Abstract
Cholesterol is important for the formation of microdomains in supported lipid bilayers and is enriched in the liquid-ordered phase. To understand the interactions leading to this enrichment, we developed an AFM-based single-lipid-extraction (SLX) approach that enables us to determine the anchoring strength of cholesterol in the two phases of a phase-separated lipid membrane. As expected, the forces necessary for extracting a single cholesterol molecule from liquid-ordered phases are significantly higher than for extracting it from the liquid-disordered phases. Interestingly, application of the Bell model shows two energy barriers that correlate with the head and full length of the cholesterol molecule. The resulting lifetimes for complete extraction are 90 s and 11 s in the liquid-ordered and liquid-disordered phases, respectively. Molecular dynamics simulations of the very same experiment show similar force profiles and indicate that the stabilization of cholesterol in the liquid-ordered phase is mainly due to nonpolar contacts.
Introduction
Eukaryotic membranes are quasi-two-dimensional, highly complex heterogeneous surfaces consisting of proteins and lipids like phospholipids, sphingolipids, and cholesterol. Cholesterol is particularly important in maintainenance of this heterogeneity, since it is probably involved in the formation of the nano- or microdomains (1, 2). These structures are suggested to play an important role in signal transduction and sorting of membrane components (3). Furthermore, it has been proposed that microdomains are in a liquid-ordered state, that is, characterized by lipids having a high degree of chain order (as in the solid state) and at the same time a high lateral mobility of the lipids (as in the liquid-disordered state) (4). Even though the existence of raftlike microdomains in cells is still controversial (1), they have been intensively studied in model membranes.
Typically, these model membranes are based on ternary lipid mixtures consisting of a lipid with a low transition temperature, like DOPC, a lipid with a high transition temperature, like sphingomyelin (SM), and cholesterol. At room temperature, a liquid-liquid phase separation is observed that is characterized by a liquid-ordered phase rich in cholesterol and a liquid-disordered phase rich in DOPC (5, 6, 7).
The different affinity of lipids for different lipid environments is important for such diverse processes as the fusion of vesicles (8), the function of peripheral membrane proteins (9), and protein sorting in the Golgi apparatus (10). For instance, the preference of cholesterol for ordered membranes seems to be essential for its passive transport from the endoplasmic reticulum via the Golgi apparatus to the plasma membrane (11, 12).
Furthermore, it is still not completely clear why and how certain lipids accumulate within a cell at different locations (11). Their anchoring strength is certainly an important parameter, which to our knowledge has not yet been determined in a phase-separated lipid bilayer. Therefore, determination of the dependence of anchor strength on different parameters, such as the phase state of the membranes or membrane composition, contributes to our understanding of these important processes.
Supported lipid bilayers (SLBs) are routinely used for lipid membrane studies (13). They can be considered as a first approximation of the lipid part of cellular membranes. SLBs opened the road for the investigation of lipid membranes with sophisticated surface-sensitive techniques like surface plasmon resonance (14), total internal reflection fluorescence microscopy (15), the surface force apparatus (16), and atomic force microscopy (AFM) (17). The surface force apparatus and AFM are also ideal for manipulating SLBs in a controlled way (18, 19). They have been used to extract lipids from SLBs, but the methods used in those studies involved pulling biotinylated lipids out of a membrane, thus having a different molecule every time and hence a different spacer length (20, 21, 22). Constant spacer length is crucial to extraction of thermodynamic parameters with high accuracy.
To complement bulk methods for studying the interaction between lipids and lipid membranes, we established a single-molecule-based method that measures the force necessary to extract a single lipid molecule out of a lipid membrane. This provides a constant spacer length and a phase-specific result. To this end, we covalently attach a single cholesterol molecule via a polyethyleneglycol (PEG) spacer to an AFM tip. This approach guarantees that we always pull one and the same molecule out of the lipid membrane. By analyzing the extraction forces at different loading rates, we derive thermal activation parameters, such as the potential width and the lifetime of a lipid molecule in a lipid membrane. By means of molecular dynamics (MD) simulations, we are able to mimic experiments and to describe at the molecular level interactions between the extracted lipid and its environment.
Materials and Methods
1,2-Dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), egg-SM (mainly 16:0 SM), cholesterol, and rhodamine-PE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol-PEG-NHS (5 kDa) was purchased from Nanocs (New York, NY). Methyl-PEG-NHS (6 kDa), NHS-PEG-NHS (5 kDa) was from Rapp (Tübingen, Germany). HPLC water and Hepes were purchased from Biochrom (Berlin, Germany). Chloroform (HPLC-grade) was purchased from Sigma-Aldrich (St. Louis, MO). Ethanol (pure) was obtained from Merck (Darmstadt, Germany).
Preparation of unilamellar vesicles
POPC or a mixture of DOPC, SM, and cholesterol (2:2:1) (PSC221 mixture) was dissolved in chloroform to a final concentration of 1 mg/mL, and 0.1 mol % of Rhodamine-PE was added. The solution was placed in a glass vial and chloroform was evaporated by a nitrogen flow followed by vacuum evaporation for at least 6 h at 0.1 mbar to ensure the absence of chloroform traces. Then, 1 mL of an aqueous buffer (10 mM Hepes and 4 mM CaCl2) was added, and after gently shaking for 30 min, multilamellar vesicles were obtained. To form unilamellar vesicles, the solution was extruded (Mini-Extruder, Avanti Polar Lipids) 31 times using a 100 nm filter (Nucleopore, Whatman, Piscataway, NJ) and allowed to equilibrate overnight at 4°C.
