Abstract
Streptococcus gordonii is a pioneer colonizer of the teeth, contributing to the initiation of the oral biofilm called dental plaque. To identify genes that may be important in biofilm formation, a plasmid integration library of S. gordonii V288 was used. After screening for in vitro biofilm formation on polystyrene, a putative biofilm-defective mutant was isolated. In this mutant, pAK36 was inserted into a locus encoding a novel two-component system (bfr [biofilm formation related]) with two cotranscribed genes that form an operon. bfrA encodes a putative response regulator, while bfrB encodes a receptor histidine kinase. The bfr mutant and wild-type strain V288 showed similar growth rates in Todd-Hewitt broth (THB). A bfr-cat fusion strain was constructed. During growth in THB, the reporter activity (chloramphenicol acetyltransferase) was first detected in mid-log phase and reached a maximum in stationary phase, suggesting that transcription of bfr was growth stage dependent. After being harvested from THB, the bfr mutant adhered less effectively than did wild-type strain V288 to saliva-coated hydroxyapatite (sHA). To simulate pioneer colonization of teeth, S. gordonii V288 was incubated with sHA for 4 h in THB with 10% saliva to develop biofilms. RNA was isolated, and expression of bfrAB was estimated. In comparison to that of cells grown in suspension (free-growing cells), bfr mRNA expression by sessile cells on sHA was 1.8-fold greater and that by surrounding planktonic cells was 3.5-fold greater. Therefore, bfrAB is a novel two-component system regulated in association with S. gordonii biofilm formation in vitro.
Dental plaque is a natural polymicrobial biofilm formed on saliva-coated tooth surfaces. One of several pioneer colonizers that initiate the formation of dental plaque, Streptococcus gordonii is an oral commensal that causes no known disease in the oral cavity (20). If it enters the blood, however, S. gordonii and other viridans group streptococci behave as pathogens to cause infective endocarditis (1, 18). S. gordonii must adhere to and colonize tissue surfaces in vivo in flowing blood or saliva. Alternatively, the microorganism will be cleared by the reticuloendothelial system or by swallowing, respectively. Many laboratories seek to understand how this organism adheres and initiates biofilm formation.
Biofilms grow and form microscopic communities of distinct and reproducible architecture (14) by a multistep process (4, 14, 33). Consistent with the differentiation of cells in biofilms, sessile cells and planktonic cells show different gene expression profiles (6, 21, 29, 30, 32, 34, 38-40). Furthermore, gene expression changes during biofilm development (21, 29). To understand the molecular mechanisms and the evolutionary basis of survival of these species, key gene regulation pathways of biofilm formation must be elucidated.
S. gordonii adheres to specific surfaces in the host to colonize and form biofilms (8). Properties of surfaces on which biofilms develop can also affect the structure of S. gordonii biofilms (17). The ability to bind and colonize requires that S. gordonii express a mechanism to sense and respond to environmental signals. Two-component systems (TCS) are common signal transduction mechanisms used by bacteria to respond to environmental signals (10). Composed of a sensor (receptor histidine kinase) and a response regulator, a TCS interacts with signals causing the autophosphorylation of the sensor. The sensor then transfers a phosphoryl group to activate the coupled response regulator.
TCS have been suggested to regulate biofilm formation by Escherichia coli (5, 24), Pseudomonas aeruginosa (25), Staphylococcus aureus (7), and Streptococcus mutans (2, 15). In this paper, we identify a novel TCS in S. gordonii, bfr (biofilm formation related). Compared with the wild type, the bfr mutant showed decreased biofilm formation on polystyrene surfaces and adhered poorly to saliva-coated hydroxyapatite (sHA). In addition, sessile and planktonic cells associated with early biofilms on sHA expressed higher levels of bfr than did free-growing cells in the absence of sHA. These data suggest that bfr may play an important role in early biofilm development by S. gordonii.
MATERIALS AND METHODS
S. gordonii V288 integration library.
The S. gordonii V288 integration library (35) was constructed with the integration vector pAK36, which contains two promoterless reporter genes, amy and cat, as we have reported previously (12). To construct this library, chromosomal DNA of S. gordonii V288 was digested and inserted upstream of the amy and cat genes of pAK36. The ligation mixture was used to transform S. gordonii V288. The resulting transformants with tetracycline (TC) resistance were pooled.
Bacterial strains and culture conditions.
S. gordonii V288 was grown in Todd-Hewitt broth (THB) at 37°C in 5% CO2. The S. gordonii V288 integration library was grown in THB supplemented with TC at 10 μg ml−1. E. coli JM 109 was grown aerobically in LB medium at 37°C. The concentrations of the antibiotics used in medium for selection were as follows: TC, 10 μg ml−1 (E. coli or S. gordonii); chloramphenicol, 5 μg ml−1 (E. coli).
Biofilm formation screening assay.
