Abstract
Cerebral microbleeds (CMB) on gradient-recalled echo (GRE) magnetic resonance imaging (MRI) are rarely seen in children, yet have been described following vascular procedures in adults. Extracorporeal membrane oxygenation (ECMO) has been associated with vascular injury and neurological events in children, but there have been no reports to date of GRE MRI findings in children treated with ECMO. We reviewed MRI scans for all vascular neurology consultations in children treated with ECMO at an academic medical center over a 5-year period. In 6 of 12 cases, GRE was acquired as others were unstable or had contraindications to MRI. All 6 of 6 (100%) GRE cases (mean age 2.1 years, 7 female, 5 male) demonstrated CMB. CMB were multiple (>3 lesions), situated in cortical or lobar regions, with a striking predominance (5/6 cases) for the right carotid distribution. Other than CMB, no cases demonstrated intracranial hemorrhage. CMB may be noted on GRE MRI after ECMO and may reflect vascular damage from gaseous emboli.
Keywords: Microbleed, GRE, ECMO, MRI
Introduction
Cerebral microbleeds (CMB) noted on gradient-recalled echo (GRE) or T2*-weighted sequences provide additional dimension beyond routine delineation of ischemic or hemorrhagic cerebrovascular lesions with magnetic resonance imaging (MRI). These isolated, circumscribed, and hypointense foci are typically subtle, extending less than 5 mm in diameter. Such microvascular lesions have been associated with cerebral amyloid angiopathy, hypertension, infective endocarditis, and an expanding differential of disorders.1–4 The imaging characteristics have also been revisited to account for distinctions in lesion size, noting macrobleeds as a potentially separate entity from microbleeds.5 Others have emphasized that variability in diagnostic technique, including magnetic field strength, slice thickness, and other factors may impact CMB detection.6
Despite these considerations, CMB have largely been categorized as increasingly frequent in older age, with a presumed link to hemorrhagic diatheses. The appearance of new CMB after endovascular or surgical procedures and increasingly frequent reports in younger individuals questions the unequivocal link with age-dependent disorders and points to other methods of microvascular injury. Even the terminology of “microbleeds” implies a tendency to hemorrhage but our understanding of the underlying pathophysiology continues to evolve.
MRI has transformed the management of cerebrovascular disorders in children. In pediatric stroke, delayed diagnosis often results from limited historical complaints, variable presentation, and the relative rarity of the condition, yet MRI may reveal subtle features that are essential to disclose a broad spectrum of underlying disorders. CMB have never been seen in routine cases of pediatric ischemic stroke. There may be certain situations, such as extracorporeal membrane deoxygenation in children, where mechanisms to produce neurological injury and CMS are present.7,8
We describe a case series of CMB after extracorporeal membrane oxygenation (ECMO) in children, extending our initial observation in a systematic, retrospective review of consecutive cases at a single center. The clinical features and imaging characteristics on GRE sequences are detailed with respect to surveillance rates, association with hemorrhage and neurological sequelae.
Case Series
A retrospective analysis was conducted to investigate CMB on GRE MRI following ECMO, used at an academic medical center over a 5-year period. The pediatric age group was selected to exclude potential confounding causes of CMB such as chronic hypertension or cerebral amyloid angiopathy. Systematic review of consecutive records was conducted to identify cases where patients less than 18 years old were referred for vascular neurology consultation after ECMO treatment. Clinical details were gleaned from a comprehensive review of the medical charts and electronic databases, including detailed collection of demographics, medical history, treatments, and neurological examinations. The local Institutional Review Board granted approval for this retrospective study.
Neuroimaging analyses focused on GRE MRI findings and were therefore restricted to the subset of cases able to undergo MRI. All GRE MRI datasets were acquired after ECMO at variable intervals on a 1.5-Tesla magnet acquired using an identical protocol. GRE sequences used TR 800 ms, TE 15 ms, 30° flip angle, 5 mm slice thickness with no gap, FOV of 240 mm, and a matrix size of 256 × 144. Two neurologists with neuroimaging expertise (DSL and NS) independently reviewed the GRE MRI sequences to identify CMB, specifically defined as focal, hypointense lesions. Artifacts such as larger areas of hemosiderin or mineral deposition causing signal loss and cross-sectional views of vessels near the cortical surface were excluded. Neuroimaging review was conducted in blind fashion with respect to any clinical details of the case.
