Abstract
Protein kinase A (PKA) phosphorylation of myofibril proteins constitutes an important pathway for β-adrenergic modulation of cardiac contractility and relaxation. PKA targets the N-terminus (Ser-23/24) of cardiac troponin I (cTnI), cardiac myosin-binding protein C (cMyBP-C) and titin. The effect of PKA-mediated phosphorylation on the magnitude of contraction has been studied in some detail, but little is known about how it modulates the kinetics of thin filament activation and myofibril relaxation as Ca2+ levels vary. Troponin C (cTnC) interaction with cTnI (C-I interaction) is a critical step in contractile activation that can be modulated by cTnI phosphorylation. We tested the hypothesis that altering C-I interactions by PKA, or by cTnI phosphomimetic mutations (S23D/S24D-cTnI), directly affects thin filament activation and myofilament relaxation kinetics. Rat ventricular myofibrils were isolated and endogenous cTn was exchanged with either wild-type cTnI, or S23D/S24D-cTnI recombinant cTn. Contractile mechanics were monitored at maximum and submaximal Ca2+ concentrations. PKA treatment of wild-type cTn or exchange of cTn containing S23D/S24D-cTnI resulted in an increase in the rate of early, slow phase of relaxation (kREL,slow) and a decrease in its duration (tREL,slow). These effects were greater for submaximal Ca2+ activated contractions. PKA treatment also reduced the rate of contractile activation (kACT) at maximal, but not submaximal Ca2+, and reduced the Ca2+ sensitivity of contraction. Using a fluorescent probe coupled to cTnC (C35S-IANBD), the Ca2+-cTn binding affinity and C-I interaction were monitored. Ca2+ binding to cTn (pCa50) was significantly decreased when cTnI was phosphorylated by PKA (ΔpCa50 = 0.31). PKA phosphorylation of cTnI also weakened C-I interaction in the presence of Ca2+. These data suggest that weakened C-I interaction, via PKA phosphorylation of cTnI, may slow thin filament activation and result in increased myofilament relaxation kinetics, the latter of which could enhance early phase diastolic relaxation during β-adrenergic stimulation.
Introduction
Cardiac muscle contractile activation is initiated by Ca2+ binding to troponin C (cTnC) and a subsequent increased interaction with troponin I (cTnI), leading to increased tropomyosin mobility and myosin interaction with actin (1). Relaxation ensues as Ca2+ is released from cTnC and is pumped back into the sarcoplasmic reticulum, resulting in reestablishment of cross-bridge inhibition via tropomyosin-mediated steric blocking as myosins detach from the thin filament (2). We (3, 4, 5, 6, 7, 8) and others (9, 10) have demonstrated that Ca2+ binding to cardiac troponin C (cTnC) is less effective than Ca2+ binding to skeletal TnC in activating contraction, primarily due to weaker affinity of N-terminal Ca2+ binding and a weaker affinity (KC-I) of Ca2+-cTnC for the switch domain of cTnI. This suggests cTnC-cTnI (C-I) interaction as a potential point for modulation in the thin filament activation pathway to regulate systolic performance at rest (basal activity) and particularly during β-adrenergic stimulation. cTnI, along with cardiac myosin binding protein C (cMyBP-C) and titin are myofilament protein targets for phosphorylation by β-adrenergic stimulation (11, 12, 13, 14).
For cTnI, β-adrenergic stimulation results in phosphorylation of Ser-23 and -24 by protein kinase A (PKA). Phosphorylation at these sites has been shown to reduce C-I interaction strength (15), reduce the Ca2+ sensitivity (pCa50) of cardiac muscle tension production (11), increase cross-bridge cycling kinetics, and accelerate cardiac muscle cell relaxation (11, 12). However, little is known about how PKA phosphorylation of cTnI modulates the kinetics of thin filament activation or myofibril relaxation, and how this kinetic modulation may be affected with changing Ca2+ levels that occur during β-adrenergic stimulation.
In this study, we tested the hypothesis that PKA phosphorylation of cTnI Ser-23/24 reduces C-I interaction and that this directly impacts thin filament activation and myofibril relaxation kinetics in cardiac muscle. We also hypothesized that the modulatory effects of cTnI phosphorylation are greater at the submaximal Ca2+ levels present in cardiomyocytes during a cardiac twitch. To test this, rat ventricular myofibrils were isolated and endogenous cTn was exchanged with recombinant wild-type (WT) cTn ± PKA treatment. Considering PKA targets cTnI, cMyBP-C, and titin, we studied the specific role of cTnI phosphorylation plays in myofilament tension regulation and relaxation kinetics by exchanging the endogenous cTn of cardiac myofibrils with recombinant cTn containing a constitutively phosphorylated state cTnI (S23D/S24D-cTnI).
Using this combination of PKA treatment and the phosphomimetic cTnI mutant (S23D/S24D-cTnI), we found that the Ca2+ sensitivity of contraction is reduced by phosphorylation of cTnI Ser-23/24, whereas the maximum tension production (TMAX) is maintained. The maximal (pCa 4.0) rate of Ca2+-mediated activation and myofibril tension generation (kACT) was slower with PKA treatment, although the rate of tension redevelopment (kTR) following a release-restretch cycle during steady-state activation was not, suggesting that PKA treatment affects the maximal rate of thin filament activation. The PKA treatment of WT cTn or exchange of cTn containing S23D/S24D-cTnI also made the early phase of relaxation (kREL,slow) faster and this effect was greater at submaximal Ca2+ activation. Interestingly, the larger, faster phase of relaxation (kREL,fast) was unaffected. Steady-state fluorescence measurements demonstrated that PKA phosphorylation of cTnI or phosphomimetic S23D/S24D-cTnI resulted in reduced Ca2+-binding affinity (KCa) and C-I interaction (KC-I), as previously reported (15, 16). These results provide evidence that PKA phosphorylation of cTnI can modulate the kinetics of both thin filament activation and myofibril relaxation and the effect may be greater at submaximal Ca2+ levels seen in cardiomyocytes during twitch activity.
