Abstract
Background
A new species of phlebotomine sand flies belonging to Trichophoromyia Barretto, 1962 genus is described, based on males collected in Jaú National Park, Amazonas state, Brazil.
Methods
The Sand flies were mounted in Canada balsam. They were measured with a binocular Olympus CH-2 microscope with the aid of a micrometer objective and the drawings were done with the help of a camera lucida.
Results
This new species named Trichophoromyia uniniensis sp. nov. is closely related to Trichophoromyia omagua (Martins, Llanos & Silva, 1976). The former can be distinguished from the latter by the shape of its paramere that has the lower apical region turned up in the new species.
Conclusion
With the new species here described a total of 39 species belonging to the Trichophoromyia genus are now known, most of them present in the Amazon rainforest.
Keywords: Trichophoromyia uniniensis sp. nov, Sand fly, Jaú National Park, Leishmaniases
Background
Sand flies are natural vectors of some disease agents, especially those of the leishmaniases, affecting many thousands of people worldwide [1]. The taxonomy of phlebotomines is complex due to the diversity of their morphological structures and the small differences between the species that permit precise identification. Other problems related to the taxonomy of this group are the complexes of species, morphological variations and anomalies [2–5]. Despite these difficulties, some species of sand flies have been described recently [6–13].
The genus Trichophoromyia is a large group of species, found mainly in rain forest [14]. Females of several species Trichophoromyia are morphologically similar and many species are known only by the males [15]. The medical importance of this genus is little understood, but Trichophoromyia ubiquitalis (Mangabeira, 1942) has been incriminated as a vector of Leishmania (Viannia) lainsoni Silveira, Shaw, Braga & Ishikawa, 1987 by Lainson et al. [16] and more recently Trichophoromyia auraensis (Mangabeira) has been incriminated as vector of both L. lainsoni and L. braziliensis Vianna [17].
A new species of sand flies, named Trichophoromyia uniniensis sp. nov., collected in Jaú National Park, Amazonas State, Brazil is described here.
Methods
The Jaú National Park (JNP) is the largest continuous area of protected tropical rain forest in the world (2.27 million hectares). It is situated on the right bank of the Negro river, 200 km northwest of Manaus, the capital of the state of Amazonas, Northwestern Brazil (1°40’ 3°00’S, 61°26’ 64°00’W). The average annual temperature is between 26°C and 27°C, with an average annual rainfall of 2,000-2,250 mm; most of it occurring between December and April. The local population is grouped into 15 riverine communities, 9 along the Unini river (559 people, 116 dwellings) and 6 along the Jaú river (218 people, 41 dwellings).
Phlebotomine sand flies were collected during six surveys conducted between February 2009 and September 2010 in communities of JNP. CDC light traps were installed 1 meter above ground level, between 6.00 pm and 6.00 am and manual aspiration was undertaken with a Castro aspirator at the foot of trees and on other surfaces in the morning and at night.
The sand flies were mounted in Canada balsam. They were measured with a binocular Olympus CH-2 microscope with the aid of a micrometer objective and the drawings were done with the help of a camera lucida. The measurements are given in micrometers. The classification is that proposed by Galati [14].
In accordance with section 8.5 of the ICZN's International Code of Zoological Nomenclature, details of the new species have been submitted to ZooBank with the life science identifier (LSID) zoobank.org/References/A1273A26-8EF9-49CF-9309-35B83DD53E64.
The description of Trichophoromyia uniniensis nov. sp. is based on eight males. After the measurements of the holotype male, we give, in brackets, the mean, standard deviations and number of paratypes examined for each structure.
Description
Trichophoromyia uniniensissp. nov. Ladeia-Andrade, Fé, Sanguinette & Andrade Filho (Figures 1, 2, 3, 4and 5)
Figure 1.
