Abstract
Clostridium sordellii lethal toxin (LT), a 250-kDa protein which is the bacteria's major virulence factor, belongs to a family of large clostridial cytotoxins which glucosylate small GTP-binding proteins. Here, we report the results of our ex vivo analysis of the structure and function of skeletal neuromuscular tissue obtained from mice at various times after intramuscular injection of a sublethal dose of LT (0.25 ng/g of body wt). The toxin caused, within 24 h, pronounced localized edema, inflammation, myofibril disassembly, and degeneration of skeletal muscle fibers in the injected area, and it glucosylated the muscle tissue's small GTPases. Regeneration of the damaged fibers was evident 6 to 9 days postinjury and was completed by 60 days. The expression of dystrophin, laminin, and fast and neonatal myosin in regenerating fibers, detected by immunofluorescence microscopy, confirmed that LT does not impair the high regenerative capacity of murine skeletal muscle fibers. Functional studies revealed that LT affects muscle contractility and neuromuscular transmission. However, partial recovery of nerve-evoked muscle twitches and tetanic contractions was observed by day 15 postinjection, and extensive remodeling of the neuromuscular junction's nerve terminals and clusters of muscle acetylcholine receptors was still evident 30 days postinjection. In conclusion, to the best of our knowledge, this is the first report to characterize the degeneration and regeneration of skeletal neuromuscular tissue after in vivo exposure to a large clostridial cytotoxin. In addition, our data may provide an explanation for the severe neuromuscular alterations accompanying wound infections caused by C. sordellii.
The genus Clostridium includes anaerobic species that cause soft tissue-necrotizing infections (i.e., cellulitis and gas gangrene) and toxic shock by virtue of their ability to produce numerous tissue-damaging enzymes and protein toxins (37, 38, 41). Infections with Clostridium sordellii often result in toxic shock and multiorgan failure (1, 4) because of the toxin-induced necrosis of various cell types. The bacteria's major virulence factor is a high-molecular-weight (Mr, ca. 250,000) lethal toxin (LT) that belongs to a family of large clostridial cytotoxins (5, 6, 23) and is antigenically related to toxin B of Clostridium difficile (28, 32), a major cause of pseudomembranous colitis after oral clindamycin therapy (14, 21).
The C. sordellii LT is a glucosyltransferase which uses a UDP-glucose cofactor (9) to glucosylate and inactivate small-molecular-mass, GTP-binding proteins (9, 22), i.e., the Ras superfamily (33). The toxin also inhibits exocytosis in chromaffin cells (15) and neurotransmitter release when injected into presynaptic neurons at identified synapses of the Aplysia central nervous system, with a potency comparable to that of the Clostridium botulinum neurotoxins (11). The observed inhibition of neurotransmitter release has been proposed (19) to be caused by the inactivation of the Rac GTPase associated with synaptic vesicles in nerve terminals. Interestingly, LT has been reported (12) to inhibit phospholipase D1, and the introduction of inactive phospholipase D1 into neurons has recently been found (20) to reproduce the exocytosis blockade caused by the toxin.
The present ex vivo study was designed to characterize the effects of the C. sordellii LT on skeletal muscle and neuromuscular transmission in order to improve our understanding of its mode of action. To the best of our knowledge, this is the first published study to describe the degeneration and regeneration of skeletal muscle tissue and the remodeling of the neuromuscular junction after in vivo exposure to a potent clostridial cytotoxin.
MATERIALS AND METHODS
C. sordellii LT, antibodies, and reagents.
C. sordellii LT produced by strain IP82 was purified as previously described (32). Our immunofluorescence studies used monoclonal antibodies directed against (i) the C-terminal domain of dystrophin (1:500 dilution; Novocastra laboratories Ltd., Newcastle upon Tyne, United Kingdom), (ii) sarcomeric α-actinin (1:25 dilution; Sigma, Saint-Quentin Fallavier, France), (iii) the heavy chain of fast myosin (1:10 dilution; Novocastra), (iv) troponin T (clone JLT-12; 1:100 dilution; Sigma), and (v) the 160-kDa isoform of neurofilaments (1:75 dilution; Sigma). Rabbit polyclonal antibodies against neonatal-myosin (1:50 dilution) were kindly provided by C. Cifuentes-Diaz (Laboratoire de Neurogenetique Moleculaire, INSERM, Evry, France), and antibodies directed against laminin (1:500 dilution) were purchased from Sigma. Alexa 488- and Alexa 633-conjugated anti-mouse immunoglobulin G (1:1,500 and 1:1,000 dilutions, respectively), Alexa 488-conjugated anti-rabbit immunoglobulin G (1:1,000 dilution), fluorescein isothiocyanate (FITC)- and tetramethylrhodamine isothiocyanate (TRITC)-conjugated α-bungarotoxin (α-BTX; 1:1,000 dilution), and TOTO-3 dye (1:500 dilution) were from Molecular Probes Europe BV, (Leiden, The Netherlands). TRITC-conjugated fasciculin-2 (1:500 dilution; in phosphate-buffered saline [PBS]) was prepared by using the FluorReporter protein labeling kit (Molecular Probes) and was kindly provided by Eric Krejci (Ecole Normale Supérieure, Paris, France). Paraformaldehyde, Procion Orange, and Evans blue dye were from Sigma. Antifading mounting media (Vectashield and Vectashield with 4′,6′-diamidino-2-phenylindole [DAPI]) were from Vector Laboratories Inc. (Burlingame, Calif.). Other chemicals were of the highest purity available.
Injection of mice with C. sordellii LT.
All experiments were performed in accordance with French and European Community guidelines for laboratory animal handling. The anterolateral region of the left hindlimb or the immediate vicinity of the levator auris longus (LAL) muscle (3) of 62 adult female Swiss-Webster mice (20 to 25 g) lightly anesthetized with ether was injected intramuscularly with C. sordellii LT (0.25 ng/g body weight; in 50 μl of PBS), and control mice were injected with PBS. Several doses of the toxin initially were tested, and we chose the above-mentioned dose for the studies described in this report because it was not lethal for mice and because its local effects were reproducible and moderate. Control mice were similarly injected with PBS. The sublethal dose of LT injected intramuscularly produced pronounced localized edema and inflammation in the area of injection but little or no disturbance in the general condition of the animals. Food and water were provided ad libitum, and the animals were housed in the animal care facility, under standard conditions at a constant temperature of 22°C with a cycle of 12 h of darkness and 12 h of daylight. At various times postinjection, mice were killed by dislocation of their cervical vertebrae, and the tibialis anterior (TA) and extensor digitorum longus (EDL) muscles were removed from the injected legs, together with their associated motor nerves. A similar procedure was used for ex vivo analysis of the actions of LT on the LAL muscles.