Preparation of SLBs
SLBs were formed on mica via the vesicle fusion method (23). As model lipids, we chose the PSC221 mixture. To form an SLB, the vesicles have to fuse with the surface of a mica plate (1 cm2) glued to a temperature-controllable fluid cell. To that aim, the vesicle solution was diluted 1:10 using the same buffer as before. Then, 50 μL of solution was applied to the freshly cleaved mica sheet for an incubation time of 45 min. After incubation, the fluid cell was gently rinsed with 200 mL of water and incubated at 50°C for 30 min. The fluid cell was then allowed to slowly cool down to room temperature and rinsed again with at least 200 mL of pure water and then with the buffer used for the experiments (50 mM NaCl and 10 mM Hepes, pH 7.1). Finally, the quality of the bilayer was optically checked with fluorescence microscopy. If too many nonfused vesicles were present, the sample was discarded. In addition, the fluorescence image of the PSC221-SLB should display a coexistence of two types of regions, one with a high and the other with a low fluorescence intensity, corresponding to the liquid-disordered (LD) and liquid-ordered (LO) states, respectively.
AFM tip functionalization
Covalent attachment of a single lipid (POPC or cholesterol) molecule (via a PEG spacer) was achieved by applying the following protocol.
First, silicon nitride cantilevers (MLCT, Bruker, Santa Barbara, CA) are placed in pure ethanol for 30 s. Chips are carefully dried with filter paper and oxygen plasma is then used to form OH groups on the surface of the tips (surface activation).
To form NH2 groups on the tip surface (amination), chips were incubated in an APTES (amino-propyl-tri-etoxy silane) solution (Vectabond/dry Aceton, 1:100, v/v) for 15 min immediately after the activation process and were then thoroughly rinsed with acetone. The formation of stable NH2 groups was completed by baking the chips at 70°C for 15 min. During baking, a solution consisting of a 1:10 mixture of lipid-PEG-NHS/methyl-PEG-NHS (50 mg/mL) in chloroform/triethylamine (5%) was prepared. Cholesterol functionalization of the cantilever was finally achieved by placing the cantilevers overnight in this solution in a chloroform saturated atmosphere. Right before the experiment, cantilevers were rinsed with chloroform, then ethanol, then ultrapure water.
AFM imaging and force spectroscopy
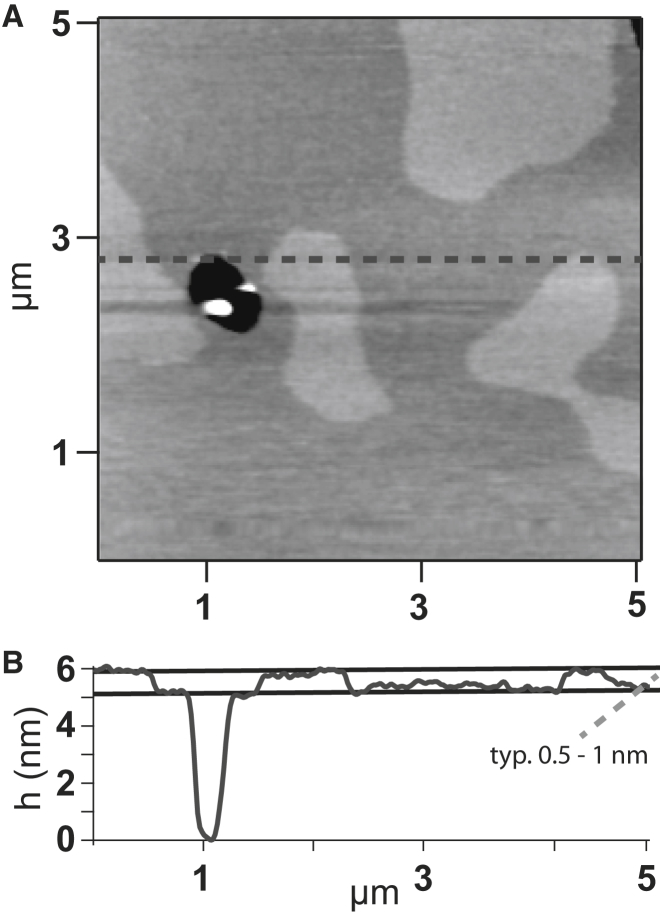
The experiments were carried out with AFM imaging and force spectroscopy performed using an MFP-3D AFM (Oxford Instruments/Asylum Research, Santa Barbara, CA). All experiments were done in aqueous buffer (50 mM NaCl and 10 mM Hepes, pH 7.2). MLCT cantilevers were used for both imaging and force spectroscopy. Nominal spring constants were between 0.01 and 0.1 nN/nm and were determined by applying the thermal noise method (24, 25). Imaging was performed in intermittent contact mode, before and after force measurements, to make sure that all extraction curves were made on the same lipid phase (Fig. 1, A and B).
Figure 1.
(A) Tapping-mode AFM image of a PSC221 membrane. Dark gray areas correspond to the LD phase and light gray areas to the LO phase. The black spot shows an area which is not covered by a lipid membrane. The white spot is a nonfused vesicle. (B) Section corresponding to the dotted line in A.
