Abstract
Clostridium perfringens iota-toxin is a binary toxin composed of an enzymatic component (Ia) and a binding component (Ib). The oligomer of Ib formed in membranes induces endocytosis. We examined the binding and internalization of Ib by using Cy3-labeled Ib. Labeled Ib was retained at the membranes of MDCK cells for 60 min of incubation at 37°C, and later it was detected in cytoplasmic vesicles. To determine whether Ib associates with lipid rafts, we incubated MDCK cells with Ib at 4 or 37°C and fractionated the Triton-insoluble membranes. An Ib complex of 500 kDa was localized at 37°C to the insoluble fractions that fulfilled the criteria of lipid rafts, but it did not form at 4°C. The amount of complex in the raft fraction reached a maximum after 60 min of incubation at 37°C. When the cells that were preincubated with Ib at 4°C were incubated at 37°C, the complex was detected in the raft fraction. The treatment of MDCK cells with methyl-β-cyclodextrin reduced the localization of the Ib complex to the rafts and the rounding of the cells induced by Ia plus Ib. When 125I-labeled Ia was incubated with the cells in the presence of Ib at 37°C, it was localized in the raft fraction. Surface plasmon resonance analysis revealed that Ia binds to the oligomer of Ib. We conclude that Ib binds to a receptor in membranes and then moves to rafts and that Ia bound to the oligomer of Ib formed in the rafts is internalized.
Clostridium perfringens type E, which produces an iota-toxin consisting of an enzyme component (Ia) and a binding component (Ib), causes antibiotic-associated enterotoxemia in rabbits (28). The involvement of an iota-like toxin is implicated in sudden outbreaks of enteritis caused by Clostridium spiroforme in rabbits (6). Therefore, iota-toxin may also be an important agent of enterotoxemia. Ia ADP-ribosylates skeletal muscle α-actin and nonmuscle β/γ-actin (3), and Ib binds to the cell, forming oligomers to create ion-permeable channels (15, 24, 32). Each component lacks toxic activity when it is injected alone, but together they act in binary combinations to produce cytotoxic, lethal, and dermonecrotic activities (28).
Sequencing of the genes encoding Ia and Ib has revealed that Ia belongs to a family of bacterial ADP-ribosylating toxins, along with the enzyme components of Clostridium botulinum C2 toxin, C. spiroforme iota-like toxin, Bacillus cereus vegetative insecticidal protein, cholera toxin, and Escherichia coli heat-labile enterotoxin (8). Crystallography of Ia complexed with NADH and site-directed mutagenesis of Ia revealed that it can be divided into two domains, an N domain (1 to 210 residues) and a C domain (211 to 413 residues) which has a cavity that binds NADH, and that Ia cleaves the N-glycoside bond of NAD+ and transfers the ADP-ribose moiety to Arg-177 of actin in the cavity (29, 33). The amino acid sequence of Ib is similar to that of the protective antigen (PA) of Bacillus anthracis (26). It has been reported that iota-toxin and C2 toxin enter cells by receptor-mediated endocytosis (4). Furthermore, K. Aktories' group has reported that C2II oligomers bind to a carbohydrate receptor, assemble C2I, enter cells by endocytosis, and release C2I into the cytosol after acidification of the endosomal compartment (4, 10). B. Stiles and M. Popoff's group has reported that Ib strongly binds to the cell surface receptors of Vero and MDCK cells and that the C-terminal domain of Ib is responsible for binding to the cell surface receptor and the N-terminal domain is important for Ia docking (17, 18, 31). Furthermore, Blocker et al. reported that acidification of the endosomal compartments is required for the uptake of iota-toxin (5). Crystallography showed that PA is characterized by a four-domain structure (27). Milne et al. (19) reported that PA inserted into membranes forms oligomers to create ion-conductive channels and that PA associates with other factors and enters cells with other factors via endocytosis. Abrami et al. (2) reported that PA induces endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. Several studies have provided evidence for the specificity of binding of Ib to eukaryotic cells (5, 17, 18, 31). We reported that Ib binds to Vero cells, forming oligomers itself to create ion-permeable channels, and that oligomer-mediated endocytosis allows Ia to internalize in the cells (24). However, little is known about the binding and internalization of Ib and the role of Ib in the entry of Ia into cells. Here we present evidence for the binding of Ib to lipid rafts of MDCK cells and the internalization of Ib with Ia into cells.
MATERIALS AND METHODS
Materials.
Methyl-β-cyclodextrin (MβCD), cholesterol, dioleoyl-l-α-phosphatidylcholine (DOPC), carboxyfluorescein (CF), and a protease inhibitor mixture were obtained from Sigma (St. Louis, Mo.). Mouse anti-caveolin-1 and -Lyn antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). A Cy3 reactive dye pack, horseradish peroxidase-labeled sheep anti-mouse immunoglobulin G, and an ECL Western blotting kit were purchased from Amersham Pharmacia Biotech (Tokyo, Japan). Dulbecco's modified Eagle medium (DMEM) was purchased from GIBCO BRL (New York, N.Y.). The pEGFP vector was purchased from BD Bioscience Clontech (Palo Alto, Calif.). All other chemicals were of the highest grade available from commercial sources.
Expression and purification of Ia and Ib.
Recombinant Ia was purified from culture supernatants of Bacillus subtilis ISW1214 carrying a plasmid containing the Ia gene, as described previously (23). Recombinant plasmids of pHY300PLK harboring the structural genes of the wild-type Ia gene were introduced into B. subtilis ISW1214 by transformation. Transformants were grown in Luria-Bertani broth at 37°C for 8 h with continuous aeration. The culture was centrifuged (18,000 × g for 20 min), and ammonium sulfate (313 g/liter) was added to the culture supernatant fluid. The ammonium sulfate fraction was dialyzed against 0.02 M Tris-HCl buffer (pH 7.5) and loaded onto a DEAE-Sepharose CL-6B column that was previously equilibrated with the same buffer. Elution of the column was done with a 0 to 0.1 M NaCl linear gradient (300-ml total volume) in 0.02 M Tris-HCl buffer (pH 7.5). Ia eluted as a sharp peak at a NaCl concentration of about 0.03 M (23). Ib was expressed as a fusion protein with glutathione S-transferase (GST) in E. coli BL21, as described previously (24). After the growth (at 30°C) and induction (with isopropyl-β-d-thiogalactopyranoside to 1 mM) of a large culture, the cells were centrifuged and disrupted by a sonicator on ice in a short burst. Centrifugation of the lysate and passaging of the soluble fraction through a glutathione-Sepharose (Amersham Pharmacia Biotech) column yielded the GST-Ib fusion protein at about 2 mg/liter. After the cleavage of purified GST-Ib with chymotrypsin, as described previously (24), the cleaved protein was passed through a glutathione-Sepharose column and then subjected to anion-exchange chromatography (Mono-Q HR10/10; Amersham Pharmacia Biotech). The protein was eluted with a 0 to 1.0 M NaCl linear gradient, and Ib eluted as a peak at a NaCl concentration of about 0.5 M. Fractions containing Ib were collected (24).