Detection of in vivo glucosylation of small GTPases in skeletal muscle tissue.
Control and LT-injected EDL muscles were harvested at various times postinjection, frozen with liquid nitrogen, and homogenized with a tissue grinder, and the preparations' proteins were examined for C. sordellii LT-mediated glucosylation as previously described (33). Briefly, samples of the muscle preparations were dissolved in glucosylation buffer (50 mM triethanolamine [pH 7.5], 2 mM MgCl2, 1 mM dithiothreitol, 0.3 mM GDP) containing 10 μg of leupeptin/ml, 1 μM pepstatin, 0.1 mM phenylmethylsulfonyl fluoride, and 0.5% Triton X-100. After centrifugation (1,000 × g for 5 min), the supernatant fluids were collected, and the amount of protein in each cell lysate was estimated by Coomassie blue staining (Bio-Rad, Verrières-Le-Buisson, France). Samples containing 50 μg of total protein were used for in vitro glucosylation assays with 7 μM UDP-[14C]glucose (DuPont NEN, Les Ulis, France) and 1 μg of LT in a final volume of 20 μl. After incubation (1 h at 37°C), the mixtures were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% monomer concentration), and the gel was stained, destained, dried, and analyzed for radioactivity by autoradiography. The relative intensities of the radiolabeled bands were quantified by using Densylab 2.5.2 software (Microvision Instruments, Evry, France).
Twitch tension measurements.
Isolated mouse EDL nerve-muscle preparations were mounted in Rhodorsil-lined organ baths superfused with an oxygenated standard solution containing 154 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES (pH 7.4), and 11 mM d-glucose. For mechanical recordings, one tendon of the muscle was tied with silk thread via an adjustable stainless steel hook to an FT03 isometric transducer (Grass Instruments, West Warwick, R.I.), and the other tendon was pinned to the silicone-lined chamber. Twitches were evoked by stimulating the motor nerve via a suction electrode, with supramaximal current pulses of 0.15-ms duration at 0.1 Hz or 60 Hz, or via an electrode assembly placed along the length of the muscle. The resting tension was adjusted for each preparation to obtain maximal contractile responses upon indirect- and direct-muscle stimulation. Signals from the isometric transducer were amplified, collected, and digitized with a computer equipped with a DT2821 analogue-to-digital interface board (Data Translation, Marlboro, Mass.). Computerized data acquisition and analysis were performed with a program kindly provided by John Dempster (University of Strathclyde, Glasgow, United Kingdom).
Electrophysiological recordings to measure the membrane potential of muscle fibers and synaptic potentials were made at room temperature (22 to 24°C) with intracellular microelectrodes filled with 3 M KCl (8 to 12 MΩ resistance) by using conventional techniques and an Axoclamp-2A system (Axon Instruments, Union City, Calif.). Electrical signals after amplification were collected and digitized, at a sampling rate of 25 kHz, with a computer equipped with an analogue-to-digital interface board.
Morphological and immunofluorescence experiments.
A histological examination was performed and the histochemistry of skeletal muscles was determined with unfixed tissue frozen in isopentane precooled in liquid nitrogen. Cryosections (7 to 10 μm thick) cut in the middle part of the muscle (in order to obtain the largest sectional area) were placed on slides, air dried, and stained with hematoxylin and eosin. For immunofluorescence analysis, cryosections were fixed in cold methanol (10 min at 4°C) and rinsed (10 min) with PBS (0.1 M, pH 7.4) supplemented with Triton X-100 (0.03%). After washing and incubation (30 min) in blocking solution (PBS containing 3% bovine serum albumin and 3% goat serum), the sections were incubated (2 h at room temperature) with primary antibodies diluted with blocking solution, washed three times (each washing was for 10 min) with PBS supplemented with Tween-20 (0.1%), incubated (1 h at room temperature) with fluorescent secondary antibodies, rinsed with PBS-Tween-20 (0.1%), and examined by fluorescence microscopy.
The sarcolemmal integrity of the skeletal muscle fibers was determined by harvesting the muscles from toxin-injected and control mice 12 h after the animals were injected intraperitoneally with a solution (0.1 ml/10 g body weight) of Evans blue dye (10 mg/ml of PBS), followed by examining the tissues according a method previously described (39). Sarcolemmal integrity also was determined by incubating (15 min) the muscles in standard Krebs-Ringer solution containing 1% Procion Orange, freezing them in isopentane precooled in liquid nitrogen, and examining their cryostat tissue sections by fluorescence microscopy (40). The presence of the fluorescent tracer in the cytoplasm of the cells in the cryostat sections indicated membrane damage.
Immunocytochemical studies of whole-mount preparations utilized the thin, flat LAL muscle (3). The muscle was fixed (25 min at room temperature) with 4% paraformaldehyde in 0.1 M PBS (pH 7.4), treated (2 h) with 0.5% Triton X-100 in PBS, rinsed three times for 10 min, and incubated (at 4°C overnight) with the primary antibody in a blocking solution containing 3% bovine serum albumin. After washing, the muscle was incubated (2 h at room temperature) with secondary antibodies, rinsed in PBS, and mounted on a glass slide with Vectashield antifading mounting medium. In all experiments, the specificity of the immunostaining was determined with preparations that were not incubated with the primary antibody.
Preparations were observed either with a Leica DM-RXA2 upright microscope equipped with a CDD camera (CoolSNAP FX; Princeton Scientific Instruments, Monmouth Junction, N.J.) and OpenLab software (Improvision, Conventry, United Kingdom) or with a TCS SP2 confocal laser scanning system (Leica Microsystems, Mannheim, Germany) mounted on an upright microscope and controlled through the manufacturer-supplied software and workstation. Images were collected by using either a 10× or 40× oil-immersion lens (numerical aperture, 0.75 and 1.25, respectively). The 488-nm wavelength line of an Argon-ion laser and the 543- and 633-nm wavelength lines of a HeNe laser were used for FITC, TRITC, and TOTO-3 excitation, respectively. Images were digitized into a maximal array of 1024 by 1024 pixels.