Extraction measurements were performed by vertically directing the cholesterol-functionalized AFM tip toward the SLB (Fig. S1 (1 and 2) in the Supporting Material). When the tip is close to the bilayer it starts to contact the bilayer (Fig. S1, 2 and 3). Then a small force (∼100 pN) is kept constant by a feedback loop for ∼4 seconds (Fig. S1, 3 and 4). If the cholesterol inserts into the bilayer during this dwell time, it is pulled out or extracted from the bilayer upon retracting the AFM tip from the bilayer (Fig. S1, 4 and 5). The tip was moved with vertical velocities, , of 50, 500, or 5000 nm/s.
Data evaluation
Typically, all analysis steps were carried out automatically by a home-written algorithm based on the software IGOR Pro 6 (Wavemetrics, Portland, OR).
Extraction curves were first recorded as cantilever deflection versus piezo extension. Then, the cantilever sensitivity was determined by measuring the slope of the deflection-extension curve after the experiment by pressing the tip onto the hard mica surface. Together with the spring constant (see above), this allowed for calculating the force-versus-distance curves. The force at rupture was recorded as the extraction force. Each data point consisted of 200–400 extraction curves taken from either the LO or the LD phase. After correction for hydrodynamic effects (26), the extraction forces were plotted in histograms for each tip velocity and either of the lipid phases.
After normalization, these probability distributions (p(f), where f is the force) can be directly transformed into lifetime-force distributions, τ(F), which are normally the result of constant-force (force-clamp) experiments. To this aim, we applied Dudko’s formula (27),
| (1) |
where is the force-dependent loading rate,
| (2) |
It was assumed that the stretching curve can be described by a wormlike chain, where L is the contour length of the linker and Lp its persistence length, is the thermal energy, is the pulling velocity, and is the cantilever spring constant. For the data evaluation, we used Lp = 0.3 nm and L = 27 nm.
Finally, these force-dependent lifetime distributions were fitted with the Bell model,
| (3) |
to get the thermal activation parameters of the lipid extraction process, namely, the width of the potential, xβ, and the lifetime, (0), at zero force (natural lifetime) (see Fig. S2 for a definition of xβ). Here, a semilogarithmic representation for the force-lifetime distribution was chosen to give the Bell model a linear appearance.
To get an estimate for the activation free energy connected to the extraction process, we employed the Arrhenius law:
| (4) |
where is the lifetime of the cholesterol at zero force in the LO phase, is the lifetime at zero force in the LD phase, and A is the Arrhenius prefactor. For lipids, we can assume A = 107 (28).
Therefore, the ratio of the two lifetimes can be written as
| (5) |
It follows for the difference between the Gibbs activation free energies of a cholesterol molecule in both phases at zero force
| (6) |
Since the final state is the same for extraction from both the the LO and LD phases, the difference in activation Gibbs free energies, , must be equal to the difference of the standard parts of the Gibbs free energies (see Fig. S2). This difference is again equivalent to the standard part of the free-energy change, ΔG0,LO→LD, when cholesterol is transferred from the LO phase directly to the LD phase. We will use this in the Discussion section to compare our results with those of others.
MD simulations
The process of lipid extraction was modeled at the atomistic level employing MD simulations that included either 64 or 128 lipids and 5000 water molecules. Both extraction of POPE from the POPC bilayer and extraction of cholesterol from model LD and LO membranes were simulated. Both equilibrium (i.e., without pulling a POPE or cholesterol molecule out of the bilayer but with the extracted molecule equilibrated at a certain distance from the bilayer midplane) and nonequilibrium MD simulations (i.e., with pulling of POPE or cholesterol out of the bilayer) were performed. The united-atom Berger’s force field was used for lipids. In the case of SM, the parameterization developed for 16:0 SM by Niemelä et al. was employed (29). The LD and LO phases were modeled by tricomponent (DOPC, SM, cholesterol) bilayers with varying ratio of the components. The LD and LO phases were modeled employing DOPC/SM/cholesterol ratios of 72:48:8 and 12:56:60, respectively. The buffer used in the extraction experiments was not included in MD simulations, because its effect would be negligible due to low concentration in comparison with the number of particles included in the simulated systems (the concentration corresponds to 2.5 NaCl and half a Hepes molecule per bilayer leaflet). The equilibrium free-energy profile of extracting a molecule of POPE from the POPC lipid bilayer was calculated employing potential of mean force (PMF) calculations with the umbrella sampling scheme. The force profiles of lipid extraction were calculated employing nonequilibrium simulations with pulling of POPE and cholesterol. In the case of cholesterol extraction, a detailed evaluation of cholesterol-membrane interaction for the pulled cholesterol was performed. Additional simulations of cholesterol in LO and LD bilayers under equilibrium were performed to elucidate the nature of cholesterol stabilization in both systems. Further computational details are given in the Supporting Material.