Construction of domain 4 of Ib.
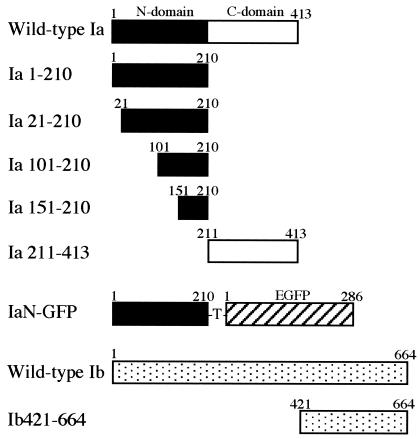
To prepare domain 4 of Ib, we constructed Ib421-664, with a deletion of the N-terminal 420 amino acids of Ib (residues 1 to 420) (Fig. 1), by a PCR using the DNA template pTIB, containing the entire Ib gene (24), a forward primer (5′-GGAGATCTAGTAATATTGATACTAA-3′) encoding a BglII site (in bold), DNA encoding amino acids 421 to 425 of Ib, and a reverse primer (5′-CCCTCGAGCTAGCTTTATTAATTTT-3′) encoding a downstream XhoI restriction site (in bold). PCR products were digested with BglII and XhoI and ligated into BamHI- and XhoI-digested pGEX-4T-1 (Amersham Pharmacia Biotech) so that the correct reading frame was maintained with the thrombin cleavage site under the GST gene (pGEX-Ib421-664). The expression and purification of Ib421-644 were performed as described above for Ib.
FIG. 1.
Schematic representation of deleted Ia and Ib proteins and IaN-GFP fusion protein.
Construction of Ia N domain-green fluorescent protein (IaN-GFP) fusion.
The IaN (residues 1 to 210 of Ia) gene was amplified by a PCR using pT-IA (23), containing the entire Ia gene, a forward primer (5′-GGAGATCTGCTTTTATTGAAAGACC-3′) encoding a BglII site (in bold), and a reverse primer (5′-CACCATGGTACTGTTTACAATAGAAGCTTC-3′) encoding a downstream NcoI restriction site (bold). PCR products were digested with BglII and NcoI and ligated with NcoI- and EcoRI-digested pEGFP, containing the entire GFP gene (16). The fused gene was ligated to BamHI- and EcoRI-digested pGEX-4T-1. The expression and purification of IaN-GFP (Fig. 1) were performed as described above for Ib.
Construction and purification of Ia deletion mutants.
Ia deletion mutants (Ia1-210, Ia21-210, Ia101-210, Ia151-210, and Ia211-413) (Fig. 1) were amplified from pT-IA by the use of specific primers. The oligonucleotide primers introduced BglII and SalI sites at the intended new 5′ and 3′ ends, respectively. Amplified fragments were ligated into BamHI- and SalI-digested pGEX-4T-1. The expression and purification of Ia deletion mutants were performed as described above for Ib.
Assay of cytotoxicity.
MDCK cells were obtained from the Riken Cell Bank (Tsukuba, Japan). They were cultured in DMEM supplemented with 10% fetal calf serum (FCS), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM glutamine (FCS-DMEM). All incubation steps were performed at 37°C in a 5% CO2 atmosphere.
The test for cytotoxicity was done with MDCK cells. The cells were cultivated in FCS-DMEM. For cytotoxicity assays, the cells were inoculated in 48-well tissue culture plates (Falcon, Oxnard, Calif.). Various concentrations of Ia and 500 ng of Ib/ml were mixed in FCS-DMEM and inoculated onto cell monolayers. The cells were observed for morphological alterations 8 h after inoculation, as described previously (23). For a measurement of the effect of MβCD on the cytotoxicity of iota-toxin, MDCK cells were preincubated with this agent at 37°C for 1 h and then incubated with Ia and Ib at 37°C for 8 h. Cholesterol levels were assayed spectrophotometrically by the use of a diagnostic kit (Cholesterol C-Test; Wako Pure Chemicals, Osaka, Japan).
Internalization of Cy3-labeled Ib and IaN-GFP.
Ib was labeled with Cy3 by using a reactive dye pack according to the manufacturer's instructions (Amersham Pharmacia Biotech). MDCK cells on poly-l-lysine-coated glass coverslips were incubated with Cy3-labeled Ib (1 μg/ml) in FCS-DMEM at 37°C for various times. For investigations of the internalization of IaN-GFP, the cells were incubated with IaN-GFP (1 μg/ml) in the presence or absence of Ib (500 ng/ml) at 37°C for 60 min. They were then washed three times with ice-cold phosphate-buffered saline and fixed with 3% paraformaldehyde in phosphate-buffered saline for 20 min before being examined under a Nikon TE300 fluorescence and phase microscope (Nikon Co., Tokyo, Japan). Images were captured and digitized with a charge-coupled device camera (Hamamatsu Photonics Co., Hamamatsu, Japan) and then were edited with Adobe Photoshop 5.0 (Adobe Systems Inc., Mountain View, Calif.).
Iodination of toxin components.
125I-labeled Ia, Ib, and Ib421-644 were prepared with Bolton-Hunter reagent (2,000 Ci/mmol; Amersham Pharmacia Biotech) as described previously (24). Ia, Ib, and Ib421-644 (50 μg) were incubated with 250 μCi of 125I-labeled Bolton-Hunter reagent. Labeled Ib plus labeled Ia retained over 90% of the original cytotoxicity of cold Ia plus cold Ib. The binding of 125I-Ib and 125I-Ib421-644 to MDCK cells was dose-dependently inhibited by cold Ib421-644 and cold Ib, respectively (data not shown), indicating that Ib and Ib421-644 bind to the same receptor on the cell surface.
Sucrose gradient fractionation.
The separation of lipid rafts was done by flotation-centrifugation on a sucrose gradient (25, 34). MDCK cells were incubated with 125I-labeled Ib, 125I-labeled Ia in the presence or absence of Ib, or 125I-labeled Ib421-664 in fresh medium at 37°C for various times. The cells were rinsed with Hanks balanced salt solution (HBSS) and then lysed by exposure to 1% Triton X-100 for 30 min at 4°C in HBSS containing a protease inhibitor mixture. The lysates were scraped from the dishes with a cell scraper and homogenized by being passed through a 22-gauge needle. The lysates were adjusted to 40% (wt/vol) sucrose, overlaid with 2.4 ml of 36% sucrose and 1.2 ml of 5% sucrose in HBSS, centrifuged at 45,000 rpm (250,000 × g) for 18 h at 4°C in an SW55 rotor (Beckman Instruments, Inc., Palo Alto, Calif.), and fractionated from the top (0.4 ml/fraction). The aliquots were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiographed.
