Abstract
Helicobacter pylori is a gram-negative bacterium that colonizes the human gastric mucosa causing gastritis and peptic ulcer and increasing the risk of gastric cancer. The efficacy of current antibiotic-based therapies can be limited by problems of patient compliance and increasing antibiotic resistance; the vaccine approach can overcome these limits. The present study describes the therapeutic vaccination of experimentally H. pylori-infected beagle dogs, an animal model that reproduces several aspects of the human infection with H. pylori. The vaccine consisted of three recombinant H. pylori antigens, CagA, VacA, and NAP, formulated at different doses (10, 25, or 50 μg each) with alum and administered intramuscularly either weekly or monthly. No adverse effects were observed after vaccination and a good immunoglobulin G response was generated against each of the three antigens. Bacterial colonization and gastritis were decreased after the completion of the vaccination cycle, especially in the case of the monthly immunization schedule. In conclusion, therapeutic vaccination in the beagle dog model was safe and immunogenic and was able to limit H. pylori colonization and the related gastric pathology.
Helicobacter pylori is a spiral-shaped, gram-negative bacterium that infects the stomach of >50% of the population worldwide, with higher prevalence in the developing countries. H. pylori induces chronic inflammation of the stomach mucosa, causing chronic gastritis and peptic ulcer (9, 33); moreover, H. pylori infection is related to gastric mucosa-associated lymphoid tissue lymphoma (4) and to an increased risk of gastric cancer (36), as also proved in animal models (13, 38).
Current therapies, based on one antisecretory agent plus antibiotics, although effective in 80 to 90% of cases, face problems of patient compliance, increasing antibiotic resistance, and possible recurrence or reinfection; in spite of continuous effort to improve these treatments, no major breakthroughs have been achieved in the most recent years (30).
To overcome the limits of antibiotic-based therapies, the vaccine approach has been undertaken since the last decade, leading us to identify some relevant bacterial antigens as candidates for vaccines (2). On the other hand, animal models of H. pylori infection have been developed to study the interaction between the bacterium and the host, the mechanisms of immune response to either infection or vaccination, and to determine the efficacy of both prophylactic and therapeutic vaccination (2, 17, 26, 34). Among these animal models, that of the beagle dog reproduces several aspects of the human infection with H. pylori. In fact, in the beagle dog model, intragastric administration of H. pylori results in a long-term chronic infection, characterized by gastritis, epithelial alterations, superficial erosions, and the appearance of macroscopic follicles in the gastric mucosa, mainly in the antral region of the stomach (28, 29).
Most of the examples of vaccination against H. pylori in animal models reported in the literature concern the use of either whole-cell preparation or single purified antigens, administered mucosally. Previous work in our laboratories has shown the feasibility of both prophylactic and therapeutic vaccination in mice with a mixture of three H. pylori toxins—CagA, VacA, and NAP—relevant in the pathogenesis of infection (8, 22, 32). Moreover, parenteral vaccination of beagle dogs with these H. pylori antigens gave good rate of protection against subsequent H. pylori experimental challenge (unpublished data).
We report here data from experiments aimed at evaluating the therapeutic approach of vaccination in beagle dogs experimentally infected with H. pylori, using recombinant CagA, VacA, and NAP, administered intramuscularly at different doses and vaccination schedules.
MATERIALS AND METHODS
Animals.
Conventional, pure-bred beagle dogs were supplied by Morini SpA (S. Polo D'Enza, Reggio Emilia, Italy), already vaccinated against hepatitis, leptospirosis, and distemper. The dogs were selected at the age of approximately 2 months on the basis of the lowest reactivity of their serum against bacterial lysate, as evaluated by enzyme-linked immunosorbent assay (ELISA). Their mothers were previously selected by the same criterion. The dogs were housed in individual boxes under standard conditions and received 300 g/day of pelleted dog diet (Altromin H; Altromin, Lage, Germany) and tap water ad libitum. Since the gastric mucosa of the dogs can be naturally colonized by other Helicobacter spp., to reduce the risk of such an unwanted infection all of the dogs, including the mothers, were subjected to preventive antibiotic therapy consisting of 250 mg of amoxicillin (Zimox; Pharmacia Upjohn, Milan, Italy) given twice daily, 60 mg of bismuth citrate (De-Nol; Yamanouchi Pharma) given twice daily, and 10 mg of doxycycline (Ronaxan; Merial Italia, Milan, Italy) once per day for 10 days. Either 2 (study 1) or 4 (study 2) weeks elapsed between the end of the antibiotic therapy and the experimental infection. An anthelmintic treatment (Drontal-Plus; Bayer, Leverkusen, Germany) was also done.
Bacterial strain and culture.
SPM326, a type I (CagA+ VacA+) H. pylori strain obtained from a human isolate and adapted to the mouse as previously described (21), was used to infect the dogs. Bacteria were grown and harvested as previously described (8) with minor modifications.
Bacterial lysate preparation.
Bacteria were harvested, washed, and resuspended in phosphate-buffered saline (PBS) and then were disrupted by sonication as previously described (8). The protein concentration of bacterial lysate was determined by Bio-Rad Protein Assay (Bio-Rad, Hercules, Calif.) according to the manufacturer's instruction with bovine serum albumin as a standard.
Antigens and vaccine formulation.
The H. pylori antigens CagA, VacA, and NAP were expressed in Escherichia coli, with a sequence corresponding to that of the mature protein. All of the antigens were obtained with a purity above 95%. The purification procedures were based on previously reported methods (1, 20, 32) and will be described in detail elsewhere.