From 2003 to 2008, 12 patients under 18 years of age were treated with venoarterial ECMO and evaluated with postprocedure vascular neurology consultation. All consultation requests were to rule out the possibility of either new ischemic or hemorrhagic strokes. In only 6 of 12 cases, GRE was acquired as others were too unstable or had MRI contraindications including possible concerns regarding compatibility of the extracorporeal circuit with MRI. All 6 of 6 (100%) cases with GRE (mean age 2.1 years, 7 female, 5 male) demonstrated CMB. Interestingly, none of the cases with CT acquired before GRE revealed any abnormalities or calcifications. CMB were noted at various ages, including the neonatal period (Fig 1). CMB were always multiple (in excess of three lesions) and situated in cortical or lobar regions. CMB were not identified in the posterior circulation or in deep subcortical zones. There was a striking predominance in 5 of 6 cases for CMB detection in the right hemisphere at locations attributable to borderzone territory in the right internal carotid artery (Fig 2). All cases in our series underwent venoarterial bypass into the right carotid artery and in 3 of 6 cases, right carotid occlusion was noted after therapeutic ligation. CMB were variable in size with no apparent spatial distribution associated with lesion size (Fig 3). Diffusion-weighted (DW) MRI revealed ischemic lesions remote from the CMB that corresponded to clinical symptoms in 3 of 6. Interestingly, the CMB GRE lesions in our study were asymptomatic and not accompanied by corresponding DW imaging (DWI) lesions. All cases were treated with full-dose anticoagulation during ECMO, yet other than the observed CMB no cases demonstrated any form of intracranial hemorrhage.
Fig. 1.
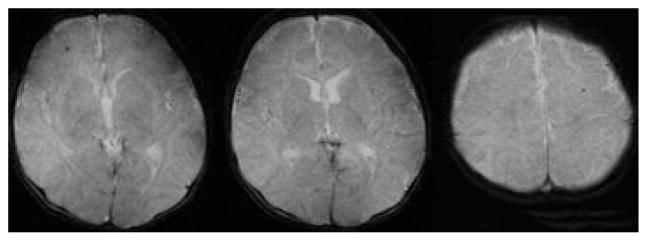
GRE MRI reveals bilateral CMB noted after venoarterial ECMO during the neonatal period in a 22-day-old boy.
Fig. 2.
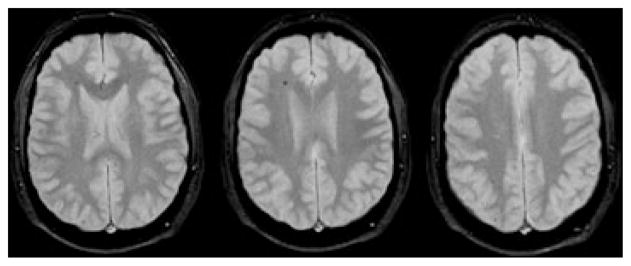
Right hemispheric CMB on GRE MRI exhibits a pattern demarcating the anterior and posterior borderzones within the carotid territory of a 21-month-old girl.
Fig. 3.
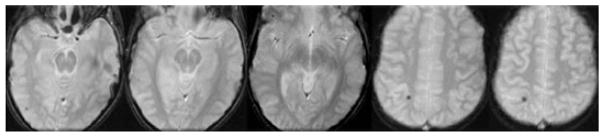
Variable size of CMB is illustrated on GRE MRI within the right hemisphere of a 26-month-old boy treated with venoarterial ECMO.
Discussion
CMB may be seen in children receiving ECMO. Exclusion of potential artifacts on GRE and the retrospective inclusion of GRE datasets, not even tailored for optimal lesion detection, suggest that the association between ECMO and CMB is unequivocal. The lesions noted in our case series demonstrated the established appearance of other reports on CMB, including a recent report of periprocedural lesions in adult cardiac valve surgery.9
Our study yielded a consistent finding of multiple CMB in all cases with GRE evaluated in vascular neurology consultation. These often subtle lesions on GRE exhibited predominance for right hemispheric sites, although contralateral lesions were occasionally noted. The CMB were most commonly situated at the borderzones within the right internal carotid artery distribution. This spatial distribution potentially implicates embolic phenomena via the recipient site of venoarterial bypass into the right common carotid artery used in all of our cases. Prior reports on neurologic injury after ECMO have engendered controversy regarding lateralization of lesions, initially suggesting a right-sided predominance with numerous subsequent reports failing to detect such hemispheric asymmetry.10–15 Therapeutic right carotid ligation after ECMO has also been implicated in the evolution of CT/MRI lesions and cerebral palsy,16 whereas other studies have failed to demonstrate potentially deleterious perfusion defects downstream.17 It should be noted that most studies in the past delineated overt ischemic or hemorrhagic lesions on ultrasound, CT, and conventional MRI, whereas our demonstration of asymptomatic CMB on GRE is distinct.11 Despite right hemispheric preponderance of CMB in our series, clinical symptoms and ischemic foci on DWI were located elsewhere. There was also no association with hemorrhage despite therapeutic anticoagulation in all cases, confirming prior findings of relatively few primary hemorrhages after ECMO.18 Detection of asymptomatic CMB in our study was prompted by symptoms eliciting vascular neurology consultation, and the prevalence of such CMB after ECMO may therefore be much higher on routine surveillance. CMB may be a marker of relatively subtle or subclinical neurologic injury, yet the mechanism remains unfounded.