Immunoblot analysis of lipid raft marker proteins.
Aliquots of the flotation sucrose gradient fractions were heated in 2% SDS sample buffer at 99°C for 3 min. The samples were electrophoresed in an SDS-PAGE gel and then transferred to a polyvinyl difluoride membrane. The membrane was blocked with Tris-buffered saline containing 2% Tween 20 and 5% skim milk and was incubated first with a primary antibody in Tris-buffered saline containing 1% skim milk, next with a horseradish peroxidase-conjugated secondary antibody, and finally with an enhanced chemiluminescence analysis solution.
Liposomes.
DOPC-cholesterol (1:1) liposomes containing CF were prepared, and CF release was monitored by a previously described procedure (25). The binding of 125I-Ib to liposomes was performed as described previously (25).
Surface plasmon resonance (SPR) analysis.
All experiments were performed with BIAcore 3000 system sensor chips and their software evaluation package (Biacore KK, Tokyo, Japan). The Ib oligomer was purified from trypsin-treated Ib by Mono Q chromatography with the fast-performance liquid chromatography system, as described previously (24). Oligomers purified by this method are in a heptameric state, although the presence of monomers or lower order oligomers has not been excluded. For CM5 chips, the system was maintained with a constant flow (10 μl/min) of HBS buffer (10 mM HEPES, pH 7.4, and 150 mM NaCl) at 25°C. The preconcentration step of this coupling requires the lowering of the pH of the protein to be immobilized to a value below its pKa, which was calculated to be 4.7 for Ib. The highest pH at which this preconcentration step was effective was pH 4.5. Ib oligomers and Ib monomers were covalently bound to the carboxylated dextran matrix by amine coupling according to the manufacturer's directions (Biacore), except that they were diluted in sodium acetate buffer, pH 4.5, to a concentration of 500 nM. These dilutions were injected onto the activated surface at a flow rate of 2 μl/min until the desired baseline level was reached (about 2,000 relative units) and then were blocked with ethanolamine according to the manufacturer's directions. The interaction between immobilized Ib and Ia was examined at 25°C. Ia diluted in HBS was injected over the Ib oligomer surface at 10 μl/min for 170 s, allowing association to take place. Dissociation was then monitored in a constant flow of HBS for at least 150 s. Bound analyte was removed, and the Ib oligomer baseline was regenerated with a 10-μl pulse of 0.2 M glycine-HCl buffer (pH 2.0). The baseline decay was <3% per cycle. The association and dissociation rate constants kon and koff were determined from sensorgram data by using the BiaEvaluation 3.0 software package.
RESULTS
Ib binding to and internalization into MDCK cells.
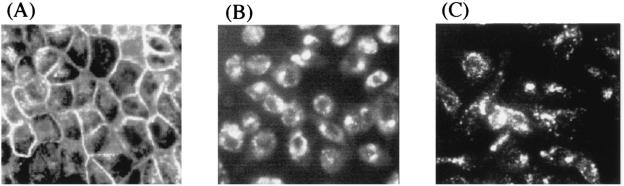
To test whether Ib binds to the plasma membrane and enters the cell by endocytosis, we incubated MDCK cells with Cy3-labeled Ib (1 μg/ml) at 37°C for various periods of time or at 4°C for 30 min, washed them, and then fixed them with 4% paraformaldehyde for 15 min. The specificity of the binding of Ib to MDCK cells was examined by fluorescence microscopy, as shown in Fig. 2. After the incubation for 30 min at 4°C, a strong signal was localized to the plasma membrane, but no fluorescent signal was detected in the cytosol (data not shown). Within 15 min at 37°C, Cy3-Ib was confined to the plasma membrane (Fig. 2A). No fluorescent signal was detected on the surface when the cells were preincubated with Ib (30 μg/ml) for 30 min at 4°C and then additionally incubated with Cy3-Ib at 37°C for 15 min, suggesting that Cy3-Ib binds to a specific receptor on MDCK cells (data not shown). After 60 min at 37°C, Cy3-Ib was present on the cell surface and in numerous small vesicles underlying the cell surface, and after 120 min, was evident in cytoplasmic vesicles in the cytosol, as shown in Fig. 2B and C. When cells that were preincubated with Cy3-Ib at 4°C for 30 min were washed and warmed to 37°C for 120 min, the fluorescent signal for Cy3-Ib was found in the cytosol (data not shown). This process seems uniform, since all cells in the field progressed at the same rate.
FIG. 2.
Internalization of Cy3-labeled Ib to endosomal compartment of MDCK cells. MDCK cells were incubated with Cy3-labeled Ib (1 μg/ml) for 15 min (A), 60 min (B), and 120 min (C) at 37°C. The cells were fixed, and the localization of Cy3-Ib was detected by fluorescence microscopy.
Binding site of Ib on membrane of MDCK cells.
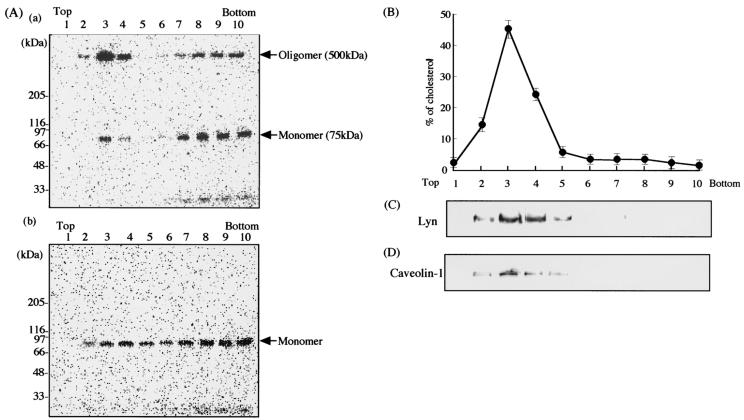
It has been reported that several toxins, such as perfringolysin O (34), C. perfringens beta-toxin (25) and epsilon-toxin (20), Shiga toxin (13), tetanus toxin (12), and Helicobacter pylori vacuolating toxin (30), bind to lipid rafts and that some bacteria and viruses enter cells via lipid rafts (9). For an investigation of the possible interaction of Ib with lipid rafts of MDCK cells, 125I-Ib was incubated with MDCK cells in DMEM containing 10% fetal bovine serum at 37°C for 30 min and the cells were treated with 1% Triton X-100 at 4°C for 60 min. Membrane components treated with Triton X-100 were fractionated by sucrose density gradient centrifugation. The fractions were subjected to SDS-PAGE and autoradiography. The Triton X-100-insoluble fractions (no. 2 to 4), which were low-density fractions, showed two bands, a minor band of about 75 kDa, which is the expected size of the monomeric toxin, and a major band of about 500 kDa, which is the expected size of the heptameric toxin, as shown in Fig. 3A. The results suggest that the oligomer of Ib is associated with the detergent-insoluble fraction. The soluble fractions (no. 6 to 10) also showed two bands of about 75 and 500 kDa. When the toxin was incubated with MDCK cells at 4°C for 60 min, only the band of 75 kDa was detected in the insoluble and soluble fractions, and no oligomer of the toxin was detected in any fractions (Fig. 3A). However, when MDCK cells that were preincubated with the toxin at 4°C for 60 min were washed and then incubated at 37°C for 60 min, the oligomer of the toxin was detected in the insoluble fractions (data not shown), showing that the oligomer is formed on the plasma membranes of cells at 37°C.
