Abstract
Collateral circulation and associated potential compensation in downstream perfusion have been recognized long before arterial occlusions were known to cause ischemic stroke. Arterial aspects and the venous capacity of collaterals to offset potentially devastating effects of blocking a cerebral artery have been studied in various animal species and even human populations with stroke, providing a framework for translational research. The time has come for collaterals to move from the periphery to a central position in stroke therapeutics, propelled by the momentum of imaging data and culminating in novel paradigms with respect to time, imaging approaches and treatment strategies. It is time for a concerted focus on collateral perfusion to harness potential therapeutic advances from acute stroke to chronic cerebrovascular disorders.
Keywords: endovascular, imaging, ischemia, stroke, thrombectomy, thrombolysis
Collateral circulation and associated potential compensation in downstream perfusion have been recognized long before arterial occlusions were known to cause ischemic stroke. Extensive anatomical and subsequent functional studies detailed the influential role of this evolutionary counterpart to normal flow in the brain. Arterial aspects and the venous capacity of collaterals to offset potentially devastating effects of blocking a cerebral artery have been studied in various animal species and even human populations with stroke, providing a framework for translational research. Recent advances in imaging of thrombolysis and endovascular thrombectomy have also consistently established that collateral perfusion is an incredibly potent prognostic factor. Collaterals enhance recanalization, improve downstream reperfusion, reduce the extent of infarct core and ischemic lesion growth, decrease hemorrhagic transformation, and improve outcome with thrombolysis or endovascular revascularization (1,2). Collaterals also radically decrease the risk of recurrent stroke in traditionally defined chronic disorders such as intracranial atherosclerosis and moyamoya, linking mechanisms of diverse cerebrovascular disorders prevalent across the globe. Indeed, vast extent of knowledge on collaterals has been culled from these ischemic disorders, yet such information is routinely sidelined in therapeutic decisions with overzealous attention placed on arterial revascularization techniques for clot disruption and plaque alteration. The time has come for collaterals to move from the periphery to a central position in stroke therapeutics, propelled by the momentum of imaging data and culminating in novel paradigms with respect to time, imaging approaches, and treatment strategies.
Collaterals determine time from vascular occlusion to symptom onset. Robust collateral perfusion may even provide eternal protection from blocked arteries in the brain, defining asymptomatic individuals that evade medical attention. It is time to abandon strict definitions of minutes on the clock shortly after stroke onset and the artificial separation of acute from chronic disorders now that routine imaging can gauge the extent of collateral perfusion. Even with the relatively simplistic selection of thrombolytic candidates based on collateral sparing on noncontrast computed tomography (CT), traditional time windows were extended from 3 to 4·5 h after symptoms became manifest (3). Collaterals are clearly an element in the equation that defines stroke severity with respect to time. Furthermore, the effect of time on ischemic injury cannot simply adjust for stroke severity as this flawed approach ignores the pivotal influence of collaterals. Future paradigms for acute stroke should increase focus on those cases with vigorous collaterals that afford minor stroke symptoms or fluctuating patterns and perhaps alter strategies with malignant profiles where collaterals have failed. Early into the subacute phase after stroke, risk also diminishes asymptotically, likely influenced by collateral perfusion. Collaterals exhibited a dramatic effect on outcome of intracranial stenosis in the first 30 days in the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial and up to five-years later in Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) (4).
The dynamic nature of collateral perfusion may be incompletely characterized with current imaging approaches that ignore temporal features. Snapshots of the brain and vascular status are inferior to serial imaging studies. Such isolated depictions at a single time point, however, also contain footprints of evolving ischemia that may be captured with novel postprocessing techniques. For instance, changes in collateral perfusion may be predicted from the topography embedded within current perfusion imaging techniques (Fig. 1). The use of thresholds with even the most sophisticated techniques such as positron emission tomography (PET) may not capture the daily or hourly fluctuations of ischemia, as with the symptomatic lesions of the Carotid Occlusion Surgery Study (COSS) trial (5). Imaging analyses of collateral perfusion patterns may also have to consider the underlying mechanism of arterial occlusion, as patterns may vary from cardio embolism to intracranial atherosclerosis (6). Thresholded volumes of hypoperfusion on Tmax may not be as informative as voxel-based measures that depict the heterogeneity of penumbra (7). A more effective strategy may also be aimed at understanding the sources of penumbral flow, or collateral perfusion. Such research may target causes of collateral failure and the process of collateralization or arteriogenesis. This vibrant process of growing collaterals is driven by elevated fluid shear stress, reaching homeostasis once collaterals have dilated. These studies will also provide insight on regulation of fluid shear stress across intracranial stenoses, expanding our understanding of hemodynamic factors paramount to a single measure in the degree of luminal stenosis.