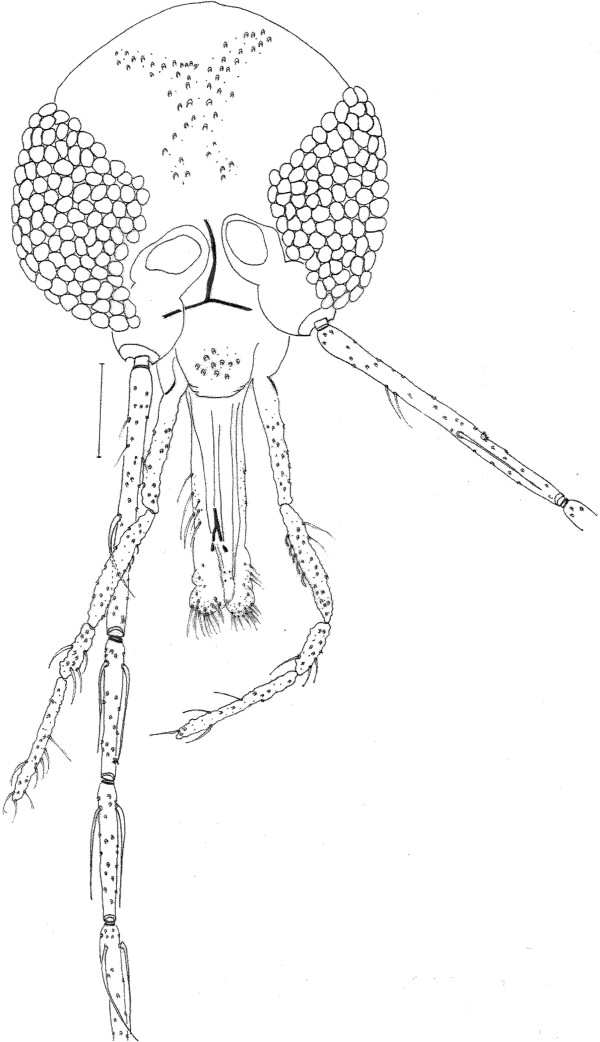
Trichophoromyia uniniensis sp. nov. (Holotype male N° 90,072). Head, frontal view. Bar = 100 μm.
Figure 2.
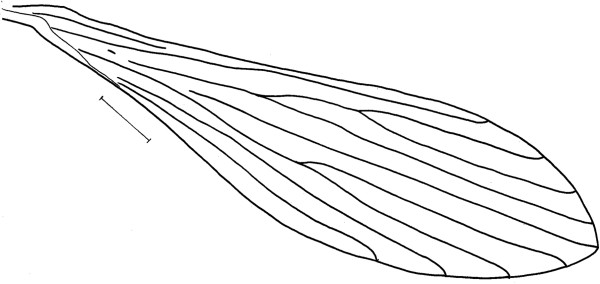
Trichophoromyia uniniensis sp. nov. (Holotype male N° 90,072). Wing. Bar = 250 μm.
Figure 3.
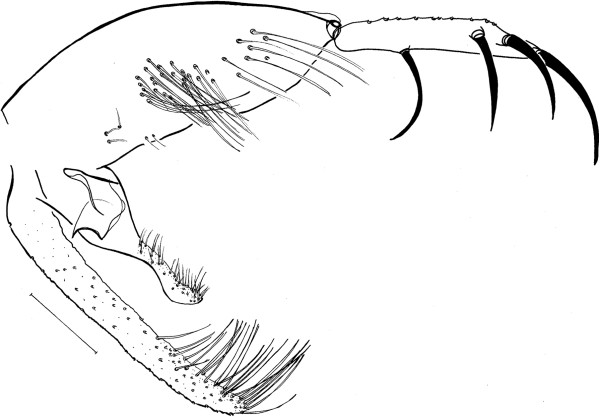
Trichophoromyia uniniensis sp. nov. (Holotype N° 90,072). Terminalia. Bar = 100 μm.
Figure 4.
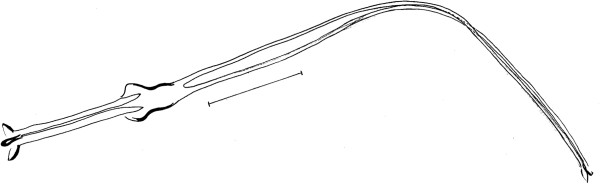
Trichophoromyia uniniensis sp. nov. (Holotype male N° 90,072). Genital pump and filaments. Bar = 100 μm.
Figure 5.