Two vaccination studies were performed with different amounts of the three antigens formulated with Al(OH)3. Each dose of vaccine used in the study 1 was composed of either 50 or 10 μg of each antigen with 1 mg of Al(OH)3, in a final volume of 1 ml. In study 2, one dose of vaccine consisted of 25 μg of each antigen, with 0.5 mg of Al(OH)3, in a final volume of 0.5 ml.
Infection and immunization of dogs.
Infection was carried out as previously described (29). Briefly, at the time of infection, 3- to 4-month-old dogs were starved for about 18 h. At 1 to 2 h before infection the dogs received intramuscularly 10 mg of cimetidine (Tagamet; Smith Kline & French, Philadelphia, Pa.)/kg. The dogs were then anesthetized intravenously with 5 μg of ketamine (Ketavet; Gellini, Latina, Italy)/kg plus 40 μg of medetomidine chloridrate (Domitor; Centralvet-Vetem SpA, Milan, Italy)/kg, and 15 min before infection they received intragastrically 0.2 M bicarbonate buffer (pH 9) to reduce the gastric acidity and favor H. pylori colonization. Each animal was then challenged intragastrically with 1010 CFU of SPM326 bacteria in 3 ml of saline. Control, naive dogs received only 3 ml of saline. After challenge, the dogs were treated with 200 μg of the anesthetic antagonist atipamezole (Antisedan; Centralvet-Vetem SpA)/kg and fed after 2 h. The challenge was performed every other day for a total three (study 1) or four (study 2) times.
After we checked for the establishment of the infection as described below, the dogs were divided into treatment groups. The vaccinations began 11 and 18 weeks after challenge in studies 1 and 2, respectively. The vaccine was administered intramuscularly three times weekly in study 1 and monthly (4-week intervals) in study 2 by injection into a shaved area of the right femoral biceps. The injection site was inspected 6, 24, and 48 h after each immunization to evaluate the possible erythema or eschar and edema formation. In both studies the elapsed time after vaccination was calculated starting from the first immunization (i.e., week 0).
In study 1, 12 dogs were infected with H. pylori and then divided into three groups of four animals. Group 1 received Al(OH)3 only, while groups 2 and 3 received weekly a vaccine containing 50 and 10 μg of each antigen, respectively. The study was carried on until the 29th week postvaccination. Study 2 included 17 animals: 14 H. pylori-infected dogs were divided into four groups, whereas the remaining three dogs (group 5) did not, as a naive control, receive challenge or subsequent treatments and were housed separately to avoid any possible contamination. Groups 1 and 2, with four dogs each, received monthly a vaccine containing 25 μg of each antigen: group 1 received additionally 20 mg omeprazole per os/day, beginning 2 days before the first vaccine administration and ending 2 weeks after the last one. Group 3 (three dogs) received Al(OH)3 instead of vaccine, plus the above-described omeprazole treatment. Group 4 (three infected control dogs) did not receive any further treatment. The study was carried on until the 23th week postvaccination. Groups and treatments of both studies are summarized in Table 1.
TABLE 1.
Treatment groups
| Study (protocol) | Group | No. of dogsa | Challengeb | Antigen amt (μg)c | Al(OH)3 (mg) | Otherd | Vol (ml) |
|---|---|---|---|---|---|---|---|
| Study 1 (weekly immunization: wk 0, 1, and 2) | 1 | 4 (2M+2F) | Y | 1 | 1 | ||
| 2 | 4 (2M+2F) | Y | 50 | 1 | 1 | ||
| 3 | 4 (2M+2F) | Y | 10 | 1 | 1 | ||
| Study 2 (monthly immunization: wk 0, 4, and 8) | 1 | 4 (2M+2F) | Y | 25 | 0.5 | PPI | 0.5 |
| 2 | 4 (2M+2F) | Y | 25 | 0.5 | 0.5 | ||
| 3 | 3 (1M+2F) | Y | 0.5 | PPI | 0.5 | ||
| 4 | 3 (2M+1F) | Y | 0.5 | ||||
| 5 | 3 (1M+2F) | N | 0.5 |
The numbers in parentheses indicate the numbers of male (M) and female (F) dogs.
Y or N indicates whether the dogs received the challenge with H. pylori or not, respectively.
The amount indicated refers to each of the three antigens (CagA, VacA, and NAP).
For details on proton pump inhibitor (PPI) (omeprazole), treatment see Materials and Methods.
Blood samples were taken periodically from the jugular vein for serological assays. Hematological and clinical chemistry analyses were also performed. The dogs were periodically subjected to physical examination and inspected for clinical signs. Body weight, food consumption, and body temperature were recorded.
To assess the bacterial infection and the level of gastric mucosal inflammation, biopsies were taken periodically under endoscopy from gastric antrum, corpus, and fundus and then processed and analyzed as described below.
All experimental protocols were approved by the Italian Ministry of Health.
Titration of specific antibodies against H. pylori antigens.
Antibodies to H. pylori antigens were quantitated by ELISA by using as a solid phase either H. pylori lysate or the same antigen preparations that composed the vaccine. Maxisorp 96-well plates (Nalge Nunc International, Roskilde, Denmark) were coated overnight at 4°C with H. pylori lysate (0.5 μg/well) or with purified recombinant CagA, VacA, or NAP (0.2 μg/well) diluted in PBS (pH 7.4) and then saturated with PBS containing 1% nonfat milk (France Lait, St.-Martin-Belle-Roche, France). Twofold dilutions of sera were added, followed by incubation for 2 h at 37°C, and the plates were then washed three times with PBS containing 0.05% Tween 20. Horseradish peroxidase-conjugated goat anti-dog immunoglobulin G (IgG) polyclonal antibody (Bethyl Laboratories, Inc., Montgomery, Tex.) was then added, followed by incubation for 2 h at 37°C. After further washes, the binding of secondary antibody was revealed by using o-phenylenediamine dihydrochloride (Sigma-Aldrich, St. Louis, Mo.) as a substrate. Antibody titers, expressed as ELISA units (EU), were calculated by comparing the response curve of test serum with that of the reference serum by using a reference line calculation program. The reference serum was a pool of sera obtained from beagle dogs previously immunized with the same antigens used in the present studies, to which the following arbitrary titers were assigned: 4,000 EU/ml for CagA, 2,000 EU/ml for NAP, 2,000 EU/ml for VacA, and 100 EU/ml for H. pylori lysate.
