Abstract
Background and purpose:
Fractional flow may identify hemodynamic effects and ischemic risk beyond percent stenosis of an artery. We hypothesized that diminished TOF-MRA signal intensity distal to an intracranial stenosis predicts stroke risk.
Methods:
TOF-MRA was acquired prospectively in the SONIA-WASID trials. The distal/proximal signal intensity ratio (SIR) was calculated from 3 mm regions of interest, blinded to outcome. Univariate and multivariate analyses included clinical variables, SIR, and invasive angiography measures to identify predictors for risk of stroke in the territory.
Results:
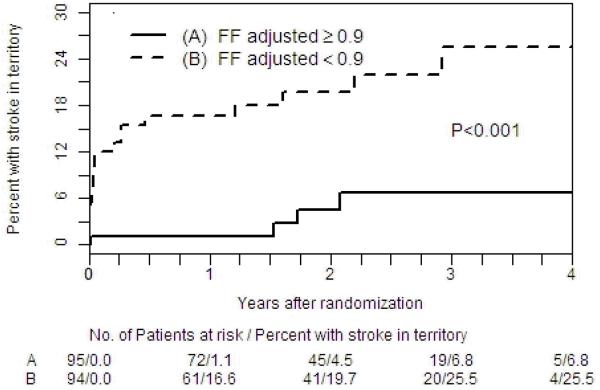
189 patients with 50-99% symptomatic intracranial stenosis in SONIA-WASID had TOF-MRA available. In univariate analysis, the hazard ratio (HR) for stroke in the territory of the symptomatic artery with SIR < 0.9 was 5.2 (1.8, 15.3; p<0.001) as compared to SIR ≥ 0.9. Multivariate analysis correcting for baseline systolic blood pressure, LDL, centrally measured percent stenosis, recency of symptoms, TICI and downstream collaterals, the HR for SIR <0.9 was 10.9 (2.0, 58.9; p<0.001). In those with <70% stenosis, a SIR <0.9 maintained a significant association with recurrent stroke in the territory (p=0.006), with a two year event rate of 17.3%.
Conclusions:
Fractional flow assessed by TOF-MRA SIR may be a useful noninvasive tool to identify high-risk intracranial lesions.
Keywords: Intracranial atherosclerosis, stenosis, fractional flow, MRA, stroke
Introduction
Intracranial atherosclerotic disease (ICAD) may be the world’s most prevalent cause of ischemic stroke.[1] Treatment trials focused on percent stenosis and increasingly on severe stenoses to identify disease.[1, 2] While the WASID trial showed that the recurrent stroke risk during follow-up was low in patients with < 70% stenosis, 40% of strokes in the territory occurred in patients with moderate stenosis.[3] These patients were excluded from the subsequent SAMMPRIS trial evaluating endovascular intervention.
Recent experience with coronary disease illustrates that percent stenosis is not an optimal marker for identifying disease of physiological significance compared with fractional flow reserve (FFR), which utilizes a pressure gradient across stenoses to identify hemodynamic significance.[4-12] FFR better identifies ischemic risk, can be measured noninvasively with conventional CT techniques and can identify the riskiest coronary lesions for percutaneous intervention, whether the stenosis is severe or moderate by anatomic measures. This approach has improved outcomes and lowered costs while resulting in fewer interventional procedures.[5, 12]
A noninvasive tool to reliably identify patients with hemodynamically significant ICAD has not been established, although several imaging techniques may provide information on flow changes due to ICAD. Time-of-flight magnetic resonance angiography (TOF-MRA) is noninvasive and routinely performed in patients suspected of having ICAD. TOF-MRA signal intensity correlates with blood flow velocity and may serve as a useful, noninvasive risk marker. We hypothesized that diminished TOF-MRA signal intensity distal to an intracranial stenosis predicts a high risk of stroke distal to the stenotic artery. The goal of this specific approach was to utilize noninvasive imaging alone as a hemodynamic marker of subsequent stroke risk, without utilizing information available from subsequent conventional angiography.
Methods
Subjects underwent TOF-MRA and were enrolled in the WASID /SONIA collaboration. The lesion location was determined purely on a noninvasive basis on MRA, as per protocol in the SONIA trial, as it was the lesion seen on MRA that identified patients as candidates for subsequent angiography and enrollment in WASID/SONIA.