To screen the integration library for strains defective in biofilm formation, a modification of the methods of O'Toole et al. (23) and Loo et al. (17) was used. The S. gordonii V288 integration library was plated on THB agar containing 10 μg of TC ml−1 and incubated for 20 h. Colonies were picked and transferred to wells on duplicate polystyrene microtiter plates (Costar 3799) containing 100 μl of THB with TC. Both plates were incubated at 37°C for 20 h, and then 25 μl of a 1% solution of crystal violet was added to each well. The plates were incubated at room temperature for 15 min and rinsed thoroughly with water to remove planktonic cells and unincorporated stain. Biofilm formation was quantified by the addition of 200 μl of 95% ethanol to each crystal violet-stained well, of which 125 μl was transferred to a new polystyrene microtiter plate. The A568 was determined with an enzyme-linked immunosorbent assay plate reader. Bacterial clones with low absorbance were picked as putative biofilm formation-defective mutants.
DNA manipulations.
Standard recombinant DNA techniques, as described by Sambrook et al. (28), were used. A QIAprep Spin Miniprep kit (Qiagen) was used to isolate plasmid from E. coli, a QIAquik PCR purification kit was used to purify PCR products, and a QIAamp DNA mini kit was used to isolate S. gordonii chromosomal DNA. DNA restriction and modification enzymes were used under the conditions recommended by the manufacturer (Promega, Madison, Wis.).
Oligonucleotide primers.
The sequences of the primers used in this study are listed in Table 1.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence | Source or reference |
|---|---|---|
| ORIEXT | 5′-AGCTCAGAGAACCTTCGAAAAAACC-3′ | This study |
| ARB1 | 5′-GGCCACGCGTCGACTAGTACNNNNNN NNNNGATAT-3′ | 23 |
| ORIINT | 5′-CAAGAGATTACGCGCAGACC-3′ | 35 |
| ARB2 | 5′-GGCCACGCGTCGACTAGTAC-3′ | 23 |
| BFRAF | 5′-CGCGGATCCTGGTAAGAATGGTGAC CA-3′ | This study |
| BFRAR | 5′-ACATGCATGCGCTTCTTCCCATACAG AT-3′ | This study |
| BGLF | 5′-GGCTATGTAGCTGATGATTAC-3′ | This study |
| BFRAR2 | 5′-CAACATACTCTTCGGATGGA-3′ | This study |
| BFRPP1 | 5′-CTGCCCGGGGTGCTTTGTCCCTATT CCGAT-3′ | This study |
| This study | ||
| BFRPP2 | 5′-AATGAGCTCCTTCGCTCCATTTTTA CT-3′ | This study |
| CATPCR | 5′-AACACTAATATCAATTTCTGTGG-3′ | 19 |
| CATSEQ | 5′-CTAAAAGTCGTTTGTTGG-3′ | 19 |
| BFRF | 5′-CCATCCGAAGAGTATGTTGA-3′ | This study |
| BFRR | 5′-CATGTAGCCTGTTGACGCT-3′ | This study |
| 16RNAF | 5′-TCCATGTGTAGCGGTGAAATG-3′ | This study |
| 16RNAR | 5′-TCCTTTGAGTTTCAACCTTGC-3′ | This study |
Arbitrarily primed PCR and sequence analysis.
The DNA sequences flanking the insertion vector were determined by arbitrary PCR (3, 23). In the first round of PCR, ORIEXT, a primer unique to the ori sequence of pACYC184, and arbitrary primer 1 (ARB1) were added to a 50-μl PCR mixture (1× Vent polymerase buffer, 1 mM MgSO4, 0.25 mM deoxynucleoside triphosphates [dNTPs], 1 U of Vent [exo+] DNA polymerase [New England Biolabs, Boston, Mass.]). The mixture of ORIEXT and ARB1 was added to the DNA template of 5 μl of a culture grown overnight in THB. The first-round PCR conditions were (i) 95°C for 5 min; (ii) 6 cycles of 94°C for 30 s, 30°C for 30 s, and 72°C for 1 min; (iii) 30 cycles of 94°C for 30 s, 43°C for 30 s, and 72°C for 1 min; and (iv) 72°C for 10 min. The components of the second-round PCRs were similar to those of the first round, except that 5 μl of the first-round PCR product was used as template DNA and the primers were ARB2 and ORIINT. The ARB2 sequence is identical to the 5′ end of primer ARB1. The ORIINT sequence was derived from the ori sequence of pACYC184 (accession no. X06403) closer to the BamHI site than ORIEXT, near the junction between pAK36 (35) and the insert fragments. The conditions of the second-round PCR were (i) 30 cycles of 94°C for 30 s, 43°C for 30 s, and 72°C for 1 min and (ii) 72°C for 10 min. The PCR products were purified with the QIAquick PCR purification kit. The purified PCR products were sequenced at the University of Minnesota DNA Sequencing Facility with the ORIINT primer. With the BLAST program, the sequences were compared with the GenBank database and the partial S. gordonii genome sequence provided by TIGR (The Institute for Genomic Research, http://www.tigr.org).
Construction of a bfr-cat fusion strain.