Material and Methods
Proteins, cTnC labeling, cTnI phosphorylation, and cTn complex reconstitution
Construction and expression of WT rat cTnC, cTnI, and cTnT in the pET24 vector has been previously described (17) (see the Supporting Material for details). C35S-cTnC and S23D/S24D-cTnI were constructed by a site-directed mutagenesis kit from WT cTnC and cTnI, respectively. The C35S-cTnC mutation was introduced to allow site-specific attachment of a fluorescent probe (IANBD ({N-[2-(iodoacetoxy)ethyl]-N-methyl}amino-7-nitrobenz-2-oxa-1,3-diazole (Mw = 406.14))) at C84 to monitor Ca2+ binding to cTn as well as the C-I interaction. The labeling efficiency was determined to be 90% using Bio-Rad protein assay (18, 19). Purified cTnI was phosphorylated by the catalytic subunit of PKA, using a cTnC affinity column (20), and the phosphorylated product was designated as pS23/pS24-cTnI. Whole cTn complexes were formed using rat cTnC (WT or IANBD-cTnCC35S), rat cTnI (WT, pS23/pS24, or S23D/S24D), and rat cTnT (WT) at a 1:1:1 molar ratio (21, 22). The cTn complexes with IANBD-cTnCC35S and/or pS23/pS24-cTnI forms were only used for solution biochemistry measurements.
cTn complexes exchange into myofibrils
cTn complexes (final concentration ∼1.0 mg/ml) containing either WT or S23D/S24D cTnI were passively exchanged into isolated myofibrils in a buffer containing (in mM): 200 KCl, 20 MOPS, 5 MgCl2, 2 EGTA, 1 DTT, and 4 ATP for overnight on a slow rocker at 4°C. Following this, myofibrils were washed twice for 30 min with gentle mixing in relaxing solution containing 1 mg/ml bovine serum albumin to remove any nonspecifically bound exogenous cTn.
Solutions for myofibril mechanics
Solution composition was computed by an iterative algorithm that calculates the equilibrium concentration of ligands and ions based on published affinity constants (23). Relaxing solutions contained (in mM): 80 MOPS, 15 EGTA, 1 Mg2+, 5 MgATP, 83 K+, 52 Na+, 15 creatine phosphate, and 20 units/ml creatine phosphokinase, pH 7.0, solution ionic strength was 170 mM. The inorganic Pi concentration determined by NMR measurement was 0.5 mM (24). Experimental temperature was 15°C. For activation solutions, the Ca2+ level (expressed as pCa = −log [Ca2+]) was set by adjusting with CaCl2. For PKA treatment, myofibrils were exposed to 200 μL relaxing solution containing 100 units of the catalytic subunit of PKA and 6 mM DTT for 45 min at 20°C.
SDS-PAGE and Western blots
To monitor the extent of exogenous cTn incorporation into myofibrils, we used cTn in which cTnT contained a 9 amino acid myc-tag at the N-terminus, similar to previous studies (25). The exchange efficiency was determined through Western blot analysis after the proteins were extracted by SDS sample buffer and separated by 12.5% SDS PAGE. The presence of the myc-tag allowed us to visibly separate the exchanged protein from endogenous. Exchange efficiency was determined by calculating the percent of myc-tagged cTnT (top band) and endogenous cTnT (bottom band) present in the sample.
Ethical approval and tissue preparation
All animal procedures were performed in accordance with the U.S. National Institutes of Health Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Washington (UW) Institutional Animal Care and Use Committee (IACUC). Rats were housed in the Department of Comparative Medicine at UW and cared for in accordance with UW IACUC procedures. Male, Sprague-Dawley rats (150–250 g) were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg) after initial exposure to isoflurane (3–5% in oxygen). When the animal had no reflexive response, the heart was rapidly excised and dissected in oxygenated physiological salt solution containing (in mM): 100 NaCl, 2.5 KCl, 24 NaHCO3, 1 Na2HPO4, 1 MgSO4⋅7H2O, and 1 CaCl2 (26). Spliced left ventricles were demembranated for 24 h at 4°C in relaxing solutions containing (in mM): 100 KCl, 9 MgCl2, 4 Na2ATP, 5 K2EGTA, 10 MOPS, 1% nonionic detergent Triton X-100, pH 7.0, and 50% v/v glycerol (8, 27).
Myofibril mechanics measurements
Single or small bundles of cardiac myofibrils were prepared from ventricular tissue and experiments were performed as previously described (28) (see the Supporting Material for details). The sarcomere length was initially set at ∼2.3 μm. Activation and relaxation data were collected at 15°C and fit with either single-exponential curves or linear coefficients as previously described, and the solution change was complete in ∼10 ms (28, 29, 30). Briefly, the activation rate (kACT; with rapid increase in Ca2+) was estimated from a single-exponential rise to a maximum. Once tension reached steady state, a release-restretch protocol was performed to measure the time course of tension redevelopment. A sudden decrease in length (20% of optimal length) was imposed on the myofibrils, and after 25 ms of unloaded shorting, myofibrils were rapidly stretched back to their original length. Relaxation was measured following a step decrease in solution Ca2+ back to pCa 9.0. Relaxation rate for the early, slow phase (kREL,slow) was determined from the slope of a regression line fit to the tension trace, normalized to the tension amplitude. The duration of the slow phase was measured from the onset of solution change at the myofibril to the shoulder marking the beginning of the fast phase. Transition from slow to rapid phase was determined through multiple factors. An apparent change in the slope of the data or a change in the signal/noise ratio was often apparent at the transition. The subsequent fast phase of relaxation (kREL,fast) to baseline (resting) tension was measured from a single exponential decay fitted to the data. A t1/2 estimation was made in cases where the decay was not well described by a single exponential, and this was converted to a rate τ = ln (2)/t1/2.
Steady-state fluorescence measurements
Steady-state fluorescent measurements have been previously described in detail (31). All steady-state fluorescence measurements were taken using a Perkin-Elmer LS50B luminescence spectrometer at 15°C. IANBD fluorescence was excited at 490 nm and monitored at ∼530 nm. Protein buffer solutions contained (in mM): 20 MOPS, 150 KCl, 3 MgCl2, 2 EGTA, and 1 DTT (pH 7.0). The fluorescence signal of 2 mL of IANBD cTnCC35S or IANBD cTnC35S (0.6 μM) was monitored with the titration of microliter amounts of cTnI (WT, pS23/pS24, or S23D/S24D) or Ca2+ in the presence (100 μM) or absence of Ca2+. The free Ca2+ concentration was calculated using Maxchelator (32). The Ca2+ sensitivity of conformational changes (pCa50, pCa value at half-maximal fluorescence signal change) were obtained by fitting the binding curve with the sigmoid Hill equation as previously described (33). The reported values are the means of three to six successive titrations.