Our experience revealing a very high rate of CMB detection after ECMO in this limited cohort and detailed observation of predominantly right hemispheric borderzone lesions distinct from ischemic foci spur questions about etiology or mechanism in the evolution of these unusual imaging findings.19 Diverse considerations may include particulate or gaseous emboli, microvascular trauma, hypoperfusion, or simply focal hemorrhages consistent with microbleed terminology.20,21 Distal, borderzone lesions in the right carotid territory unassociated with ischemia or other forms of hemorrhage raises the possibility of gaseous emboli.22 Overt hypoperfusion has not been substantiated and gaseous emboli may produce lesions devoid of either ischemia or hemorrhage.23 Furthermore, variable size and imaging features on GRE are consistent with other manifestations of gaseous emboli.24 Recent work on such lesions in the adult cardiac valve surgery population also implicated gaseous emboli as a cause.9 These findings provide an interesting link with the subtle neuropsychological deficits and small capillary arteriolar dilatations noted after cardiac bypass surgery more than 2 decades ago.25,26 Transcranial Doppler ultrasound helped confirm the subtle yet deleterious effects of such gaseous emboli and a recent study also suggested similar pathophysiology during ECMO.27,28
Determining influential mechanisms of neurologic injury after ECMO may have broad implications for the use of this life-sustaining therapy. ECMO provides critical life support for infants and children with cardiac or respiratory failure, yet neurologic injury is common.29 Venoarterial bypass with ECMO may elicit thrombosis or hemorrhage in up to 86% of cases.30 Microemboli may occur due to gas in central venous catheters and may vary with the degree of ECMO support.27,28 Biomarkers such as elevated glial fibrillary acidic protein have been promoted to presage neurologic injury as bedside evaluation and ultrasound come first due to logistic difficulties with MRI and even CT.31 Although transport to the scanner and image acquisition with CT or MRI is feasible during ECMO, the goal is early prediction of complications before more extensive injury.7,32,33 Seemingly innocuous or asymptomatic CMB may not deter ECMO strategies for cardiopulmonary or respiratory support, but such lesions may impair cognitive function or impart other long-term neurological sequelae. Discerning the pathogenesis of CMB and confirming gaseous emboli would alleviate concerns regarding anticoagulation and prompt interventions that target gaseous emboli with different procedural techniques and the use of alternative filtration methods. Close correlation has been noted between prior imaging findings and cognitive impairment noted years after ECMO use, eliciting particular concern for pediatric subjects.34,35 Whereas overt hemorrhages may be noted with most imaging modalities, the subtle correlates of CMB may become apparent only after GRE discloses these lesions.8 Although we have continued to use the designation “microbleeds” based on prior imaging definitions, such terminology may actually defy the true nature of these lesions. Persistence of CMB for many years may later cause erroneous diagnoses such as amyloid angiopathy and faulty decisions regarding antithrombotic regimens.36
Numerous limitations influenced our findings, including the retrospective nature and extensive selection bias. The prevalence of CMB in children in the general population remains unknown and our findings of relatively high prevalence of CMB may have been driven by focal neurological symptoms and severity eliciting neurological consultation. Acquisition of GRE MRI remains a challenge in patients on ECMO, which was likely also prompted by more concerning symptoms and vascular neurology consultation. Referral and selection bias may also have eliminated cases with findings evident solely on CT. It remains possible that inconsistent times to imaging and absence of GRE before ECMO limit our ability to comment further on causality, ECMO duration thresholds for injury, or other temporal features. The GRE MRI was not optimally tailored for CMB detection and artifacts remain possible despite precautionary measures.