Urease test.
One set of biopsies taken from the three gastric sites (antrum, corpus, and fundus) were immediately dipped in 0.5 ml of urea broth (39) at room temperature and then inspected periodically; we recorded the time when the medium color changed from yellow-orange to pink, since this is a distinctive effect of urease activity. A sample was considered positive if such a change occurred within 24 h.
Histopathology.
One set of biopsies was fixed in 4% buffered formaldehyde and then embedded in paraffin. Sections 4 μm thick were cut, dewaxed, and stained with hematoxylin and eosin (HE). Adjacent sections were subjected to immunohistochemical (IHC) analysis with a mouse anti-VacA monoclonal antibody as previously described (29); the antibody binding was revealed by using biotinylated anti-mouse secondary antibody and avidin-biotin complex-peroxidase (Vector Laboratories, Burlingame, Calif.) with 3-3′diaminobenzidine-hydrochloride (Sigma Chemical Co., St. Louis, Mo.) as a chromogen substrate.
The histological examination of HE-stained sections included the assessment of the number of inflammatory cells using a visual analogue scale based on the updated Sydney System modified for canine specimens (3, 14). Briefly, in each biopsy phlogosis, follicles, neutrophils, and fibrosis were evaluated and scored from 0 to 3, and the overall gastritis score assigned to each biopsy resulted from the highest value obtained among the above-mentioned scores. The significance of the variations of gastritis scores was evaluated by applying the Fisher exact test in the antrum, since this site is more involved in H. pylori-induced gastritis both in dogs and in humans.
H. pylori selective culture from biopsies.
Freshly taken biopsies from gastric antrum, corpus, and fundus were immersed in 0.1 ml of brain heart infusion (BHI) medium, and streaked onto selective agar-blood plates made as described above, with the addition of 0.2 mg of Bacitracin/ml (Sigma-Aldrich). After 5 to 8 days of culture under microaerophilic conditions, plates were examined for the presence of H. pylori colonies.
RESULTS
Two separate studies were performed, with different vaccination schedules and antigen doses, administered intramuscularly with Al(OH)3 as an adjuvant. In study 1 the dogs were immunized weekly with either 10 or 50 μg of each antigen. In study 2 vaccine contained 25 μg of each antigen and was administered monthly, with or without the administration of omeprazole during the vaccination period.
Evaluation of the infection.
In both vaccination studies urease test was performed on gastric biopsies from each dog before the challenge; all of the selected dogs were urease negative.
In study 1, the urease positivity of all of the biopsies taken after challenge (not shown), together with their IHC positivity to VacA, and the visualization of bacteria morphologically identifiable as H. pylori, proved a successful infection.
In study 2, all of the biopsies analyzed after challenge were urease positive (results not shown); however, the naive controls, which had not been challenged and were VacA negative, were also urease positive. The histological analysis revealed in almost all of these samples the presence of large, helical Helicobacter spp. other than H. pylori (Gastrospirillum-like organisms [GLO]), which are known to be able to colonize the canine stomach, are morphologically well distinct from H. pylori and possess urease activity but lack peculiar H. pylori antigens such as VacA. This natural GLO infection did not prevent establishment of the experimental infection with H. pylori but led to discontinue the use of the urease test to evaluate the H. pylori infection in study 2, since the GLO urease activity is indistinguishable from that of H. pylori. Thus, the H. pylori infection was evaluated on the basis of IHC positivity to VacA. To avoid any uncertainty about the establishment of the experimental infection, another set of biopsies was taken after a further 4 weeks, and only the dogs that were determined to be VacA positive in both of the samplings, with visualization of some well-defined spiral bacteria with the typical H. pylori morphology, in addition to a positive stain detectable within the gastric glands and in mucus or in some epithelial cells, were considered H. pylori-infected and enrolled into the treatment groups.
Culture of the biopsies taken from infected dogs originated only in few cases a very few and small colonies showing the typical morphology of H. pylori colonies. These colonies were scarcely vital; thus, further cultivation and their unequivocal characterization were unsuccessful. The IHC analysis therefore remains the best method for detecting H. pylori presence in the gastric mucosa. It must be pointed out that cultures of gastric biopsies from dogs that were VacA negative by IHC never yielded H. pylori colonies.
Immunization does not produce adverse side effects.
No clinical signs were observed in both vaccinated and control groups. All dogs had a body weight increment within the normal range throughout both studies, a finding consistent with regular food consumption. The injection site did not show any reaction. No abnormal variations of body temperature were observed after vaccination, no abnormalities were generally found upon physical examination, and no vaccine-related variations in hematological or clinical chemistry parameters were observed during and after vaccination in both the studies. All of these data indicate that the vaccine was well tolerated by dogs.
Immunization induces specific antibody response.