The bfr promoter region was amplified by PCR with primers BFRPP1 (containing a SmaI restriction site) and BFRPP2 (containing a SacI restriction site). After digestion with SmaI and SacI, the PCR-amplified product (362 bp) was cloned into pMH109 (11), which had been previously digested with SmaI and SacI. pMH109 contains a promoterless gram-positive cat gene. The ligation mixture was transformed into E. coli JM109 with selection for resistance to TC and chloramphenicol. Recombinant plasmid pMH109 was then digested with BamHI to release a 1.2-kb bfr-cat fragment, which was cloned into BamHI-digested pSF143 (36). The resulting recombinant plasmid, pSFbfrCAT, was introduced into the S. gordonii chromosome as described by Tao and Herzberg (35) and selected for TC resistance. The resultant recombinant strain was named MG288-1026. To confirm that the correct fusion construct on the chromosome had been generated, primers BGLF and CATPCR (Table 1) were used to amplify the promoter-cat fusion from MG288-1026. The PCR product was sequenced directly with BGLF and a cat-defined nested primer, CATSEQ (Table 1).
Preparation of cell extracts and CAT assay.
After preculture to stationary phase, harvesting, and 1:10 dilution in fresh prewarmed medium, S. gordonii cells grown in THB (10 ml) were harvested by centrifugation (4,000 × g, 15 min, 4°C) and washed once with TPE buffer (100 mM Tris/HCl [pH 7.8] containing 1 mM phenylmethylsulfonyl fluoride and 1 mM EDTA) (19). Bacterial cells were resuspended in 1 ml of TPE buffer, transferred into a tube containing glass beads (FastProtein Blue kit; Bio 101, Savant, N.Y.), and disrupted with a FastPrep FP120 machine (vortex mixing for two periods of 1 min at speed 6). Suspensions were centrifuged (13,000 rpm [Micromax PF120; IEC, Mass.], 3 min, 4°C) to remove glass beads and cell debris, and the supernatants were removed for enzyme assays. Protein concentrations were determined with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.) with bovine serum albumin as the standard. Chloramphenicol acetyltransferase (CAT) enzyme activities in 50 μl of cell extracts were measured by a 5,5′-dithiobis(2-nitrobenzoic acid) assay with a recording spectrophotometer with a temperature-controlled cuvette (31).
Preparation of sHA.
Human whole saliva was collected from several donors and pooled as described previously (9), and an informed-consent protocol that was reviewed and approved by the University of Minnesota Institutional Review Board was used. After clarification by centrifugation, the supernatant was sterilized by filtration through a 0.22-μm-pore-size polystyrene filter (Corning Glass Works, Corning, N.Y.). The sterile whole saliva was stored at −80°C until use. To prepare sHA for the biofilm assay, 100 mg of hydroxyapatite (catalog no. 159814; ICN, Aurora, Ohio) was equilibrated for 1 h with 1 mM KH2PO4-K2HPO4 buffer (pH 6.8) with 50 mM KCl, 1 mM CaCl2, and 0.1 mM MgCl2 (modified Gibbons' buffer) at ambient temperature. Equilibrated hydroxyapatite was then incubated with 3 ml of sterile whole saliva for 1 h at 37°C in 5% CO2 and washed three times with ice-cold modified Gibbons' buffer. To prepare sHA for the adhesion assay in buffer, 10 mg of hydroxyapatite (ICN) was equilibrated for 1 h with modified Gibbons' buffer at ambient temperature. Immediately before use, equilibrated hydroxyapatite was incubated with 1 ml of sterile whole saliva for 1 h at ambient temperature and then washed three times with modified Gibbons' buffer.
Early biofilm assay with sHA.
S. gordonii V288 cells (3 ml) were harvested from an overnight THB culture by centrifugation, resuspended in an equal volume of fresh THB with 10% sterile whole saliva, mixed with 100 mg of fresh sHA, and then incubated with continuous inversion on a roto-torque at 37°C in 5% CO2. After 4 h, the tubes were placed on ice until sHA beads settled at the bottom. The supernatant containing planktonic cells was aspirated and transferred to a new tube. Loosely associated cells were removed by washing three times with ice-cold modified Gibbons' buffer; sessile cells remained associated with sHA. In parallel, cells (3 ml) were cultured in suspension without sHA in THB with 10% saliva under the same incubation conditions. These cells grown in the absence of an adhesion surface were termed free-growing cells. Planktonic cells (unattached to sHA) and free-growing cells (incubated in the sHA-free THB) were harvested by centrifugation.
sHA adhesion assay.
The sHA adhesion assay used was a modification of methods used by Liljemark et al. (16) and Tellefson and Germaine (37). S. gordonii V288 was cultured overnight with [3H]thymidine (10 μCi/ml) in THB medium, centrifuged, and diluted to an optical density at 620 nm of 0.3 (109 cells/ml) with modified Gibbons' buffer. The cells (1 ml) were then incubated with 10 mg of sHA for 30 min at ambient temperature with continuous inversion on a roto-torque. The unattached cells were removed by aspiration. The sHA with attached bacteria was washed three times to remove additional unattached cells. The radioactivity associated with sHA was monitored by liquid scintillation counting. The percentage of adherent or sHA-associated bacteria was calculated as the ratio of radioactivity in counts per minute associated with sHA to the total counts per minute in the 1-ml input suspension × 100.
