Abstract
Background and Purpose
We hypothesized that a favorable vascular profile (FVP) defined as anatomic intactness of the Circle of Willis combined with a stable cerebral perfusion pressure (mean arterial blood pressure>65 mm Hg) is a prerequisite for collateral recruitment and maintenance and may improve outcome. We performed post hoc analyses of a subset of the Safety and Efficacy of NeuroFlo Technology in Ischemic Stroke (SENTIS) trial data set to identify whether FVP is associated with independent outcome.
Methods
SENTIS was a randomized, controlled device trial comparing hemodynamic augmentation with the NeuroFlo device to best medical treatment. We identified all patients from the primary dataset (n=515 patients) with available intracranial vascular imaging at baseline. Vascular imaging data were read blind to clinical and treatment data. We performed univariate and multivariate analyses to identify predictors of independent outcome (modified Rankin Scale 0–2) at 90 days.
Results
A total of 192/515 SENTIS subjects had available baseline vascular imaging (91 treated/101 controls). Baseline characteristics did not differ between groups. Overall, FVP was seen in 89.6% of patients and predicted independent outcome in univariate (odds ratio, 7.46; 95% confidence interval, 1.68–33.18; P=0.0082) and multiple logistic regression analyses (odds ratio, 10.22; 95% confidence interval, 1.78–58.57; P=0.0091). Aside from FVP, only baseline National Institutes of Health Stroke Scales (NIHSS; odds ratio, 0.74; 95% confidence interval, 0.67–0.82, P<0.0001) entered the predictive model. There was no interaction with randomization to treatment or control.
Conclusions
FVP and baseline NIHSS independently predicted outcome in this subset of the SENTIS population. FVP is a novel parameter to predict outcome of acute stroke patients and further studies will establish its potential role for selection of optimal candidates for hemodynamic augmentation.
Keywords: aortic occlusion, brain perfusion augmentation, clinical trials, ischemic stroke, SENTIS
Recently, the primary results of the Safety and Efficacy of NeuroFlo Technology in Ischemic Stroke (SENTIS) trial were published.1 NeuroFlo therapy involves partial occlusion of the abdominal aorta that results in a prompt increase in blood volume above the peripheral arterial occlusion and has been shown to increase cerebral blood flow specifically.2–4 In the intent-to-treat analysis, the SENTIS results did not achieve statistical significance for the primary efficacy end point; however, safety of the procedure was established and favorable trends, especially with regard to stroke-related mortality, were observed.
We hypothesized that a favorable vascular profile (FVP) combined with a stable cerebral perfusion pressure is a prerequisite for collateral recruitment and maintenance. We performed post hoc analyses of the SENTIS trial to identify whether a FVP is associated with independent outcome.
Methods
For the detailed methods of the trial, we refer to the original publication of the SENTIS trial (ClinicalTrials.gov, #NCT00119717).1 The trial was funded by CoAxia, Inc. All authors vouch for the accuracy and completeness of the data and analysis. All authors had access to all the data in the study and had final responsibility for submission of this publication. Briefly, patients were allocated to NeuroFlo treatment with standard medical management (treatment) or standard medical management alone (control) using a 1:1 randomization scheme stratified by site, baseline National Institutes of Health Stroke Scales (NIHSS) score, and the time from symptom onset. All patients were followed for safety and efficacy through 90 days. Follow-up imaging was performed at 24 hours and, in cases of neurological worsening, at anytime up to 90 days.