In summary, CMB may be noted after ECMO in children. These CMB patterns suggest that gaseous emboli in the extracorporeal circuit may cause asymptomatic lesions, distinct from ischemic and hemorrhagic findings. Increasing routine use of MRI may reveal detailed, subtle MRI findings that go beyond gross detection of hemorrhagic or ischemic lesions to uncover mechanistic aspects of what happens to the brain on ECMO. These observations on CMB also evoke questions that challenge the universal idea that CMB are synonymous with hemorrhage-prone lesions of hypertension or amyloid.3 Our limited initial observations of CMB in children warrant further study in larger cohorts and prospective investigation, including long-term evaluation of neurological sequelae.
Acknowledgments
Funding support: Grant support included funding from the National Institutes of Health-NINDS K23NS054084 (DSL) and P50NS044378 (DSL, JLS).
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
This work was described as a scientific presentation at the International Stroke Conference 2009 in San Diego, CA, USA.
References
- 1.Yakushiji Y, Yokota C, Yamada N, et al. Clinical characteristics by topographical distribution of brain microbleeds, with a particular emphasis on diffuse microbleeds. J Stroke Cerebrovasc Dis. 2011;20:214–121. doi: 10.1016/j.jstrokecerebrovasdis.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Klein I, Iung B, Labreuche J, et al. Cerebral microbleeds are frequent in infective endocarditis: a case-control study. Stroke. 2009;40:3461–3465. doi: 10.1161/STROKEAHA.109.562546. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregoire SM, Brown MM, Kallis C, et al. MRI detection of new microbleeds in patients with ischemic stroke: five-year cohort follow-up study. Stroke. 2010;41:184–186. doi: 10.1161/STROKEAHA.109.568469. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40:2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lidegran MK, Frenckner BP, Mosskin M, et al. MRI of the brain and thorax during extracorporeal membrane oxygenation: preliminary report from a pig model. ASAIO J. 2006;52:104–109. doi: 10.1097/01.mat.0000194058.62228.56. [DOI] [PubMed] [Google Scholar]
- 8.Bulas DI, Taylor GA, O’Donnell RM, et al. Intracranial abnormalities in infants treated with extracorporeal membrane oxygenation: update on sonographic and CT findings. AJNR Am J Neuroradiol. 1996;17:287–294. [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon SB, Lee JW, Kim SJ, et al. New cerebral lesions on T2*-weighted gradient-echo imaging after cardiac valve surgery. Cerebrovasc Dis. 2010;30:194–199. doi: 10.1159/000317108. [DOI] [PubMed] [Google Scholar]
- 10.Orr RA, Dalton HJ. Extracorporeal membrane oxygenation and right-sided brain lesions. Pediatrics. 1989;83:635–636. [PubMed] [Google Scholar]
- 11.Taylor GA, Fitz CR, Miller MK, et al. Intracranial abnormalities in infants treated with extracorporeal membrane oxygenation: imaging with US and CT. Radiology. 1987;165:675–678. doi: 10.1148/radiology.165.3.3317499. [DOI] [PubMed] [Google Scholar]
- 12.Lago P, Rebsamen S, Clancy RR, et al. MRI, MRA, and neurodevelopmental outcome following neonatal ECMO. Pediatr Neurol. 1995;12:294–304. doi: 10.1016/0887-8994(95)00047-j. [DOI] [PubMed] [Google Scholar]
- 13.Mendoza JC, Shearer LL, Cook LN. Lateralization of brain lesions following extracorporeal membrane oxygenation. Pediatrics. 1991;88:1004–1009. [PubMed] [Google Scholar]
- 14.Schumacher RE, Barks JD, Johnston MV, et al. Right-sided brain lesions in infants following extracorporeal membrane oxygenation. Pediatrics. 1988;82:155–161. [PubMed] [Google Scholar]
- 15.Wiznitzer M, Masaryk TJ, Lewin J, et al. Parenchymal and vascular magnetic resonance imaging of the brain after extracorporeal membrane oxygenation. Am J Dis Child. 1990;144:1323–1326. doi: 10.1001/archpedi.1990.02150360047018. [DOI] [PubMed] [Google Scholar]
- 16.Desai SA, Stanley C, Gringlas M, et al. Five-year follow-up of neonates with reconstructed right common carotid arteries after extracorporeal membrane oxygenation. J Pediatr. 1999;134:428–433. doi: 10.1016/s0022-3476(99)70199-x. [DOI] [PubMed] [Google Scholar]
- 17.Raju TN, Kim SY, Meller JL, et al. Circle of Willis blood velocity and flow direction after common carotid artery ligation for neonatal extracorporeal membrane oxygenation. Pediatrics. 1989;83:343–347. [PubMed] [Google Scholar]