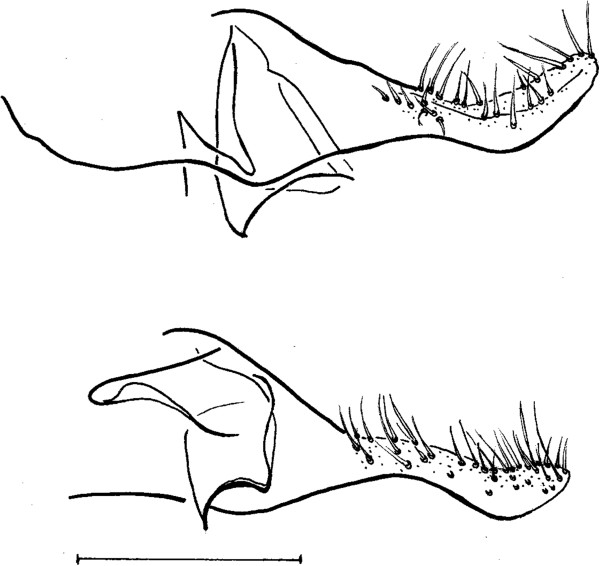
Trichophoromyia omagua (above) (from Martins et al. [19]); Trichophoromyia uniniensis (below) sp. nov. (Holotype male N° 90,072). Paramere. Bar = 100 μm.
Holotype (male) Sand fly of small size, ca. 2,774 (2,994 ± 272.6; n = 7) in length. Scutum and paratergite light brown, contrasting with pale scutellum and pleura.
Head (Figure 1)
552 (548 ± 6.8; n = 7) long and 345 (353 ± 11.0; n = 7) wide. Head length/head width ratio 1.60: 1 (1.53 ± 0.04; n = 7). Clypeus 82 (82 ± 3.5; n = 7) long; clypeus length/head length ratio 0.15: 1 (0.15 ± 0.01; n = 7). Eye 238 (230 ± 5.0; n = 7) long and 126 (123 ± 13.3; n = 7) wide; eye length/head length 0.43: 1 (0.42: 1 ± 0.00; n = 7). Interocular distance 112 (122 ± 7.0; n = 7). Labrum-epipharynx (LE) 201 (198 ± 3.3; n = 7). LE/head length 0.36: 1 (0.36 ± 0.01; n = 7). Antenna with simple and long ascoid, reaching the basis of the next flagellomere. Ascoids on AIII implanted at the same level. Antennal formula 2/III-XIII. Antennomere lengths: AIII 255 (260 ± 7.5; n = 6); AIV 129 (130 ± 6.4; n = 6); AV 126 (126 ± 3.1; n = 6); AXV > AXVI (AXV > AXVI; n = 4). Papilla present on AIII (pre-apical), AIV, AXIV-AXVI. Ratios: AIII/head length 0.46: 1 (0.48 ± 0.01; n = 6); AIII/LE 1.27: 1 (1.32 ± 0.04; n = 6). Palpomere lengths: P1 34 (31 ± 1.1; n = 7); P2 85 (90 ± 4.9; n = 7); P3 112 (119 ± 2.3; n = 6); P4 51 (50 ± 1.1; n = 5); and 119 (119 ± 2.4; n = 4). Palpal formula 1.4.2.3.5. [(1.4.2.3.5.; n = 1; 1.4.2.5.3.; n = 1; 1.4.2.(3.5.); n = 2]. Newstead's spines inserted medially on palpomere 3 and not visible on palpomere 2.
Cervix
Ventrocervical sensillae absent.
Thorax
Proepimeral setae 3–3 (3–3; n = 2; 4–4; n = 2) and anepisternal superior setae present, 18–18, in the paratypes ranging from 14 to 18, setae in the anterior region of the katepisternum absent. Wing (Figure 2) 1,863 (1,977 ± 44.6; n = 7) long and 524 (530 ± 12.5; n = 5) at maximum width. Length/width ratio 3.56: 1 (3.60: 1 ± 0.3; n = 5). Length of the vein sections: R5 1,325 (1,306 ± 21.1; n = 6); alpha 607 (568 ± 26.9; n = 6); beta 235 (270 ± 15.5; n = 7); gamma 248 (251 ± 13.3; n = 6); delta 400 (352 ± 22.7; n = 6). The anterior and posterior legs of the hollotype were lost. Anterior femur, tibia, tarsomere I and tarsomeres II + III + IV + V 773 ± 11.4 (n = 4), 1,007 ± 19.3 (n = 4), 645 ± 20.5 (n = 4) and 683 ± 23.8 (n = 4), respectively. Femur, tibia, tarsomere I and tarsomeres II + III + IV + V of the median leg measurement: 718 (725 ± 7.5; n = 4), 1,270 (1,256 ± 19.3; n = 4), 745 (735 ± 30.6; n = 4) and 731 (718 ± 11.0; n = 4), respectively. The mean and standard deviation of the paratypes for femur, tibia, tarsomere I and tarsomeres II + III + IV + V of the posterior leg were 798 ± 10.1 (n = 6), 1,424 ± 10.2 (n = 6), 840 ± 16.4 (n = 6) and 791 ± 14.0 (n = 6), respectively.