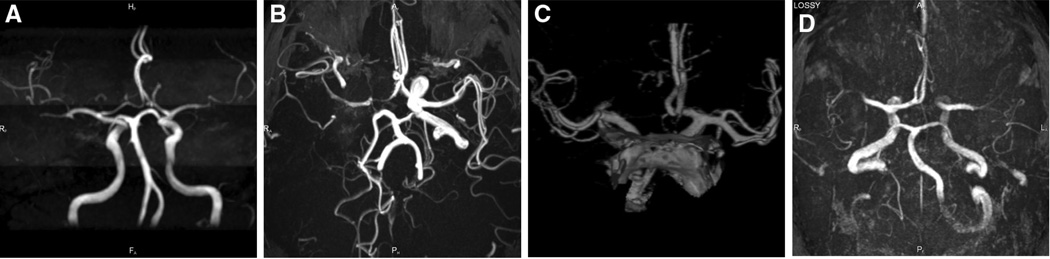
We identified all patients from the primary dataset (n=515 patients) with available intracranial vascular imaging (magnetic resonance angiography, computer tomography angiography, or conventional angiography) at baseline. Two independent readers (P.D.S., D.S.L.) evaluated the vascular imaging blind to clinical and treatment data. FVP was defined as the combination: (1) intact Circle of Willis including visualization of the communicators, and (2) mean arterial blood pressure>65 mm Hg at all time points within the first 12 hours of the NeuroFlo procedure. The later cutoff value was arbitrarily chosen as the widely accepted upper limit of cerebral perfusion pressure below which ischemic brain may progress to infarction (Figure).
Figure.
Examples of favorable (A and B) and nonfavorable vascular (C and D) profile anatomy. A, Intact Circle of Willis (CoW). B, Occlusion of intracranial internal carotid artery with intact CoW. C, D, Missing anterior cerebral artery A1 segment.
Baseline demographics were summarized for treated and non-treated subjects. There were no statistical differences between groups in this cohort and the complete dataset. P values presented are from a Fisher exact tests (categorical variables) and 2-sample Wilcoxon tests (continuous variables). We performed univariate and multivariate analyses to identify predictors of independent outcome (modified Rankin Scale [mRS] 0–2) at 90 days. We also analyzed the data by shift in trichotomized (mRS 0–2, 3–4, 5–6) outcomes of the mRS. Cochran–Mantel–Haenszel tests were run for each shift analysis. All statistical tests were 2-sided; statistical analyses were conducted in SAS version 9.1 or above (SAS Institute, Cary, NC).
Results
Between October 2005 and January 2010, 515 patients were enrolled in the SENTIS trial at 68 centers. A total of 257 patients were randomized to the control group and 258 patients were randomized to the treatment group (intent-to-treat population). Twenty-eight patients randomized to treatment were excluded because of prespecified criteria, 5 patients randomized to treatment did not receive treatment, and 1 patient randomized to the control group received NeuroFlo treatment (both were protocol deviations) resulting in 261 nontreated patients and 226 treated patients in the modified as treated analysis.1
A total of 192/515 SENTIS subjects had available baseline vascular imaging (91 treated/101 controls). Baseline characteristics did not differ between groups (Table 1).
Table 1.
Baseline Characteristics of FVP Subset
| Baseline Stroke Presentation Characteristics | Treated | Nontreated | P Value | |
|---|---|---|---|---|
| FVP (CoW intact and MAP>65 mm Hg) | % (n/N) | 94.5% (86/91) | 85.1% (86/101) | 0.0562 |
| Time from symptom onset to BL, h | Mean±SD | 7.7±2.7 | 8.1±3.0 | 0.2817 |
| Time from symptom onset to Rz, h | Mean±SD | 8.2±2.6 | 8.4±2.8 | 0.5530 |
| Baseline NIHSS | Mean±SD | 11.5±4.3 | 10.7±4.4 | 0.2737 |
| Systolic blood pressure, mm Hg | Mean±SD | 155.6±22.9 | 157.3±25.9 | 0.6420 |
| Diastolic blood pressure, mm Hg | Mean±SD | 83.5±16.4 | 86.2±16.9 | 0.2725 |
| Glucose, mg/dL | Mean±SD | 136.9±51.9 | 128.5±38.9 | 0.2653 |
| Temperature, °C | Mean±SD | 36.7±0.5 | 36.6±0.5 | 0.3882 |
| Heart rate, bpm | Mean±SD | 82.1±16.4 | 78.4±17.4 | 0.1077 |
| Respiratory rate | Mean±SD | 18.3±3.2 | 17.5±3.1 | 0.1395 |
| Side of infarct (right) | % (n/N) | 45.1% (41/91) | 55.4% (56/101) | 0.1932 |
Baseline characteristics of the FVP subset of the Safety and Efficacy of NeuroFlo Technology in Ischemic Stroke trial. BL indicates baseline; CoW, Circle of Willis; FVP, favorable vascular profile; and MAP, mean arterial blood pressure; NIHSS, National Institutes of Health Stroke Scales.
