaints, and inability to speak English were excluded. Using our EMR, we retrospectively identified patients across all four time periods, from January 1, 2010 through March 31, 2012, and created a dataset of all patient visits. We then applied the inclusion/exclusion criteria to this dataset to define the eligible patients of interest across the four time periods. This dataset also allowed us to define the patients missed by our RAs during the ACASI intervention period, including those the RAs were unable to approach during the patient's visit (e.g., if they were with another patient) or because the patient visited the ED at a time of day when no RA was present.
Study Procedures
During the ACASI period, patients were approached for enrollment after they completed triage, either in the waiting room or patient care rooms. We obtained written assent from minors and consent from patients 18 years of age or older. The IRB granted a waiver of parental consent if guardians were absent at time of enrollment, and we asked guardians for consent if present. To protect participant confidentiality, guardians provided consent for a general health questionnaire without knowing the study's STI focus. Participants confirmed participation after reading an introductory page and learning the study focus. We considered them enrolled if they progressed to the first page of questions.
Participants who completed the ACASI had an answer summary with testing recommendations and contact information integrated into the EMR for review by healthcare providers (Appendix 2), who could order STI testing using standard ED methods. Gonorrhea and chlamydia testing is done through commercially available testing kits that use nucleic acid amplification testing (NAAT), either using urine samples or urethral/penile swabs. NAAT tests are run by our hospital laboratory once daily, Monday-Friday, resulting in turnaround times of less than 72 hours for all tests. Our ED has an opt-out program for rapid HIV testing, with results available within twenty-minutes using an oral swab. Additionally, we have traditional serum tests for syphilis and hepatitis available. While these diseases were discussed briefly in our educational lectures, the focus was on gonorrhea and chlamydia.
Participants who did not reach the end of the questionnaire did not have a summary page created. An electronic flag in the EMR notified doctors and nurses these data were available, and it was the responsibility of the treating physicians and NP's to review the recommendation and order testing. Periodic reminders to use the ACASI information were distributed using email and signs in the ED. A preliminary review of the data in July, 2011 showed that the treating physicians and nurse practitioners sometimes failed to review the ACASI information and order tests for youth meeting criteria for STI testing. To improve this, beginning September 1, 2011, we created a protocol that enabled ED staff nurses to order tests for participants with a Group 1 recommendation.
Test results were followed-up using existing ED protocol. Nurse practitioners (NP) review test results, notify patients of positive tests, create treatment plans for patients not empirically treated in the ED, and notify the health department in accordance with Missouri reporting requirements. Patients needing treatment return to our ED or receive an outpatient plan, most commonly transmitting prescriptions by telephone to a pharmacy of the patients’ choosing. When the NP cannot contact patients by phone, they send certified letters. The phone numbers provided by patients via the ACASI (Appendix 2) enabled NPs to successfully notify patients of test results and create treatment plans.
Outcomes
The primary outcome was the proportion of chlamydia and gonorrhea ED testing among all patients eligible to use the ACASI had it been continuously available during the study periods. Secondary outcomes for ACASI participants included testing recommendations, proportion of participants receiving testing and with positive tests, and participant evaluation of the ACASI system. To create a planned subgroup analysis of patients whose chief complaints were not indicative of STI, we excluded ED patients with genitourinary or gynecological chief complaints, and women with a chief complaint of abdominal or pelvic pain. Participants also rated the ACASI for ease of use and understanding, comfort with system confidentiality, and preference for method of disclosure of sensitive information using 5-point Likert scales or categorical responses.
Sample Size
We estimated a target sample of 1556 ACASI participants in order to detect an increase of 5% in the overall STI testing percentages in the ACASI + Education period compared to the initial Education period, with an alpha of 0.05 and a power of 80% (assumes 50% of ACASI participants would qualify for testing and 50% of those who qualified for testing would receive it during their ED visit). For patients with multiple visits during the study period, only first visits were analyzed to maintain independence of testing results.
Statistical Analysis
We used chi-squared tests to compare the percentage of patients receiving STI testing across all four time periods, and for pairwise comparisons to examine STI testing between two adjacent time periods. We used the Bonferroni adjustment when comparing testing across all four time periods. We divided our alpha of 0.05 by four, so that each period had an alpha of 0.0125. We did this to correct for potentially increased risk of Type I error due to multiple testing. We calculated Bonferroni-adjusted 95% CIs for the percentage of patients receiving testing within each of the four time periods. For the pairwise testing we used an alpha of 0.05 and calculated unadjusted 95% CIs around the change in percentage of patients tested between the two time periods. Given many uncertainties in the assumptions used for sample size calculation, we planned an interim efficacy analysis to occur in December 2012, after approximately 800 participants had used the ACASI. The purpose of the interim analysis was to provide the opportunity to stop the study early because efficacy had already been established, to stop early because of futility, or to increase the sample size if the observed effect size was slightly smaller than anticipated in the original power computations. Stopping early due to established efficacy was based on the O'Brien-Fleming stopping rule22 and required a P value < 0.001. Stopping early because of futility required a conditional power of less than 0.2. Analysis was done using SPSS version 18.