Results
Recombinant cTn complex exchange profiles and phosphorylation profile
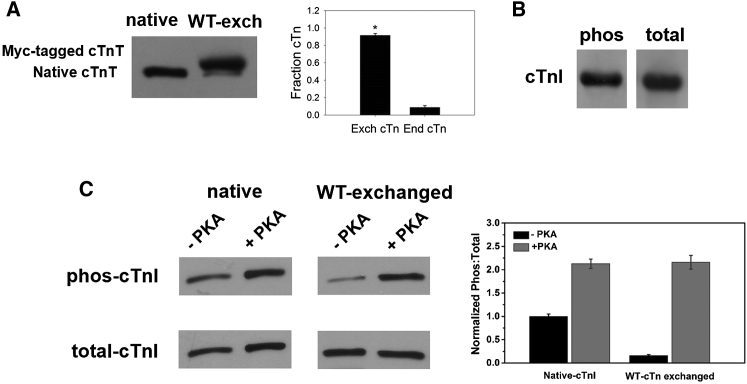
Recombinant cTn complex containing WT or S23D/S24D-cTnI were exchanged into rat ventricular myofibrils to determine the effects of isometric tension development and relaxation kinetics. To quantify the extent of the exchange, cTn containing cTnT labeled at the N-terminus with a c-myc tag was exchanged into myofibrils. Densitometry analysis of Western blots using cTnT-specific antibodies indicated that this procedure typically results in over 90% endogenous cTn replacement by cTn containing the c-myc tagged cTnT (Fig. 1 A), similar to the exchange efficiencies reported by our group and others in myofibrils and demembranated trabeculae (24, 34). This suggests that the exchange protocol was efficient and changes in contractile parameters should be attributed to the exchanged cTn containing either WT cTnI or cTnI phosphomimetic variants.
Figure 1.
(A) Exchange efficiency of recombinant rat cardiac troponin complex (cTn) into rat cardiac myofibrils. (B) PKA phosphorylation profile for WT-cTnI using a cTnC column. (C). PKA phosphorylation profile of cTnI for native and WT-cTn exchanged myofibrils before and after PKA treatment.
WT-cTnI was phosphorylated by PKA using a cTnC column. To quantify the extent of cTnI phosphorylation, the phosphorylation profile was determined by Western blot using antibodies of rabbit polyclonal to cTnI (phospho S22 + S23) and goat antirabbit IgG-HRP, whereas the total amount of cTnI was determined using antibodies of rabbit polyclonal lgG to cTnI (H170) and goat antirabbit IgG-HRP (Fig. 1 B). These measurements suggested the phosphorylation protocol was very efficient with 87% of cTnI successfully phosphorylated.
Endogenous cTn in isolated myofibrils was passively exchanged for recombinant rat cTn containing either WT-cTnI or S23D/S24D-cTnI overnight, the myofibrils were then washed with relaxing solution containing bovine serum albumin twice. The extent of phosphorylation for cMyBP-C and titin was not measured, however, it should be the same for each group of WT-cTnI and S23D/S24D-cTnI exchanged myofibrils, as these preparations were made from the same hearts. For all WT-cTnI treated groups, myofibril samples were divided into two aliquots, one treated with PKA and the other with an equal amount of buffer, for measurement of the kinetics of thin filament activation and myofibril relaxation. The extent of phosphorylation for cTnI in WT myofibrils (before PKA-treatment) was related to the exchange efficiency. Because exchange of recombinant cTn into rat myofibrils for replacing native cTn was not 100% (but close to it), some small amount of residual cTnI phosphorylation was likely present in every exchange batch. Here, we measured the cTnI phosphorylation profile before and after PKA treatment in both native and WT-cTn exchanged myofibrils. It is clear that the endogenous cTnI phosphorylation levels in native myofibrils were ∼40%, and the cTnI phosphorylation signal after PKA treatment was highly increased, showing over 85% cTnI was phosphorylated. Following exchange of recombinant WT-cTn into native myofibrils, the phosphorylation level of cTnI was almost completely eliminated, further confirming the exchange protocol was nearly complete. Similar to the native myofibrils, PKA treatment also dramatically increased the cTnI phosphorylation signaling, resulting in over 85% cTnI phosphorylated.
Myofibril activation kinetics
Myofibrils exchanged with recombinant cTn complex containing either WT or S23D/S24D cTnI were exposed to continually flowing solutions that were rapidly switched for step increases and decreases in [Ca2+], from pCa 9.0 to either maximal (pCa 4.0) or submaximal [Ca2+], and finally back to 9.0. With this protocol, the magnitude and rate of tension generation and relaxation at 15°C were then collected, as shown in an example tension trace in Fig. 2 A. Table 1 and Fig. 2, B and C, summarize the tension magnitude and kinetic parameters for rat ventricular myofibrils exchanged with cTn containing WT cTnI ± PKA treatment. The kinetic results of native rat ventricular myofibrils are summarized in Table S1.
Figure 2.
(A) Sample trace for a rat cardiac myofibril after exchanging recombinant cTn complex. The inset is a close-up of a slow phase of relaxation. Contraction and relaxation kinetics parameter for rat cardiac myofibril after exchanging recombinant rat WT cTn complex ± PKA treatment at (B) maximal (pCa = 4.0) and (C) submaximal (pCa = 5.4) Ca2+ levels. To see this figure in color, go online.
Table 1.
Tension generation and relaxation parameters after recombinant rat WT cTn or rat cTn contained S23D/S24D-cTnI exchange into rat ventricular myofibrils at 15°C
| Myofibril batches (n) | pCa | Tension generation |
kTR (s−1) | Relaxation |
|||
|---|---|---|---|---|---|---|---|
| Slow phase |
Fast phase |
||||||
| Tmax (mN/mm2) | kACT (s−1) | tREL,slow (ms) | kREL,slow (s−1) | kREL,fast (s−1) | |||
| WT (16) | 4.0 | 76 ± 9 | 3.7 ± 0.3 | 5.6 ± 1.3 | 85 ± 8 | 0.9 ± 0.2 | 18 ± 3 |
| (12) | 5.4 | 30 ± 4 | 1.6 ± 0.2 | 3.4 ± 0.6 | 94 ± 14 | 2.1 ± 0.3 | 17 ± 2 |
| WT+PKA (14) | 4.0 | 73 ± 12 | 2.6 ± 0.4a | 5.8 ± 1.3 | 56 ± 4a | 1.7 ± 0.3b | 24 ± 3b |
| (10) | 5.4 | 19 ± 6a | 1.4 ± 0.1 | 2.9 ± 0.5 | 60 ± 4a | 7.1 ± 2.1a | 16 ± 3 |
| S23D/S24D (20) | 4.0 | 61 ± 5a | 2.7 ± 0.3a | 7.1 ± 0.4 | 65 ± 5a | 2.7 ± 0.6b | 22 ± 3 |
| (8) | 4.8 | 46 ± 13 | 3.1 ± 0.4 | 7.2 ± 0.4 | 55 ± 5 | 4.5 ± 0.5 | 23 ± 2 |
| (7) | 5.2 | 26 ± 6 | 3.0 ± 0.4 | 6.7 ± 0.4 | 49 ± 7 | 6.1 ± 1.9 | 18 ± 2 |
| (7) | 5.4 | 17 ± 3a | 3.1 ± 0.3b | 6.5 ± 0.4a | 52 ± 6a | 6.1 ± 1.6b | 20 ± 3 |
| (7) | 5.6 | 8 ± 2 | 3.2 ± 0.3 | 4.5 ± 1.6 | 67 ± 12 | 6.4 ± 2.1 | 17 ± 4 |
Values given are mean ± SE. Number in parentheses is number of myofibrils. ap < 0.05 vs. WT, and bp < 0.01 vs. WT.