Results
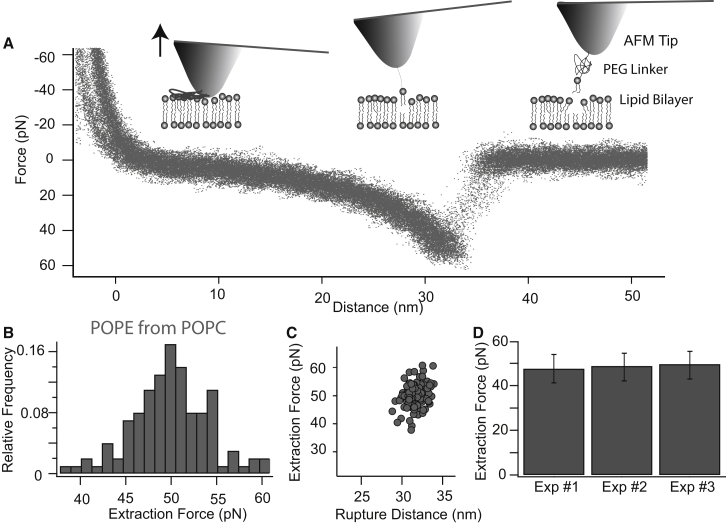
To guarantee the reliability and reproducibility of our experiments, we started out by coupling phospholipids (POPE) to AFM tips and measured the force needed to extract a single POPE molecule from a POPC lipid bilayer. Fig. 2 A shows the superposition of >100 extraction curves. Fig. 2, B–D, shows that the results within one experiment, as well as the results of different experiments with the same system, are reproducible. The obtained extraction forces are 50 ± 5 pN, which is consistent with previous results (see Discussion).
Figure 2.
Extraction of a POPE molecule from a POPC bilayer at 1 μm/s. (A) Superposition of >100 extraction curves. (B) Histogram of extraction forces. (C) Scatter plot of extraction force versus rupture distance. (D) Forces (±SD) needed to extract POPE molecules from POPC bilayers for three independent experiments.
In nonequilibrium MD simulations of POPE extraction from the POPC bilayer, we varied the pulling rates between 0.0025 and 1 m/s. Rates as low as these used in the experiment cannot be achieved due to computational costs, which increase with the reduction of the pulling rate. Representative force profiles are shown in Fig. 3. By comparing the resulting nonequilibrium and equilibrium force profiles (the latter obtained as equilibrium forces at constrained positions, approximately corresponding to an infinitely slow pulling rate), we conclude that a pulling rate of at most 0.05 m/s should be used in nonequilibrium MD simulations. The mean extraction force values (± SD) are 69 ± 2 pN and 70 ± 2 pN for pulling rates of 0.0025 and 0.05 m/s, and 70 ± 2 pN for the equilibrium pulling. These values are in a reasonable accord with the force obtained by the AFM experiment.
Figure 3.
Force profiles calculated during nonequilibrium MD simulations of POPE extraction from POPC (upper row) and cholesterol extraction from LO and LD bilayers (lower row). For POPE, two extraction rates are depicted, with the equilibrium force profile shown as circles. Low extraction rates (0.05 m/s at most) are required in nonequilibrium simulations to reproduce the force profile calculated under equilibrium conditions. One extraction rate is presented for cholesterol extracted from disordered and ordered lipid bilayers. The corresponding force versus time dependencies are shown in Fig. S4.
To study the extraction of a single cholesterol molecule from a phase-separated PSC221 bilayer, the cholesterol molecule was attached to the AFM tip via a PEG spacer. Stretching curves were associated with single lipid extractions when we observed a single rupture event (a drop to the baseline) and when the distance from the surface at the time of rupture was less than or equal to the contour length, L, of the PEG-molecule (L ∼ 27 nm). Longer events or multiple ruptures represent multiple molecules in parallel or unspecific adhesion. Roughly 20% of all force curves were identified as single extraction events. From these traces, we determined the forces that were necessary to pull a single cholesterol molecule out of the LO and the LD regions of a phase-separated lipid membrane made from the PSC221 mixture. To obtain the potential width and the lifetime, measurements were carried out at three different pulling speeds (50, 500, and 5000 nm/s) for each phase.
Fig. 4 shows representative extraction force histograms for both LO and LD phases at different pulling speeds. All measurements were carried out in duplicate and were fully reproducible. Negative controls with no cholesterol attached showed an insignificant number (<1%) of (false-positive) extraction events. For the extraction of cholesterol from the LD phase, we get mean (± SD) extraction forces of 12 ± 5, 20 ± 5, and 30 ± 5 pN for 50, 500, and 5000 nm/s, respectively. For the extraction of cholesterol from the LO phases, we get mean (± SD) extraction forces of 22 ± 5, 28 ± 5, and 36 ± 5 pN for 50, 500, and 5000 nm/s, respectively.
Figure 4.
Force distribution for the extraction of cholesterol from LO phases and LD phases. The LO phases are rich in cholesterol, and the LD phases are not.