Results
Overall ED Testing
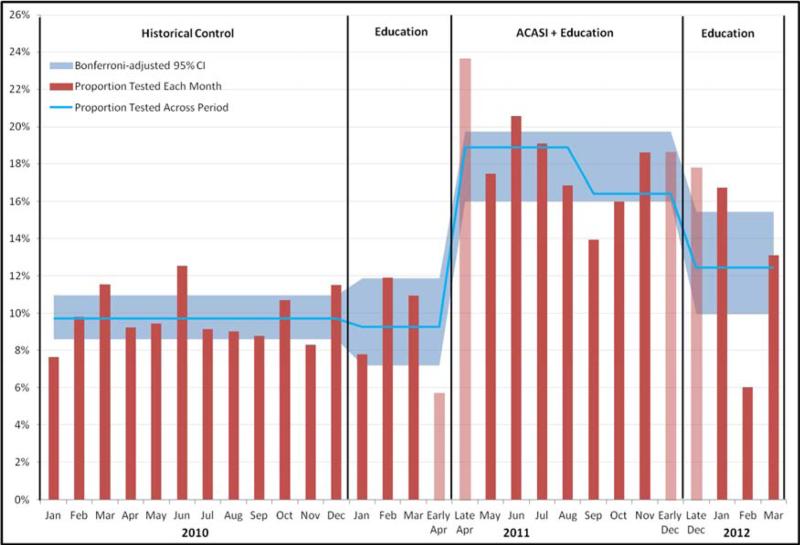
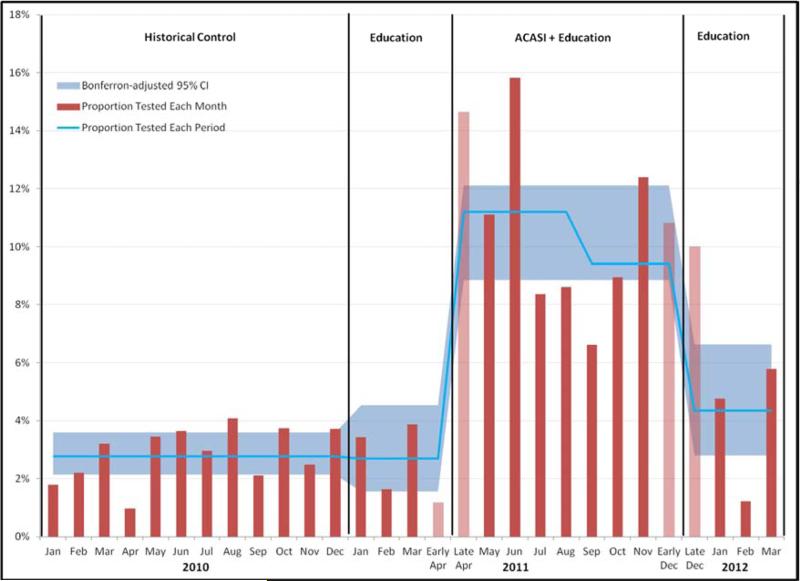
There were 15,840 total visits from patient's 15-21 years old during the twenty-five month study period, and 8421 unique patients who met the inclusion/exclusion criteria. Characteristics of these patients are shown in Table 1. Figure 1 shows the percentage of ED patients who were tested for STI's in each time period and the associated Bonferroni-adjusted 95% CIs. The change in overall testing across all four study time periods was significant (P< 0.001). In pairwise contrasts, testing percentages did not differ significantly between historical controls and the addition of educational lectures, (9.7% to 9.3%, difference -0.4%, 95% CI [-1.7%, 2.4%)]). Interim analysis in December 2011 (n=800) showed testing increased (9.3% to 17.8%, difference 8.5%, 95% CI [6.1%, 10.8%]) and met our criterion for early stopping of enrollment (p < 0.001). In the three months following ACASI withdrawal, testing decreased (17.8% to 12.4%, difference -5.4%, 95% CI [2.7%, 7.9%]). Figure 2 shows the percentage of ED patients receiving testing across the four time periods and the associated Bonferroni-adjusted 95% CI's for the subgroup whose chief complaints were not indicative of STI; it showed similar levels of significance to the overall group.
Table 1.