There were also no major differences between patients with (n=172) and without (n=20) FVP except for higher NIHSS scores in the latter subgroup (mean 10.8±4.3 versus 12.9±5.0; median 11 versus 14.5; P=0.0401). Overall, FVP was seen in 89.6% of patients with a trend in favor of treated patients (94.5% versus 85.2%; P=0.0562). Variables used in univariate logistic regression models to determine if they are associated with mRS 0 to 2 at 90 days are listed in Table 2. In the univariate models, the following were associated with the outcome (all P values<0.10): FVP, baseline NIHSS, and history of atrial fibrillation. These variables were used in a multivariable model. Mortality and severe disability were higher in the group without FVP (17.4% versus 45%). The presence of FVP predicted independent outcome in univariate (odds ratio, 7.46; 95% confidence interval, 1.68–33.18; P=0.0082) and multiple logistic regression analyses after adjustment for all variables (odds ratio, 10.22; 95% confidence interval, 1.78–58.57; P=0.0091). Aside from FVP, only baseline NIHSS (odds ratio, 0.74; 95% confidence interval, 0.67–0.82; P<0.0001) and presence of atrial fibrillation (odds ratio, 0.48; 95% confidence interval, 0.21–1.12; P=0.0909) entered the predictive model. The addition of treated arm and the interaction term did not add significant information to the model (likelihood ratio χ2 test; P=1.000; ie, there was no interaction with randomization to treatment or control).
Table 2.
Logistic Regression Analysis
| Variables | β (SE) | OR [95% CI] | P Value |
|---|---|---|---|
| Intercept | 1.67 (0.65) | … | 0.0098 |
| FVP vs. no FVP | 1.16 (0.45) | 10.22 [1.78–58.57] | 0.0091 |
| Baseline NIHSS* | −0.30 (0.05) | 0.74 [0.67–0.82] | <0.0001 |
| History of AFib | 0.36 (0.22) | 2.07 [0.89–4.81] | 0.0909 |
List of variables in univariate logistic regression model (association with mRS 0–2 at 90 days): treated arm, FVP, baseline NIHSS, age, infarct side, glucose, time from symptom onset to randomization, sex, race (black, white, other), SBP, DBP, AFib, diabetes mellitus, hypertension, hyperlipidemia, CII, TIA, seizures, valvular disease, myocardial infarction, angina, peripheral vascular disease, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, and smoking status.
AFib indicates atrial fibrillation; CI indicates confidence interval; DBP, diastolic blood pressure; FVP, favorable vascular profile; NIHSS, National Institutes of Health Stroke Scales; and SBP, systolic blood pressure.
All depicted variables were associated with the outcome (all P values<0.10) and included into the multivariate model. There was no interaction of FVP and treated arm (likelihood ratio χ2 test; P=1.000).
One unit increase.
For the shift analysis, stratification by baseline NIHSS and age, stratification by baseline NIHSS and infarct side, and stratification by baseline NIHSS and history of atrial fibrillation, there were no statistical differences between treated groups, whether subjects had FVP or not (all P values>0.48).