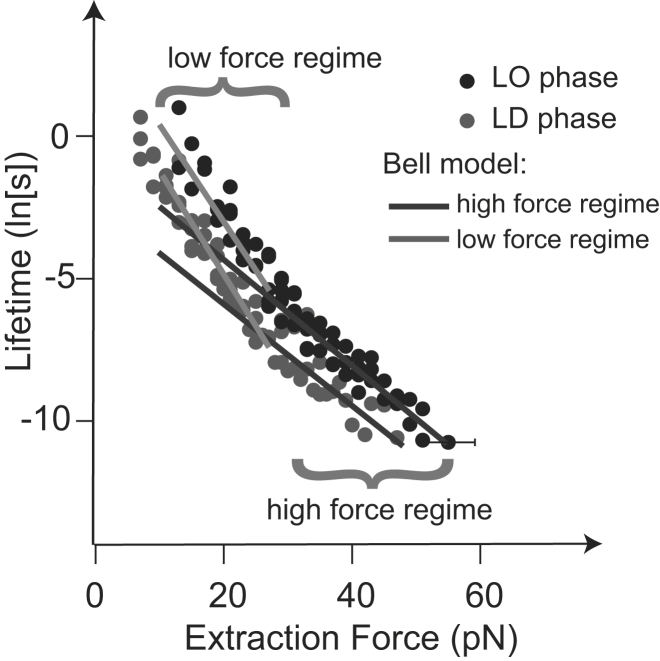
Fig. 5 shows the measured data after model-free transformation of the histograms into force-dependent lifetimes, as described in Materials and Methods. Neither extraction from the LO phase nor that from the LD phase can be described with a single Bell model. Therefore, following Evans (22), we fit the data in a piecewise manner. The lifetime-force distribution for each phase is divided in a low- and a high-force regime, and Bell models are fitted in each regime separately. The resulting thermal activation parameters are given in Table 1. Utilizing Eqs. 4 and 6 results in activation free energies of and for the LO and LD phases, respectively, and therefore in an activation free energy difference, , between the phases of −2.1 kBT (−5.2 kJ/mol).
Figure 5.
Lifetime versus extraction force from the data given in Fig. 4. Both LO and LD phases show a biphasic behavior; therefore, a Bell model is fitted to the distributions in the high- and low-force regimes, respectively. Forces <10 pN are not considered, because this is the sensitivity of our instrument.
Table 1.
Thermal activation parameters obtained from fitting the Bell model to the low- and high-force regimes for the LO and LD phases
| Phase state | Potential width, xβ | Natural lifetime, τ0,Bell | |
|---|---|---|---|
| Low-force regime | LO | 1.5 nm | 90 s |
| LD | 1.5 nm | 11 s | |
| High-force regime | LO | 0.8 nm | 1 s |
| LD | 0.7 nm | 0.1 s |
See Fig. 5 for a visual representation of the fit.
MD simulations of cholesterol extraction from LO and LD phases were performed at pulling rates of 0.0025 and 0.05 m/s. Each value was determined from five independent simulations in both LD and LO cases, with varying lateral localization of the pulled cholesterol molecule with regard to other lipid components. The resulting mean extraction force values are reported in Table 2 and representative force profiles are shown in Fig. 3 (lower plots). Note that although there are minor discrepancies between the forces calculated at the two pulling rates, the difference between the pulling from the LO and that from the LD phases is evident. Namely, in all simulated systems, the force required for pulling cholesterol out of the membrane is higher in the LO than in the LD phase. Assuming standard velocity dependence (30), the force range of∼90–120 pN from the MD simulation agrees well with forces obtained from AFM experiments. In the course of the simulations in the LD phase, the number of contacts between the pulled cholesterol and SM increased during the pulling, whereas contacts with DOPC became less frequent. The pulled cholesterol was also in contact with other cholesterol molecules. In LO membranes, different possibilities were observed, as some of the cholesterol molecules resided for whole simulations in either pure SM or mixed DOPC/SM clusters. We pulled cholesterol from both environments, with no differences observed in extraction force.
Table 2.
Mean extraction force for cholesterol extraction from model LO and LD bilayers calculated employing nonequilibrium MD simulations
| Pulling rate (m/s) | FLO phase (pN) | FLD phase (pN) |
|---|---|---|
| 0.0025 | 118 (± 5) | 92 (± 5) |
| 0.05 | 123 (± 5) | 112 (± 5) |
We based our detailed analysis of cholesterol-membrane interactions on equilibrium MD simulations of both membrane types to determine the molecular basis of the difference between cholesterol extraction from the LO and that from the LD phases. In general, cholesterol is stabilized in lipid membranes by both polar and nonpolar interactions (31). This stabilization can be characterized by numbers of contacts formed between a cholesterol molecule and molecules of lipids and water. Regarding polar interactions, three types of contact can be observed: hydrogen bonds between the 3OH groups of cholesterol and water, hydrogen bonds between the 3OH group of cholesterol and carbonyl oxygen atoms of lipid, and polar pairs between the 3OH group of cholesterol and choline groups of lipids. Nonpolar interactions can be quantified as close contacts between nonpolar atoms of the cholesterol ring system and nonpolar groups in acyl chains of lipids. Table 3 shows average numbers of H-bonds, charge pairs, and nonpolar contacts formed by a cholesterol molecule in the LD and LO bilayers of a cholesterol molecule. A clear effect in the transition from the LD to the LO phase is a significant (∼9%) increase in the number of nonpolar contacts, although changes in the number of polar interactions around the 3OH group also occur. Hence, the stabilization effect for cholesterol in the LO compared to the LD membrane is due to a combination of nonpolar interactions between cholesterol and other membrane components, H-bonds, and other polar contacts. In addition, we tested whether the rupture of SM-SM hydrogen bonds plays a role during cholesterol extraction from the LO phase. Namely, we monitored the number of SM-SM hydrogen bonds in the nearest neighborhood of the extracted cholesterol molecule during the extraction of cholesterol from the LO phase in one of the MD trajectories (see Fig. S5). We observe no dependence of the number of SM-SM hydrogen bonds on the position of the extracted molecule, confirming that the rupture of SM-SM hydrogen bonds does not significantly contribute to the value of the extraction force.