Patient Characteristics Across Four Time Periods (N=8421)
| Dates | Historical Control | Education | ACASI+Education | Education |
|---|---|---|---|---|
| 01/01/10 - 12/31/10 | 01/01/11-04/17/11 | 04/18/11-12/20/11 | 12/21/11-03/31/12 | |
| Number of Patients | 3929 | 982 | 2601 | 909 |
| Patients Whose Chief Complaint Not Indicative of STI*, % | 83.4 | 83.5 | 84.1 | 83.6 |
| Median age, years (Interquartile Range) | 16.8 (15.9-17.7) | 16.6 (15.8-17.6) | 16.8 (15.8-17.7) | 16.5 (15.7-17.6) |
| Female, % | 54.7 | 56.4 | 54.9 | 56.5 |
| African-American, % | 68.9 | 67.2 | 61.7 | 59.1 |
Excludes all genitourinary or gynecologic chief complaints and abdominal pain in women
Figure 1. ACASI Eligible ED Patients Receiving Chlamydia and/or Gonorrhea Testing Regardless of Chief Complaint.
Data are the proportions of youth eligible to take the ACASI who received chlamydia/gonorrhea testing (urine, cervical, or penile) each month (red bars), overall proportion tested that period (blue line), and the Bonferroni-adjusted 95% confidence interval (CI) limits for testing proportions in each period (blue shading). Pink bars represent partial months. In the ACASI period, the blue line represents testing before and after the nursing standing order was available on September 1, 2011. In the control period, 9.7% (381/3929; Bonferroni-adjusted 95% CI [8.6- 10.9]) received testing. During the initial education-only period, 9.3% (91/982; Bonferroni-adjusted 95% CI [7.2-11.9]) received testing. After ACASI introduction, 17.8% (463/2601; Bonferroni-adjusted 95% CI [16.0-19.8]) received testing. After ACASI withdrawal, 12.4% (113/909; Bonferroni-adjusted 95% CI [9.9-15.4]) received testing.
Figure 2. ACASI Eligible ED Patients Receiving Chlamydia and/or Gonorrhea Testing if Chief Complaints not Indicative of STI.
Data are the proportions of youth eligible to take the ACASI who received chlamydia/gonorrhea testing (urine, cervical, or penile) each month (red bars), overall proportion tested that period (blue line), and Bonferroni-adjusted 95% confidence interval (CI) limits for testing proportions in each period (blue shading). Pink bars represent partial months. In the ACASI period the blue line represents before and after the nursing standing order was available on September 1, 2011. It excludes patient visits with ED chief complaints that are genitourinary or gynecologic, and chief complaints of abdominal/pelvic pain in women. In the control period, 2.8% (91/3281; Bonferroni-adjusted 95% CI [2.1-3.6]) received testing. During the initial education only period, 2.7% (22/820; Bonferroni-adjusted 95% CI [1.6-4.5]) received testing. After ACASI introduction, 10.4% (227/2188; Bonferroni-adjusted 95% CI 8.9-12.1]) received testing. After ACASI withdrawal, 4.3% (33/760; Bonferroni-adjusted 95% CI [2.8-6.6]) received testing.
ACASI Participants
During the ACASI intervention period, we approached 51.4% (1337/2601) of eligible patients, and enrolled 59.8% (800/1337) of those approached. Participants had a median age of 16.7 years (IQR 15.8-17.6 years) vs. 16.8 years (IQR 15.8-17.7 years) for non-participants. The participants were 68.0% African-American (544/800) vs. 58.9% (1061/1801) for non-participants, and 58.3% (466/800) of participants were women vs. 53.1% (957/1801) of non-participants. All participants progressed past the introduction to the first page of questions.
We analyzed data for all 800 ACASI participants (Table 2). The ACASI recommended testing for 50.2% of women, of whom 56.0% received testing, and 21.4% of those tested were positive for chlamydia and/or gonorrhea (Table 3). Testing was recommended for 55.4% of men, of whom 48.6% received testing, and 13.3% of those tested were positive for chlamydia and/or gonorrhea. During the ACASI period, 33.1% (265/800) of all ACASI participants received testing, and 11.0% (198/1801) of eligible non-participants received testing. Eight participants had tests ordered but no sample was sent for analysis (reasons not documented), and five participants provided insufficient urine for lab analysis. We classified these participants as not receiving testing.
Table 2.
ACASI Participant Characteristics (N=800)
| N (%) | |
|---|---|
| Median age, yrs | 16.9 |
| Interquartile range | 15.9-17.7 |
| Female | 466 (58.3) |
| Hispanic/Latino ethnicity | 33 (4.1) |
| Race* | |
| African-American | 507 (63.3) |
| White | 224 (28.0) |
| Asian | 6 (7.5) |
| Mixed | 58 (7.2) |
| Native American or Alaskan | 5 (6.2) |
| Insurance Status | |
| Medicaid | 498 (62.3) |
| Private/Military | 261 (32.6) |
| None | 41 (5.1) |
| Chief Complaint Not Indicative of STI† | 667 (83.4) |
| Access to Regular Medical Care | 676 (84.5) |
| Ever had sexual intercourse | |
| Anal | 78 (9.7) |
| Oral | 318 (39.8) |
| Vaginal | 479 (59.9) |
| Any Type | 509 (63.6) |
| Willing to be tested in ED | 698 (87.3) |
| Provided phone number(s) | 621 (77.6) |
| Provided email address | 552 (69.0) |
Race data self-reported via ACASI, differs from registration race data in manuscript
Excludes all genitourinary or gynecologic chief complaints and abdominal pain in women
ACASI – Audio-enhanced Computer-Assisted Self-Interview; ED – emergency department; STI – sexually transmitted infection
Table 3.