We first compared contraction during maximal (pCa 4.0) Ca2+ activation. TMAX did not differ between untreated and PKA-treated myofibrils (76 ± 9 vs. 73 ± 12 mN/mm2, respectively) containing WT-cTnI, consistent with our previous results on the demembranated trabeculae (34). The rate of tension rise with rapid switching from pCa 9.0 to 4.0 solution (kACT) includes the kinetic processes of Ca2+-dependent thin filament activation, myosin cross-bridge binding, and subsequent development of tension. kACT was 3.7 ± 0.3 s−1 for untreated myofibrils and was significantly slower (2.6 ± 0.4 s−1) for PKA-treated myofibrils. To differentiate between the cross-bridge versus thin filament contributions to kACT, once activation was complete (tension in steady state), we also measured the rate of tension redevelopment (kTR) following rapid release and restretch of myofibrils. The kTR protocol is thought to measure the rate of myosin cross-bridge attachment and subsequent tension generation (27), following myofibril release and restretch, with Ca2+ binding to cTn and thin filament activation in near steady state. The kTR (5.6 ± 1.3 s−1) was faster than kACT (3.7 ± 0.3), suggesting the thin filament activation process may be rate limiting for rat cardiac myofibrils at 15°C, similar to what we have reported for mouse cardiac myofibrils (24). Following PKA treatment, kTR (5.8 ± 1.3 s−1) did not differ from nontreated myofibrils. This suggests that the slowing of kACT with PKA treatment likely resulted from a slowing of the thin filament activation process during maximal Ca2+ activation.
In addition to maximal Ca2+ activation, it is important to determine how the kinetics of contraction are affected by PKA treatment during submaximal Ca2+ activations, which is more reflective of what occurs during a cardiac twitch. For our initial assessment, we chose a Ca2+ level (pCa 5.4 ∼4 μM), which was below the half-maximal (pCa50) activation of the rat ventricular myofibrils, producing 0.41 TMAX (30 ± 4 mN/mm2). In contrast to TMAX, tension was lower (19 ± 6 mN/mm2) in PKA-treated myofibrils at pCa 5.4, reflecting the previously reported reduction in the Ca2+ sensitivity of tension that occurs with PKA treatment (11). The contraction kinetics for these activations is summarized in Fig. 2 C. At pCa 5.4, both kACT and kTR were considerably slower than during maximal Ca2+ activations (no PKA treatment), as previously reported for mouse cardiac myofibrils (24), demonstrating the Ca2+ sensitivity of contraction kinetics. Furthermore, similar to maximal Ca2+ activation, kACT was significantly slower than kTR, again suggesting thin filament activation kinetics are limiting to the rate of tension development. However, in contrast to maximal Ca2+ activations, there was no difference in kACT between untreated and PKA-treated myofibrils (1.6 ± 0.2 vs. 1.4 ± 0.1 s−1, respectively). This suggests that for activations with subsaturating levels of Ca2+, the influence of PKA-mediated phosphorylation of myofilament protein to attenuate the kinetics of thin filament activation is reduced or eliminated. This will be discussed in more detail below.
Myofibril relaxation kinetics
Because β-adrenergic modulation of cardiac function also includes elevated heart rate, faster relaxation is important to ensure maintained or increased diastolic ventricular filling. Thus, we also measured how the kinetics of relaxation are affected by PKA treatment at maximal and submaximal Ca2+ levels. For myofibrils producing isometric tension, rapid switching back to Ca2+ free solution (pCa 9.0) results in an early, slow phase of relaxation followed by a more rapid (fast) phase back to baseline tension (Fig. 2 A). The rate (kREL,slow) of slow phase relaxation (0.9 ± 0.2 s−1), which is thought to be determined primarily by the cross-bridge detachment rate (24, 29, 35, 36, 37, 38), was almost twice as fast for PKA-treated myofibrils (1.7 ± 0.3 s−1). The duration (85 ± 8 ms) of the slow phase (tREL,slow) was also significantly shorter for PKA-treated myofibrils (56 ± 4 ms). Here, we also analyzed the contribution of the slow phase relaxation, and found that the contributions before and after PKA phosphorylation at pCa 4.0 were 6% and 10%, respectively. We previously demonstrated that tREL,slow is sensitive to the properties of cTn and that a reduction in Ca2+-binding affinity and/or C-I interaction can result in a shorter duration of the slow phase (24). The much larger, fast phase of relaxation (kREL,fast) is thought to be reflective of several sarcomere properties and also uneven relaxation kinetics between sarcomeres in series (29, 36, 37, 38). This rate (18 ± 3 s−1) was also faster for PKA-treated myofibrils (24 ± 3 s−1).
During relaxation from contractions at pCa 5.4, kREL,slow was >twofold faster (2.1 ± 0.3 s−1) than during maximal Ca2+ activation, but tREL,slow of the slow phase (94 ± 14 ms) and the fast phase rate kREL,fast (17 ± 2 s−1) were not significantly affected. Similar to pCa 4.0, PKA-treated myofibrils had significantly reduced tREL,slow (60 ± 4 ms) at pCa 5.4 compared to nontreated myofibrils. Interestingly, PKA treatment greatly increased kREL,slow at pCa 5.4 (7.1 ± 2.1 s−1) compared with nontreated myofibrils, whereas kREL,fast was not affected (16 ± 3 s−1). At pCa 5.4, the contributions of slow phase relaxation before and after PKA phosphorylation were 10% and 18%, respectively. These data suggest the primary effect of PKA treatment during submaximal Ca2+ activation is to reduce myofibril tension and the slow phase of relaxation, which speeds overall relaxation.