Abdomen (Figures 3, 4 and 5)
Papillae absent on abdominal tergites. Gonostyle 197 (188 ± 2.3; n = 7) long, with four spines: one apical, one upper external, one lower external and one internal implanted in the basal third. Sub terminal seta absent. Gonocoxite 330 (326 ± 4.9; n = 7) long and 116 (123 ± 8.9; n = 7) wide, about 33–35 (32–37 in paratypes) setae implanted in the middle of the structure. Paramere broad at its base, narrowing in its middle region and dilating at its apex. Distal third with ventral elbow and dorsal margin with a slight curve upwards (Figure 5). Lateral lobe 306 (300 ± 6.0; n = 7) long and 34 (29 ± 2.9; n = 7) wide, without persistent setae at its apex. Lateral lobe/gonocoxite ratio 0.93: 1 (0.90 ± 0.02; n = 7). Conical and pigmented aedeagus. Genital filament 428 (420 ± 15.2; n = 7) long and genital pump 160 (169 ± 8.0; n = 7). Genital filament/genital pump ratio 2.68: 1 (2.59 ± 0.25; n = 7). Apex of genital filaments with slight dilatation.
Female
Unknown
Type-material
Male holotype and all male paratypes were collected in the Jaú National Park, near the Unine river, Municipality of Barcelos, Amazonas state, Brazil with CDC light traps (Ladeia-Andrade S, Fé NF, et al. Col.). Holotype (N. 90,072) collected on 07/02/2009 in the Vila Nunes community; two males paratypes (N. 90,073 and 90,074) collected on 30/05/2009 in the Vista Alegre community; two paratypes male (N. 90,075 and 90,076) collected on 07/02/2009, in the Vila Nunes community; three paratypes male (N. 90,077, 90,078 and 90,079) collected on 06/02/2009 in the Vista Alegre community.
Holotype (90,072) and three paratypes (N. 90,073, 90,075 and 90,077) deposited in the "Coleção de Flebotomíneos" of the "Instituto René Rachou/FIOCRUZ" (FIOCRUZ-COLFLEB), Belo Horizonte, Brazil. Two paratypes (N. 90,074 and 90,078) deposited in the collection of "Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Brazil. Two paratypes (90,076 and 90,079) deposited in the collection of "Instituto Oswaldo Cruz/FIOCRUZ, Rio de Janeiro, Brazil.
Etymology
The name Trichophoromyia uniniensis sp. nov. alludes to the Unini river, located in the Jaú National Park, the type locality of this species.
Results and discussion
To date, 38 species have been formally described for the genus Trichophoromyia [13, 14, 18] which can be divided into two groups, based on the ratio between the genital filaments/genital pump. Some species have this ratio lower than 3 and others greater than 4. The new species, with a ratio of 2.68: 1 (2.59 ± 0.25; n = 7) is included in the first group. In Trichophoromyia genus, only four species have this characteristic: Trichophoromyia meirai (Causey & Damasceno, 1945), Trichophoromyia omagua (Martins, Llanos & Silva, [19]), Trichophoromyia reburra (Fairchild & Hertig, 1961) and T. ubiquitalis. Trichophoromyia meirai present the lateral lobe longer than the gonocoxite, while in the new species the opposite occurs. The paramere can be used to distinguish T. ubiquitalis and T. reburra from T. uniniensis. This structure is slightly curved towards the gonocoxite in the new species and straight in the others species. Trichophoromyia omagua also presents the curved paramere [19]. However, this species can be distinguished from the T. uniniensis n. sp. by the shape of the paramere (see Figure 5). The dorsal margin of the paramere in T. omagua is concave while in the new species in its median it is slightly convex, followed by a small pre-apical concavity. Further, the elbow in the ventral region of T. omagua is closer to the middle of the paramere while in the new species it is situated near its fourth apical region.