RNA isolation.
Collected sessile, planktonic, and suspension cells were resuspended in lysing reagents (FastRNA Blue kit; Bio 101) containing 0.6 M sodium phosphate (pH 6.8), transferred to FastPrep Blue tubes, and processed in a FastPrep FP120 machine (Bio 101) at a speed rating of 6 for 2 min. Disrupted cells were incubated for 5 min at 60°C to permit RNA to desorb from hydroxyapatite and then maintained for an additional 5 min at room temperature. After centrifugation (12,000 × g, 10 min), the supernatant was extracted with chloroform-isoamyl alcohol and passed through Spin-30 diethyl pyrocarbonate-H2O columns (BD Biosciences) to remove sodium phosphate. Each column was loaded with 50 μl of supernatant and spun for 5 min at 700 × g at room temperature. Desalted RNA was precipitated from solution with isopropanol. The integrity of the RNA was checked by electrophoresis through 1.0% (wt/vol) nondenaturing agarose gels. The RNA obtained was then digested with DNase I (Takara Shuzo Co., Ltd.) for 2 h at 37°C and finally purified with an RNeasy mini kit (Qiagen Inc.) in accordance with the manufacturer's instructions. The concentration of RNA was determined by measuring the A260 in a spectrophotometer.
Reverse transcription.
RNA (10 μg) was mixed in water with 500 pmol of random hexamer primers in a final volume of 68 μl, incubated at 80°C for 5 min, and placed on ice for 5 min. A reverse transcriptase (RT) enzyme master mixture (32 μl) containing 20 μl of 5× reaction buffer, 5 μl (100 U) of anti-RNase (Ambion, Houston, Tex.), 5 μl of 10 mM dNTP, and 2 μl (100 U) of Moloney murine leukemia virus RT (Promega) was then added to the primer-annealed RNA. The reaction mixture was incubated at 40°C for 1 h and at 92°C for 10 min. As negative controls, all RNA samples were also incubated without RT.
Differential gene expression.
The bfr mRNAs in sessile, planktonic, and suspension cells of S. gordonii were quantified and compared by a PCR method. Template cDNA obtained by reverse transcription in 1 μl was mixed with 24 μl of a PCR mixture in a final reaction volume of 25 μl containing 5 μl of 5× Green Goto Taq buffer (Promega), 2 mM dNTP, 25 pmol (each) of the specific forward and reverse primers, and 2.5 U of Taq polymerase (Promega). The PCR conditions were (i) 95°C for 5 min; (ii) 30 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 1 min; and (iii) 72°C for 10 min. Each specific PCR product (5 μl) was analyzed by electrophoresis in a 1.8% agarose gel containing ethidium bromide. After electrophoresis, the gel was exposed to UV light (312 nm) and specific PCR products were quantified for the absolute intensity of each band with Kodak 1D image analysis software (Eastman Kodak, Rochester, N.Y.).
To estimate the amount of specific mRNA transcript expressed under a set of conditions, serial PCRs with templates of 100, 10, 1, 0.1, 0.01, and 0.001 ng of purified S. gordonii V288 genomic DNA were performed for bfr and the 16S rRNA gene. For each gene, the intensity of a specific band was plotted against the log10 of the amount of starting template. A specific equation for each gene was derived from the linear range of each plot and used to estimate the relative amount of that gene in the starting templates for RT-PCR. Relative amounts of bfr in sessile, planktonic, and suspension cells were normalized by comparison with the absolute amount of 16S rRNA.
RESULTS
Identification of bfr in S. gordonii V288.
With a plasmid integration library of S. gordonii V288 constructed with pAK36 (35), biofilm-defective mutants were isolated from the polystyrene surface of 96-well microtiter plates. About 7,500 transformants were screened for biofilm defects; 21 different mutants were identified. One mutant, named MG288-1015, showed a 35% ± 2.1% (mean ± standard error [SE], n = 3) reduction in biofilm formation. In this mutant, pAK36 was inserted into a novel TCS as shown by sequence analysis. This TCS was designated bfr.
The bfr locus shows close homology with a putative TCS in S. mutans UA159 (NC_004350). BfrA shows 62% identity with the putative S. mutans response regulator (GenBank accession number NP_721431.1); BfrB and the putative histidine kinase (GenBank accession number NP_721431.1) are 46% identical. The bfrA gene encodes a 25.7-kDa putative response regulator consisting of 226 amino acids. The bfrB gene encodes a 32.9-kDa putative histidine kinase consisting of 291 amino acids. A possible start codon for bfrA was determined by context (Fig. 1A). The putative start codon is GTG, which follows an upstream ribosomal binding site, GGAGA. The start codon of bfrB immediately follows the stop codon of bfrA, and a potential ribosome binding site for bfrB is located within the 3′-terminal part of bfrA (Fig. 1A). The proximity of the bfrA and bfrB genes suggests that they may be cotranscribed.