Table 3.
Phase-specific average numbers of H-bonds, charge pairs, and nonpolar contacts in a cholesterol molecule
| LO phase | LD phase | |
|---|---|---|
| H-bonds | 1.5 (± 0.1) | 1.24 (± 0.2) |
| Other polar pairs | 3.9 (± 0.1) | 4.6 (± 0.6) |
| Nonpolar contacts | 83 (± 1) | 76 (± 2) |
Average numbers were calculated using equilibrium MD simulations (i.e., without the pulling of cholesterol out of the bilayer).
Since the cholesterol is coupled to the AFM tip via its 3OH group, we estimated to what extent this attachment geometry influences the observed differences between cholesterol extraction from LO and that from LD phases in the SLB. Based on Table 3, the number of H-bonds formed by the cholesterol 3OH group changes by only a fraction of an H-bond between the LD and LO phases, whereas the number of nonpolar contacts changes by almost 10. This suggests that the attachment of cholesterol to the AFM tip does not significantly affect the observed differences between the binding of cholesterol to the LO and LD phases.
Discussion
Cholesterol is a key component in determining the physical state of biomembranes of eukaryotic cells. In addition, cholesterol can be used as a lipid anchor, e.g., for studying DNA; and its ability to modify the biophysical properties of biomembranes is used in biomimetic systems. To our knowledge, this is the first AFM single-lipid-extraction (SLX) study to provide insight into the forces experienced by a single cholesterol molecule during extraction from defined phases in SLBs. The general validity of our approach is tested by extracting POPE from a POPC SLB. The obtained extraction force (∼50 pN) fits well with previous results (22). This is also in accord with the forces estimated employing MD simulations.
In the following, we discuss in detail the extraction of single cholesterol molecules from a phase-separated lipid bilayer. The loading-rate-dependent extraction forces lie between 12 and 36 pN and are therefore smaller but of the same order of magnitude as the extraction forces of phospholipids (22). In addition, this range is consistent with our nonequilibrium MD pulling simulations, where we obtain forces of ∼100 pN at loading rates a factor of ∼105 higher.
Note that in the MD simulations in the case of POPE extraction from the POPC bilayer, the pulling-rate dependence plays a relatively small role. This is consistent with experimental observations, where the pulling-rate dependence for POPE extraction is significantly less pronounced than that for cholesterol extraction (see Fig. S3). Surprisingly, the pulling-rate dependence for cholesterol extraction force in the LO and LD phases is similar, but the differences in force are robust and consistent with experiments. Therefore, we believe that our MD simulations provide a sensible molecular-level explanation of the observed greater stabilization of cholesterol in the LO lipid phase.
Further molecular information from the experiments was deduced by transforming the probability distributions at different force loading rates into a force-dependent lifetime distribution. The observed two regimes suggest that there is an inner and an outer barrier in the energy landscape, where the width of the outer barrier should in this case correspond to the full length of the molecule (22). Indeed, the obtained value corresponds well with the full length of the cholesterol molecule (∼1.6 nm).
Forces of ≥25 pN could cause a deformation of the energy landscape in such a way that the inner barrier now becomes the dominating one, resulting in a change of the slope of the fit in Fig. 5. The location of the inner barrier (i.e., the width of the inner potential) agrees, very well with the length of the iso-octyl group of cholesterol (∼0.7 nm) (see Fig. 6).
Figure 6.

The length of a cholesterol molecule is ∼1.6 nm. The length of its iso-octyl part is ∼0.7 nm. Interestingly, these dimensions match the widths of the full potential and the widths of the inner potential obtained from the Bell model (Fig. 5). The PEG (which links the cholesterol to the AFM tip) is attached to the oxygen atom of the 3-OH group (asterisk).
The Bell model also directly yields natural lifetimes, i.e., the average time the cholesterol molecule would stay in the lipid bilayer at zero force. A comparison between the two lipid phases (LO and LD) shows that the cholesterol lifetime in the LO phase is ∼10 times higher than in the LD phase for both high- and low-force regimes (see Table 1). Other experiments with lipid membranes show the same trends in different lipid phases. For example, in one study the lifetime of cholesterol was estimated to be 38 h on (saturated) SM vesicles and 4 h on unsaturated diacyl phospholipids (32). In a asimilar way, cell membranes exposed to an efficient cholesterol acceptor (cyclodextrin) showed two kinetic pools with half-lifetimes of 20 min and 20 s (33). These results are consistent with the concept of microdomains (slow pool, LO phase) being surrounded by the rest of the plasma membrane (fast pool, LD phase). Thus, our ratio for the cholesterol lifetime in LO and LD phase is consistent with measurements made by others.
Since the obtained lifetime of 90 s for the whole cholesterol molecule (in the low-force regime) in the cholesterol-rich LO phase seems to be relatively low, we compare it with the situation in cholesterol micelles. To that end, we use an approximation from Israelachvili (28) to calculate critical micelle concentrations (CMCs):
| (7) |
Here, the residence time, , corresponds to the mean lifetime in our experiments and the collision time, , corresoponds to a typical motional correlation time, on the order of for lipids (28).
Inserting into Eq. 7 our measured lifetime from the low-force regime (Fig. 5) for the cholesterol-rich LO phase results in
| (8) |
This corresponds well to the value of 25–40 nM reported in (34) for the CMC of cholesterol with micelles. Thus, thermodynamic parameters from micelles and SLBs seem to be rather similar.