Measurement of muscle fibers.
ImageJ, a public domain image analysis software package (http://rsb.info.nih.gov/ij/download/), was used to measure the cross-sectional area of fibers in unfrozen sections stained with hematoxylin and eosin.
Statistical analysis.
Data were compared by using Student's t test, and a P value of <0.05 was considered statistically significant. Unless otherwise stated, the values in the text are expressed as means ± standard deviations (SD).
RESULTS
Degeneration and regeneration of skeletal muscle fibers after in vivo exposure to C. sordellii LT.
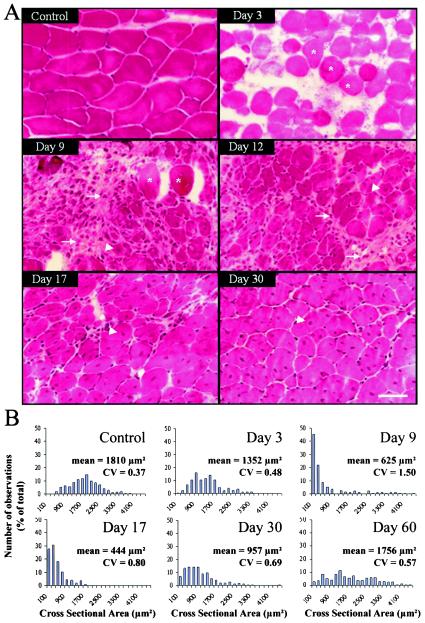
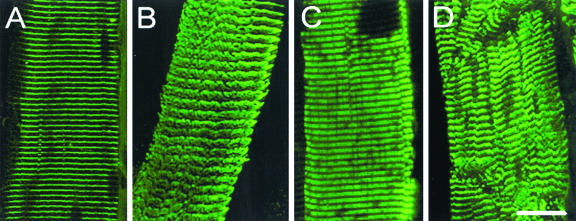
A single sublethal injection of C. sordellii LT (0.25 ng/g of body weight) into the immediate vicinity of the TA, LAL, or EDL muscles induced, within 24 h, pronounced localized edema and inflammation in the injected area, which was not observed in control animals injected with PBS. A histological examination of muscle sections prepared from the toxin-injected animals revealed degeneration and necrosis of muscle fibers (Fig. 1A). In addition, the presence of many mononuclear cells and phagocytes in the interstitial connective tissue indicated an active phagocytic process. One day after LT injection, immunostaining of the skeletal muscle fibers with α-actinin antibody revealed disorganization of the muscle Z line (Fig. 2A and B). Also, immunostaining of troponin T (a specific marker of thin filaments of the striated muscle sarcomere) indicated myofibril disassembly; that is, the well-organized parallel bands observed in control fibers (Fig. 2C) were not seen in the LT-treated fibers (Fig. 2D). These findings were confirmed by electron microscopy observation of the vacuolization of the necrotic muscle fibers associated with sarcolemmal disruption (data not shown).
FIG. 1.
Representative transverse TA muscle sections stained with hematoxylin and eosin (A) and distribution of myofiber cross-sectional area (B) from control mice and at various times after mice received a sublethal intramuscular injection of C. sordellii LT. In panel A, note the presence of only necrotic fibers (asterisks) and abundant inflammatory infiltrates (arrows) by 3 days postinjection. Regenerating small myocytes with central nuclei are evident (arrowheads) by 9 to 17 days postinjection, but necrotic fibers and connective tissue are not present, and skeletal muscles possess large regenerating fibers with central nuclei by 17 to 30 days postinjection. Muscle structure and fiber size are almost completely restored by 30 days postinjection, but myofibers still contain central nuclei. Bar = 50 μm. In panel B, the mean value and coefficient of variation (CV = SD/mean) are indicated for each distribution of myofiber cross-sectional area based on data obtained from three independent experiments (n > 250 in each histogram).
FIG. 2.
Disorganization of muscle fiber cytoskeleton observed 1 day after mice received a sublethal intramuscular injection of C. sordellii LT. Single-teased fibers from control TA muscles (panels A and C) and from LT-injected muscles (panels B and D) were immunostained with anti-α-actinin (panels A and B) and anti-troponin T (panels C and D) antibodies. Note the striated and regular parallel lines of the two immunostained muscle proteins in the control muscles (panels A and C) and the loss of the striated appearance associated with a disoriented meshwork of myofilaments in LT-injected muscles (panels B and D). Bar = 20 μm.
The beginning of regeneration of the LT-damaged skeletal muscle fibers was evident 6 to 9 days postinjection of the toxin, as demonstrated by the appearance of many small muscle cells (cross-sectional area of <500 μm2) exhibiting central nuclei (Fig. 1). During the development of the regeneration process (12 to 30 days postinjection), the number of newly formed cells grew over time and increased in diameter (Fig. 1). Interstitial fibrosis was not observed, but persistent central myonuclei were still evident 30 and 60 days after LT-induced injury. These results indicate that LT does not impair skeletal muscle regeneration.
In vivo glucosylation of skeletal muscle GTPases by C. sordellii LT.
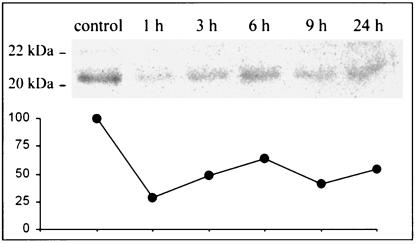
In order to monitor the toxin's possible in vivo ability to glucosylate small GTPases in skeletal muscle tissue, lysates of control and LT-injected EDL muscles were incubated in vitro with LT in the presence of UDP-[14C]glucose. The control muscle preparations (four different experiments) exhibited the radiolabeled glucosylation of proteins with a mass in the range of 20 to 22 kDa, which is in the molecular mass range for low-molecular-weight GTPases (other proteins were not labeled).