A previously described method was used to measure the relative distal:proximal signal intensity ratio (SIR) on TOF-MRA as a surrogate marker of fractional flow.[13] Diminished SIR distal to a stenosis occurs with worsening hemodynamic impairment of flow. Feasibilityand intra-rater agreement of 0.975 (Pearson correlation coefficient) have been previously reported[13], with an associated inter-observer variability of 0.847 (Pearson correlation coefficient).[14]
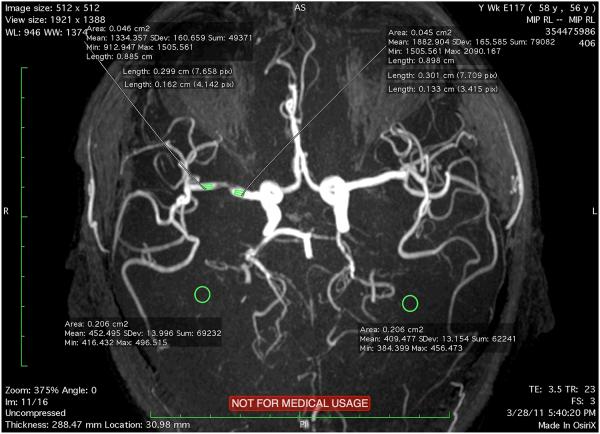
Using a DICOM viewer, a 3 mm region of interest (ROI) distal and proximal to a stenosis is visualized on maximum-intensity projection (MIP) views. Each ROI covering the entire vessel lumen is outlined and mean SI for the ROI is measured. We specifically avoided branch points to ensure that we limited the potential influence of caliber or vessel diameter changes. We placed the volumes of interest where the vessel calibers were visually approximately equivalent at locations proximal and distal to the lesion (Figure 1).
Figure 1.
Illustration of placement of regions of interest proximal and distal to a stenosis and potential areas of background measurement.
To adjust for varying background intensity, we measured the background intensity in close proximity to the stenosis and subtracted this value from both the proximal and distal signal intensity (Figure 1). The adjusted SIR reported in this study was termed FF (adjusted) and is calculated as:
In this study, we digitized the entire SONIA-WASID MRA film archive. We measured the SIR, adjusted for background, of the symptomatic artery in each case.
Analyses here included clinical variables (SBP, LDL, recency of symptoms), SIR (FF adjusted) below vs. above median, and invasive angiography measures (centrally measured luminal stenosis, TICI score of antegrade flow, collateral grade) to identify predictors of stroke in the territory (SIT). Univariate and multivariate Cox regression analyses were performed and hazard ratios (HR) with confidence intervals reported. Kaplan-Meier curves were compared with the log-rank test. P-value < 0.05 indicates statistical significance and no adjustment for multiplicity of comparisons was considered.
Results
297 MRA film datasets acquired at the time of SONIA subject enrollment from 1999-2003 were located and subsequently digitized from hard film copies to DICOM files. A total of 189 MRA cases of sufficient quality were available. Demographic data on this cohort have been previously reported and no differences were noted amongst those in this subset with respect to other subjects enrolled in SONIA. Baseline variables are displayed in Table 1. In univariate analysis (Table 2 and Figure 3), the hazard ratio (HR) (FFR < 0.9: FFR ≥ 0.9 for the symptomatic artery) for stroke in the territory of the stenotic artery was 5.2 (1.8, 15.3; p<0.001). In multivariate analysis correcting for baseline blood pressure, LDL, percent stenosis, recency of symptoms, TICI and downstream collaterals, the HR (FFR < 0.9: FFR ≥ 0.9) for stroke in the territory of the stenotic artery was 10.9 (2.0, 58.9; p<0.001) (Table 2). Only collaterals also had a significant independent association with stroke risk, HR 13.8 (3.4, 55.5; p<0.001). In the subset of patients with <70% stenosis, a SIR<0.9 maintained a significant association with recurrent stroke in the territory (p=0.006), with a 2-year event rate of 17.3%, showing that even moderate stenoses can pose substantial ischemic risk. The results for patients with ≥70% stenosis showed a similar trend but were not statistically significant, possibly because of the small sample size of this subgroup.
Table 1.
Patient characteristics.
| Baseline Characteristic | Total n=189 N (%) |
|---|---|
|
FF adjusted Mean ± SD Median Range IQR |
0.89 ± 0.28 0.90 0.29 – 2.61 0.74 – 1.03 |
|
FF adjusted FF adjusted >= 0.9 FF adjusted < 0.9 |
95 (50.3) 94 (49.7) |
|
Percent Stenosis Stenosis < 70 Stenosis >=70 |
n = 188 123 (65.4) 65 (34.6) |
|
TICI TICI 1/2a/2b TICI 3 |
n = 155 30 (19.4) 125 (80.6) |
|
Collateral None or Complete (delayed or early) Marginal or Partial |
n = 114 96 (84.2) 18 (15.8) |
|
Symptoms within 17 days
from enrollment Symptoms <=17 days Symptoms > 17 days |
101 (53.4) 88 (46.6) |
|
Systolic Blood Pressure SBP >=140 SBP < 140 |
106 (56.1) 83 (43.9) |
|
LDL Cholesterol LDL < 100 LDL >= 100 |
n = 164 44 (26.8) 120 (73.2) |
Table 2.