FIG. 1.
The bfr operon and its flanking genes. (A) Putative ribosome binding sites (RBS) of bfrA and bfrB. The arrows show translation directions. GTG serves as a putative start codon of bfrA. aa, amino acids. (B) ORFs in the bfr locus. The arrows show transcription directions. The triangle shows the site of pAK36 insertion into bfr. From the GenBank database, the protein most homologous to each numbered S. gordonii ORF is as follows: 1, pyruvate oxidase of Streptococcus oralis (accession no. AAC69578); 2, hypothetical protein of Streptococcus pneumoniae R6 (accession no. NP_358237); 3, hypothetical protein of Lactobacillus gasseri (accession no. ZP_00046833); 4, conserved hypothetical protein of S. mutans UA159 (accession no. NP_721848); 5,6-phospho-β-glucosidase of S. mutans UA159 (accession no. NP_721491); 6, hypothetical protein of S. mutans UA159 (accession no. NP_721429); 7, putative ABC transporter, ATP-binding protein of S. mutans UA159 (accession no. NP_721428).
bfr locus.
The flanking open reading frames (ORFs) of bfr were assembled on the basis of the TIGR unfinished genome sequence of S. gordonii, and the site of insertion of the pAK36 vector within bfrA is indicated in Fig. 1B. A bgl gene, encoding a 6-phospho-β-glucosidase, is located immediately upstream of the bfr operon and transcribed in the same direction. There are 383 nucleotides between the start codon of bfrA and the stop codon of the bgl gene. The stop codon of the bgl gene was followed by an inverted repeat sequence, which may form a transcriptional terminator. A typical upstream promoter was not found. A poxA gene, encoding a pyruvate oxidase, is located upstream of bgl and transcribed in the same direction. Three ORFs, encoding hypothetical proteins, exist between the poxA gene and the bgl gene. An ORF encoding a hypothetical protein is located immediately downstream of bfr. This ORF is transcribed in the opposite direction of bfr and may form an operon with the ORF immediately upstream, which encodes a putative ABC transporter.
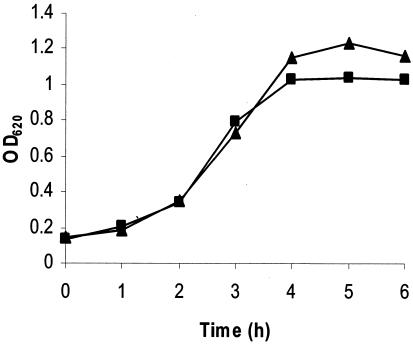
Growth of S. gordonii bfr mutant.
bfr mutant strain MG288-1015 and parent strain S. gordonii V288 showed similar growth rates in THB broth maintained in air with 5% CO2 (Fig. 2).
FIG. 2.
Growth of S. gordonii V288 and MG288-1015 in THB. Symbols: ▴, S. gordonii V288; ▪, strain MG288-1015. The data shown are representative of three independent experiments. OD620, optical density at 620 nm.
sHA adhesion assay.
As a pioneer colonizer, S. gordonii relies on its ability to adhere to saliva-coated tooth surfaces to initiate the formation of dental plaque. To test whether a bfrAB mutation affects the adhesion of S. gordonii to saliva-coated surfaces, an sHA adhesion assay was performed with modified Gibbons' buffer. Under these conditions, S. gordonii neither synthesizes detectable trichloroacetic acid-precipitable protein nor shows apparent growth (M. C. Herzberg and H. F. Jenkinson, unpublished data). Under these conditions, 14.2% ± 3.2% (mean ± SE, n = 4) of bfrAB mutant cells adhered to sHA, in contrast to wild-type V288 cells, which showed 34.0% ± 5.1% (mean ± SE, n = 4) adhesion.
bfr expression profile.
To estimate the activity of the bfr promoter during growth, bfr-cat fusion strain MG288-1026 was cultured in THB supplemented with TC at 37°C with 5% CO2. Cell extracts were prepared at intervals for measurement of CAT activity. CAT activity in MG288-1026 cultures was detected at mid-exponential phase and reached a maximum at stationary phase (Fig. 3). CAT activity appeared to be stable through the first several hours of stationary phase (data not shown).
FIG. 3.
Expression of bfrAB by S. gordonii MG288-1026 during growth. CAT activity (⧫) was the reporter for the bfr promoter activity, and growth was determined spectrophotometrically (▵, culture density). The data shown are representative of three independent experiments. OD600, optical density at 600 nm.
Expression of bfr in early biofilms on sHA.