In contrast, only weak glucosylation of proteins was observed in muscles previously injected in vivo with the LT and removed 1, 3, 6, 9, and 24 h postinjection (Fig. 3). These results indicated that the C. sordellii LT glucosylated the muscle's small GTPases in vivo, thus inhibiting incorporation of [14C]glucose into those GTP-binding proteins during the subsequent in vitro incubation with the toxin.
FIG. 3.
LT injection in skeletal muscle induces GTPase glucosylation in vivo. Samples of EDL muscle tissue, harvested at various times after in vivo injection of LT and containing 50 μg of protein, were incubated with LT (50 ng/μl) and UDP-[14C]glucose, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and autoradiographed. The intensities of the bands estimated by densitometry are plotted on the graph. The value of the control was assigned to 100%. Notice the decrease in the intensities at 1 to 24 h after LT injection, indicating an in vivo modification of the LT substrates. Data shown are representative of four independent determinations made at the times indicated.
Changes in the expression of muscle proteins after in vivo exposure to C. sordellii LT.
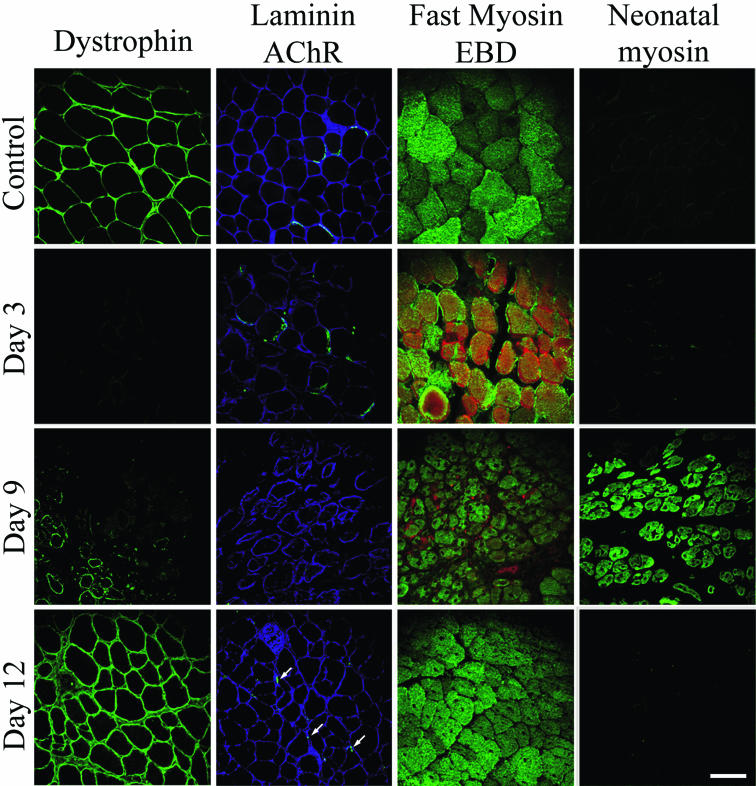
The kinetics of expression of various muscle proteins was followed by immunofluorescence microscopy during the above-described regeneration process. The proteins included (i) dystrophin, a cytoskeletal protein located in the inner face of the plasma membrane of muscle fibers, (ii) the extracellular matrix protein laminin, and (iii) fast and neonatal myosin in degenerating and regenerating skeletal muscle fiber sections.
Several observations confirmed that, under our experimental conditions, the C. sordellii LT did not impair the high regenerative capacity of murine skeletal muscle fibers. First, dystrophin immunostaining was absent on day 3 postinjection of LT but was detected in small-diameter muscle cells on day 9 postinjection (Fig. 4). The intensity of the immunostaining thereafter increased and was homogeneously distributed among the fibers by day 9 to day 12 postinjection. Second, immunolabeling of the extracellular matrix protein α-laminin, which was markedly reduced at day 3 postinjection, was faintly observed in small regenerating fibers on day 9 but was increased by day 12 postinjection. Third, although immunolabeling of nicotinic acetylcholine receptors (AChRs) with FITC-conjugated α-BTX was diffuse in necrotic fibers on day 3 postinjection and was not detected on day 9, it was observed on day 12 (Fig. 4, arrows). Fourth, immunostaining of fast myosin (only the fast myosin heavy chain isoforms are present in adult mouse TA muscles [31]) revealed homogeneous labeling of regenerating cells (characterized by their unstained central nuclei) at 9 and 12 days after the LT injection. Fifth, permeabilization of necrotic muscle fibers was detected by a bright red Evans blue dye staining of fibers during the first few days after toxin injection. However, muscle fiber permeabilization was not evident on day 9 postinjection, and staining was only observed in some intramuscular blood vessels (Fig. 4). Sixth, immunostaining to determine the distribution of neonatal myosin, which is expressed in developing but not in adult skeletal muscle tissue (7, 27), revealed regenerating muscle fibers expressing the neonatal myosin isoform on day 9 postinjection of toxin but not on day 3. At 12 days postinjection there is no abnormal persistence of neonatal myosin (Fig. 4).
FIG. 4.
Changes in protein expression patterns (revealed by immunostaining of cryosections) during degeneration and regeneration after mice received a sublethal injection of C. sordellii LT into their TA muscles. The immunolabeling of dystrophin, laminin, fast myosin, and neonatal myosin was followed at the times indicated post-LT injection. Staining of acetylcholine receptors (AChRs) was performed with FITC-conjugated α-BTX, and staining with Evans blue dye (EBD) was used to assess muscle membrane injury (red). For details, see Materials and Methods. Note the transient expression of neonatal myosin at day 9 postinjection. Bar = 50 μm.
Blockade of indirectly and directly elicited skeletal muscle contractions after in vivo exposure to C. sordellii LT.
Single and repetitive nerve stimulation of isolated EDL nerve-muscle preparations removed from control animals evoked typical twitch and tetanic contractions (Fig. 5). In contrast, EDL muscles (n = 3) isolated from animals exposed to LT exhibited about a 60% blockade of nerve-evoked muscle twitch (Fig. 5A) and tetanic responses (Fig. 5B) at 1 day postinjection, and a complete block was observed between 3 to 5 days after local LT injection (Fig. 5). In addition to the blockade of indirectly elicited muscle twitches, directly elicited twitches also were blocked in LT-treated EDL muscles (data not shown). Our results indicated that the C. sordellii LT affects muscle contractility as well as neuromuscular transmission. Thus, we subsequently performed studies to determine whether the effects we observed were a function of LT-induced changes in muscle membrane permeability.