Univariate and multivariate analysis for prediction of recurrent stroke in the territory.
| Univariate HR (95%CI) |
P | Multivariate HR (95% CI) |
P | |
|---|---|---|---|---|
|
FFadj >=0.9
FFadj <0.9 |
1.00 5.2 (1.8,15.3) |
<0.001 | 1.00 10.9 (2.0,58.9) |
<0.001 |
|
%stenosis <70
%stenosis >=70 |
1.00 2.3 (1.01,5.2) |
0.048 | 1.00 1.5 (0.5,4.6) |
0.51 |
|
TICI 1/2a/2b
TICI 3 |
1.00 0.5 (0.2,1.4) |
0.20 | 1.00 2.0 (0.5,8.6) |
0.33 |
|
Collateral no or
complete Marginal or partial |
1.00 8.5 (3.0,23.5) |
<0.001 | 1.00 13.8 (3.4,55.5) |
<0.001 |
|
Symptoms <=17
days Symptoms > 17 days |
1.00 1.4 (0.6,3.2) |
0.42 | 1.00 1.04 (0.3, 3.4) |
0.95 |
|
SBP >=140mm Hg
SBP <140mm Hg |
1.00 1.1 (0.5,2.5) |
0.83 | 1.00 0.4 (0.1,1.4) |
0.16 |
|
LDL <100
LDL >=100 |
1.00 1.3 (0.4,4.0) |
0.62 | 1.00 1.3 (0.3,6.3) |
0.75 |
Discussion
Our data suggest that fractional flow assessed by TOF-MRA SIR may be a useful noninvasive tool to identify high-risk intracranial lesions even in patients with moderate stenosis, and may be suitable for selection of high-risk patients for future clinical trials evaluating new therapies for ICAD.
Three NIH-funded multicenter trials of ICAD in the US have defined the research landscape of this disorder over the last 2 decades. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial revealed that magnetic resonance angiography (MRA) and other noninvasive techniques are sensitive but not specific tests for identifying disease.[15] The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial showed aspirin to be as effective and safer than warfarin for patients with ICAD measured by 50-99% maximal stenosis.[1] The SAMMPRIS trial enrolled patients with 70-99% stenoses only and showed that aggressive medical therapy was superior to Wingspan stenting.[2] Because the 1- year rate of stroke in the medical arm of SAMMPRIS was still 12.2%,[16] this suggests that there are patients with ICAD who have a very high risk of stroke despite aggressive medical therapy. Currently there are no reliable imaging techniques to identify those patients. Our data suggest that TOF-MRA SIR may be a powerful predictor of recurrent stroke in the territory and that hemodynamic predictors such as SIR may allow stroke risk stratification across a wide range of lesion severity, including moderate levels of stenosis.
The experience with coronary artery disease illustrates that percent stenosis is not an optimal marker for identifying disease of physiological significance. Noninvasive methods to measure coronary FFR have also been introduced using CTA.[9, 10] Computational fluid dynamics (CFD) of noninvasive CTA can determine FFR.
While severity of stenosis may have different predictive value in the cerebral circulation than it does in the coronary tree, it is also possible that there are better predictors of stroke risk than severity of stenosis (as measured by percent stenosis). In WASID, a retrospective analysis suggested that impaired collaterals on angiography may be a better predictor of stroke in the territory than percent stenosis, suggesting that impaired hemodynamics related to a stenosis may be the key prognostic factor. The current study using TOF MRA also suggests a strong relationship between impaired hemodynamics related to an intracranial stenosis and increased stroke risk and, if confirmed in subsequent studies, will enable the identification of hjgh-risk patients with a relatively simple non-invasive technique. Additional information from future MRA strategies with respect to collateral status could yield a combined noninvasive algorithm to predict recurrent stroke risk.
Our study has limitations. The MRAs analyzed here were not digital but collected on film, potentially leading to suboptimal measurement. Only studies of sufficient quality could be used for this study, representing selection bias. Sufficient quality was determined by the availability of maximum-intensity projections that incorporated the lesion, including proximal and distal vessel segments, in the field of view. Placement of ROI and the view chosen for measurement are subjective. However, such subjectivity is commonly encountered in routine imaging measurements in cerebrovascular patients. This includes placement of sampling volume in carotid ultrasound, probe “angle” in blind transcranial Doppler ultrasound, locating the normal reference segments on DSA of distal or proximal vessels in NASCET calculations of carotid percent stenosis or WASID measurements of intracranial stenosis. In addition, signal intensity is affected by other factors such as vessel diameter and turbulence. We utilized measurements at arterial locations between branch points in vessels where the vast majority of all vessel diameter changes take place, in order to minimize this factor. Prospective validation in other datasets is needed. Despite these limitations, the acceptable intra- and inter-observer variability of this technique and the strong correlation of SIR with a clinical endpoint suggests that it is an imaging parameter of potential value.