FIG. 3.
Sucrose density gradient analysis of 125I-Ib-bound MDCK cells. (A) MDCK cells were incubated with 125I-Ib (500 ng/ml) in DMEM containing 10% fetal bovine serum at 37°C (a) or 4°C (b) for 30 min, extracted with HBSS containing 1% Triton X-100 at 4°C for 30 min, and sonicated. The extracts were mixed with 40% sucrose and then loaded at the bottom of a centrifuge tube. After sucrose gradient ultracentrifugation, 0.4-ml gradient fractions were collected from the tops of the tubes. Aliquots of the gradient fractions were dissolved in 2× SDS sample buffer and incubated at 37°C for 10 min. Samples were subjected to SDS-PAGE, followed by autoradiography. (B) The distribution of cholesterol in the sucrose gradient fractions was determined as described in Materials and Methods. The data are the means and standard deviations from four experiments. The aliquots of gradient fractions were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. After the transfer, the blots were treated with an anti-Lyn (C) or anti-caveolin-1 (D) antibody. Peroxidase-conjugated secondary antibodies bound to the membrane were detected by enhanced chemiluminescence as described in Materials and Methods.
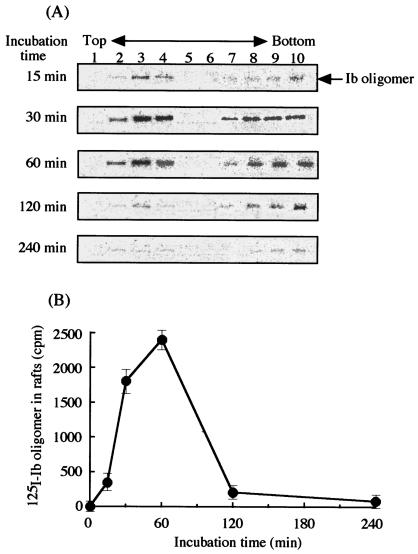
When raft markers in fractions obtained by sucrose density gradient centrifugation were analyzed with anti-caveolin-1 and anti-Lyn antibodies, caveolin-1 and Lyn were only detected in the insoluble fractions (no. 2 to 4) (Fig. 3C and D). The cholesterol in these fractions was then measured, and >85% of the cholesterol was detected in these fractions (no. 2 to 4) (Fig. 3B). Therefore, the results show that lipid rafts are contained in the insoluble fractions (no. 2 to 4), suggesting that the oligomer of Ib is specifically located within lipid rafts of MDCK cells. We determined the time course of oligomer formation in lipid raft fractions under our experimental conditions. As shown in Fig. 4, the 500-kDa band of the oligomer reached a maximum intensity after 60 min of incubation, later decreased in a time-dependent manner, and disappeared at 240 min.
FIG. 4.
Oligomer formation of 125I-labeled Ib in lipid rafts of MDCK cells. (A) MDCK cells were incubated with 125I-labeled Ib at 37°C for the indicated times. Triton X-100-insoluble cell extracts were subjected to SDS-PAGE, followed by autoradiography, as described in Materials and Methods. (B) The radioactivity of the 125I-Ib oligomer in MDCK cell lipid rafts was determined by using the Fuji BAS2000 system. The data are the means and standard deviations from three experiments.
Ia itself nonspecifically bound to MDCK cells at 4 and 37°C, while Ib bound specifically (data not shown). When MDCK cells that were preincubated with Ib at 4°C for 60 min were incubated with Ia at 4°C for 60 min, washed, and then incubated at 37°C for 240 min, cell rounding did not occur (Table 1). This result suggests that cell rounding did not take place, although Ia and Ib bound to the cells. However, when MDCK cells that were preincubated with Ib at 4°C for 60 min were incubated with Ia at 37°C for 60 min, washed, and then incubated at 37°C for 240 min, cell rounding did occur (Table 1). It therefore appears that the incubation of Ib at 37°C, i.e., the formation of the oligomer, is essential for the activity induced by Ia plus Ib. MDCK cells were also preincubated with Ib (500 ng/ml) at 37°C for 30, 60, and 120 min, washed, and incubated with various amounts of Ia at 37°C for 60 min. They were then washed and incubated at 37°C for 240 min (Table 1). Preincubation with Ib for 30 and 60 min caused rounding of the cells, but preincubation for 120 min did not. This result indicates that the Ib oligomer exists on the surface of the cell membrane within 60 min, but not for 120 min.
TABLE 1.
Cell rounding activity of iota-toxin under various conditions
| Condition seta | Cell rounding with indicated dose of Ia (ng/ml)
|
||
|---|---|---|---|
| 50 | 100 | 500 | |
| A | − | − | + |
| B | + | +++ | +++ |
| C | |||
| 30-min first step | + | ++ | +++ |
| 60-min first step | + | ++ | +++ |
| 120-min first step | − | − | − |
For condition set A MDCK cells were incubated with Ib (500 ng/ml) at 4°C for 60 min. The washed cells were treated with various amounts of Ia at 4°C for 60 min, washed, and incubated at 37°C for 240 min. For condition set B, MDCK cells were incubated with Ib (500 ng/ml) at 4°C for 60 min. The washed cells were treated with various amounts of Ia at 37°C for 60 min, washed, and incubated at 37°C for 240 min. For condition set C, MDCK cells were incubated with Ib (500 ng/ml) at 37°C for 30, 60, or 120 min. The washed cells were treated with various amounts of Ia at 37°C for 60 min, washed, and incubated at 37°C for 240 min. Cell rounding activity was scored as follows: +++, 100% rounding; ++, 50 to 80% rounding; +, 20 to 40% rounding; −, no rounding. The experiments were repeated three times, and data from a representative experiment are shown.
Effect of MβCD on binding and biological activity of Ib.