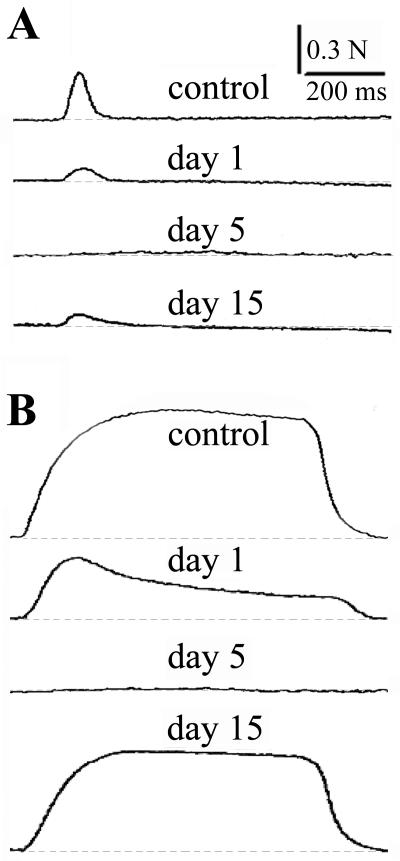
FIG. 5.
Nerve-evoked muscle twitches and tetanic responses of EDL nerve-muscle preparations isolated from controls and from mice that received a sublethal intramuscular injection of C. sordellii LT. (A) Typical muscle twitches evoked by nerve stimulation of control muscle and of muscles injected with LT 1, 5, and 15 days previously. (B) Tetanic responses evoked by nerve stimulation (60 Hz) under control conditions and 1, 5, and 15 days postinjection of LT. Note the blockade of muscle twitching and tetanic responses at day 5 after LT injection and the partial recovery of the contractile responses at day 15 postinjection.
Intracellular recordings performed with EDL muscles removed from animals 1 and 5 days postinjection of LT revealed a significant (P < 0.01) decrease in the resting membrane potential of muscle fibers (Table 1). This membrane depolarization, which was more pronounced at 5 days than at 1 day postinjection, indicates that LT significantly alters the muscle fibers' membrane permeability. Electrophysiological recordings performed with EDL neuromuscular junctions 5 days after LT injection revealed that nerve stimulation was unable to trigger endplate potentials and action potentials in the depolarized fibers (n = 4) and that miniature endplate potentials could not be recorded in the depolarized fibers (data not shown).
TABLE 1.
Resting membrane potential of skeletal muscle fibers from control and LT-injected EDL muscles at various times postinjection of LT
| Day(s) postinjection | Membrane potential (mV)a
|
|
|---|---|---|
| Controlb | LT-injected | |
| 1 | −72.1 ± 2.4 | −56.5 ± 2.1c |
| 5 | −70.3 ± 1.4 | −16.8 ± 2.4c |
| 15 | −71.5 ± 3.4 | −62.7 ± 2.9c |
Values represent the mean ± SD of 18 measurements performed under each condition (in triplicate).
Controlateral EDL muscles were used as controls.
Values is statistically significant (P < 0.01) compared to control.
Muscle membrane damage induced by LT was further confirmed by assessing the uptake of Procion Orange dye by the toxin-exposed muscle fibers. One day after LT injection, ca. 50% of the fibers in the cryosections contained the dye (data not shown), and almost all of the fibers contained the dye by 5 days postinjection. These results are consistent with the marked depolarization of the muscle membrane detected in our electrophysiological recordings.
The observed LT-induced blockade of nerve-evoked muscle twitches and tetanic contractions partially recovered by day 15 postinjection of toxin. Interestingly, although tetanic stimulation at 60 Hz elicited a maximal tetanic force that was 73% ± 10.8% (n = 3) of that recorded in control muscles (P < 0.01), twitches evoked by nerve stimulation at 0.1 Hz developed a peak force that was only about 33% ± 4.7% (n = 3) of that recorded in the control preparations (P < 0.01).
Neuromuscular junction remodeling after in vivo exposure to C. sordellii LT.
FITC-conjugated α-BTX was used to characterize the distribution of AChRs in skeletal muscle endplates after in vivo exposure to a sublethal dose of LT. We found that AChRs were distributed in continuous and dense clusters in control muscle fibers (Fig. 6A) and that their distribution was more diffuse and less dense, as revealed by the intensity profiles, in LT-exposed muscle fibers (Fig. 6B). The LT-induced distribution pattern persisted for several days, until the endplates were barely detectable by α-BTX staining. Staining with α-BTX (revealing the presence of AChRs) was again evident in regenerating muscle fibers ca. 12 days postinjection of the toxin (data not shown). At 30 days postinjection, muscle fibers still exhibited central myonuclei (Fig. 6D), and their distribution of AChRs was either similar to that of the controls (Fig. 6C and D, asterisks) or was observed in small, closely spaced membrane patches (Fig. 6C and E), thus suggesting the formation of new endplate regions. A strong similarity was observed in the distribution pattern of AChRs and of acetylcholinesterase staining with TRITC-conjugated fasciculin-2, a specific inhibitor of the enzyme (13, 24). The distribution of acetylcholinesterase in neuromuscular junctions 30 days after in vivo exposure to LT is shown in Fig. 6F.
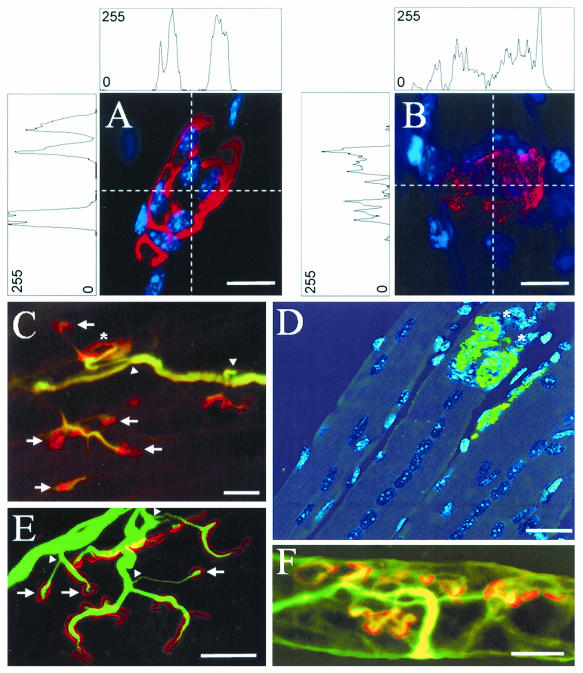
FIG. 6.