Fig. 1.
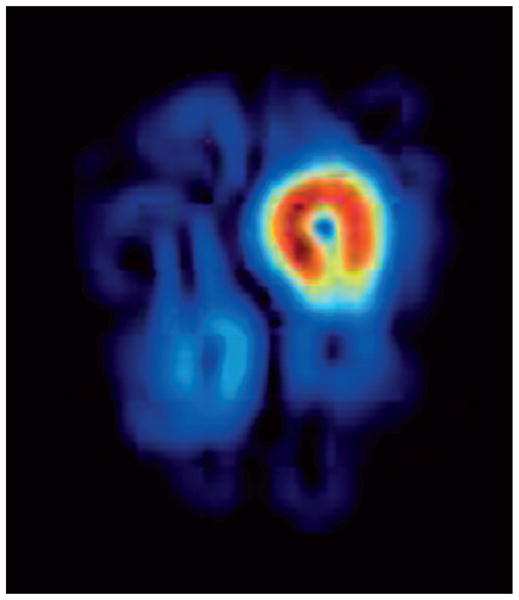
Cerebral blood volume gradient map illustrating regional variations in this critical perfusion parameter during acute stroke because of left middle cerebral artery occlusion. The circumscribed area of increased gradients around the core offers insight on the potential expansion of ischemic injury into the surrounding penumbra during ensuing hours.
Collateral therapeutics will depend on such future studies, providing alternatives beyond the currently limited portfolio of arterial revascularization options (8). Adjunctive hemodynamic interventions may be warranted when arterial revascularization may be limited or too risky because of hemorrhagic transformation. Poor collateral perfusion followed by hyper-perfusion after opening an artery may precipitate bleeding. When a malignant profile is identified, curtailed arterial revascularization may be augmented by collateral therapeutics. In other cases, an optimal balance between reperfusion and collateral augmentation may also be identified. More thorough understanding of limited reperfusion after recanalization must incorporate downstream elements that influence no reflow such as increased resistance, cerebral venous steal, and interventions that augment venous capacity or diastolic flow. Beyond the acute phase, the contribution of hemodynamics to optimal medical therapy deserves study, and the arteriogenic potential of statins should be investigated. In sum, collateral therapeutics for the brain must catch up to the pace of translational research on collaterals in the heart, from genetics to tailored interventions.
Collaterals harbor vast potential for translational research in stroke, offering perspective that extends beyond our currently limited understanding of time, imaging, and arterial recanalization strategies. Realization of such advances, however, will require significant paradigm shifts rather than pursuing the same approaches to date. It is time for a concerted focus on collateral perfusion to harness potential therapeutic advances from acute stroke to chronic cerebrovascular disorders.
Acknowledgments
Funding: The primary author was supported by the National Institute of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) (K23NS054084, P50NS044378, K24NS072272), during this work.
Footnotes
Conflict of interest: None declared.
References
- 1.Bang OY, Saver JL, Kim SJ, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–9. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 2.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–9. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4·5 h after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–74. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO, Adams HP, Jr, Derdeyn CP. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA. 2011;306:1983–92. doi: 10.1001/jama.2011.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SJ, Seok JM, Bang OY, et al. MR mismatch profiles in patients with intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. J Cereb Blood Flow Metab. 2009;29:1138–45. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31:1836–51. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebeskind DS. Collateral therapeutics for cerebral ischemia. Expert Rev Neurother. 2004;4:255–65. doi: 10.1586/14737175.4.2.255. [DOI] [PubMed] [Google Scholar]