Myofibril activation and relaxation kinetics with S23D/S24D-cTnI
To determine the specific role of cTnI phosphorylation (without phosphorylation of cMyBP-C or titin), we exchanged cTn containing the phosphomimetic mutant S23D/S24D-cTnI into myofibrils to study activation and relaxation at maximal and submaximal Ca2+ concentrations. S23D/S24D-cTnI has previously been demonstrated to mimic the effect of PKA phosphorylation of cTnI at Ser-23/24 both structurally and functionally (39, 40). Ca2+ levels were chosen to produce tensions that were <25% (pCa 5.6), ∼25% (pCa 5.4), ∼50% (pCa 5.2), and ∼75% (4.8) of maximal (pCa 4.0) activated tension. The data of tension magnitude and kinetics parameters are also summarized in Table 1. Similar to PKA treatment, myofibrils containing S23D/S24D-cTnI slowed the kinetics of maximal Ca2+ (pCa 4.0) activation (2.7 ± 0.3 s−1) compared with WT cTn exchanged myofibrils (3.7 ± 0.3 s−1), though the effect was somewhat smaller in magnitude for kACT. Also similar, kACT was significantly slower than kTR. Akin to the PKA-treated myofibril, the kREL,slow (2.7 ± 0.6 s−1) is significantly faster and the tREL,slow (65 ± 5 ms) is shorter in the myofibrils containing S23D/S24D-cTnI compared with WT cTn exchanged myofibrils, especially for submaximal Ca2+ conditions. An example tension trace demonstrating this is shown in Fig. 3. Interestingly, no clear changes were detected in kREL,fast in the S23D/S24D-cTnI exchanged myofibrils.
Figure 3.
Slow phase relaxation transient at submaximal Ca2+ level for WT-cTn (black) and S23D/S24D-cTn (red) exchanged rat cardiac myofibrils. To see this figure in color, go online.
Steady-state fluorescence measurements of KC-I and KCa
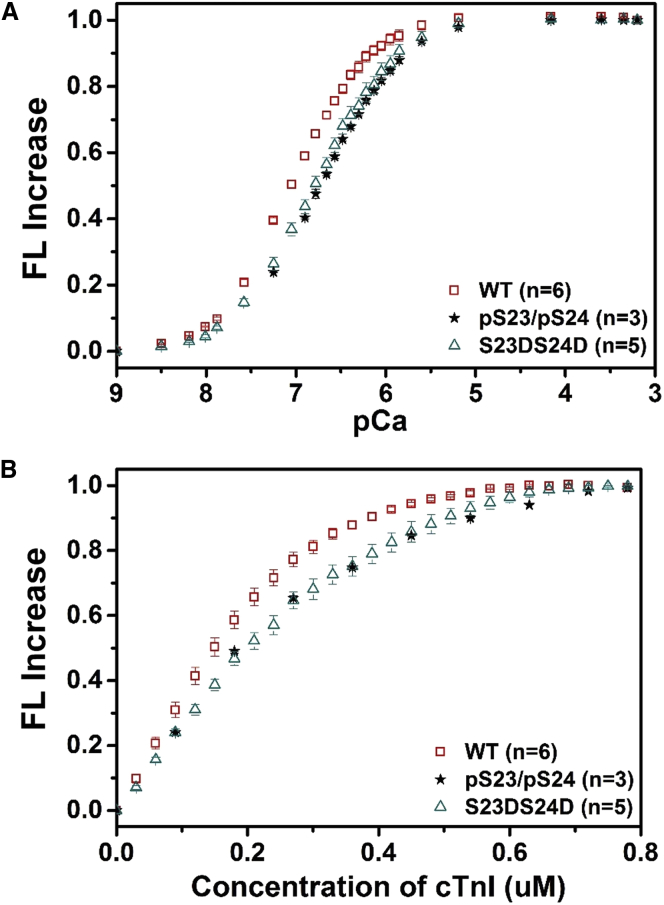
To determine the effects of PKA phosphorylation on KC-I and KCa of cTn, in the absence of confounding influences of other myofilament proteins, we performed steady-state biochemical measurements with the fluoroprobe IANBD attached at the C84 of . IANBD, serves as an environment-sensitive and sulfhydryl-reactive extrinsic fluorophore, and has been used to label protein molecules for studying the intramolecular interactions (18, 31, 41). Fluorescence labeling at C84 reports on conformational changes in NcTnC resulting from Ca2+ binding. We first compared the Ca2+-dependent conformational changes of cTn containing either WT-cTnI, phosphorylated-cTnI (pS23/pS24-cTnI), or S23D/S24D-cTnI. As shown in Fig. 4 A, phosphorylation (pS23/pS24-cTnI) reduced Ca2+-binding affinity compared to the WT-cTn complex. The Ca2+ sensitivity of the fluorescence signal (reported as pCa50) was shifted −0.31 pCa units, from 7.05 ± 0.03 (WT-cTnI) to 6.74 ± 0.03 (pS23/pS24-cTnI). cTn containing S23D/S24D-cTnI also demonstrated a similar right shift (pCa50 = 6.77 ± 0.03) compared to WT-cTn.
Figure 4.
Changes in the IANBD fluorescence emission intensity of (A) cTnCC35S in complex with cTnTWT and cTnI variants with titration of Ca2+ and (B) of cTnCC35S alone with titration of cTnI variants in the presence of 100 μM Ca2+. To see this figure in color, go online.
C-I interaction plays a gate keeper role in translating the Ca2+ signal to other myofilament proteins to initiate cardiac muscle contraction. Binding of cTnI to cTnC was measured by titrating labeled with cTnI variants in the presence of 100 μM Ca2+. Fig. 4 B shows the IANBD fluorescence change as the cTnI concentration is increased up to 0.8 μM in solutions containing 0.6 μM . The lack of further change in the fluorescence signal beyond 0.6 μM cTnI suggests a strong affinity of cTnI for cTnC and that 1:1 binding of cTnC/cTnI was achieved. Both the phosphorylated-cTnI (pS23/pS24-cTnI) and the cTnI phosphomimetic (S23D/S24D-cTnI) reduced C-I affinity compared to WT-cTnI. We are currently studying the structural basis of how phosphorylation of cTnI influences C-I interaction and Ca2+ sensitivity via long-timescale molecular dynamics simulations (42).