To simulate pioneer colonization on teeth, S. gordonii cells were incubated with sHA in THB medium supplemented with 10% saliva for 4 h at 37°C with 5% CO2. For comparison, cells were cultured in suspension as described above but without sHA. These cultures yielded free-growing cells in the absence of an adhesion target, sHA. Sessile and planktonic cells were recovered from the tubes with sHA. RNA was then isolated from each cell phenotype and analyzed for expression of bfr by RT-PCR. The expression of bfr by cells with each phenotype was compared to that by free-growing cells. After 4 h, biofilm-associated cells expressed more bfr-specific mRNA than did free-growing cells. The sessile cells expressed 1.8-fold ± 0.2-fold (mean ± SE, n = 3) more bfr-specific mRNA than did free-growing cells; planktonic cells expressed 3.5-fold ± 0.4-fold (mean ± SE, n= 3) more specific mRNA than did free-growing cells. In these experiments, the forward primer, BFRF, was located in the 3′-terminal part of bfrA (nucleotides 623 to 641) while the reverse primer, BFRR, was located in the 5′-terminal part of bfrB (nucleotides 107 to 125). Cotranscription of bfrA and bfrB was also confirmed.
DISCUSSION
S. gordonii is a pioneer colonizer of dental plaque, relying on its ability to adhere to survive and maintain its place in the oral cavity. We are interested in learning how pioneer oral streptococci control adhesion and enable the initiation of biofilms on teeth. We screened a mutational library of S. gordonii strains for the ability to form biofilms on polystyrene surfaces. We report here on the characteristics of a strain with reduced biofilm formation on polystyrene surfaces that contained a mutation in a novel TCS (designated bfrAB).
To learn if the bfrAB mutation affects adhesion to a relevant target, free-growing cells were harvested, washed in buffer, and allowed to adhere to sHA in buffer for 1 h. The bfr mutant showed reduced adhesion to sHA. Under these conditions, the cells were unable to synthesize new protein or grow. Hence, bfrAB may regulate the constitutive adhesion ability of S. gordonii.
To simulate early S. gordonii colonization in vivo, biofilms were developed on sHA incubated in THB and 10% saliva. To study bfr expression, RNA was isolated from biofilm-adherent (sessile) and nonadherent (planktonic) cells. By 4 h, bfrAB-specific mRNA was more abundant in cells forming biofilms on sHA than in free-growing cells, suggesting that bfr is involved in the development of oral biofilms. We do not know if the biomass of bfr mutant cells that accumulate in sHA biofilms is less than that of the wild type. The highly charged absorbing characteristics of hydroxyapatite make it challenging to measure the sessile biomass on sHA. These data do strongly suggest, however, that bfrAB is used to control early S. gordonii biofilm formation by regulating the ability of cells to adhere to sHA.
The sHA biofilm model reported here nicely simulates important intraoral environmental conditions appropriate to the analysis of gene regulation during the development of oral biofilms. The system presents S. gordonii (or other species) with a biologically relevant simulation of the surface of teeth, sHA, the concomitant presence of saliva, controlled temperature, and surface shear forces achieved by tumbling the incubation mixture. Conditions were developed to recover RNA reliably from sessile and planktonic cells from the same incubation mixture. For the first time, we can directly estimate the expression of selected genes (or the transcriptome when gene arrays become available) in sessile and planktonic cells simulating pioneer colonization of the tooth.
During biofilm formation in the oral environment, pioneer colonizers like S. gordonii must originate as free-growing cells in the salivary fluid that bathes the teeth. After initial adhesion, cells can detach and reattach or float freely and be swallowed. In the presence of attractive surfaces, bacteria can adhere and grow or detach and assume the planktonic phenotype (13). Some planktonic cells in this environment may then become sessile. Indeed, experiments similar to those reported here were conducted with sHA incubated in chemically defined synthetic medium (Y. Lei and M. C. Herzberg, unpublished). After sessile cells were removed, the recovered planktonic cells were tested for the ability to adhere to fresh sHA. Recovered planktonic cells do become sessile. This experiment can be repeated until the recovery of planktonic cells is exhausted and there are too few cells to manage. Hence, adhesion is a dynamic process that continues as the colonial biofilm develops on the surface (13).
In the microenvironment approximating the surface of the teeth, we reasoned that S. gordonii cells proximal to the salivary film (pellicle) represent phenotypes different from those of free-growing cells elsewhere and more distant from the teeth in the salivary fluid. Indeed, biofilm-associated cells in proximity to the target sHA surface expressed significantly higher levels of bfrAB-specific mRNA than did cells in a separate tube without an available sHA surface. Furthermore, unattached planktonic cells expressed higher levels of bfrAB mRNA than did sessile cells in the same tube. Compared to free-growing cells in the absence of sHA, sessile and planktonic phenotypes are regulated in this system by the proximity of saliva-coated surfaces. In this context, we do not know if proximity implies distance from the target surface or the probability of collisions and transient adhesion events. We do, however, have evidence that biofilm-associated cells release a cell-free factor that alters gene expression in free-growing cells (Zhang and Herzberg, unpublished data), suggesting that proximity may reflect the radius of diffusion and effective concentration of the signaling molecule. Transient adhesion to sHA appears to up-regulate the expression bfrAB in S. gordonii. The presence of 10% saliva in THB is not a sufficient signal. Planktonic cells in a sHA environment express more bfrAB than do sessile cells. A high level of the BfrA and BfrB proteins may be required for planktonic bacteria to become committed sessile cells on sHA. After cells becoming sessile, bfrAB expression appears to be reduced. Since bfrAB appears to regulate the adhesive ability of the cells, we speculate that bfr may be involved in the transition of free-growing bacteria to biofilm-associated planktonic cells to committed sessile cells on sHA.