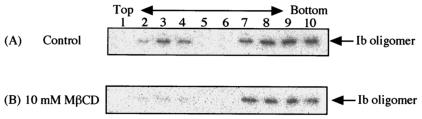
Kilsdonk et al. reported that MβCD selectively encapsulates membrane cholesterol and does not deplete lipids other than cholesterol at concentrations of 5 to 10 mM (14). The effect of MβCD on the cell rounding induced by iota-toxin (Ia plus Ib) was investigated (Table 2). When MDCK cells were incubated with 5 and 10 mM MβCD at 37°C for 60 min, the cholesterol content of the cells decreased to about 51 and 30%, respectively, of that in untreated control cells (Table 2). Incubation of the toxin with MDCK cells that were pretreated with 5 and 10 mM MβCD at 37°C for 60 min produced a decrease of about 90 and 95%, respectively, in the cell rounding induced by Ia plus Ib in untreated cells (Table 2). Next, for an investigation of whether Ib binds to cells that were treated with MβCD, the cells treated with 10 mM MβCD were incubated with 125I-Ib at 37°C for 30 min and then with 1% Triton X-100 at 4°C for 60 min. After sucrose density gradient centrifugation, the fractions were subjected to SDS-PAGE analysis. Figure 5 shows that no oligomer was detected in the insoluble fractions (no. 2 to 4). We investigated whether the cholesterol in lipid rafts affects the direct binding of the toxin or the integrity of the binding site. The addition of cholesterol (50 μg/ml) had no effect on the rounding of untreated MDCK cells induced by Ia plus Ib under the conditions used (data not shown), suggesting that Ib does not directly interact with cholesterol.
TABLE 2.
Effect of MβCD on cell rounding induced by Ia plus Iba
| Concn (mM) of MβCD | Cell rounding with indicated dose of Ia (ng/ml)
|
% Cholesterol in cellsb | ||||
|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 50 | 100 | ||
| 0 | − | + | ++ | +++ | +++ | 100.0 |
| 5 | − | − | − | + | ++ | 51.3 ± 7.6 |
| 10 | − | − | − | − | + | 29.6 ± 5.4 |
MDCK cells were treated with MβCD at 37°C for 30 min. The cells were incubated with various amounts of Ia in the presence of Ib (100 ng/ml). Cell rounding activity was scored as follows; +++, 100% rounding; ++, 50 to 80% rounding; +, 20 to 40% rounding; −, no rounding. The experiments were repeated three times, and data from a representative experiment are shown.
The amount of cholesterol in cells was determined as described in Materials and Methods. The data are means and standard deviations of three experiments.
FIG. 5.
Effect of MβCD on interaction of Ib with MDCK cells. MDCK cells were incubated in the absence (A) or presence (B) of 10 mM MβCD at 37°C for 30 min. 125I-Ib was mixed with the cells and then incubated at 37°C for 60 min. Triton X-100-insoluble cell extracts were separated by sucrose density ultracentrifugation. Gradient fractions were subjected to SDS-PAGE, followed by autoradiography, as described in Materials and Methods.
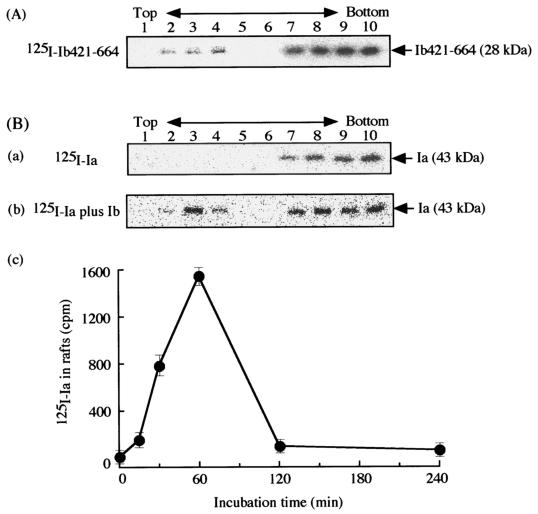
PA can be divided into four domains (27). Based on mutational and biochemical analyses, these four domains have been reported to have different functions, namely binding to other components, insertion into the membrane, oligomerization, and binding to the cell (21, 27). Domains 1 to 3 of Ib show 30 to 40% homology in amino acid sequence with those of PA (26). Domain 4 (residues 421 to 664) of Ib is not homologous in sequence to that of PA (26). In addition, Marvaud et al. reported that the receptor of Ib is different from that of PA (18). From these findings, we investigated whether domain 4 of Ib binds to lipid rafts of MDCK cells. 125I-labeled Ib421-664 (Ib with a deletion of the 420 N-terminal amino acids, i.e., Ib with domain 4 only) was incubated with MDCK cells in DMEM containing 10% fetal bovine serum at 37°C for 30 min, and then the cells were treated with 1% Triton X-100 at 4°C for 60 min. The Triton X-100-insoluble components were fractionated by sucrose density gradient centrifugation. The fractions were subjected to SDS-PAGE and autoradiography (Fig. 6A). One band of about 28 kDa, which is the expected size of Ib421-664, was detected in the insoluble fractions (no. 2 to 4), as was the oligomer of Ib, suggesting that domain 4 of Ib binds to lipid rafts.
FIG. 6.
Binding of 125I-labeled Ib421-611 and 125I-Ia to lipid rafts of MDCK cells in the presence of Ib. (A) MDCK cells were incubated with 125I-labeled Ib421-664 (500 ng/ml) at 37°C for 60 min. (B) MDCK cells were incubated with 125I-labeled Ia (500 ng/ml) in the absence (a) or presence (b) of Ib (500 ng/ml) at 37°C for 60 min. Triton X-100-insoluble cell extracts were separated by sucrose density ultracentrifugation and analyzed as described in Materials and Methods. The radioactivity of 125I-Ia binding to MDCK cell rafts was determined at 37°C for various periods by using a Fuji BAS2000 system (c). The data are the means and standard deviations from three experiments.
Effect of Ib on phosphatidylcholine-cholesterol liposomes.
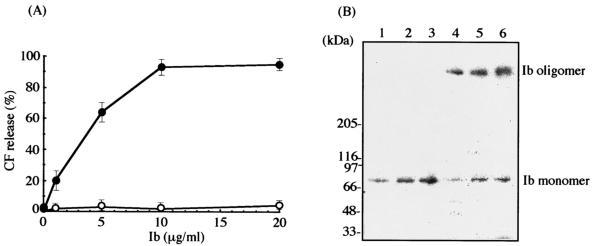
To investigate whether Ib forms a functional oligomer in artificial membranes, we incubated the toxin with DOPC-cholesterol (1:1) liposomes containing 5(6)-CF at 4 and 37°C for 3 h. Figure 7A shows that Ib (1 to 10 μg/ml) dose dependently caused a CF release from the liposomes at 37°C, but not at 4°C. However, these results indicate that the sensitivity of liposomes to the toxin is lower than that of Vero cells (24). For clarification of the binding of Ib to the liposomes, 125I-Ib was incubated with liposomes at 4 and 37°C for 3 h. Next, liposomes were subjected to SDS-PAGE analysis and autoradiography. As shown in Fig. 7B, Ib bound to liposomes in a dose-dependent manner when it was incubated at 37°C. The liposomes showed the presence of 125I-Ib migrating at 76 and 500 kDa, indicating that Ib forms a heptamer in artificial membranes. The formation of the oligomer was elevated with increasing doses of the toxin. However, when Ib and the liposomes were incubated at 4°C, Ib migrating at 76 kDa was observed, but no oligomer was present in the liposomes.