Discussion
Hemodynamic augmentation by partial aortic occlusion has been shown by several imaging techniques to increase cerebral blood flow by ≈30%, an effect that lasts beyond the procedure itself.4 The SENTIS trial tested the clinical efficacy and safety of the NeuroFlo device, that by increasing cerebral blood flow to ischemic brain was hypothesized to lead to reduced morbidity and mortality in acute stroke patients treated within 14 hours after onset of symptoms.1 SENTIS established safety for the NeuroFlo procedure but missed statistical significance for the primary clinical outcome end point. Arguably, the presence of collaterals, especially the presence of an intact Circle of Willis combined with sufficient cerebral perfusion pressure would increase the chance of an acute stroke patient to experience a better outcome. This could be mediated by establishing and stabilizing collateral perfusion to ischemic areas of the brain.5 Also, the presence of a FVP could be a prerequisite for any hemodynamic augmentative approach to work in ameliorating the sequelae of ischemic stroke by improved penumbral flow, stroke size reduction, and thereby improving clinical outcome.
FVP and baseline NIHSS independently predicted outcome in this subset of the SENTIS population. Although the presence of a FVP by itself is an independent predictor of stroke outcome, we could not detect any interaction of FVP present with a therapy effect of the NeuroFlo procedure.
Although further analysis of the acquired imaging data are necessary, it may be assumed that the procedure, by improving collateral flow to ischemic penumbral brain, reduces infarct size. Stroke size has been repeatedly established as a predictor for outcome and mortality.6–9 Whether presence of FVP in combination with hemodynamic augmentation reduces infarct size and this effect again results in improved clinical outcomes remains to be seen. FVP is a novel parameter to predict outcome of acute stroke patients and further studies will establish its potential role for selection of optimal candidates for hemodynamic augmentation.
Our analysis is limited by its nature as a post hoc calculation; however, the size of our subgroup and the nature of the source data generated from a randomized trial add to its importance. Another limitation by virtue of performed imaging is the lack of assessment of extra- to intracranial collaterals, which is mostly a domain of Doppler ultrasound (eg, the ophthalmic collateral).
At the current stage, it is not known whether the NeuroFlo device will be the subject of further study. If so, systematic assessment of FVP including extra- to intracranial collaterals may be an important protocol feature.
Acknowledgments
Sources of Funding
CoAxia, Inc provided funding for the SENTIS Trial.
Drs Schellinger, Liu, Dillon, Nogueira, Shuaib, Liebeskind have received honoraria, travel grants, and/or consulting fees from CoAxia.
Footnotes
Disclosures
Dr Köhrmann has no conflicts to report.
References
- 1.Shuaib A, Bornstein NM, Diener HC, Dillon W, Fisher M, Hammer MD, et al. SENTIS Trial Investigators. Partial aortic occlusion for cerebral perfusion augmentation: safety and efficacy of NeuroFlo in Acute Ischemic Stroke trial. Stroke. 2011;42:1680–1690. doi: 10.1161/STROKEAHA.110.609933. [DOI] [PubMed] [Google Scholar]
- 2.Hammer M, Jovin T, Wahr JA, Heiss WD. Partial occlusion of the descending aorta increases cerebral blood flow in a nonstroke porcine model. Cerebrovasc Dis. 2009;28:406–410. doi: 10.1159/000235628. [DOI] [PubMed] [Google Scholar]
- 3.Emery DJ, Schellinger PD, Selchen D, Douen AG, Chan R, Shuaib A, et al. Safety and feasibility of collateral blood flow augmentation after intravenous thrombolysis. Stroke. 2011;42:1135–1137. doi: 10.1161/STROKEAHA.110.607846. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS. Aortic occlusion for cerebral ischemia: from theory to practice. Curr Cardiol Rep. 2008;10:31–36. doi: 10.1007/s11886-008-0007-3. [DOI] [PubMed] [Google Scholar]
- 5.Liebeskind DS. Reperfusion for acute ischemic stroke: arterial revascularization and collateral therapeutics. Curr Opin Neurol. 2010;23:36–45. doi: 10.1097/WCO.0b013e328334da32. [DOI] [PubMed] [Google Scholar]
- 6.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. EPITHET Investigators. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 7.Thomalla GJ, Kucinski T, Schoder V, Fiehler J, Knab R, Zeumer H, et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke. 2003;34:1892–1899. doi: 10.1161/01.STR.0000081985.44625.B6. [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 9.Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, et al. DEFUSE-EPITHET Investigators. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]