Testing in ACASI Participants
| Recommendation Group* | Total | Received Testing (% Total) | Gonorrhea Positive† | Chlamydia Positive† | |
|---|---|---|---|---|---|
| Female N=466 | 1 | 234 (50.2%) | 131 (56.0%) | 9 (6.9%) | 25 (19.1%) |
| 2 | 72 (15.4%) | 20 (27.8%) | 1 (5.0%) | 4 (20.0%) | |
| 3 | 155 (33.2%) | 14 (9.0%) | 0 | 2 (14.3%) | |
| 4 | 5 (1.1%) | 1 (20.0%) | 0 | 0 | |
| Male N=334 | 1 | 185 (55.4%) | 90 (48.6%) | 3 (3.3%) | 10 (11%) |
| 2 | 16 (4.8%) | 3 (18.8%) | 0 | 0 | |
| 3 | 124 (37.1%) | 5 (4.0%) | 0 | 0 | |
| 4 | 9 (2.7%) | 1 (11.1%) | 0 | 0 | |
1=Patients should be offered testing, based on CDC criteria or endorsed symptoms; 2= Patient does not need testing but sexual history indicated elevated infection risk; 3= Patient does not need testing or have elevated infection risk; 4= Patient left too many answers blank to provide a recommendation.
1 man and 6 women tested positive for both chlamydia and gonorrhea
ACASI – Audio-enhanced Computer-Assisted Self-Interview; CDC – Centers for Disease Control and Prevention
Testing was recommended for 48.3% of women whose chief complaints were not indicative of STI (Table 4), of whom 43.7% were tested while in the ED, and 19.2% of those tested were positive for chlamydia and/or gonorrhea. Testing was recommended for 51.4% of men whose chief complaints were not indicative of STI, of whom 45.7% were tested while in the ED, and 10.0% of those tested were positive for chlamydia and/or gonorrhea. Testing for participants receiving a Group 1 recommendation did not change after the implementation of the nursing protocol, decreasing from 55.9% (132/236) to 48.6% (89/183) after implementation. Group 1 patients who were tested were similar regarding their gender, race, and insurance status to those who were not tested.
Table 4.
Testing in ACASI Participants with Chief Complaints not Indicative of STI*
| Recommendation Group† | Total | Received Testing (% Total) | Gonorrhea Positive‡ | Chlamydia Positive‡ | |
|---|---|---|---|---|---|
| Female N=347 | 1 | 167 (48.1%) | 73 (43.7%) | 4 (5.5%) | 14 (19.2%) |
| 2 | 54 (15.6%) | 9 (16.7%) | 1 (11.1%) | 2 (22.2%) | |
| 3 | 123 (35.4%) | 3 (2.4%) | 0 | 0 | |
| 4 | 3 (0.9%) | 0 | 0 | 0 | |
| Male N=320 | 1 | 173 (54.1%) | 79 (45.7%) | 2 (2.5%) | 7 (8.9%) |
| 2 | 16 (5.0%) | 3 (18%) | 0 | 0 | |
| 3 | 122 (38.1%) | 3 (2.5%) | 0 | 0 | |
| 4 | 9 (2.8%) | 1 (11.1%) | 0 | 0 | |
Excludes all genitourinary or gynecologic chief complaints and abdominal pain in women
1=Patients should be offered testing, based on CDC criteria or endorsed symptoms; 2= Patient does not need testing but sexual history indicated elevated infection risk; 3= Patient does not need testing or have elevated infection risk; 4= Patient left too many answers blank to provide a recommendation.
1 man and 4 women tested positive for both Chlamydia and gonorrhea
ACASI – Audio-enhanced Computer-Assisted Self-Interview; CDC – Centers for Disease Control and Prevention
Forty-seven participants, 40 with group 1 recommendations, tested positive for gonorrhea and/or Chlamydia. All were notified by telephone and had a treatment plan; 13 received empiric treatment during the initial ED visit, 7 returned to our ED for treatment, 25 received pharmacy prescriptions, and 2 received outpatient treatment elsewhere.
ACASI Use and Evaluation
Table 5 summarizes participant evaluation of the ACASI. The median time needed to read the introduction and complete the ACASI was 8.3 minutes (Interquartile Range 6.3-11.2 minutes). Among the 266 participants who received STI testing, 84.9% (225/265) provided phone numbers for test result follow-up, and 74.3% (197/265) provided an email address for informational handouts. No concerns regarding violation of participant confidentiality were reported.
Table 5.