Discussion
It is well established that cTnI is phosphorylated by PKA at sites Ser-23/24 during β-adrenergic stimulation, and that this affects Ca2+-mediated contraction of cardiac muscle. Zhang et al. (11) demonstrated that PKA phosphorylation of a cardiac skinned muscle results in decreased Ca2+ sensitivity of muscle contraction, as well as an increased rate of cardiac muscle relaxation. Kentish et al. (12) also reported that PKA phosphorylation accelerated the myofibrillar relaxation rate, and suggest that this is due, at least in part, to faster cross-bridge cycle kinetics. Both of these studies used Diazo-2, a Ca2+ chelator, which does not allow complete relaxation from maximal activation. Although faster cross-bridge cycling was implicated as a means to faster relaxation, there was no direct assessment of cross-bridge detachment rate. Additionally, the role of thin filament activation kinetics, which is modulated by Ca2+ and cTnI phosphorylation, in accelerating cross-bridge detachment and relaxation was not examined. Here, we provide evidence that thin filament activation kinetics is affected by PKA-mediated phosphorylation of cTnI (via a reduction in C-I interaction), that this can slow the kinetics of both tension development and relaxation, and that this effect is larger at the submaximal levels of Ca2+ that the heart operates during a cardiac twitch. Comparisons of kACT vs. kTR allowed us to distinguish thin filament activation kinetics (with activation defined as the availability of strong myosin binding sites on F-actin of the thin filament) from the rate of tension development (24), although measures of the initial, slow phase of relaxation provide a putative measure of the rate of cross-bridge detachment (35, 43).
The lusitropic effect of PKA phosphorylation on cTnI at Ser-23/24 appears to result from two functional mechanisms: i), a decrease in the Ca2+-binding affinity of cTnC and subsequent interactions of cTnC with cTnI to reduce the Ca2+-dependent activation of thin filament (44, 45) and ii), an increase in cross-bridge cycling rate (12, 44) that is likely due to increasing the rate-limiting process, detachment of myosin from actin. To study the role of cTnI-specific effects, separate from potential effects from other myofilament proteins phosphorylated by PKA (cMyBP-C and titin) (11, 12, 13, 14), we compared contraction and relaxation of myofibrils treated with PKA versus those exchanged with recombinant cTn containing the phosphomimic S23D/S24D-cTnI. Our solution biochemical measurements of cTn-Ca2+ binding affinity and C-I interaction gave nearly identical results with comparing PKA treatment versus the phosphomimic, validating its use to study the role of cTnI-specific effects of phosphorylation on the kinetics of myofibril contraction and relaxation.
Contractile activation kinetics
To better understand how PKA phosphorylation affects thin filament activation, we compared kACT and kTR as Ca2+ was varied. Here, kACT reflects the combined rate of Ca2+ binding to cTn, subsequent changes in interaction between cTn subunits that allow thin filament activation and tension generation. In contrast, kTR reports the rate of myosin cross-bridge attachment and subsequent tension generation when Ca2+ binding to cTn and thin filament activation are in or near steady state. We found that there is no difference for TMAX between untreated and PKA-treated myofibrils at maximal Ca2+ level (pCa 4.0), although tension was reduced by PKA treatment at submaximal Ca2+, consistent with our previous findings in rat trabeculae (34), and with other reports (11, 12).
A believed-novel finding is that kTR was faster than kACT at both maximal and submaximal Ca2+ levels. This result is different from most previous studies (29, 35, 43), where no difference between kACT and kTR has been reported. The most likely explanation for this difference with previous reports is the level of contaminant Pi in experimental solutions. Most previous reports involved using the enzyme purine nucleoside phosphorylase with the substrate 7-methyl guanosine (the phosphate mop), such that Pi contamination was reduced to <5 μM, and the solutions were referred to as Pi-free solutions. In our study, we did not use this phosphate mop and NMR measurement of our solutions determined that contaminant Pi was ∼0.5 mM (24). This level of Pi is closer to what is present in the heart. The presence of Pi influences cross-bridge cycling specifically, without affecting thin filament activation kinetics. We previously reported (24) that under phosphate mop conditions there was no difference between kACT and kTR at the maximal Ca2+ level. However, without this mop in activation solutions (0.5 mM contaminant Pi), kACT was slower than kTR. Our current results confirm those previous findings during maximal Ca2+ activation, and extend them by demonstrating a similar effect at submaximal levels of Ca2+ and at maximal Ca2+ when cTnI is phosphorylated by PKA.
Myofibril relaxation kinetics
Measurements of relaxation rates of myofibrils by rapid solution switching can provide insights into the role of cross-bridge dynamics in this process. The time course of full tension relaxation following Ca2+ removal (pCa 9.0, Fig. 2 A) is most often biphasic, beginning with an early, slow phase of relaxation followed by a more rapid (fast) relaxation phase. Here, we reported that kREL,slow was accelerated at the submaximal Ca2+ level, even without cTnI phosphorylation. This result is different from previous reports, where this rate did not differ between relaxations from maximal or submaximal Ca2+ activation (35, 43, 46). Relaxation is a complicated process, involving Ca2+ removal from cTnC and the effect of its loss and cross-bridge detachment on thin-filament deactivation. The rate and duration of the slow phase are very sensitive to the conditions that affect cross-bridge kinetics, such as temperature, muscle fiber type (myosin isoform), mechanical perturbation, and chemical interventions. During submaximal Ca2+ activations, we might expect the initial phase of relaxation to be faster than during maximal activation because there is less Ca2+ binding to thin filaments at any given time. Thus, when myosins detach, it is easier for the thin filament to become deactivated. This is especially true for cardiac muscle, where myosin binding has been shown to enhance Ca2+ binding to the thin filament. Furthermore, the presence of Pi (0.5 mM Pi in our study) should exacerbate this effect, as it results in a reduction of the tension (strain) bearing cross-bridges. This may accelerate the detachment of myosin cross-bridges from the thin filament, thus contributing to an increase in the slow phase of relaxation.
Both kTR and kREL,slow have commonly been used as a reflection of the cross-bridge cycling rate and/or the rate of cross-bridge detachment (35, 43, 47). However, it is important to remember the differences in conditions during these two measurements. For example, kTR is measured when Ca2+ in activation solutions is constant and Ca2+ binding to cTn is in a relative steady state. However, kREL,slow is measured following the abrupt removal of Ca2+ from the system. Thus, the amount of Ca2+ bound to the thin filament is more dynamic and is reduced at a rate determined by the Ca2+ dissociation rate (koff) of cTn. The change in bound Ca2+ is likely most dramatic at the beginning of the relaxation phase, when the gradient of Ca2+ bound (to cTn) versus that in solution is highest. Additionally, Ca2+ koff may be further increased as cross-bridges start to detach (48) at the beginning of relaxation. As such, it is difficult to make strong correlations between kTR vs. kREL,slow as a measure of cross-bridge cycling or detachment kinetics.