MD simulations help to exclude the possibility of significant changes in the measured lifetimes resulting from our attachment geometry (where PEG is linked to the 3OH group), because they show that there is only a minor change in the number of polar interactions around the 3OH group (where the linker is attached; see Fig. 6). Furthermore, the simulations suggest that the preference of cholesterol for the liquid-ordered phase over the liquid-disordered phase arises from nonpolar interactions between cholesterol and other membrane components. This is evidenced by a significant (∼9%) increase in the number of nonpolar contacts with the change from the LD to the LO phase.
Regardless of which molecular interaction is mainly responsible for the increased affinity of cholesterol for the LO phase, this increase results in a higher cholesterol concentration in the LO phase than in the LD phase, as well as a difference between the desorption energies in the two phases.
As mentioned, is equivalent to the standard part of the free-energy change, , when cholesterol is transferred from the LO phase directly to the LD phase (see Materials and Methods and Fig. S2). This enables us to compare our measurements to those of others.
Assuming that the cholesterol concentrations in both phases are in equilibrium, and further assuming that cholesterol concentrations can be used instead of the activities, one can calculate this difference in the standard Gibbs free energies (for the transfer of cholesterol from the LD to the LO phase) from the ratio of the two concentrations by invoking the mass action law (28).
| [9] |
Here, is the thermal energy and describes the cholesterol concentration in the LD and LO phases.
We use Gibbs’ phase diagram (35) to obtain an estimate a [Chol]LD/[Chol]LO ratio of 7:47. In this way, we get
| (10) |
Now, this value can be compared to our value based on the ratio of the lifetimes of the two phases, namely −2.1 kBT (−5.2 kJ/mol). Furthermore, and in agreement with our data, Bezlyepkina et al. (36) state a value on the order of for the difference in standard Gibbs free energies for the transfer of cholesterol from the LO to the LD phase. Also, Tsamaloukas et al. (37) state a very similar value for the transfer of cholesterol from POPC bilayers to POPC/SM/Chol (40:40:20) bilayers, supporting our observation that the membrane composition, rather than the phase, plays the dominant role in the cholesterol partition between the phases. Finally, provides additional valuable information to determine tie lines.
Conclusions
We have designed and carried out an AFM force spectroscopic experiment to determine the anchoring strength of a single cholesterol molecule in the LO and LD phases of a phase-separated lipid bilayer made from a tertiary lipid mixture. Loading-rate-dependent measurements with a constant linker length make it possible to compare extraction force, unloading lifetime, width of the Bell potential, and interaction free energy for the two phases. These phases could be imaged by AFM in advance, making feasible a direct correlation of topographic features (i.e., the LD and the LO phases) with force data.
As expected, the extraction forces for the LO phase are significantly higher than those for the LD phase, but overall, the estimated lifetimes come out rather short (by tens of seconds). We therefore provide a thorough energetic discussion and a comparison with the CMCs of similar systems, as well as with equilibrium and nonequilibrium (pulling) MD simulations, to support our findings. The MD simulations in addition show that the main difference between cholesterol interaction in the LO and that in the LD phase is due to changes in nonpolar contacts. Our experiments constitute an important step toward understanding microdomain formation and stability. We anticipate that this method will be extended toward protein-lipid interactions.
Acknowledgments
The authors thank Erich Sackmann (Technische Universität Munich) for helpful discussions. T.H. acknowledges funding from SFB863 and the European Science Foundation EuroMEMBRANE CRP OXPL (Hu 997/7-1). F.S. thanks the Hanns-Seidel-Stiftung (HSS) for financial support. P.J. thanks the Czech Science Foundation (grant P208/12/G016); he acknowledges the Academy of Sciences of the Czech Republic for the Praemium Academie award and the Academy of Finland for the Finland Distinguished Professor (FiDiPro) award.
Editor: Heiko Heerklotz.
Footnotes
Five figures, and MD Simulations Methodology are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(14)00736-X.
Contributor Information
Lukasz Cwiklik, Email: lukasz.cwiklik@jh-inst.cas.cz.
Thorsten Hugel, Email: thugel@mytum.de.
Supporting Citations
References (38, 39, 40, 41, 42, 43, 44, 45, 46, 47) appear in the Supporting Material.