ACASI Evaluation by Participants (N=800)
| Survey Time | N (%) |
|---|---|
| Very Short | 302 (37.8) |
| Short | 192 (24.0) |
| Moderate | 244 (30.5) |
| Long | 40 (5.0) |
| Too Long | 7 (0.9) |
| No Answer | 15 (1.9) |
| Survey Ease of Use | |
| Very Easy | 621 (77.6) |
| Easy | 90 (11.3) |
| Moderate | 64 (8.0) |
| Difficult | 5 (0.6) |
| Very Difficult | 4 (0.5) |
| No Answer | 16 (2.0) |
| Survey Wording | |
| Very Easy | 647 (80.9) |
| Easy | 68 (8.5) |
| Moderate | 51 (6.4) |
| Difficult | 7 (0.9) |
| Very Difficult | 9 (1.1) |
| No Answer | 18 (2.3) |
| Survey Confidentiality | |
| Very Comfortable | 481 (60.1) |
| Comfortable | 155 (19.4) |
| Neutral | 93 (11.6) |
| Uncomfortable | 35 (4.4) |
| Very uncomfortable | 18 (2.3) |
| No Answer | 18 (2.3) |
| Survey Audio | |
| Very Helpful | 351 (43.9) |
| Helpful | 83 (10.4) |
| Moderate | 242 (30.2) |
| Unhelpful | 31 (3.9) |
| Very Unhelpful | 65 (8.1) |
| No Answer | 28 (3.5) |
| Method Preferred | |
| Computer | 554 (69.3) |
| Paper | 20 (2.5) |
| Face To Face | 122 (15.3) |
| Doesn't Know | 85 (10.6) |
| No Answer | 19 (2.4) |
ACASI -- Audio-enhanced Computer-Assisted Self-Interview
Limitations
Our study had several limitations. We could not directly compare how testing in at-risk youth changed with the introduction and removal of the ACASI, because we used the ACASI to determine which youth were at-risk and in need of testing. As a proxy outcome measure we chose overall ED testing of all eligible participants, which allowed us to compare testing as we added and withdrew the ACASI. As a single-center study in a city with high incidence of STIs, the results may not be generalizable to other settings. We relied on accuracy and veracity of participants’ recall to create STI-testing recommendations, and some at-risk youth may not have received a recommendation to be tested. Some screening opportunities were missed when physicians and nurses did not respond to the EMR prompts to review available ACASI information. We believe the failure to review information is the most likely reason we did not see an improvement in testing with the nursing standing order. A ‘hard stop’ in the EMR to require review of the ACASI findings, or automatic test ordering for those who screen positive, might increase program effectiveness. Though all participants who tested positive were notified and had a treatment plan, we did not verify receipt of outpatient treatment in every case. Formal cost comparison between the ACASI and other screening strategies was beyond the scope of this research. We recognize the ultimate outcome of this work is to increase the number of patients receiving appropriate treatment. While increasing testing is the first and necessary step, we plan to further evaluate the effect of the improved ACASI system (under development) to increase rates of receipt of treatment.
Discussion
Our findings have implications for the public health management of STIs in youth. Implementation of the ACASI in our ED led to a near doubling of testing for chlamydia and gonorrhea in this population of at-risk youth. The intervention was well accepted by participants and did not interfere with workflow in the ED. Over half of ACASI participants who qualified for STI testing received it during their ED visit, and all who tested positive were notified and received treatment plans. The high percentage of notification of positive test results was enhanced by participants providing contact information via the ACASI. We were able to notify a greater percentage of patients of their test results than other ED-based efforts intended to improve this practice.23 This finding is clinically significant, especially considering results of an earlier study, where many patients with chlamydia and gonorrhea who had been tested in a large urban ED were never notified of their test results, with many not receiving empiric treatment in the ED.24 While our ED does not provide partner notification or expedited partner therapy for patients with a positive test, modifying the ACASI to allow collection of partner contact information could facilitate adoption of this practice in the ED.
Of note, most patients who used the ACASI presented with complaints that were not indicative of STI. Of those who were tested from this group, 10% of men and 19% of women were positive for chlamydia and/or gonorrhea. These youth would be unlikely to receive STI testing in the ED using conventional interviewing techniques. Approximately 19 million youth 15-24 years of age sought ED care in 2007,25 many of whom might have benefited from STI testing and could have been identified using our method. The ACASI also could be used to identify at-risk youth in other settings, such as clinics, schools, and youth drop-in centers.26 The utility of this system can be summarized by the concept “number-needed-to-screen” (NNS), the number of patients needed to complete the ACASI to detect one additional STI among patients. We had 800 participants complete the ACASI, of whom 40 participants classified as group 1 tested positive for gonorrhea and/or chlamydia (Table 3), giving an NNS of 20 for the overall cohort (800 screened/40 captured). Had all group 1 patients received testing as indicated and we captured twice the number of infections, the NNS could have dropped as low as 10. Of the 800 participants who completed the ACASI, 667 had a chief complaint that was not indicative of STI (Table 4). Of these 667, 22 patients who were classified as group 1 tested positive for gonorrhea and/or chlamydia, leading to a NNS of 30 for this subgroup (667 screened/22 captured). In a system where all eligible patients complete the ACASI and all at-risk patients receive testing, the NNS will be dependent primarily on the disease prevalence in the screened population.