Only 22 species of Trichophoromyia are described, based on both sexes. This because females of several species present similar morphology. [15]. In fact, of the 22 species whose females have been described, only those of T. reburra, Trichophoromyia cellulana (Young & Duncan, 1979), T. omagua and T. ubiquitalis can be differentiated morphologically [14]. Some females of Trichophoromyia were collected in the Jaú National Park, however, we prefer not to describe them as females of Trichophoromyia uniniensis since other species of Trichophoromyia were also collected in sympatry with the new species.
Conclusion
With the new species here described a total of 39 species belonging to the Trichophoromyia genus are now known, most of them present in the Amazon rainforest.
Acknowledgments
The authors wish to thank the inhabitants of the Jaú National Park for granting then access to their communities; Chico Mendes Institute for Biodiversity Conservation (ICMBio/SISBio) for granting access of the research team to Jaú National Park; the Health Department of Barcelos and the malaria control team of Barcelos for help with fieldwork; Rui Alves de Freitas and Artêmio Coelho da Silva of Instituto Nacional de Pesquisas da Amazônia, for their valiable assistence; Dr Eunice Aparecida Bianchi Galati and Dr Andrey José de Andrade of the Faculty of Public Health (University of São Paulo) for their valuable orientation and advice. JD Andrade Filho is a research fellow of CNPq.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SLA, NFF, CCS and JDAF participated in the morphological analysis of the specimens and taxonomic discussion. CCS and JDAF did the drawings and measurements of the new species. SLA, NFF CCS JDAF drafted the manuscript. All the authors read and agreed with this manuscript.
Contributor Information
Simone Ladeia-Andrade, Email: sladeia@ioc.fiocruz.br.
Nelson Ferreira Fé, Email: nelson@fmt.am.gov.br.
Cristiani de Castilho Sanguinette, Email: cristiani@cpqrr.fiocruz.br.
José Dilermando Andrade Filho, Email: jandrade@cpqrr.fiocruz.br.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;5:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanzaro GC, Ostrovska K, Herrero MV, Lawyer PG, Warburg A. Lutzomyia longipalpis is a species complex: genetic divergence and interspecific hybrid sterility among three populations. Am J Trop Med Hyg. 1993;48:839–847. doi: 10.4269/ajtmh.1993.48.839. [DOI] [PubMed] [Google Scholar]
- 3.Costa PL, Silva FJ, Andrade Filho JD, Shaw JJ, Brandão Filho SP. Bilateral anomaly in Evandromyia evandroi (Diptera: Psychodidae: Phlebotominae) captured in Vicência Municipality, Northern Rainforest Region of Pernambuco State, Brazil. J Am Mosq Cont Assoc. 2012;28:128–130. doi: 10.2987/11-6218R.1. [DOI] [PubMed] [Google Scholar]
- 4.Scarpassa VM, Alencar RB. Lutzomyia umbratilis, the main vector of Leishmania guyanensis, represents a novel species complex? PloS One. 2012;7:e37341. doi: 10.1371/journal.pone.0037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanguinette CC, Faustino JX, Serra e Meira PCL, Botelho HA, Carvalho GML, Gontijo CF, Andrade Filho JD. Anomalies in the sand fly Lutzomyia longipalpis (Lutz and Neiva), the main vector of Leishmania infantum chagasi in Brazil. J Am Mosq Cont Assoc. 2013;2013(29):54–58. doi: 10.2987/12-6290R.1. [DOI] [PubMed] [Google Scholar]
- 6.Andrade AJ, Galati EAB. A new species of Evandromyia (Diptera: Psychodidae: Phlebotominae) from Minas Gerais State, Brazil. J Med Entomol. 2012;49:445–450. doi: 10.1603/ME11128. [DOI] [PubMed] [Google Scholar]