Remodeling of the neuromuscular junction in whole-mount LAL muscles obtained from mice at various times after they received a sublethal injection of C. sordellii LT. (A) Control neuromuscular junction in which AChRs are stained with TRITC-conjugated α-BTX (red) and subsynaptic nuclei are stained with DAPI (blue). (B) Staining of AChRs and subsynaptic nuclei (as in panel A) in a junction from a muscle 1 day after LT injection. Note the different intensity profiles for the AChR staining shown in panels A and B, with fluorescence intensity presented as an arbitrary unit of values between 0 and 255 color levels. (C and E) Nodal nerve sprouting (arrowheads) revealed by immunostaining with an antineurofilament antibody (green), and AChRs labeled with TRITC-conjugated α-BTX (red) 30 days post-LT injection. Note the presence of neoformed (arrows) and mature-like AChR clusters (asterisk). (D) Staining of AChRs with FITC-conjugated α-BTX (green), and TOTO-3 dye (blue) staining of nuclei in a regenerated muscle 30 days post-LT injection. Note the presence of large, aligned central nuclei in various fibers. (F) Staining of acetylcholinesterase with TRITC-conjugated fasciculin-2 and axons labeled with an antineurofilament antibody 30 days post-LT injection. Note the similarity between the staining of AChRs in panel C and of acetylcholinesterase in panel F. Bars = 4 μm (A and B), 20 μm (C and D), and 15 μm (E and F).
Morphological examination of the motor innervation of muscles 30 days postinjection with LT revealed many thin filaments emerging from the nodes of Ranvier of the intramuscular myelinated axons (Fig. 6C and E, arrowheads). The collateral sprouting (revealed by neurofilament immunostaining) abutted clusters of AChRs labeled with TRITC-conjugated α-BTX (Fig. 6C and E), at a time when acetylcholinesterase was present in the neuromuscular junctions (Fig. 6F). Our results indicate that the recovery of neuromuscular transmission after in vivo exposure to a sublethal dose of C. sordellii LT injected intramuscularly correlates with the significant remodeling of the neuromuscular junction's constitutive elements.
DISCUSSION
The present study demonstrates that the intramuscular injection of a single sublethal dose of C. sordellii LT causes significant damage to skeletal muscle fibers. The mechanism by which LT elicits necrosis of skeletal muscle fibers has not been completely elucidated, but it appears to be related to its ability to glucosylate small GTP-binding proteins. Data obtained in the present study indicate that the LT-induced degeneration of muscle fibers in vivo correlates with the toxin's effect on small GTP-binding proteins in skeletal muscle. More specifically, we observed (Fig. 3) that LT glucosylates these skeletal muscle proteins in vivo, as previously reported for other eukaryotic cells (9, 22, 33). Therefore, our results support the idea that, within 24 h after the toxin's internalization into skeletal muscle fibers, it exerts an action on small GTPases in the fibers. Also, to the best of our knowledge, this report is the first time that a large clostridial cytotoxin or LT has been shown to produce similar in vivo and in vitro effects in skeletal muscle fibers. However, further studies are needed to identify precisely the small GTP-binding proteins and GTPases targeted by LT in skeletal muscle. Also, it is likely that the toxin has an effect on endothelial cells located at the interface between blood and skeletal muscle, leading to the expression of proinflammatory mediators, vascular leakage, and subsequent ischemia of skeletal muscle tissue. Indeed, C. sordellii LT has been shown (42) to disrupt the actin microfilament system and the monolayer integrity of primary cultured human endothelial cells. LT-induced endothelial dysfunction and vascular injury could (i) cause electrolytes and water to be released into the interstitial space, thus contributing to the marked localized edema we observed, and (ii) impair oxygen delivery to and alter the pH of muscle tissue. Thus, a combination of events triggered by LT could lead to rapid anoxia and the necrosis of skeletal muscle.
A few days after the injection of LT, some muscle fibers were necrotic, while others exhibited various degrees of myofibrillar degeneration. Immunostaining of troponin T, α-actinin, and dystrophin in the control and toxin-treated muscles detected myofibril disorganization 1 day after exposure to LT, and it revealed that dystrophin was absent in necrotic fibers by day 3 postinjection of LT. The severity of the LT-induced muscle membrane injury was indicated by the high percentage of fibers with a fluorescent cytoplasm as revealed by inward leakage of Procion Orange dye or Evans blue dye (17) and by the marked muscle fiber depolarization (Table 1) detected by our electrophysiological recordings. Five days postinjection of LT, the large number of fibers exhibiting membrane injury resulted in the loss of synaptic responses and a complete blockade of neuromuscular transmission and muscle force evoked by nerve stimulation.
We observed a relatively rapid (by ca. 30 days) regeneration of neuromuscular components after the initial LT-induced necrosis of muscle fibers. This observation is not surprising, since skeletal muscle is known to have a remarkable capacity to regenerate after being subjected to a variety of injuries resulting in either partial damage to or necrosis of its muscle fibers. The events that must occur in order to achieve the complete regeneration of skeletal muscle tissue include the phagocytosis of necrotic muscle debris, revascularization, activation, proliferation and differentiation of muscle precursor cells, and reinnervation (8, 25). The onset of muscle regeneration is triggered by events that occur during muscle degeneration, and it is dependent on the proliferation and differentiation of myogenic progenitors called satellite cells and on muscle-derived stem cells that have been suggested to be pluripotent (18, 35, 36). Satellite mononucleated cells are usually quiescent. However, after skeletal muscle damage they must be activated in order to proliferate and differentiate into myotubes and myofibers. Thus, despite the muscle and vascular alterations that may be caused by LT (42), we found that muscle satellite cells were able to initiate an effective and successful myogenic program allowing muscle repair. The regeneration is rapidly completed in LT-treated muscles, in contrast to the poor regenerative responses of muscles exposed to the blood vessel-damaging metalloproteases present in some snake venoms (reviewed in reference 16). Also, although the recovery of injured skeletal muscle often is hindered by the development of interstitial fibrosis (26), we found that the regeneration of LT-treated muscles occurs without fibrosis.