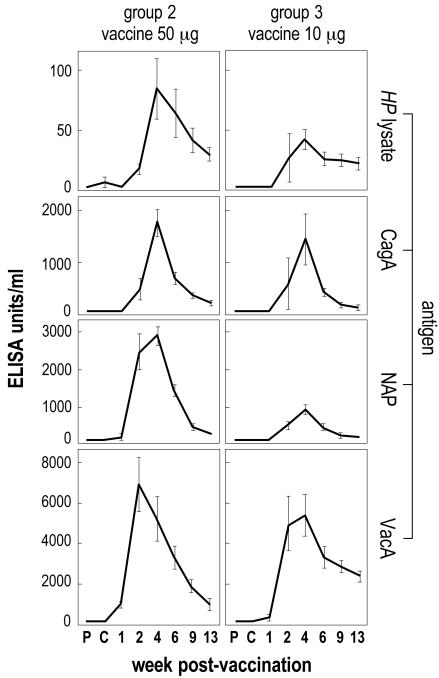
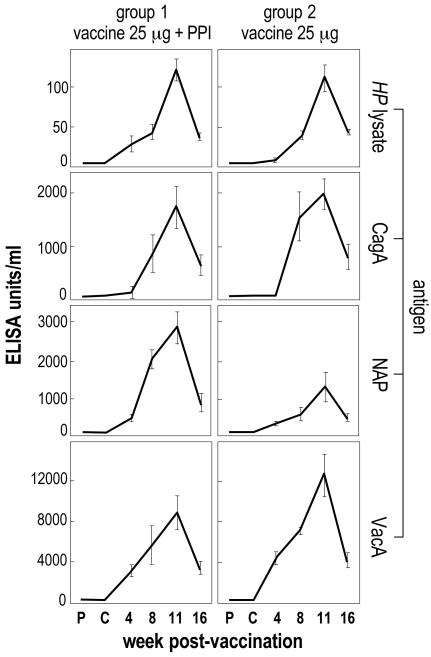
Figures 1 and 2 show the kinetics of serum IgG antibody response throughout studies 1 and 2, respectively.
FIG. 1.
Study 1 (weekly immunization): kinetics of serum IgG antibody response against H. pylori antigens in vaccinated groups. The mean value of each group is reported. The error bars indicate ±1 standard error. P, prechallenge; C, postchallenge (prevaccination).
FIG. 2.
Study 2 (monthly immunization): kinetics of serum IgG antibody response against H. pylori antigens in vaccinated groups. The mean value of each group is reported. Error bars indicate ±1 standard error. P, prechallenge; C, postchallenge (prevaccination).
In study 1 (weekly immunization), specific IgGs against each of the immunizing antigens were detected after the second dose of vaccine. Such an antibody response was dose dependent and further augmented after the third (and last) dose. Specific antibodies were still detectable between the 9th and 13th weeks postvaccination.
Similar results were obtained in study 2 (monthly immunization). Also, in this case, the second dose of vaccine produced a well-detectable increase of specific IgG, and the peak of the antibody production was reached after the third (and last) dose of vaccine. Appreciable levels of IgG were still measured at week 16 postvaccination (Fig. 2).
In neither study were specific antibodies detected in the nonvaccinated groups (results not shown).
Therapeutic vaccination reduces the severity of gastric pathology.
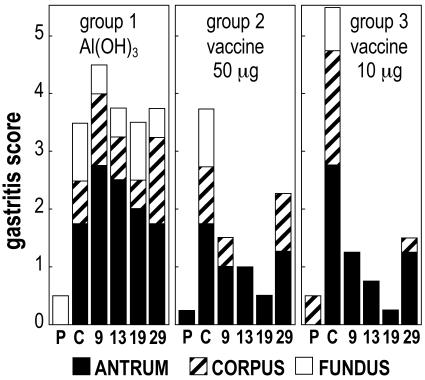
Figure 3 shows the course of the gastritis throughout study 1 (weekly immunization). In this experiment, the gastritis score increased after the challenge, mainly in the antrum. Compared to antral prechallenge scores, the postchallenge scores showed a significant increase in all groups (P < 0.05). Histology of the gastric biopsies revealed a mild degree of edema and hyperemia of the lamina in all three gastric regions examined. The inflammatory infiltrate localized in the antrum was characterized by the presence of mono- and polymorphonuclear leukocytes infiltrating the lamina propria among the glands in a diffuse form, with or without microfollicular aggregates of mononuclear cells in the deep glandular corion. In most of the samples, several polymorphonuclear leukocytes were interspersed in the epithelial cell layer and also in the mucus, suggesting that neutrophil transcytosis was elicited by the infection. These signs are consistent with those previously described in H. pylori-infected dogs and are also typical of chronic active gastritis in infected humans (29). After vaccination with both high and low doses, corpus and fundus gastritis rapidly disappeared, and a progressive decrease of the gastritis score to very low values occurred in vaccinated dogs (groups 2 and 3), until the sampling before the last sampling. At this penultimate sampling, the antrum gastritis scores remained similar to those of postchallenge in the unvaccinated controls (group 1), whereas they significantly decreased in vaccinated dogs (groups 2 and 3, P < 0.05) to levels very close to those of the prechallenge time points. At the last sampling the gastritis score of vaccinated dogs increased, suggesting that the effects of weekly vaccination were partial and transient.
FIG. 3.
Study 1 (weekly vaccination): course of gastritis score. P, prechallenge; C, postchallenge; 9, 13, 19, and 29, weeks postvaccination. Each column is composed by distinct sections corresponding to the score of each gastric site (antrum, corpus, and fundus). For each gastric site, the mean value of the gastritis score of the group is reported.