- 18.Lazar EL, Abramson SJ, Weinstein S, et al. Neuroimaging of brain injury in neonates treated with extracorporeal membrane oxygenation: lessons learned from serial examinations. J Pediatr Surg. 1994;29:186–190. doi: 10.1016/0022-3468(94)90315-8. discussion 190-181. [DOI] [PubMed] [Google Scholar]
- 19.Dejode JM, Antonini F, Lagier P, et al. Capgras syndrome: a clinical manifestation of watershed cerebral infarct complicating the use of extracorporeal membrane oxygenation. Crit Care. 2001;5:232–235. doi: 10.1186/cc1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingyinn M, Rais-Bahrami K, Viswanathan M, et al. Altered cerebrovascular responses after exposure to venoarterial extracorporeal membrane oxygenation: role of the nitric oxide pathway. Pediatr Crit Care Med. 2006;7:368–373. doi: 10.1097/01.PCC.0000225372.38460.12. [DOI] [PubMed] [Google Scholar]
- 21.Vogler C, Sotelo-Avila C, Lagunoff D, et al. Aluminum-containing emboli in infants treated with extracorporeal membrane oxygenation. N Engl J Med. 1988;319:75–79. doi: 10.1056/NEJM198807143190203. [DOI] [PubMed] [Google Scholar]
- 22.Forster A, Szabo K, Hennerici MG. Pathophysiological concepts of stroke in hemodynamic risk zones: do hypoperfusion and embolism interact? Nat Clin Pract Neurol. 2008;4:216–225. doi: 10.1038/ncpneuro0752. [DOI] [PubMed] [Google Scholar]
- 23.Regan JD, Gibson KD, Rush JE, et al. Clinical significance of cerebrovascular gas emboli during polidocanol endovenous ultra-low nitrogen microfoam ablation and correlation with magnetic resonance imaging in patients with right-to-left shunt. J Vasc Surg. 2011;53:131–137. doi: 10.1016/j.jvs.2010.06.179. [DOI] [PubMed] [Google Scholar]
- 24.Jeon SB, Kang DW. Neurological picture. Cerebral air emboli on T2-weighted gradient-echo magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2007;78:871. doi: 10.1136/jnnp.2006.102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moody DM, Bell MA, Challa VR, et al. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990;28:477–486. doi: 10.1002/ana.410280403. [DOI] [PubMed] [Google Scholar]
- 26.McKhann GM, Grega MA, Borowicz LM, Jr, et al. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37:562–571. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 27.Zanatta P, Forti A, Bosco E, et al. Microembolic signals and strategy to prevent gas embolism during extracorporeal membrane oxygenation. J Cardiothorac Surg. 2010;5:5. doi: 10.1186/1749-8090-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furui E, Hanzawa K, Ohzeki H, et al. “Tail sign” associated with microembolic signals. Stroke. 1999;30:863–866. doi: 10.1161/01.str.30.4.863. [DOI] [PubMed] [Google Scholar]
- 29.Barrett CS, Bratton SL, Salvin JW, et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10:445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 30.Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010;13:385–392. doi: 10.2350/09-09-0704-OA.1. [DOI] [PubMed] [Google Scholar]
- 31.Bembea MM, Savage W, Strouse JJ, et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12:572–579. doi: 10.1097/PCC.0b013e3181fe3ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lidegran MK, Mosskin M, Ringertz HG, et al. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: clinical benefits in diagnosis and treatment. Acad Radiol. 2007;14:62–71. doi: 10.1016/j.acra.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Lidegran M, Palmer K, Jorulf H, et al. CT in the evaluation of patients on ECMO due to acute respiratory failure. Pediatr Radiol. 2002;32:567–574. doi: 10.1007/s00247-002-0756-x. [DOI] [PubMed] [Google Scholar]
- 34.Glass P, Bulas DI, Wagner AE, et al. Severity of brain injury following neonatal extracorporeal membrane oxygenation and outcome at age 5 years. Dev Med Child Neurol. 1997;39:441–448. doi: 10.1111/j.1469-8749.1997.tb07463.x. [DOI] [PubMed] [Google Scholar]
- 35.Jarjour IT, Ahdab-Barmada M. Cerebrovascular lesions in infants and children dying after extracorporeal membrane oxygenation. Pediatr Neurol. 1994;10:13–19. doi: 10.1016/0887-8994(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 36.Benbassat J, Baumal R, Herishanu Y. Treatment of acute ischemic stroke in patients with cerebral microbleeds: a decision analysis. QJM. 2011;104:73–82. doi: 10.1093/qjmed/hcq119. [DOI] [PubMed] [Google Scholar]