Similar to most previous findings for rodent cardiac muscle, we found that PKA phosphorylation of cTnI reduces the Ca2+ sensitivity of tension, and speeds overall relaxation. However, Walker et al. (49) reported that PKA phosphorylation does not change cross-bridge kinetics in human cardiac myofibrils. The reason for this difference may be due to the different myosin isoforms in human and rat cardiac myofibrils. Another possible reason is the inorganic Pi concentration of solutions. The Walker study did not report the Pi concentration, however, in an earlier report, their Pi concentration is <5 μM. Perhaps more interesting is our finding that the modulatory effect of cTnI phosphorylation on myofibril relaxation is greater at the submaximal Ca2+ and that this is correlated with a reduction in C-I interaction. Thus, it may be that conditions that reduce C-I interaction (here either reduce Ca2+ or cTnI phosphorylation) result in reduced contractile activation, a more rapid deactivation of the thin filament when Ca2+ is removed and a more rapid initiation of the relaxation process.
Conclusion
The most significant findings of the current study are i), the Ca2+ sensitivity of tension is decreased by PKA phosphorylation, although the maximal tension production is maintained, as previously reported (34); ii), the phosphorylation of cTnI, rather than that of cMyBP-C and titin, is responsible for the reduction of slow phase relaxation, thus speeding overall relaxation; and iii), PKA phosphorylation of cTnI decreases C-I interaction (as well as the binding affinity of Ca2+ to cTnC). These findings suggest a connection between C-I interaction and the kinetics of thin filament activation, tension development, and the initial phase of myofibril relaxation. Thus, the degree of C-I interaction may act as a regulator that is modulated by changes in Ca2+ and cTn phosphorylation during β-adrenergic stimulation of the heart.
Acknowledgments
We thank Drs. An-yue Tu and Charles Luo for preparations of cTnI mutant proteins and protein isolation. We appreciate the help and support of Prof. Rommie Amaro, Dr. Maria V. Razumova, and Dr. Peter Kekenes-Huskey. We are indebted to Martha Mathiason for the development of data acquisition and analysis software.
This research was supported by National Institutes of Health (NIH) R01 HL-65497 & HL-11197 (M.R.), American Heart Association (AHA) 11POST7400069 (V.S.R.), and AHA 12POST11570005 (S.L.). Funding and support from the National Biomedical Computation Resource (NBCR) is provided through NIH P41 GM103426. Work in the JAM group is supported in part by National Science Foundation (NSF), NIH, Howard Hughes Medical Institute (HHMI), and the NSF Supercomputer Centers.
Editor: Bernhard Brenner.
Footnotes
Vijay Rao and Yuanhua Cheng contributed equally to this work.
One table and detailed Material and Methods section are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(14)00745-0.
Supporting Material
References
- 1.Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 2.Zaugg M., Schaub M.C. Cellular mechanisms in sympatho-modulation of the heart. Br. J. Anaesth. 2004;93:34–52. doi: 10.1093/bja/aeh159. [DOI] [PubMed] [Google Scholar]
- 3.Regnier M., Martin H., Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys. J. 2004;87:1815–1824. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regnier M., Martyn D.A., Chase P.B. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys. J. 1998;74:2005–2015. doi: 10.1016/S0006-3495(98)77907-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillis T.E., Martyn D.A., Regnier M. Investigation of thin filament near-neighbour regulatory unit interactions during force development in skinned cardiac and skeletal muscle. J. Physiol. 2007;580:561–576. doi: 10.1113/jphysiol.2007.128975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Racca A.W., Beck A.E., Regnier M. Contractility and kinetics of human fetal and human adult skeletal muscle. J. Physiol. 2013;591:3049–3061. doi: 10.1113/jphysiol.2013.252650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regnier M., Rivera A.J., Gordon A.M. Thin filament near-neighbour regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca(2+) relations. J. Physiol. 2002;540:485–497. doi: 10.1113/jphysiol.2001.013179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regnier M., Rivera A.J., Chase P.B. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ. Res. 2000;86:1211–1217. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- 9.Li M.X., Spyracopoulos L., Sykes B.D. Binding of cardiac troponin-I147-163 induces a structural opening in human cardiac troponin-C. Biochemistry. 1999;38:8289–8298. doi: 10.1021/bi9901679. [DOI] [PubMed] [Google Scholar]
- 10.Parvatiyar M.S., Pinto J.R., Potter J.D. Predicting cardiomyopathic phenotypes by altering Ca2+ affinity of cardiac troponin C. J. Biol. Chem. 2010;285:27785–27797. doi: 10.1074/jbc.M110.112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R., Zhao J., Potter J.D. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ. Res. 1995;76:1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 12.Kentish J.C., McCloskey D.T., Solaro R.J. Phosphorylation of troponin I by protein kinase A accelerates relaxation and cross-bridge cycle kinetics in mouse ventricular muscle. Circ. Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 13.Colson B.A., Bekyarova T., Moss R.L. Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium. Circ. Res. 2008;103:244–251. doi: 10.1161/CIRCRESAHA.108.178996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamasaki R., Wu Y., Granzier H. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ. Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 15.Solaro R.J., Rosevear P., Kobayashi T. The unique functions of cardiac troponin I in the control of cardiac muscle contraction and relaxation. Biochem. Biophys. Res. Commun. 2008;369:82–87. doi: 10.1016/j.bbrc.2007.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward D.G., Brewer S.M., Trayer I.P. Characterization of the interaction between the N-terminal extension of human cardiac troponin I and troponin C. Biochemistry. 2004;43:4020–4027. doi: 10.1021/bi036128l. [DOI] [PubMed] [Google Scholar]
- 17.Köhler J., Chen Y., Chase P.B. Familial hypertrophic cardiomyopathy mutations in troponin I (K183D, G203S, K206Q) enhance filament sliding. Physiol. Genomics. 2003;14:117–128. doi: 10.1152/physiolgenomics.00101.2002. [DOI] [PubMed] [Google Scholar]