The regeneration of adult mouse skeletal muscles is a well-described phenomenon (2), and it has been studied after muscle fiber necrosis produced by the most potent Elapidae snake toxins known, e.g., notexin from Notechis scutatus venom (10). However, since the molecular steps required to recruit the satellite cells needed to regenerate damaged muscle tissue are still poorly characterized, the C. sordellii LT may be a useful reagent in studies to elucidate the factors responsible for the migration, proliferation, and differentiation of the precursor cells.
We deduced the remodeling of the neuromuscular junction at 30 days postinjection of C. sordellii LT from observations of whole-mount LAL muscles (Fig. 6). Presynaptic and postsynaptic elements underwent a remodeling process characterized by a profuse nodal sprouting that restored synaptic connections and by a normalization of the distribution of nicotinic AChRs and acetylcholinesterase. The results of our functional studies (Fig. 5) also demonstrated that motor nerve terminals rapidly regenerated after exposure to C. sordellii LT. This remodeling is quite different from that observed after exposure to various serotypes of C. botulinum neurotoxins (BoNTs), which are highly specific Zn2+-dependent endoproteases that cleave single peptide bonds (except for BoNT/C1) in one of the three proteins (the 25-kDa synaptosomal-associated protein, syntaxin 1, and synaptobrevin) constituting the essential components for synaptic vesicle docking and fusion during regulated exocytosis (reviewed in references 30 and 34). Thus, these neurotoxins block acetylcholine release and produce profound but transient skeletal muscle paralysis (29) and atrophy in vivo, while leaving the viability of nerve endings unaltered (reviewed in reference 30). In contrast, the C. sordellii LT, which also blocks the regulated exocytosis of neurotransmitters (11, 15, 19), causes microscopically observable skeletal myonecrosis and degenerative changes in the neuromuscular junction.
In conclusion, to the best of our knowledge, this is the first published study to describe the degeneration and regeneration of skeletal muscle tissue and the remodeling of the neuromuscular junction after in vivo exposure to a large clostridial cytotoxin, i.e., the C. sordellii LT. Our data may provide an explanation for the severe myonecrosis and neuromuscular alterations accompanying wound infections caused by C. sordellii.
Acknowledgments
We thank Sabine De la Porte (C.N.R.S. Gif-sur-Yvette) for sharing equipment during our studies and Arnold Kreger (University of Maryland School of Medicine) for helpful suggestions during the revisions of the manuscript.
Our studies were supported by the Direction des Systèmes de Forces et de la Prospective (grant 026065093 to J.M.), the Programme de Recherche Fondamentale en Microbiologie from the Ministère Délégué à la Recherche et aux Nouvelles Technologies (to M.R.P. and J.M.), and the Association Française contre les Myopathies (to J.M.). J.B. was supported by a fellowship from the DGA-CNRS.
Editor: J. T. Barbieri
REFERENCES
- 1.Abdulla, A., and L. Yee. 2000. The clinical spectrum of Clostridium sordellii bacteraemia: two case reports and a review of the literature. J. Clin. Pathol. 53:709-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. E. 1998. Studies of the dynamics of skeletal muscle regeneration: the mouse came back. Biochem. Cell Biol. 76:13-26. [PubMed] [Google Scholar]
- 3.Angaut-Petit, D., J. Molgó, A. Connold, and L. Faille. 1987. The levator auris longus muscle of the mouse: a convenient preparation for studies of short- and long-term presynaptic effects of drugs or toxins. Neurosci. Lett. 82:83-88. [DOI] [PubMed] [Google Scholar]
- 4.Bitti, A., P. Mastrantonio, P. Spigaglia, G. Urru, A. I. Spano, G. Moretti, and G. B. Cherchi. 1997. A fatal postpartum Clostridium sordellii associated toxic shock syndrome. J. Clin. Pathol. 50:259-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boquet, P. 1999. Bacterial toxins inhibiting or activating small GTP-binding proteins. Ann. N. Y. Acad. Sci. 886:83-90. [DOI] [PubMed] [Google Scholar]
- 6.Busch, C., and K. Aktories. 2000. Microbial toxins and the glycosylation of rho family GTPases. Curr. Opin. Struct. Biol. 10:528-535. [DOI] [PubMed] [Google Scholar]
- 7.Butler-Browne, G. S., and R. G. Whalen. 1984. Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev. Biol. 102:324-334. [DOI] [PubMed] [Google Scholar]
- 8.Carlson, B. M., and J. A. Faulkner. 1983. The regeneration of skeletal muscle fibers following injury: a review. Med. Sci. Sports Exerc. 15:187-198. [PubMed] [Google Scholar]
- 9.Chaves-Olarte, E., I. Florin, P. Boquet, M. Popoff, C. von Eichel-Streiber, and M. Thelestam. 1996. UDP-glucose deficiency in a mutant cell line protects against glucosyltransferase toxins from Clostridium difficile and Clostridium sordellii. J. Biol. Chem. 271:6925-6932. [DOI] [PubMed] [Google Scholar]
- 10.Dixon, R. W., and J. B. Harris. 1996. Myotoxic activity of the toxic phospholipase, notexin, from the venom of the Australian tiger snake. J. Neuropathol. Exp. Neurol. 55:1230-1237. [DOI] [PubMed] [Google Scholar]
- 11.Doussau, F., S. Gasman, Y. Humeau, F. Vitiello, M. Popoff, P. Boquet, M. F. Bader, and B. Poulain. 2000. A Rho-related GTPase is involved in Ca2+-dependent neurotransmitter exocytosis. J. Biol. Chem. 275:7764-7770. [DOI] [PubMed] [Google Scholar]
- 12.El Hadj, N. B., M. R. Popoff, J. C. Marvaud, B. Payrastre, P. Boquet, and B. Geny. 1999. G-protein-stimulated phospholipase D activity is inhibited by lethal toxin from Clostridium sordellii in HL-60 cells. J. Biol. Chem. 274:14021-14031. [DOI] [PubMed] [Google Scholar]