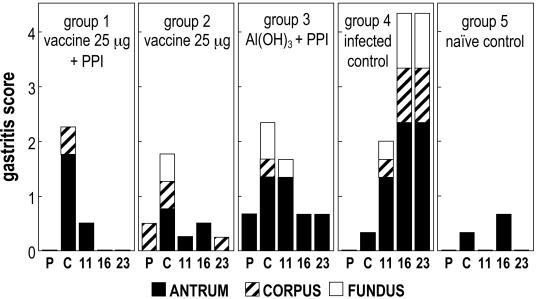
Figure 4 shows the course of the gastritis score throughout study 2 (monthly immunization). Also in the present study, the challenge resulted in an increase of gastritis, mainly in the antrum, but with a slower rate compared to that of the previous study; in fact, in the infected controls (group 4) the gastritis score continued to increase significantly (P < 0.05) from the postchallenge sampling to the last two time points. The monthly immunization schedule applied in study 2, with a vaccine containing an amount of antigens (25 μg) intermediate between the two doses used in study 1 (10 and 50 μg), showed better efficacy in reducing the gastritis induced by H. pylori infection. In fact, after vaccination, a decrease of the gastritis was observed in all of the vaccinated dogs (groups 1 and 2), the antrum gastritis scores reaching the zero value at the last sampling, identical to that of prechallenge level. Control group 3, which received Al(OH)3 plus PPI, also showed a trend toward an amelioration of inflammation, conceivably due to PPI effect, but less marked than that of the vaccinated groups, since at the last two samplings some follicular gastritis was still detected. Conversely, an increasing inflammation trend was evident in the infected controls (group 4), which at the last sampling showed chronic follicular gastritis, with the characteristic presence of small lymphoplasmacytic aggregates among the glands of corpus and antrum, or true lymphoid follicles in the antrum. These follicles were associated with degenerative processes in the epithelial cells, the presence of scattered neutrophilic granulocytes, and an increase in the exocytosis of mononuclear cells. PPI administered in parallel with vaccine to the group 1 seemed to produce an additional little effect in terms of reduction of inflammation, as the gastritis scores decreased more evidently than in group 2, that received vaccine without PPI. In the naive controls (group 5), small, casual fluctuations of antrum gastritis scores were noted throughout the study.
FIG. 4.
Study 2 (monthly vaccination): course of gastritis score. P, prechallenge; C, postchallenge; 11, 16, and 23, weeks postvaccination. Each column is composed by distinct sections corresponding to the score of each gastric site (antrum, corpus, and fundus). For each gastric site the mean value of the gastritis score of the group is reported.
Therapeutic vaccination reduces H. pylori colonization.
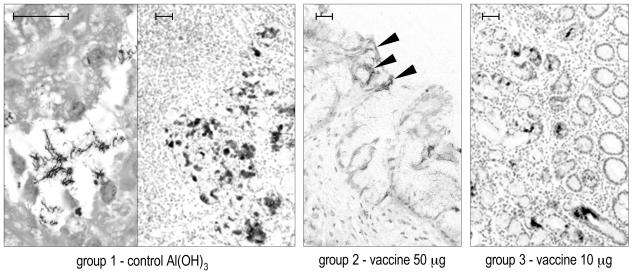
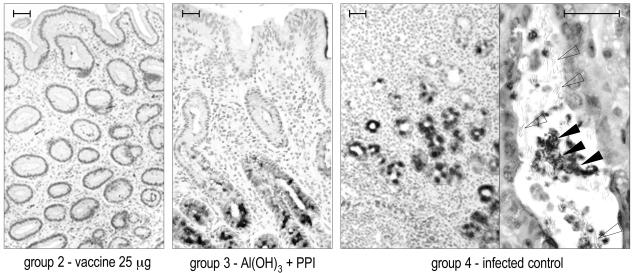
Table 2 summarizes the IHC results obtained in both studies with an antibody to VacA as a specific marker of H. pylori presence. Figure 5 and 6 show representative IHC results obtained with biopsies taken at the last sampling of studies 1 and 2, respectively. The IHC substantially confirmed the histopathologic observations.
TABLE 2.
IHC resultsa
| Dog | Study 1 (weekly immunization)
|
Study 2 (monthly immunization)
|
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 [Al(OH)3] at wk:
|
Group 2 (vaccine [50 μg]) at wk:
|
Group 3 (vaccine [10 μg]) at wk:
|
Group 1 (vaccine [25 μg + PPI]) at wk:
|
Group 2 (vaccine [25 μg]) at wk:
|
Group 3 [Al(OH)3 + PPI] at wk:
|
Group 4 (infected control)
|
Group 5 (uninfected control)
|
||||||||||||||||||||||||||||
| C | 9 | 13 | 19 | 29 | C | 9 | 13 | 19 | 29 | C | 9 | 13 | 19 | 29 | C | 11 | 16 | 23 | C | 11 | 16 | 23 | C | 11 | 16 | 23 | C | 11 | 16 | 23 | C | 11 | 16 | 23 | |
| 1 | + | + | + | + | + | − | + | − | − | − | + | + | + | − | + | + | − | − | − | + | − | − | − | + | + | + | + | + | + | + | + | − | − | − | − |
| 2 | + | + | + | + | + | + | + | − | − | + | + | − | − | + | − | + | − | − | − | + | − | − | − | + | + | + | − | + | + | + | + | − | − | − | − |
| 3 | − | + | + | + | + | + | − | + | + | + | + | + | + | + | − | + | − | − | − | + | − | − | − | + | + | + | + | + | + | + | + | − | − | − | − |
| 4 | + | + | + | + | + | + | − | − | + | − | + | − | − | − | − | + | + | + | − | + | + | − | − | ||||||||||||
C, postchallenge (preimmunization); week 0, administration of first dose of vaccine. Results: +, VacA positivity was found in at least one of the biopsies examined (gastric antrum, corpus, or fundus); −, all biopsies were VacA negative. Within each group, each line refers to the IHC results of an individual dog.
FIG. 5.
Study 1 (weekly immunization): representative IHC results obtained with anti-VacA antibody on 4-μm-thick sections of formalin-fixed, paraffin-embedded gastric antral biopsies at the last sampling (29th week postvaccination). The higher magnification at the left side of the left panel (group 1) shows positively stained H. pylori within a glandular lumen. The solid arrowheads in the central panel indicate some weak, superficial positivity in a dog of group 2. Bar, 50 μm.