FIG. 7.
Ib-induced CF release from DOPC-cholesterol liposomes and oligomer formation of Ib. (A) CF-loaded DOPC-cholesterol (1:1) liposomes were incubated with various amounts of Ib at 4°C (○) and 37°C (•) for 3 h. CF release was determined as described in Materials and Methods. Data are given as means ± standard errors (n = 5). (B) DOPC-cholesterol (1:1) liposomes were incubated with 125I-Ib at 4°C (lanes 1 to 3) and 37°C (lanes 4 to 6) for 3 h. The membrane-bound toxin was solubilized and subjected to SDS-PAGE, followed by autoradiography. The concentrations of 125I-Ib were as follows: lanes 1 and 4, 5 μg/ml; lanes 2 and 5, 10 μg/ml; lanes 3 and 6, 20 μg/ml.
Binding to MDCK cells and internalization of Ia.
To investigate the effect of Ib on the binding of Ia to cells, we incubated the cells with 125I-Ia in the presence or absence of Ib at 37°C for 30 min and then treated them with 1% Triton X-100 at 4°C for 60 min. After sucrose density gradient centrifugation, the fractions were subjected to SDS-PAGE analysis (Fig. 6B). One labeled band of about 43 kDa, which is the expected size of Ia, was detected in the low-density fractions (no. 2 to 4) in the presence of Ib, but no band was detected in the absence of Ib, indicating that Ia binds to lipid rafts in the presence of Ib. Furthermore, a time course of 125I-Ia binding to the lipid raft fraction in the presence of Ib showed the same pattern as that of the 125I-Ib oligomer binding to the lipid raft fraction (Fig. 6B).
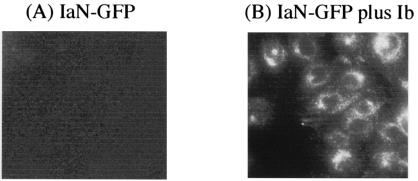
From the crystallography of Ia, we concluded that the C domain (residues 211 to 412) of Ia has a cavity in which NAD+ is folded, corresponding to the catalytic domain (33). Marvaud et al. (18) reported that the N domain of Ia plays a role in the binding of Ia to Ib. Therefore, to examine the effect of the N domain (residues 1 to 210) of Ia on the binding of Ia to Ib bound to cells, we incubated the cells with wild-type Ia (10 ng/ml) and Ib (500 ng/ml) in the presence of various Ia deletion mutants (Fig. 1) at 37°C for 8 h. As shown in Table 3, the N domain (1 to 210 residues) and the Ia 21-210 deletion mutant at >1.0 μg/ml completely inhibited cell rounding induced by Ia plus Ib. The Ia 101-210 and Ia 151-210 deletion mutants, used at >5.0 and 10 μg/ml, respectively, blocked the activity. However, the C domain (Ia 211-413) had no effect on the rounding activity. These results suggest that the central region (residues 151 to 210) of Ia is responsible for the interaction with Ib bound to cells. To investigate whether the N domain of Ia is internalized in MDCK cells in the presence of Ib, we incubated cells grown on glass dishes with the IaN-GFP fusion protein in the presence of Ib at 37°C. As shown in Fig. 8, after 120 min of incubation, IaN-GFP was present in cytoplasmic vesicles (Fig. 8B). However, when the cells were incubated with the fusion protein in the absence of Ib, no fluorescence was observed in the cytosol (Fig. 8A).
TABLE 3.
Inhibitory effect of Ia deletion mutants on cytotoxic activity induced by Ia plus Iba
| Deletion mutant | Cell rounding with indicated dose of mutant (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.05 | 0.1 | 0.5 | 1 | 5 | 10 | |
| Ia1-210 | +++ | +++ | ++ | + | − | − | − |
| Ia21-210 | +++ | +++ | ++ | + | − | − | − |
| Ia101-210 | +++ | +++ | +++ | ++ | + | − | − |
| Ia151-210 | +++ | +++ | +++ | +++ | ++ | + | − |
| Ia211-413 | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
Confluent monolayers of MDCK cells (2 × 106 cells) were exposed to various doses of variant Ia deletion mutants in the presence of 10 ng of wild-type Ia/ml and 500 ng of Ib/ml at 37°C. Cell rounding was recorded after 8 h. Cell rounding activity was scored as follows: +++, 80 to 100 % rounding; ++, 50 to 80% rounding; +, 20 to 40% rounding; −, no rounding. The experiments were repeated three times, and data from a representative experiment are shown.
FIG. 8.
Internalization of IaN-GFP to endosomal compartment of MDCK cells in the presence of Ib. MDCK cells were incubated with IaN-GFP (1 μg/ml) in the absence (A) or presence (B) of Ib (1 μg/ml) for 120 min at 37°C. The cells were fixed, and the localization of IaN-GFP was detected by fluorescence microscopy.
SPR analysis with amine-coupled Ib.
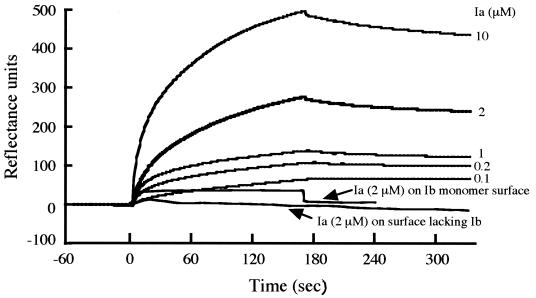
We examined whether monomeric or oligomeric Ib specifically interacts with Ia. SPR analysis showed that the serial injection of Ia at various concentrations results in a strong binding of Ia to the oligomer of Ib (Fig. 9). The association rate constant (kon) and the dissociation rate constant (koff) for Ia were calculated to be 6.53 × 103 M−1 s−1 and 5.61 × 10−3 s−1, respectively, and the equilibrium constant, Kd, calculated from these kinetic constants, was 8.59 × 10−7 M for Ia. However, as shown in Fig. 9, Ia did not bind to the immobilized monomer of Ib. Furthermore, Ia did not bind to a protein-free surface blocked with ethanolamine.
FIG. 9.
SPR analysis of binding of Ia to Ib immobilized on a dextran matrix flow cell surface by primary amine coupling. Injections of Ia (at the indicated concentrations) in HBSS were made onto the Ib oligomer surfaces. The binding of Ia was recorded in real time. Injections were done at 10 μl/min for 170 s, followed by HBSS alone. The experiments were repeated three times, and results for a representative experiment are shown.