Many studies have examined universal screening for chlamydia and gonorrhea in the ED,5-7,27-30 with some arguing that age-based screening is cost-effective.31,32 Despite these studies, we are unaware of any ED's doing routine age-based gonorrhea/chlamydia screening, though our ED and others now conduct routine age-based HIV screening.33,34 Unlike HIV screening, there is no low-cost or rapid test available for gonorrhea/chlamydia. Although we designed our decision algorithm to maximize testing, only 52% of participants met criteria for immediate testing. Using the ACASI to guide targeted screening allows us to focus resources on patients at highest risk of infection while avoiding unnecessary tests. Though some patients may be missed using this approach, particularly if they fail to report sexual activity,15,35 our data suggest this was infrequent using the ACASI. Few ACASI participants identified as Group 2 or Group 3 were considered at sufficient risk based on their traditional interview or physical examination to receive immediate STI testing, with only a very small proportion having an STI. The ACASI elicits a concise sexual history in a confidential manner with contact information that can be integrated into the EMR, and the ability to email informational material.
Although we used the ACASI in a study context and it is not currently in use, we are designing a sustainable version for use in our ED and other ED's. Our pilot study demonstrated the feasibility of using our system to improve STI testing in at-risk patients during ED visits. Moving forward we will focus more on integration of the system into our ED workflow, increasing test ordering in at-risk patients, and additional educational opportunities. As part of this effort we are attempting to decrease the number of interview questions and the time required for completion. We plan to integrate our existing HIV testing program into future iterations of the ACASI, converting the ACASI from ‘opt-in’ to ‘opt-out’ to help increase its use to improve our sexual history data for patients receiving HIV testing and to improve testing for gonorrhea and chlamydia. The next version of the ACASI will automatically order STI testing via our ED EMR when a patient is classified at-risk for infection and in need of testing. While the ACASI summary will still be viewable in the EMR, a review of the information will not be required to initiate STI testing. Future iterations will provide access to educational videos and more opportunities for feedback on how to improve our system. We may also expand the scope of gonorrhea and chlamydia testing to include oral and anal testing when indicated.36
In summary, implementation of an ACASI in our pediatric ED significantly increased the proportion of chlamydia and gonorrhea testing above education alone. Participants favorably evaluated the ACASI as easy to use and preferred the ACASI over other methods of data collection. The ACASI is a promising method both to collect sensitive information and to provide real-time interventions in at-risk youth.
Supplementary Material
Acknowledgments
We wish to thank Rob Brown, Ian Lackey, Ken Kenney, and Steve Wilmas (Washington University School of Medicine Pediatric Computing Facility) for creating the software program for the ACASI, Jack Baty (Washington University School of Medicine) for his support managing the patient databases and aiding statistical analysis, Ericka Hayes, MD (Washington University School of Medicine) and Stephen Porter, MD, MSc (The Hospital for Sick Children) for their conceptual contributions, Indi Trehan, MD, MPH (Washington University School of Medicine) for his manuscript review, the students of the Washington University Pediatric Emergency Medicine Research Associates’ Program (PEMRAP) for assisting in study enrollment, and the physicians and nurses of Washington University School of Medicine and St. Louis Children's Hospital for help in implementing our intervention.
Funding support:
Research reported in this publication was supported by a grant from National Institute of Child Health & Human Development (T32HD049338-01A2) and the Washington University Institute of Clinical and Translational Sciences grant (UL1 TR000448) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Mo., for the use of the Health Behavior, Communication and Outreach Core, which provided study design and survey development services and is supported in part by a National Cancer Institute Cancer Center Support Grant (P30 CA091842).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings: Preliminary data from this manuscript were presented at the Pediatric Academic Society Meeting in May 2012, in Boston, MA, and at the Society for Academic Emergency Medicine Conference in May, 2012, in Chicago, IL.
Financial Disclosures: The authors have no financial relationships to disclose
Conflicts of Interest: The authors have no conflicts of interest to disclose
Author Contributions:
FAA conceived the study. All authors contributed to the study methodology. KBS provided statistical advice. FAA and DMJ obtained research funding. FAA created the survey instruments. FAA, DBJ, KKC, KP, and DMJ provided substantial revisions to the survey instruments. FAA supervised the conduct of the study, data collection, managed the data, and did quality control. FAA and KBS analyzed the data. FAA, DBJ, KBS, JG, DMJ interpreted the data. FAA drafted the manuscript. FAA, DBJ, JG, DMJ provided substantial revisions to the drafts of the manuscript. All authors reviewed and approved the final manuscript as submitted. FAA takes responsibility for the paper as a whole.