FIG. 6.
Study 2 (monthly immunization): representative IHC results obtained with anti-VacA antibody on 4-μm-thick sections of formalin-fixed, paraffin-embedded gastric antral biopsies at the last sampling (week 23 postvaccination). The higher magnification at the right side of the right panel (group 4) shows positively stained H. pylori (solid arrowheads) within a glandular lumen, mostly aggregated, surrounded by several Gastrospirillum-like organisms VacA negative (empty arrowheads). Bar, 50 μm.
In study 1 (weekly immunization), at the sampling before the last, H. pylori was not detectable by IHC, and the urease test (not shown) was negative in 50% of the vaccinated dogs (groups 2 and 3), whereas all of the infected controls remained positive. At the last sampling, the low level of H. pylori detection by IHC was confirmed, but all of the samples had become urease positive, a finding consistent with the increase of gastritis score observed by histopathology. Interestingly, in group 2, which received the highest dose of vaccine, the IHC positivity was generally very weak and localized superficially (Fig. 5, central picture) compared to the other groups.
The vaccination regimen of study 2 (monthly immunization) showed better efficacy against bacterial colonization than that observed in study 1 (weekly immunization), a finding consistent with the stronger reduction of gastritis described above. In fact, at the final sampling, H. pylori was undetectable by IHC in all of the vaccinated dogs (groups 1 and 2) (Fig. 6, left picture), whereas two of three dogs that received Al(OH)3 plus PPI (group 3) and all of the infected controls (group 4) were still H. pylori positive. The additional PPI treatment in group 1 did not influence the course of H. pylori colonization.
DISCUSSION
We have shown here that parenteral, alum-adjuvanted H. pylori vaccine, consisting of the well-defined antigens VacA, CagA, and NAP, all involved in the pathogenesis of H. pylori infection (24), can limit an already-established experimental infection in beagle dogs.
It is known that parenteral vaccination can confer protective immunity against mucosal infections. In the case of H. pylori this has been demonstrated in various animal models by several study groups, including ours (2, 6, 10-12). In most cases, however, the experimental vaccine consisted of a bacterial lysate or of a single recombinant antigen, such as urease. It is likely that effective immunity against bacteria could be better achieved by the combination of different antigens participating in different aspects of the pathogenesis of the infection, as with the vaccine against pertussis (27). Based on this, we decided to pursue the development of a vaccine against H. pylori consisting of various proteins, all involved in the virulence of the bacterium. Indeed, previous work in animals had shown that immunization with VacA, CagA, or NAP was protective against challenge with H. pylori (22, 32).
Several examples of therapeutic vaccination against H. pylori have been described in animal models (5, 8, 11, 16), with variable results, both in terms of bacterial eradication (ranging from a negligible efficacy to a complete eradication) and of gastritis reduction. We decided to use the dog model rather than the murine one, since it allowed us to take gastric biopsies repeatedly from the same animal, without sacrificing it, thus providing a more reliable time course of the evolution of both H. pylori colonization and gastric inflammation.
In both studies performed here, the experimental infection of dogs with H. pylori was successful. In study 2, a GLO contamination was observed in all of the biopsies taken after challenge. Conceivably, such a contamination was preexistent, in spite of the preventive antibiotic therapy performed before enrollment of the dogs, since it was found even in the naive control animals that were kept isolated from the others. The urease negativity of the prechallenge biopsies can be explained with the failure of the preventive antibiotic treatment, which at the time of the enrollment could have only lowered the GLO infection under the detection limit of urease test rather than eradicate it. Compared to the infection in study 1, the H. pylori infection in study 2 seems to have arisen and become established more slowly; this was particularly evident when we examined the inflammation trend in the infected control group 4 (Fig. 4), in which the antrum gastritis score increased significantly at late time points. This may have been due to a GLO competition with H. pylori in colonizing the gastric niche. These observations led us to conclude that, in the beagle dog model, a naturally occurring GLO infection can delay, but not prevent, the establishment of H. pylori colonization after experimental infection.
In both studies, the histological and IHC analyses of H. pylori-infected animals showed that H. pylori distribution in the gastric mucosa was not homogeneous. Although an evident IHC positivity to VacA was localized in the mucus layer or into glandular lumina, a small number of spiral-shaped and IHC-positive bacteria were detectable, also suggesting a prevalence of coccoid rather than spiral forms. The limited presence of spiral forms can well explain the scarce colony-forming ability and the poor viability of bacteria observed when biopsies from infected dogs were put in culture.
In both studies, therapeutic parenteral vaccination with CagA, VacA, and NAP was able to reduce the H. pylori colonization, as judged by IHC. In parallel, the gastritis scores were also significantly reduced in the vaccinated groups, transiently in study 1 (weekly vaccination) and stably during the period of observation in study 2 (monthly vaccination). These data indicate that vaccination counteracted the H. pylori infection and its pathological consequences. Particularly in the study 1 (weekly vaccination), the reduction of gastritis was a slow process, requiring several weeks to be achieved. This effect can also be explained with the observation, made in several vaccination studies, that a protective local immune reaction against H. pylori can produce per se a transient gastric inflammation (25). Thus, within a short time after vaccination, it is not possible to distinguish a “protective” inflammation from that induced by H. pylori. Interestingly, in study 2 (monthly vaccination), although the additional PPI treatment (group 1) did not influence the H. pylori eradication, it seems to have slightly accelerated and strengthened the decrease of gastric inflammation.