DISCUSSION
The action of bacterial protein toxins against eukaryotic cells is dependent on the interaction of the toxin with a specific receptor on the cell surface. Recently, lipid rafts have been reported to act as surface platforms during signal transduction and endocytosis (22), and some toxins (12, 13, 20, 25, 30), bacteria (9), and viruses (7) have been shown to enter cells via lipid rafts. The present study demonstrates that Ib specifically binds to a receptor on the cytoplasmic membrane of MDCK cells and accumulates in lipid rafts and that Ia bound to the oligomer formed on the rafts then enters the cell.
Cy3-Ib was detected on the cell surface up until 60 min of incubation at 37°C and was also found in vesicles in the cytosol. However, it was not found on the membrane after 120 min of incubation, indicating that Ib binds to a receptor on MDCK cells and is then internalized via endocytosis.
Only the monomer of Ib was detected in the Triton X-100-soluble and -insoluble fractions of cells incubated with Ib at 4°C, suggesting that the receptor of Ib is distributed around cytoplasmic membranes. Thus, it is unlikely that the receptor is confined to lipid rafts. The oligomer of Ib was detected in lipid rafts after incubation of the washed cells at 37°C, suggesting that the oligomer is formed in lipid rafts. In addition, the C-terminal region of Ib (Ib421-664) blocked the binding of Ib to the cells, as reported by Marvaud et al. (17), and was detected in lipid raft fractions from MDCK cells. It therefore appears that the C-terminal region of Ib binds to a receptor that is distributed over the entire membrane surface of the cell, that a receptor which is linked with Ib gathers in lipid rafts at 37°C, and that Ib forms oligomers in the lipid rafts.
Lipid rafts are postulated to be cholesterol- and glycosphingolipid-enriched microdomains of the cell plasma membrane that are different from the rest of the membrane. The treatment of MDCK cells with MβCD reduced the cholesterol content of lipid raft fractions, the binding of Ib to the cells, and the rounding activity induced by Ia plus Ib. However, cholesterol had no effect on the activity induced by Ia plus Ib, showing that Ib does not directly interact with cholesterol in lipid rafts. It has been speculated that the functional properties of lipid rafts that are relevant to the intracellular trafficking of Ib may be especially susceptible to treatment with MβCD. It has been reported that the disruption or depletion of cell membrane-associated cholesterol causes major changes in the function and/or distribution of raft-associated membrane components (20, 30). It therefore appears that a function of lipid rafts is to gather Ib so that it can form oligomers and enter cells.
In the present study, Ia was detected in the raft fraction with, but not without, Ib oligomers. Furthermore, SPR analysis showed that Ia binds specifically to the Ib oligomer, not the monomer. Milne et al. (19) reported that B. anthracis lethal factor can bind to the PA oligomer, but not the PA monomer. Thus, it appears that Ia binds to the oligomers of Ib in the rafts. It was reported previously that the Ib oligomer itself induces endocytosis (24). The deletion of amino acid residues from the N domain of Ia provided evidence of a binding site for Ib in the N domain of Ia. Furthermore, IaN-GFP was observed in vesicles in the cytosol in the presence of Ib, but not in its absence, showing that residues 151 to 210 of Ia are critical for the recognition of the oligomer. We therefore concluded that Ia bound to Ib oligomers formed in the lipid rafts is internalized in the cells by endocytosis induced by the oligomers.
The pretreatment of MDCK cells with MβCD resulted in a reduction in the cell rounding induced by Ia plus Ib and in the binding of Ib oligomers to lipid raft fractions of the cells, but there was no effect on the binding of Ib oligomers to nonlipid raft fractions, suggesting that the activity induced by Ia plus Ib is dependent on the binding of Ib oligomers to lipid rafts. The levels of Ib monomer bound to lipid raft fractions at 4°C decreased after an additional incubation at 37°C. However, the Ib oligomer was not detected in lipid raft fractions at 4°C but appeared after an additional incubation at 37°C. In addition, Ia specifically bound to the Ib oligomer in vitro, but not the Ib monomer. Members of our laboratory reported that Ib spontaneously self-associates to form oligomers (500-kDa heptamers) and that the incubation of cells with Ib oligomers plus Ia results in no effect on the cells (24). The incubation of liposomes with the toxin at 37°C caused the formation of a complex (500 kDa) of the toxin and the release of CF from the liposomes, showing that the complex is a functional oligomer (heptamer). It therefore appears that the toxin forms a functional heptamer as a pore in the biological membrane. Furthermore, it is likely that no membrane protein was included in the oligomer that was formed on the surface of the cells. These results suggest that Ib monomers bind to receptors in lipid rafts and form functional oligomers in the membrane without any change in Ib, although we cannot rule out the possibility that the Ib monomer may translocate to lipid raft fractions from nonlipid raft fractions.
Several bacterial pore-forming toxins have been reported to utilize lipid rafts to intoxicate cells. Aerolysin and Clostridium septicum alpha-toxin bind to glycosylphosphatidylinositol-anchored proteins in lipid rafts (1, 11), and C. perfringens epsilon-toxin and perfringolysin bind to cholesterol in lipid rafts (20, 34). It has been proposed that lipid rafts serve as concentrating platforms to promote pore formation by toxins that form oligomers. Our findings indicate that the internalization of iota-toxin is mediated through lipid rafts (cholesterol-rich microdomains) at the plasma membrane, suggesting that the lipid rafts contain all of the necessary components for the mediation of toxin endocytosis. The findings that Ib is concentrated in lipid rafts, where it forms oligomers, and that it induces endocytosis have provided useful information on the cytotoxicity induced by bacterial toxins.
Acknowledgments
This research was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by the Open Research Center Fund for Promotion, and by the Mutual Aid Corporation for Private Schools of Japan.