References
- 1.Centers for Disease Control and Prevention . Sexually Transmitted Disease Surveillance 2010. Department of Health and Human Services; 2011. [Google Scholar]
- 2.Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010 Dec 17;59(RR-12):1–110. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Chlamydia screening among sexually active young female enrollees of health plans--United States, 2000-2007. MMWR Morb Mortal Wkly Rep. 2009 Apr 17;58(14):362–365. [PubMed] [Google Scholar]
- 4.Bureau of HIV, STD, and Hepatitis Division of Community and Public Health. Missouri Department of Health and Senior Services. 2010 Epidemiologic Profiles of HIV, STD, and Hepatitis in Missouri. 2011 [Google Scholar]
- 5.Todd CS, Haase C, Stoner BP. Emergency department screening for asymptomatic sexually transmitted infections. Am J Public Health. 2001 Mar;91(3):461–464. doi: 10.2105/ajph.91.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva A, Glick NR, Lyss SB, et al. Implementing an HIV and sexually transmitted disease screening program in an emergency department. Ann Emerg Med. 2007 May;49(5):564–572. doi: 10.1016/j.annemergmed.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Embling ML, Monroe KW, Oh MK, Hook EW., 3rd. Opportunistic urine ligase chain reaction screening for sexually transmitted diseases in adolescents seeking care in an urban emergency department. Ann Emerg Med. 2000 Jul;36(1):28–32. doi: 10.1067/mem.2000.105930. [DOI] [PubMed] [Google Scholar]
- 8.Monroe KW, Weiss HL, Jones M, Hook EW., 3rd. Acceptability of urine screening for Neisseria gonorrheae and Chlamydia trachomatis in adolescents at an urban emergency department. Sex Transm Dis. 2003 Nov;30(11):850–853. doi: 10.1097/01.OLQ.0000086600.71690.14. [DOI] [PubMed] [Google Scholar]
- 9.Mehta SD, Rompalo A, Rothman RE, Londner MS, Zenilman JM. Generalizability of STD screening in urban emergency departments: comparison of results from inner city and urban sites in Baltimore, Maryland. Sex Transm Dis. 2003 Feb;30(2):143–148. doi: 10.1097/00007435-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SD, Hall J, Lyss SB, Skolnik PR, Pealer LN, Kharasch S. Adult and pediatric emergency department sexually transmitted disease and HIV screening: programmatic overview and outcomes. Acad Emerg Med. 2007 Mar;14(3):250–258. doi: 10.1197/j.aem.2006.10.106. [DOI] [PubMed] [Google Scholar]
- 11.Williams JR, Ho ML, Grupp-Phelan J. The acceptability of mental health screening in a pediatric emergency department. Pediatr Emerg Care. 2011 Jul;27(7):611–615. doi: 10.1097/PEC.0b013e318222554e. [DOI] [PubMed] [Google Scholar]
- 12.Fein JA, Pailler ME, Barg FK, et al. Feasibility and effects of a Web-based adolescent psychiatric assessment administered by clinical staff in the pediatric emergency department. Arch Pediatr Adolesc Med. 2010 Dec;164(12):1112–1117. doi: 10.1001/archpediatrics.2010.213. [DOI] [PubMed] [Google Scholar]
- 13.Porter SC, Mandl KD. Data quality and the electronic medical record: a role for direct parental data entry. Proc AMIA Symp. 1999:354–358. [PMC free article] [PubMed] [Google Scholar]
- 14.Porter SC, Silvia MT, Fleisher GR, Kohane IS, Homer CJ, Mandl KD. Parents as direct contributors to the medical record: validation of their electronic input. Ann Emerg Med. 2000 Apr;35(4):346–352. doi: 10.1016/s0196-0644(00)70052-7. [DOI] [PubMed] [Google Scholar]
- 15.van Griensven F, Supawitkul S, Kilmarx PH, et al. Rapid assessment of sexual behavior, drug use, human immunodeficiency virus, and sexually transmitted diseases in northern thai youth using audio-computer-assisted self-interviewing and noninvasive specimen collection. Pediatrics. 2001 Jul;108(1):E13. doi: 10.1542/peds.108.1.e13. [DOI] [PubMed] [Google Scholar]
- 16.Vanable PA, Carey MP, Brown JL, et al. Test-retest reliability of self-reported HIV/STD-related measures among African-American adolescents in four U.S. cities. J Adolesc Health. 2009 Mar;44(3):214–221. doi: 10.1016/j.jadohealth.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyitray AG, Kim J, Hsu CH, et al. Test-retest reliability of a sexual behavior interview for men residing in Brazil, Mexico, and the United States: the HPV in Men (HIM) Study. Am J Epidemiol. 2009 Oct 15;170(8):965–974. doi: 10.1093/aje/kwp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams ML, Freeman RC, Bowen AM, et al. A comparison of the reliability of self-reported drug use and sexual behaviors using computer-assisted versus face-to-face interviewing. AIDS Educ Prev. 2000 Jun;12(3):199–213. [PubMed] [Google Scholar]