- 13.Feng, G., E. Krejci, J. Molgó, J. M. Cunningham, J. Massoulie, and J. R. Sanes. 1999. Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function. J. Cell Biol. 144:1349-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost, F., G. F. Craun, and R. L. Calderon. 1998. Increasing hospitalization and death possibly due to Clostridium difficile diarrheal disease. Emerg. Infect. Dis. 4:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasman, S., S. Chasserot-Golaz, M. R. Popoff, D. Aunis, and M. F. Bader. 1999. Involvement of Rho GTPases in calcium-regulated exocytosis from adrenal chromaffin cells. J. Cell Sci. 112:4763-4771. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez, J. M., and A. Rucavado. 2000. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie 82:841-850. [DOI] [PubMed] [Google Scholar]
- 17.Hamer, P. W., J. M. McGeachie, M. J. Davies, and M. D. Grounds. 2002. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J. Anat. 200:69-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawke, T. J., and D. J. Garry. 2001. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91:534-551. [DOI] [PubMed] [Google Scholar]
- 19.Humeau, Y., M. R. Popoff, H. Kojima, F. Doussau, and B. Poulain. 2002. Rac GTPase plays an essential role in exocytosis by controlling the fusion competence of release sites. J. Neurosci. 22:7968-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humeau, Y., N. Vitale, S. Chasserot-Golaz, J. L. Dupont, G. Du, M. A. Frohman, M. F. Bader, and B. Poulain. 2001. A role for phospholipase D1 in neurotransmitter release. Proc. Natl. Acad. Sci. USA 98:15300-15305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, S., C. R. Clabots, F. V. Linn, M. M. Olson, L. R. Peterson, and D. N. Gerding. 1990. Nosocomial Clostridium difficile colonisation and disease. Lancet 336:97-100. [DOI] [PubMed] [Google Scholar]
- 22.Just, I., J. Selzer, F. Hofmann, G. A. Green, and K. Aktories. 1996. Inactivation of Ras by Clostridium sordellii lethal toxin-catalyzed glucosylation. J. Biol. Chem. 271:10149-10153. [DOI] [PubMed] [Google Scholar]
- 23.Just, I., F. Hofmann, and K. Aktories. 2000. Molecular mode of action of the large clostridial cytotoxins. Curr. Top. Microbiol. Immunol. 250:55-83. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson, E., P. M. Mbugua, and D. Rodriguez-Ithurralde. 1984. Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angusticeps. J. Physiol. Paris 79:232-240. [PubMed] [Google Scholar]
- 25.Lefaucheur, J. P., and A. Sebille. 1995. The cellular events of injured muscle regeneration depend on the nature of the injury. Neuromuscul. Disord. 5:501-509. [DOI] [PubMed] [Google Scholar]
- 26.Li, Y., and J. Huard. 2002. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am. J. Pathol. 161:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marechal, G., K. Schwartz, G. Beckers-Bleukx, and E. Ghins. 1984. Isozymes of myosin in growing and regenerating rat muscles. Eur. J. Biochem. 138:421-428. [DOI] [PubMed] [Google Scholar]
- 28.Martinez, R. D., and T. D. Wilkins. 1992. Comparison of Clostridium sordellii toxins HT and LT with toxins A and B of C. difficile. J. Med. Microbiol. 36:30-36. [DOI] [PubMed] [Google Scholar]
- 29.Meunier, F. A., G. Schiavo, and J. Molgó. 2002. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J. Physiol. Paris 96:105-113. [DOI] [PubMed] [Google Scholar]
- 30.Meunier, F. A., J. Herreros, G. Schiavo, B. Poulain, and J. Molgó, J. 2002. Molecular mechanism of action of botulinal neurotoxins and the synaptic remodeling they induce in vivo at the skeletal neuromuscular junction, p. 305-347. In E. J. Massaro (ed.), Handbook of neurotoxicology, vol. 1. Humana Press, Totowa, N.J.
- 31.Pellegrino, M. A., M. Canepari, R. Rossi, G. D'Antona, C. Reggiani, and R. Bottinelli. 2003. Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J. Physiol. 546:677-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popoff, M. R. 1987. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect. Immun. 55:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popoff, M. R., E. Chaves-Olarte, E. Lemichez, C. von Eichel-Streiber, M. Thelestam, P. Chardin, D. Cussac, B. Antonny, P. Chavrier, G. Flatau, M. Giry, J. de Gunzburg, and P. Boquet. 1996. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J. Biol. Chem. 271:10217-10224. [DOI] [PubMed] [Google Scholar]
- 34.Schiavo, G., M. Matteoli, and C. Montecucco. 2000. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80:717-766. [DOI] [PubMed] [Google Scholar]
- 35.Schultz, E., and K. M. McCormick. 1994. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 123:213-257. [DOI] [PubMed] [Google Scholar]
- 36.Seale, P., A. Asakura, and M. A. Rudnicki. 2001. The potential of muscle stem cells. Dev. Cell 1:333-342. [DOI] [PubMed] [Google Scholar]
- 37.Stevens, D. L., and A. E. Bryant. 1999. The pathogenesis of shock and tissue injury in clostridial gas gangrene, p. 623-636. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxin, 2nd ed. Academic Press, New York, N.Y.
- 38.Stevens, D. L., and A. E. Bryant. 2002. The role of clostridial toxins in the pathogenesis of gas gangrene. Clin. Infect. Dis. 35:S93-S100. [DOI] [PubMed] [Google Scholar]
- 39.Straub, V., J. A. Rafael, J. S. Chamberlain, and K. P. Campbell. 1997. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139:375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinsley, J., N. Deconinck, R. Fisher, D. Kahn, S. Phelps, J. M. Gillis, and K. Davies. 1998. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat. Med. 4:1441-1444. [DOI] [PubMed] [Google Scholar]
- 41.Titball, R. W. 1999. Membrane-damaging and cytotoxic phospholipases, p. 310-329. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxin, 2nd ed. Academic Press, New York, N.Y.
- 42.Vouret-Craviari, V., D. Grall, G. Flatau, J. Pouyssegur, P. Boquet, and E. Van Obberghen-Schilling. 1999. Effects of cytotoxic necrotizing factor 1 and lethal toxin on actin cytoskeleton and VE-cadherin localization in human endothelial cell monolayers. Infect. Immun. 67:3002-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]