We thank C. Matsubara, T. Kojima, M. Nakanishi, A. Tanabe, and M. Harui for their competent technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Abrami, L., M. Fivaz, P.-E. Glauser, R. J. Parton, and F. G. van der Goot. 1998. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J. Cell Biol. 140:525-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aktories, K., and A. Wegner. 1989. ADP-ribosylation of actin by clostridial toxins. J. Cell Biol. 109:1385-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth, H., D. Blöcker, J. Behlke, W. Bergsma-Schutter, A. Brisson, R. Benz, and K. Aktories. 2000. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J. Biol. Chem. 275:16478-16483. [DOI] [PubMed] [Google Scholar]
- 5.Blöcker, D., J. Behlke, K. Aktories, and H. Barth. 2001. Cellular uptake of the Clostridium perfringens binary iota-toxin. Infect. Immun. 69:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borriello, S. P., and R. J. Carman. 1983. Association of iota-like toxin and Clostridium spiroforme with both spontaneous and antibiotic-associated diarrhea and colitis in rabbits. J. Clin. Microbiol. 17:414-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chazal, N., and D. Gerlier. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domenighini, M., and R. Rappuoli. 1996. Three conserved consensus sequences identify the NAD+-binding site of ADP-ribosylating enzymes, expressed by eukaryotes, bacteria and T-even bacteriophages. Mol. Microbiol. 21:667-674. [DOI] [PubMed] [Google Scholar]
- 9.Duncan, M. J., J. S. Shin, and S. N. Abraham. 2002. Microbial entry through caveolae: variations on a theme. Cell. Microbiol. 4:783-791. [DOI] [PubMed] [Google Scholar]
- 10.Eckhardt, M., H. Barth, D. Blöcker, and K. Aktories. 2000. Binding of Clostridium botulinum C2 toxin to asparagine-linked complex and hybrid carbohydrates. J. Biol. Chem. 275:2328-2334. [DOI] [PubMed] [Google Scholar]
- 11.Gordon, V. M., K. L. Nelson, J. T. Buckley, V. L. Stevens, R. K. Tweten, P. C. Elwood, and S. H. Leppla. 1999. Clostridium septicum alpha toxin uses glycosylphosphatidylinositol-anchored protein receptor. J. Biol. Chem. 274:27274-27280. [DOI] [PubMed] [Google Scholar]
- 12.Herreros, J., T. Ng, and G. Schiavo. 2001. Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol. Biol. Cell 12:2947-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katagiri, Y. U., T. Mori, H. Nakajima, C. Katagiri, T. Taguchi, T. Takeda, N. Kiyokawa, and J. Fujimoto. 1999. Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J. Biol. Chem. 274:35278-35282. [DOI] [PubMed] [Google Scholar]
- 14.Kilsdonk, E. P. C., P. G. Yancey, G. W. Stoudt, F. W. Bangerter, W. J. Johnson, M. C. Phillips, and G. H. Rothbalt. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 270:17250-17256. [DOI] [PubMed] [Google Scholar]
- 15.Knapp, O., R. Benz, M. Gibert, J. C. Marvaud, and M. R. Popoff. 2002. Interaction of Clostridium perfringens iota-toxin with lipid bilayer membranes. Demonstration of channel formation by the activated binding component Ib and channel block by the enzyme component Ia. J. Biol. Chem. 277:6143-6152. [DOI] [PubMed] [Google Scholar]
- 16.Magalhaes, A. C., J. A. Silva, K. S. Lee, V. R. Martins, V. F. Prado, S. S. Ferguson, M. V. Gomez, R. R. Brentani, and M. A. Prado. 2002. Endocytic intermediates involved with the intracellular trafficking of a fluorescent cellular prion protein. J. Biol. Chem. 277:33311-33318. [DOI] [PubMed] [Google Scholar]
- 17.Marvaud, J. C., T. Smith, M. L. Hale, M. R. Popoff, L. A. Smith, and B. G. Stiles. 2001. Clostridium perfringens iota-toxin: mapping of receptor binding and Ia docking domains on Ib. Infect. Immun. 69:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marvaud, J. C., B. G. Stiles, A. Chenal, D. Gillet, M. Gibert, L. A. Smith, and M. R. Popoff. 2002. Clostridium perfringens iota toxin. Mapping of the Ia domain involved in docking with Ib and cellular internalization. J. Biol. Chem. 277:43659-43666. [DOI] [PubMed] [Google Scholar]
- 19.Milne, J. C., D. Furlong, P. C. Hanna, J. S. Wall, and R. J. Collier. 1994. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 269:20607-20612. [PubMed] [Google Scholar]
- 20.Miyata, S., J. Minami, E. Tamai, O. Matsushita, S. Shimamoto, and A. Okabe. 2002. Clostridium perfringens epsilon-toxin forms a heptameric pore within the detergent-insoluble microdomains of Madin-Darby canine kidney cells and rat synaptosomes. J. Biol. Chem. 277:39463-39468. [DOI] [PubMed] [Google Scholar]
- 21.Mourez, M., D. B. Lacy, K. Cunningham, R. Legmann, B. R. Sellman, J. Mogridge, and R. J. Collier. 2002. 2001: a year of major advances in anthrax toxin research. Trends Microbiol. 10:287-293. [DOI] [PubMed] [Google Scholar]
- 22.Nabi, I. R., and P. U. Le. 2003. Caveolae/raft-dependent endocytosis. J. Cell Biol. 161:673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagahama, M., Y. Sakaguchi, K. Kobayashi, S. Ochi, and J. Sakurai. 2000. Characterization of the enzymatic component of Clostridium perfringens iota-toxin. J. Bacteriol. 182:2096-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagahama, M., K. Nagayasu, K. Kobayashi, and J. Sakurai. 2002. Binding component of Clostridium perfringens iota-toxin induces endocytosis in Vero cells. Infect. Immun. 70:1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagahama, M., S. Hayashi, S. Morimitsu, and J. Sakurai. 2003. Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J. Biol. Chem. 278:36934-36941. [DOI] [PubMed] [Google Scholar]
- 26.Perelle, S., M. Gibert, P. Boquet, and M. R. Popoff. 1993. Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. Infect. Immun. 61:5147-5156. (Erratum, 63:4967, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-838. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai, J., M. Nagahama, and S. Ochi. 1997. Major toxins of Clostridium perfringens. J. Toxicol. Toxin Rev. 16:195-214. [Google Scholar]
- 29.Sakurai, J., M. Nagahama, J. Hisatsune, N. Katunuma, and H. Tsuge. 2003. Clostridium perfringens iota-toxin, ADP-ribosyltransferase: structure and mechanism of action. Adv. Enzyme Regul. 43:361-377. [DOI] [PubMed] [Google Scholar]
- 30.Schraw, W., Y. Li, M. S. McClain, F. G. van der Goot, and T. L. Cover. 2002. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J. Biol. Chem. 277:34642-34650. [DOI] [PubMed] [Google Scholar]
- 31.Stiles, B. G., M. L. Hale, J. C. Marvaud, and M. R. Popoff. 2000. Clostridium perfringens iota toxin: binding studies and characterization of cell surface receptor by fluorescence-activated cytometry. Infect. Immun. 68:3475-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stiles, B. G., M. L. Hale, J. C. Marvaud, and M. R. Popoff. 2002. Clostridium perfringens iota toxin: characterization of the cell-associated iota b complex. Biochem. J. 367:801-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuge, H., M. Nagahama, H. Nishimura, J. Hisatsune, Y. Sakaguchi, Y. Itogawa, N. Katunuma, and J. Sakurai. 2003. Crystal structure and site-directed mutagenesis of enzymatic components from Clostridium perfringens iota-toxin. J. Mol. Biol. 325:471-483. [DOI] [PubMed] [Google Scholar]
- 34.Waheed, A. A., Y. Shimada, H. F. Heijnen, M. Nakamura, M. Inomata, M. Hayashi, S. Iwashita, J. W. Slot, and Y. Ohno-Iwashita. 2001. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts). Proc. Natl. Acad. Sci. USA 98:4926-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]