- 19.Bachman JW. The patient-computer interview: a neglected tool that can aid the clinician. Mayo Clin Proc. 2003 Jan;78(1):67–78. doi: 10.4065/78.1.67. [DOI] [PubMed] [Google Scholar]
- 20.Cook RL, Hutchison SL, Ostergaard L, Braithwaite RS, Ness RB. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Annals of internal medicine. 2005 Jun 7;142(11):914–925. doi: 10.7326/0003-4819-142-11-200506070-00010. [DOI] [PubMed] [Google Scholar]
- 21.Gaydos CA, Quinn TC, Willis D, et al. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in female urine and endocervical swab specimens. J Clin Microbiol. 2003 Jan;41(1):304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979 Sep;35(3):549–556. [PubMed] [Google Scholar]
- 23.Huppert JS, Reed JL, Munafo JK, et al. Improving notification of sexually transmitted infections: a quality improvement project and planned experiment. Pediatrics. 2012 Aug;130(2):e415–422. doi: 10.1542/peds.2011-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yealy DM, Greene TJ, Hobbs GD. Underrecognition of cervical Neisseria gonorrhoeae and Chlamydia trachomatis infections in the emergency department. Acad Emerg Med. 1997 Oct;4(10):962–967. doi: 10.1111/j.1553-2712.1997.tb03659.x. [DOI] [PubMed] [Google Scholar]
- 25.Niska R, Bhuiya F, Xu J, National Hospital Ambulatory Medical Care Survey 2007 emergency department summary. National health statistics reports. 2010 Aug 6;(26):1–31. [PubMed] [Google Scholar]
- 26.Brener ND, Eaton DK, Kann L, et al. The Association of Survey Setting and Mode with Self-Reported Health Risk Behaviors among High School Students. Public Opinion Quarterly. 2006 Sep 21;70(3):354–374. 2006. [Google Scholar]
- 27.Mossenson A, Algie K, Olding M, Garton L, Reeve C. ‘Yes wee can’ - a nurse-driven asymptomatic screening program for chlamydia and gonorrhoea in a remote emergency department. Sex Health. May. 2012;9(2):194–195. doi: 10.1071/SH11064. [DOI] [PubMed] [Google Scholar]
- 28.Aldeen T, Haghdoost A, Hay P. Urine based screening for asymptomatic/undiagnosed genital chlamydial infection in young people visiting the accident and emergency department is feasible, acceptable, and can be epidemiologically helpful. Sex Transm Infect. 2003 Jun;79(3):229–233. doi: 10.1136/sti.79.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta SD, Rothman RE, Kelen GD, Quinn TC, Zenilman JM. Unsuspected gonorrhea and chlamydia in patients of an urban adult emergency department: a critical population for STD control intervention. Sex Transm Dis. 2001 Jan;28(1):33–39. doi: 10.1097/00007435-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Sood T, Sally D, Spencer N, Banerjee A, Hinchley G. Feasibility of screening for Chlamydia trachomatis in young men attending an emergency department. Emergency medicine journal : EMJ. 2008 Jul;25(7):428–430. doi: 10.1136/emj.2007.054155. [DOI] [PubMed] [Google Scholar]
- 31.Mehta SD, Bishai D, Howell MR, Rothman RE, Quinn TC, Zenilman JM. Cost-effectiveness of five strategies for gonorrhea and chlamydia control among female and male emergency department patients. Sex Transm Dis. 2002 Feb;29(2):83–91. doi: 10.1097/00007435-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Al-Tayyib AA, Miller WC, Rogers SM, et al. Evaluation of risk score algorithms for detection of chlamydial and gonococcal infections in an emergency department setting. Acad Emerg Med. 2008 Feb;15(2):126–135. doi: 10.1111/j.1553-2712.2008.00027.x. [DOI] [PubMed] [Google Scholar]
- 33.Lyons MS, Lindsell CJ, Ledyard HK, Frame PT, Trott AT. Emergency department HIV testing and counseling: an ongoing experience in a low-prevalence area. Ann Emerg Med. 2005 Jul;46(1):22–28. doi: 10.1016/j.annemergmed.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Haukoos JS, Hopkins E, Conroy AA, et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA. 2010 Jul 21;304(3):284–292. doi: 10.1001/jama.2010.953. [DOI] [PubMed] [Google Scholar]
- 35.DiClemente RJ, Sales JM, Danner F, Crosby RA. Association between sexually transmitted diseases and young adults' self-reported abstinence. Pediatrics. 2011 Feb;127(2):208–213. doi: 10.1542/peds.2009-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus JL, Bernstein KT, Kohn RP, Liska S, Philip SS. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis. 2011 Oct;38(10):922–924. doi: 10.1097/OLQ.0b013e31822a2b2e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.