- 7.Barata RA, Meira PCL S e, Carvalho GML. Lutzomyia diamantinensis sp. nov., a new phlebotomine species (diptera: Psychodidae) from a quartzite cave in Diamantina, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2012;107:1006–1010. doi: 10.1590/S0074-02762012000800007. [DOI] [PubMed] [Google Scholar]
- 8.Carrasquilla MC, Munstermann L, Marín D, Ocampo C, Ferro C. Description of Lutzomyia (Helcocyrtomyia) tolimensis, a new species of phlebotomine sandfly (Diptera: Psychodidae) from Colombia. Mem Inst Oswaldo Cruz. 2012;107:993–997. doi: 10.1590/S0074-02762012000800005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galati EAB, Galvis-Ovallos F. Description of two new sand fly species related to Nyssomyia antunesi (Diptera, Psychodidae, Phlebotominae) J Med Entomol. 2012;49:238–252. doi: 10.1603/ME11118. [DOI] [PubMed] [Google Scholar]
- 10.Figueira EAG, Silva G, Chagas ECS, Shimabukuro PHF. Phlebotomine sandflies (Diptera: Psychodidae) from Lábrea, state of Amazonas, Brazil, with a description of Evandromyia (Aldamyia) apurinan Shimabukuro, Figueira & Silva, sp. nov. Mem Inst Oswaldo Cruz. 2013;108:208–287. doi: 10.1590/S0074-02762013000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teles CBG, Freitas RA, Oliveira AFJ, Ogawa GM, Araújo EAC, Medeiros JF, Pessoa FAC, Camargo LMA. Description of a new phlebotomine species (Diptera: Psychodidae, Phlebotominae) and new records of sand flies from the State of Acre, northern Brazil. Zootaxa. 2013;3609:85–90. doi: 10.11646/zootaxa.3609.1.6. [DOI] [PubMed] [Google Scholar]
- 12.Sábio PB, Andrade AJ, Galati EAB. Assessment of the taxonomic status of some species included in the Shannoni complex, with the description of a new species of Psathyromyia (Diptera: Psychodidae: Phlebotominae) J Med Entomol. 2014;51:331–341. doi: 10.1603/ME13153. [DOI] [PubMed] [Google Scholar]
- 13.Santos TV, Silva FMM, Barata IR, Andrade AJ, Galati EAB. A new species of phlebotomine, Trichophoromyia adelsonsouzai (Diptera: Psychodidae) of Brazilian Amazonia. Mem Inst Oswaldo Cruz. 2014;109:140–146. doi: 10.1590/0074-0276130159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galati EAB. Morfologia e Taxonomia. Classificação de Phlebotominae. In: Rangel RF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Fiocruz; 2003. pp. 23–206. [Google Scholar]
- 15.Young DG, Duncan MA. Guide to the Identification and Geographic Distribution of Lutzomyia Sand Flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) Mem Am Entomol Inst. 1994;54:1–881. [Google Scholar]
- 16.Lainson R, Shaw JJ, Souza AAA, Silveira FT, Falqueto A. Further observations on Lutzomyia ubiquitalis (Psychodidae: Phlebotominae), the sand fly vector of Leishmania (Viannia) lainsoni. Mem Inst Oswaldo Cruz. 1992;87:437–439. doi: 10.1590/S0074-02761992000300016. [DOI] [PubMed] [Google Scholar]
- 17.Valdivia HO, De Los Santos MB, Fernandez R, Baldeviano GC, Zorrilla VO, Vera H, Lucas CM, Edgel KA, Lescano AG, Mundal KD, Graf PC. Natural Leishmania infection of Lutzomyia auraensis in Madre de Dios, Peru, detected by a fluorescence resonance energy transfer-based real-time polymerase chain reaction. Am J Trop Med Hyg. 2012;87:511–517. doi: 10.4269/ajtmh.2012.11-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barreto M, Burbano ME, Young DG. Description of Lutzomyia (Trichophoromyia) pabloi n. sp. and the female of L. howardi (Diptera: Psychodidae) from Colombia. J Med Entomol. 2002;39:601–604. doi: 10.1603/0022-2585-39.4.601. [DOI] [PubMed] [Google Scholar]
- 19.Martins AV, Llanos BZ, Silva JE. Estudos sôbre os flebotomíneos do Peru. IV. Departamento de Loreto: lista das espécies coletadas, descrição de uma espécie nova, Lutzomyia omagua n. sp. e redescrição do macho de Lutzomyia scaffi (Damasceno & Arouck, 1956) (Diptera, Psychodidae, Phlebotominae) Rev Brasil Biol. 1976;36:495–501. [Google Scholar]