Our finding of differential expression of bfr among sessile, planktonic, and free-growing cells is consistent with the distinct patterns of gene and protein expression found in other species. For example, Bacillus cereus cells show three different proteomic profiles during biofilm development on glass wool (21). After 2 h of exposure to the glass wool, both sessile and planktonic cells surrounding the glass wool produce more YhbH, a regulatory protein probably involved in the general stress response, than do cells in a culture without glass wool (21). YhbH is assumed to play a key role in the transition from the planktonic phenotype to the sessile biofilm phenotype in B. cereus (21). Comparison of associated gene and protein patterns in these three kinds of cells will further understanding of the regulation of biofilm development and give additional credence to the conclusion that planktonic cells are biofilm associated and differ in gene expression from free-growing cells distant from the target surface.
The bfr locus shows promoter activity in free-growing cells during mid-log-phase growth, maximizing in stationary phase, as shown by reporter (CAT) activity. The increase in promoter activity suggests a corresponding increase in bfr transcription. If confirmed, the expression profile would suggest that bfr is linked to cell growth. On the other hand, bfr does not apparently contribute to the growth of free-growing cells, since the growth curves are similar for wild-type S. gordonii and the bfr mutant. Consistent with the suggestion that bfr may poise free-growing cells to assume a biofilm phenotype, it is of interest that the phenotype of biofilm cells typically approximates that of stationary-phase cells in suspension cultures (33).
S. gordonii bfr and a putative TCS in S. mutans show high homology. Unlike bfr, the homologous TCS from S. mutans has no known function. Furthermore, the homologous S. mutans TCS differs in genetic organization from the other reported TCS of S. mutans, some of which may be biofilm associated (2, 15). S. mutans is found in acidogenic dental plaque associated with dental caries and may initiate biofilm formation in relatively inaccessible pits and fissures and on some protected smooth tooth surfaces (27). S. gordonii is generally found as a pioneer colonizer on the smooth surfaces of teeth. Since both species do initiate biofilms, the TCS in S. mutans and bfr in S. gordonii may be required for similar functions in biofilm development. TCS are also involved in biofilm development in E. coli (5, 24, 26), Pseudomonas aeruginosa (25), and Staphylococcus aureus (7). There is little similarity, however, among the genetic loci of these TCS to one another and S. gordonii bfr, suggesting that varied regulation mechanisms of biofilm development may have evolved in different species to facilitate selective tissue tropisms. To respond to different environmental stimuli, bacteria may require parallel response pathways that converge to enable biofilm formation (22). The existence of more than one biofilm-associated TCS in a species such as S. mutans or E. coli may facilitate biofilm development in different or alternative environments.
The bfr locus contains other genes that may participate in biofilm formation. A bgl gene, encoding a 6-phospho-β-glucosidase, is located immediately upstream of the bfr gene. This bgl gene may be a member of a regulon responsible for β-glucoside metabolism in S. gordonii (A. O. Kiliç et al., unpublished data) that is expressed during colonization of heart valves during experimental endocarditis (12). When our gene fusion library was screened for biofilm formation on polystyrene surfaces, a poxA mutant was also identified as biofilm deficient. The poxA gene is located in the bfr locus (Fig. 1B). Containing at least two other key genes, the bfr locus may be crucial for biofilm formation by S. gordonii, although it is not clear how each of these genes contributes to biofilm development.
In conclusion, bfr is a novel TCS that may be involved in biofilm development by S. gordonii on polystyrene and saliva-coated surfaces. Further study of the bfr operon will facilitate our understanding of the development of oral biofilms.
Acknowledgments
We thank Lin Tao of the University of Illinois at Chicago for helpful advice during the progress of this work and TIGR for the S. gordonii genomic data.
This work was supported by National Institutes of Health grant R01 DE08590.