- 18.Martyn D.A., Regnier M., Gordon A.M. Ca2+ - and cross-bridge-dependent changes in N- and C-terminal structure of troponin C in rat cardiac muscle. Biophys. J. 2001;80:360–370. doi: 10.1016/S0006-3495(01)76020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon A.M., Qian Y., Martyn D.A. Characterization of troponin-C interactions in skinned barnacle muscle: comparison with troponin-C from rabbit striated muscle. J. Muscle Res. Cell Motil. 1997;18:643–653. doi: 10.1023/a:1018631806182. [DOI] [PubMed] [Google Scholar]
- 20.Dong W.-J., Xing J., Cheung H.C. Structural mapping of single cysteine mutants of cardiac troponin I. Proteins. 2000;41:438–447. [PubMed] [Google Scholar]
- 21.Potter J.D. Preparation of troponin and its subunits. Methods Enzymol. 1982;85(Pt B):241–263. doi: 10.1016/0076-6879(82)85024-6. [DOI] [PubMed] [Google Scholar]
- 22.Dong W.-J., Robinson J.M., Cheung H.C. Ca2+-induced conformational transition in the inhibitory and regulatory regions of cardiac troponin I. J. Biol. Chem. 2003;278:8686–8692. doi: 10.1074/jbc.M212886200. [DOI] [PubMed] [Google Scholar]
- 23.Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- 24.Kreutziger K.L., Piroddi N., Regnier M. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J. Mol. Cell. Cardiol. 2011;50:165–174. doi: 10.1016/j.yjmcc.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tachampa K., Wang H., de Tombe P.P. Cardiac troponin I threonine 144: role in myofilament length dependent activation. Circ. Res. 2007;101:1081–1083. doi: 10.1161/CIRCRESAHA.107.165258. [DOI] [PubMed] [Google Scholar]
- 26.Adhikari B.B., Regnier M., Martyn D.A. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys. J. 2004;87:1784–1794. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. USA. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colomo F., Piroddi N., Tesi C. Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. J. Physiol. 1997;500:535–548. doi: 10.1113/jphysiol.1997.sp022039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreutziger K.L., Piroddi N., Regnier M. Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. J. Physiol. 2008;586:3683–3700. doi: 10.1113/jphysiol.2008.152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesi C., Colomo F., Poggesi C. The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys. J. 2000;78:3081–3092. doi: 10.1016/S0006-3495(00)76845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Robertson I.M., Regnier M. Structural and functional consequences of the cardiac troponin C L48Q Ca(2+)-sensitizing mutation. Biochemistry. 2012;51:4473–4487. doi: 10.1021/bi3003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patton C., Thompson S., Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004;35:427–431. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 33.George S.E., Su Z., Johnson J.D. The fourth EF-hand of calmodulin and its helix-loop-helix components: impact on calcium binding and enzyme activation. Biochemistry. 1996;35:8307–8313. doi: 10.1021/bi960495y. [DOI] [PubMed] [Google Scholar]
- 34.Rao V.S., Korte F.S., Martyn D.A. N-terminal phosphorylation of cardiac troponin-I reduces length-dependent calcium sensitivity of contraction in cardiac muscle. J. Physiol. 2013;591:475–490. doi: 10.1113/jphysiol.2012.241604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poggesi C., Tesi C., Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Arch. 2005;449:505–517. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- 36.Kress M., Huxley H.E., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J. Mol. Biol. 1986;188:325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- 37.Huxley A.F., Simmons R.M. Rapid ‘give’ and the tension ‘shoulder’ in the relaxation of frog muscle fibres. J. Physiol. 1970;210:32P–33P. [PubMed] [Google Scholar]
- 38.Luo Y., Davis J.P., Rall J.A. Myofibrillar determinants of rate of relaxation in skinned skeletal muscle fibers. Adv. Exp. Med. Biol. 2003;538:573–581. doi: 10.1007/978-1-4419-9029-7_51. discussion 581–582. [DOI] [PubMed] [Google Scholar]
- 39.Finley N., Abbott M.B., Rosevear P.R. NMR analysis of cardiac troponin C-troponin I complexes: effects of phosphorylation. FEBS Lett. 1999;453:107–112. doi: 10.1016/s0014-5793(99)00693-6. [DOI] [PubMed] [Google Scholar]
- 40.Sakthivel S., Finley N.L., Robbins J. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J. Biol. Chem. 2005;280:703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., McCully M.E., Regnier M. Structural and functional consequences of cardiac troponin C L57Q and I61Q Ca(2+)-desensitizing variants. Arch. Biochem. Biophys. 2013;535:68–75. doi: 10.1016/j.abb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng, Y., S. Lindert, …, M. Regnier. Computational Studies of S23D/S24D Troponin I Mutation on Cardiac Troponin Structural Dynamics. (In Revision). [DOI] [PMC free article] [PubMed]
- 43.Tesi C., Piroddi N., Poggesi C. Relaxation kinetics following sudden Ca(2+) reduction in single myofibrils from skeletal muscle. Biophys. J. 2002;83:2142–2151. doi: 10.1016/S0006-3495(02)73974-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hünlich M., Begin K.J., VanBuren P. Protein kinase A mediated modulation of acto-myosin kinetics. J. Mol. Cell. Cardiol. 2005;38:119–125. doi: 10.1016/j.yjmcc.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Wattanapermpool J., Guo X., Solaro R.J. The unique amino-terminal peptide of cardiac troponin I regulates myofibrillar activity only when it is phosphorylated. J. Mol. Cell. Cardiol. 1995;27:1383–1391. doi: 10.1006/jmcc.1995.0131. [DOI] [PubMed] [Google Scholar]
- 46.Stehle R., Krüger M., Pfitzer G. Does cross-bridge activation determine the time course of myofibrillar relaxation? Adv. Exp. Med. Biol. 2003;538:469–479. doi: 10.1007/978-1-4419-9029-7_43. discussion 479. [DOI] [PubMed] [Google Scholar]
- 47.Stehle R., Krüger M., Pfitzer G. Force kinetics and individual sarcomere dynamics in cardiac myofibrils after rapid ca(2+) changes. Biophys. J. 2002;83:2152–2161. doi: 10.1016/S0006-3495(02)73975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis J.P., Norman C., Tikunova S.B. Effects of thin and thick filament proteins on calcium binding and exchange with cardiac troponin C. Biophys. J. 2007;92:3195–3206. doi: 10.1529/biophysj.106.095406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker J.S., Walker L.A., de Tombe P. Protein kinase A changes calcium sensitivity but not cross-bridge kinetics in human cardiac myofibrils. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H138–H146. doi: 10.1152/ajpheart.00838.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.