This study using TOF MRA suggests a strong relationship between impaired hemodynamics related to an intracranial stenosis and increased stroke risk and, if confirmed in subsequent studies, will enable the identification of hjgh-risk patients with a relatively simple non-invasive technique.
Figure 2.
Kaplan-Meier plot for recurrent stroke in the territory for SIR above and below the median.
Acknowledgments
Sources of Funding
This work by Dr. Liebeskind has been funded by NIH-National Institute of Neurological Disorders and Stroke Awards NIH/NINDS P50NS044378, K24NS072272, R01NS077706, and R13NS082049. Dr. Chimowitz was the grant recipient for the NINDS funded WASID trial (1 R01 NS36643). Bristol Myers-Squibb supplied the warfarin and placebo warfarin for the WASID trial and Bayer supplied the aspirin and placebo aspirin for the WASID trial. Dr. Chimowitz has also received research grants from NINDS to fund the SAMMPRIS trial (U01 NS058728) and to fund other research on intracranial stenosis (1 K24 NS050307 and 1 R01 NS051688).
Footnotes
Clinical Trial Registration-URL: This trial was not registered because enrollment began prior to July 1, 2005.
Conflict(s) of Interest/Disclosure(s)
Some authors (Liebeskind, Scalzo, Fong) were employed by the University of California (UC), which holds a patent on retriever devices for stroke, at the time of this work.
Dr. Liebeskind is a scientific consultant regarding trial design and conduct to Stryker (modest) and Covidien (modest). Dr. Chimowitz currently serves on stroke adjudication committees of an industry funded osteoporosis drug trial (Merck and Co., Inc.) and an atrial fibrillation trial (Medtronics). He also serves on the DSMB of another industry funded patent foramen ovale closure trial (W.L Gore and Associates). He is compensated for these activities (modest).
References
- 1.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. The New England journal of medicine. 2005;352:1305–16. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL, Jr., Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ. Stenting versus aggressive medical therapy for intracranial arterial stenosis. The New England journal of medicine. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, Sila CA, Jovin TG, Romano JG, Cloft HJ. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 4.Berger A, Botman KJ, MacCarthy PA, Wijns W, Bartunek J, Heyndrickx GR, Pijls NH, De Bruyne B. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Journal of the American College of Cardiology. 2005;46:438–42. doi: 10.1016/j.jacc.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 5.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. The New England journal of medicine. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 6.Fischer JJ, Samady H, McPherson JA, Sarembock IJ, Powers ER, Gimple LW, Ragosta M. Comparison between visual assessment and quantitative angiography versus fractional flow reserve for native coronary narrowings of moderate severity. The American journal of cardiology. 2002;90:210–5. doi: 10.1016/s0002-9149(02)02456-6. [DOI] [PubMed] [Google Scholar]
- 7.Govindaraju K, Badruddin IA, Viswanathan GN, Ramesh SV, Badarudin A. Evaluation of functional severity of coronary artery disease and fluid dynamics’ influence on hemodynamic parameters: A review. Phys Med. 2013;29:225–32. doi: 10.1016/j.ejmp.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–60. doi: 10.1016/S0140-6736(11)61335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. Journal of the American College of Cardiology. 2011;58:1989–97. doi: 10.1016/j.jacc.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 10.Min JK, Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning AM, Defrance T, Lansky A, Leipsic J. Usefulness of noninvasive fractional flow reserve computed from coronary computed tomographic angiograms for intermediate stenoses confirmed by quantitative coronary angiography. The American journal of cardiology. 2012;110:971–6. doi: 10.1016/j.amjcard.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. The New England journal of medicine. 1996;334:1703–8. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 12.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. The New England journal of medicine. 2009;360:213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 13.Leng XY, Wong KS, Soo YO, Leung TW, Zou XY, Wang YJ, Feldmann E, Liu LP, Liebeskind DS. Signal intensity ratio as a novel measure of hemodynamic significance for intracranial atherosclerosis. Int J Stroke. 2013 doi: 10.1111/ijs.12080. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leng X, Ip HL, Soo Y, Leung TW, Liu LP, Feldmann E, Wong KS, Liebeskind DS. Inter-observer Reproducibility of Signal Intensity Ratio on MR Angiography for Hemodynamic Impact of Intracranial Atherosclerosis. J Stroke Cerebrovasc Dis. 2013 doi: 10.1016/j.jstrokecerebrovasdis.2013.07.036. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, Smith HH, Nichols F, Rogg J, Cloft HJ, Wechsler L, Saver J, Levine SR, Tegeler C, Adams R, Sloan M. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 16.Chimowitz MI, et al. Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis. 10.1056/nejmoa1105335 nejm.org. [DOI] [PMC free article] [PubMed]