Some vaccines against H. pylori have already been determined to be safe and immunogenic in humans (15, 23), although in some cases unwanted side effects have been observed due to the toxicity of the adjuvant. In particular, a vaccine consisting of the same antigens used here, formulated with alum for intramuscular injection, was recently tested in H. pylori-negative human volunteers, showing high safety and immunogenicity (19, 31), in agreement with the results described here in dogs. Unfortunately, it is known that an antibody response does not necessarily correlate with protection against H. pylori (7). In fact, in spite of the previously observed good immunogenicity in humans and both immunogenicity and efficacy in animals, when other H. pylori vaccines were assessed for therapeutic efficacy in humans (15, 23), neither H. pylori eradication nor an amelioration of gastric pathology was achieved. It is unclear whether the failure of these attempts of therapeutic vaccination was due to the antigen (soluble urease or whole-cell vaccine), to the route of administration (oral), or to the necessity of limiting the dose of the adjuvants (E. coli heat-labile enterotoxin or its mutant LTR192G) because of their intrinsic toxicity.
Numerous studies of vaccination, both in animal models and in human volunteers, indicate that the route of administration, as well as the adjuvant used, can play a crucial role in the induction of protective immune response. In particular, prime-boost vaccination protocols, combining mucosal and parenteral administration, seem to be more promising than mucosal or parenteral alone in eliciting both cellular and humoral response (18, 37), as well as in providing protection against H. pylori challenge (18). Although these protection data have been obtained by prophylactic vaccination, they should be taken in account in attempts to design a more efficacious protocol of therapeutic vaccination since, in this case, the infection can act as an effective mucosal priming (35).
In conclusion, the multicomponent vaccine against H. pylori tested intramuscularly in beagle dogs in the studies described here showed good safety and immunogenicity. The efficacy of monthly immunization schedule (study 2) was greater than the weekly immunization schedule (study 1), both in terms of H. pylori eradication and of gastritis reduction. These results not only indicate the feasibility of parenteral, therapeutic vaccination against H. pylori but also suggest that a further investigation on the most appropriate immunization schedule, including the evaluation of adding possible boosts, could further improve the vaccine efficacy.
Acknowledgments
We thank Francesco Norelli for providing H. pylori antigens, Mario Contorni for vaccinal formulations, and Lucia Luperi for managing the animal housing and treatments.
This study has been partially granted by MURST (the Ministry of University and of Scientific and Technologic Research of Italy).
Editor: A. D. O'Brien
REFERENCES
- 1.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, and R. Rappuoli. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 3.Dixon, M. F., R. M. Genta, J. F. Yardley, and P. Correa. 1996. Classification and grading of gastritis: the updated Sydney system. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 4.Du, M. Q., and P. G. Isaccson. 2002. Gastric MALT lymphoma: from etiology to treatment. Lancet Oncol. 3:97-104. [DOI] [PubMed] [Google Scholar]
- 5.Dubois, A., C. K. Lee, H. Kleanthous, P. T. Mehlman, and T. Monath. 1998. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect. Immun. 66:4340-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg, J. C., S. J. Czinn, C. A. Garhart, R. W. Redline, W. C. Bartholomae, J. M. Gottwein, J. G. Nedrud, S. E. Emancipator, B. B. Boehm, P. V. Lehmann, and T. G. Blanchard. 2003. Protective efficacy of anti-Helicobacter pylori immunity following systemic immunization of neonatal mice. Infect. Immun. 71:1820-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Meyers, J. Nedrud, and R. Weltzin. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghiara, P., M. Rossi, M. Marchetti, A. Di Tommaso, C. Vindigni, F. Ciampolini, A. Covacci, J. L. Telford, M. T. De Magistris, M. Pizza, R. Rappuoli, and G. Del Giudice. 1997. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect. Immun. 65:4996-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin, C. S., J. A. Armstrong, and B. J. Marshall. 1986. Campylobacter pyloridis, gastritis, and peptic ulceration. J. Clin. Pathol. 39:353-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottwein, J. M., T. G. Blanchard, O. S. Targoni, J. C. Eisenberg, B. M. Zagorski, R. W. Redline, J. G. Nedrud, M. Tary-Lehmann, P. V. Lehmann, and S. J. Czinn. 2001. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund adjuvant by systemic immunization. J. Infect. Dis. 184:308-314. [DOI] [PubMed] [Google Scholar]
- 11.Guy, B., C. Hessler, S. Fourage, B. Robki, M. J. Quentin Millet. 1999. Comparison between targeted and untargeted systemic immunizations with adjuvanted urease to cure Helicobacter pylori infection in mice. Vaccine 17:1130-1135. [DOI] [PubMed] [Google Scholar]
- 12.Guy, B., C. Hessler, S. Fourage, J. Haensler, E. Vialon-Lafay, B. Rokbi, and M. J. Millet. 1998. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine 16:850-856. [DOI] [PubMed] [Google Scholar]
- 13.Han, S. U., Y. B. Kim, H. J. Joo, K. B. Hahm, W. H. Lee, Y. K. Cho, D. Y. Kim, and M. W. Kim. 2002. Helicobacter pylori infection promotes gastric carcinogenesis in a mice model. J. Gastroenterol. Hepatol. 17:253-261. [DOI] [PubMed] [Google Scholar]