Editor: J. B. Bliska
REFERENCES
- 1.Bayliss, R., C. Clarke, C. M. Oakley, W. Somerville, A. G. Whitfield, and S. E. Young. 1983. The microbiology and pathogenesis of infective endocarditis. Br. Heart J. 50:513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhagwat, S. P., J. Nary, and R. A. Burne. 2001. Effects of mutating putative two-component systems on biofilm formation by Streptococcus mutans UA159. FEMS Microbiol. Lett. 205:225-230. [DOI] [PubMed] [Google Scholar]
- 3.Caetano-Anolles, G. 1993. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl. 3:85-94. [DOI] [PubMed] [Google Scholar]
- 4.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 5.Dorel, C., O. Vidal, C. Prigent-Combaret, I. Vallet, and P. Lejeune. 1999. Involvement of the Cpx signal transduction pathway of E. coli in biofilm formation. FEMS Microbiol. Lett. 178:169-175. [DOI] [PubMed] [Google Scholar]
- 6.Finelli, A., C. V. Gallant, K. Jarvi, and L. L. Burrows. 2003. Use of in-biofilm expression technology to identify genes involved in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:2700-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fournier, B., and D. C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 182:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons, R. J., and J. V. Houte. 1975. Bacterial adherence in oral microbial ecology. Annu. Rev. Microbiol. 29:19-44. [DOI] [PubMed] [Google Scholar]
- 9.Gong, K., L. Mailloux, and M. C. Herzberg. 2000. Salivary film expresses a complex, macromolecular binding site for Streptococcus sanguis. J. Biol. Chem. 275:8970-8974. [DOI] [PubMed] [Google Scholar]
- 10.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 11.Hudson, M. C., and G. C. Stewart. 1986. Differential utilization of Staphylococcus aureus promoter sequences by Escherichia coli and Bacillus subtilis. Gene 48:93-100. [DOI] [PubMed] [Google Scholar]
- 12.Kiliç, A. O., M. C. Herzberg, M. W. Meyer, X. Zhao, and L. Tao. 1999. Streptococcal reporter gene-fusion vector for identification of in vivo expressed genes. Plasmid 42:67-72. [DOI] [PubMed] [Google Scholar]
- 13.Lappin-Scott, H. M., and C. Bass. 2001. Biofilm formation: attachment, growth, and detachment of microbes from surfaces. Am. J. Infect. Control 29:250-251. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence, J. R., D. R. Korber, B. D. Hoyle, J. W. Costerton, and D. E. Caldwell. 1991. Optical sectioning of microbial biofilms. J. Bacteriol. 173:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Y. H., P. C. Lau, N. Tang, G. Svensater, R. P. Ellen, and D. G. Cvitkovitch. 2002. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 184:6333-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liljemark, W. F., C. G. Bloomquist, and G. R. Germaine. 1981. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect. Immun. 31:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manford, M., J. Matharu, and K. Farrington. 1992. Infective endocarditis in a district general hospital. J. R. Soc. Med. 85:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNab, R., and H. F. Jenkinson. 1998. Altered adherence properties of a Streptococcus gordonii hppA (oligopeptide permease) mutant result from transcriptional effects on cshA adhesin gene expression. Microbiology 144(Pt. 1):127-136. [DOI] [PubMed] [Google Scholar]
- 20.Nyvad, B., and M. Kilian. 1987. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 95:369-380. [DOI] [PubMed] [Google Scholar]
- 21.Oosthuizen, M. C., B. Steyn, J. Theron, P. Cosette, D. Lindsay, A. Von Holy, and V. S. Brozel. 2002. Proteomic analysis reveals differential protein expression by Bacillus cereus during biofilm formation. Appl. Environ. Microbiol. 68:2770-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Toole, G. A. 2003. To build a biofilm. J. Bacteriol. 185:2687-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 24.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkins, M. D., H. Ceri, and D. G. Storey. 2001. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 40:1215-1226. [DOI] [PubMed] [Google Scholar]
- 26.Prigent-Combaret, C., O. Vidal, C. Dorel, and P. Lejeune. 1999. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 181:5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quivey, R. G., W. L. Kuhnert, and K. Hahn. 2001. Genetics of acid adaptation in oral streptococci. Crit. Rev. Oral Biol. Med. 12:301-314. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schembri, M. A., K. Kjaergaard, and P. Klemm. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253-267. [DOI] [PubMed] [Google Scholar]
- 31.Shaw, W. V. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737-755. [DOI] [PubMed] [Google Scholar]
- 32.Stanley, N. R., R. A. Britton, A. D. Grossman, and B. A. Lazazzera. 2003. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J. Bacteriol. 185:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 34.Svensater, G., J. Welin, J. C. Wilkins, D. Beighton, and I. R. Hamilton. 2001. Protein expression by planktonic and biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 205:139-146. [DOI] [PubMed] [Google Scholar]
- 35.Tao, L., and M. C. Herzberg. 1999. Identifying in vivo expressed streptococcal genes in endocarditis. Methods Enzymol. 310:109-116. [DOI] [PubMed] [Google Scholar]
- 36.Tao, L., D. J. LeBlanc, and J. J. Ferretti. 1992. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene 120:105-110. [DOI] [PubMed] [Google Scholar]
- 37.Tellefson, L. M., and G. R. Germaine. 1986. Adherence of Streptococcus sanguis to hydroxyapatite coated with lysozyme and lysozyme-supplemented saliva. Infect. Immun. 51:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremoulet, F., O. Duche, A. Namane, B. Martinie, and J. C. Labadie. 2002. Comparison of protein patterns of Listeria monocytogenes grown in biofilm or in planktonic mode by proteomic analysis. FEMS Microbiol. Lett. 210:25-31. [DOI] [PubMed] [Google Scholar]
- 39.Wen, Z. T., and R. A. Burne. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 68:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]