- 14.Happonen, I., J. Linden, S. Saari, M. Karjalainen, M. L. Hanninen, K. Jalava, and E. Westmark. 1998. Detection and effects of helicobacters in healthy dogs and in dogs with signs of gastritis. J. Am. Vet. Med. Assoc. 213:1767-1774. [PubMed] [Google Scholar]
- 15.Kotloff, K. L., M. B. Sztein, S. S. Wasserman, G. A. Losonsky, S. C. Di Lorenzo, and R. I. Walker. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, C. K., K. Soike, J. Hill, K. Georgakopoulos, and T. Tibbitts. 1999. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine 17:1493-1505. [DOI] [PubMed] [Google Scholar]
- 17.Lee, C. K. 2001. Vaccination against Helicobacter pylori in non-human primate models and humans. Scand. J. Immunol. 53:437-442. [DOI] [PubMed] [Google Scholar]
- 18.Londoño-Arcilia, P., D. Freeman, H. Kleanthous, A. M. O'Dowd, S. Lewis, A. K. Turner, E. L. Rees, T. J. Tibbits, J. Greenwood, T. P. Monath, and M. J. Darsley. 2002. Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parental boosting vaccination regimen. Infect. Immun. 70:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfertheiner, P., V. Schultze, G. Del Giudice, B. Rosenkranz, S. H. E. Kaufmann, F. Winau, T. Ulrichs, E. Theophil, C. P. Jue, D. Novicki, F. Norelli, M. Contorni, D. Berti, J. S. Lin, C. Schwenke, M. Goldman, D. Tornese, J. Ganju, E. Palla, R. Rappuoli, and B. Scharschmidt. 2002. Phase I safety and immunogenicity of a three-component H. pylori vaccine. Gastroenterology 122(Suppl. 1):A585. [Google Scholar]
- 20.Manetti, R., P. Massari, D. Burroni, M. De Bernard, A. Marchini, R. Olivieri, E. Papini, C. Montecucco, R. Rappuoli, and J. L. Telford. 1995. Helicobacter pylori cytotoxin: importance of native conformation for induction of neutralizing antibodies. Infect. Immun. 63:4476-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti, M., B. Aricò, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of H. pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti, M., M. Rossi, V. Giannelli, M. M. Giuliani, M. Pizza, S. Censini, A. Covacci, P. Massari, C. Pagliaccia, R. Manetti, J. L. Telford, G. Douce, G. Dougan, R. Rappuoli, and P. Ghiara. 1998. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine 16:33-37. [DOI] [PubMed] [Google Scholar]
- 23.Michetti, P., C. Kreiss, K. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthésy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in H. pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 24.Montecucco, C., and R. Rappuoli. 2001. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell. Biol. 2:457-466. [DOI] [PubMed] [Google Scholar]
- 25.Nedrud, J. G., S. S. Blanchard, and S. J. Czinn. 2002. Helicobacter pylori inflammation and immunity. Helicobacter 7(Suppl. 1):24-29. [DOI] [PubMed] [Google Scholar]
- 26.Nedrud, J. G.2001. Helicobacter pylori vaccines: lessons from small animal models. Scand. J. Immunol. 53:429-436. [DOI] [PubMed] [Google Scholar]
- 27.Rappuoli, R. 1997. Rational design of vaccines. Nat. Med. 3:374-376. [DOI] [PubMed] [Google Scholar]
- 28.Rossi, G., D. Fortuna, L. Pancotto, G. Renzoni, E. Taccini, P. Ghiara, R. Rappuoli, and G. Del Giudice. 2000. Immunohistochemical study of lymphocyte populations infiltrating the gastric mucosa of beagle dogs experimentally infected with Helicobacter pylori. Infect. Immun. 68:4769-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi, G., M. Rossi, C. G. Vitali, D. Fortuna, D. Burroni, L. Pancotto, S. Capecchi, S. Sozzi, G. Renzoni, G. Braca, G. Del Giudice, R. Rappuoli, P. Ghiara, and E. Taccini. 1999. A conventional beagle dog model for acute and chronic infection with Helicobacter pylori. Infect. Immun. 67:3112-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggiero, P., S. Peppoloni, D. Berti, R. Rappuoli, and G. Del Giudice. 2002. New strategies for the prevention and treatment of Helicobacter pylori infection. Expert Opin. Investig. Drugs 11:1127-1138. [DOI] [PubMed] [Google Scholar]
- 31.Ruggiero, P., S. Peppoloni, R. Rappuoli, and G. Del Giudice. 2003. The quest for a vaccine against Helicobacter pylori: how to move from mouse to man? Microbes Infect. 5:749-756. [DOI] [PubMed] [Google Scholar]
- 32.Satin, B., G. Del Giudice, V. Della Bianca, S. Dusi, C. Laudanna, F. Tonello, D. Kelleher, R. Rappuoli, C. Montecucco, and F. Rossi. 2000. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 191:1467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sontag, S. J. 1997. Guilty as charged: bugs and drugs in gastric ulcer. Am. J. Gastroenterol. 92:1255-1261. [PubMed] [Google Scholar]
- 34.Sutton, P.2001. Progress in vaccination against Helicobacter pylori. Vaccine 19:2286-2290. [DOI] [PubMed] [Google Scholar]
- 35.Svennerholm, A.-M., and M. Quiding-Järbrink. 2003. Priming and expression of immune responses in the gastric mucosa. Microbes Infect. 5:731-739. [DOI] [PubMed] [Google Scholar]
- 36.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R. J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 37.Vajdy, M., M. Singh, M. Ugozzoli, M. Briones, E. Soenawan, L. Cuadra, J. Kazzaz, P. Ruggiero, S. Peppoloni, F. Norelli, G. Del Giudice, and D. O'Hagan. 2003. Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal followed by systemic immunizations. Immunology 110:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe, T., M. Tada, H. Nagai, S. Sasaki, and M. Nakao. 1998. Helicobacter pylori induces gastric cancer in Mongolian gerbils. Gastroenterology 115:642-648. [DOI] [PubMed] [Google Scholar]
- 39.Weltzin, R., H. Kleanthous, F. Guirakhoo, T. P. Monath, and C. K. Lee. 1997. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine 15:370-376. [DOI] [PubMed] [Google Scholar]