Supporting Material
References
- 1.Hancock J.F. Lipid rafts: contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pike L.J. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J. Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 4.Simons K., Ehehalt R. Cholesterol, lipid rafts, and disease. J. Clin. Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veatch S.L., Keller S.L. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 6.de Almeida R.F.M., Fedorov A., Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandit S.A., Vasudevan S., Scott H.L. Sphingomyelin-cholesterol domains in phospholipid membranes: atomistic simulation. Biophys. J. 2004;87:1092–1100. doi: 10.1529/biophysj.104.041939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lev S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:739–750. doi: 10.1038/nrm2971. [DOI] [PubMed] [Google Scholar]
- 9.Cross B., Ronzon F., Rieu J.-P. Measurement of the anchorage force between GPI-anchored alkaline phosphatase and supported membranes by AFM force spectroscopy. Langmuir. 2005;21:5149–5153. doi: 10.1021/la0470986. [DOI] [PubMed] [Google Scholar]
- 10.Sprong H., Van Der Sluijs P., Van Meer G. How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol. 2001;2:504–513. doi: 10.1038/35080071. [DOI] [PubMed] [Google Scholar]
- 11.Bennett W.F.D., Tieleman D.P. Molecular simulation of rapid translocation of cholesterol, diacylglycerol, and ceramide in model raft and nonraft membranes. J. Lipid Res. 2012;53:421–429. doi: 10.1194/jlr.M022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sackmann E. Supported membranes: scientific and practical applications. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 14.Besenicar M., Macek P., Anderluh G. Surface plasmon resonance in protein-membrane interactions. Chem. Phys. Lipids. 2006;141:169–178. doi: 10.1016/j.chemphyslip.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Oreopoulos J., Epand R.F., Yip C.M. Peptide-induced domain formation in supported lipid bilayers: direct evidence by combined atomic force and polarized total internal reflection fluorescence microscopy. Biophys. J. 2010;98:815–823. doi: 10.1016/j.bpj.2009.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benz M., Gutsmann T., Israelachvili J. Correlation of AFM and SFA measurements concerning the stability of supported lipid bilayers. Biophys. J. 2004;86:870–879. doi: 10.1016/S0006-3495(04)74162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milhiet P.-E., Gubellini F., Lévy D. High-resolution AFM of membrane proteins directly incorporated at high density in planar lipid bilayer. Biophys. J. 2006;91:3268–3275. doi: 10.1529/biophysj.106.087791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stetter F.W.S., Hugel T. The nanomechanical properties of lipid membranes are significantly influenced by the presence of ethanol. Biophys. J. 2013;104:1049–1055. doi: 10.1016/j.bpj.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clausen-Schaumann H., Seitz M., Krautbauer R., Gaub H.E. Force spectroscopy with single bio-molecules. Curr. Opin. Chem. Biol. 2000;4:524–530. doi: 10.1016/s1367-5931(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 20.Wieland J.A., Gewirth A.A., Leckband D.E. Single-molecule measurements of the impact of lipid phase behavior on anchor strengths. J. Phys. Chem. B. 2005;109:5985–5993. doi: 10.1021/jp045461b. [DOI] [PubMed] [Google Scholar]
- 21.Ounkomol C., Xie H., Heinrich V. Versatile horizontal force probe for mechanical tests on pipette-held cells, particles, and membrane capsules. Biophys. J. 2009;96:1218–1231. doi: 10.1016/j.bpj.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans E., Ludwig F. Dynamic strengths of molecular anchoring and material cohesion in fluid biomembranes. J. Phys. Condens. Matter. 2000;12:A315–A320. [Google Scholar]
- 23.Leonenko Z.V., Carnini A., Cramb D.T. Supported planar bilayer formation by vesicle fusion: the interaction of phospholipid vesicles with surfaces and the effect of gramicidin on bilayer properties using atomic force microscopy. Biochim. Biophys. Acta. 2000;1509:131–147. doi: 10.1016/s0005-2736(00)00288-1. [DOI] [PubMed] [Google Scholar]
- 24.Sader J.E., Larson I., White L.R. Method for the calibration of atomic force microscope cantilevers. Rev. Sci. Instrum. 1995;66:3789–3798. [Google Scholar]
- 25.Pirzer T., Hugel T. Atomic force microscopy spring constant determination in viscous liquids. Rev. Sci. Instrum. 2009;80:035110. doi: 10.1063/1.3100258. [DOI] [PubMed] [Google Scholar]
- 26.Alcaraz J., Buscemi L., Puig-de-Morales M. Correction of microrheological measurements of soft samples with atomic force microscopy for the hydrodynamic drag on the cantilever. Langmuir. 2002;18:716–721. [Google Scholar]
- 27.Dudko O.K., Hummer G., Szabo A. Theory, analysis, and interpretation of single-molecule force spectroscopy experiments. Proc. Natl. Acad. Sci. USA. 2008;105:15755–15760. doi: 10.1073/pnas.0806085105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Israelachvili J.N. 3rd ed. Academic Press; New York: 2010. Intermolecular and Surface Forces: With Applications to Colloidal and Biological Systems (Colloid Science) [Google Scholar]
- 29.Niemelä P., Ollila S. Assessing the nature of lipid raft membranes. PLoS Comput. 2007;3:e34. doi: 10.1371/journal.pcbi.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans E., Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys. J. 1997;72:1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Róg T., Pasenkiewicz-Gierula M., Karttunen M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta. 2009;1788:97–121. doi: 10.1016/j.bbamem.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Kan C.C., Bittman R., Hajdu J. Phospholipids containing nitrogen- and sulfur-linked chains: kinetics of cholesterol exchange between vesicles. Biochim. Biophys. Acta. 1991;1066:95–101. doi: 10.1016/0005-2736(91)90256-8. [DOI] [PubMed] [Google Scholar]
- 33.Haynes M.P., Phillips M.C., Rothblat G.H. Efflux of cholesterol from different cellular pools. Biochemistry. 2000;39:4508–4517. doi: 10.1021/bi992125q. [DOI] [PubMed] [Google Scholar]
- 34.Haberland M.E., Reynolds J.A. Self-association of cholesterol in aqueous solution. Proc. Natl. Acad. Sci. USA. 1973;70:2313–2316. doi: 10.1073/pnas.70.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith A.K., Freed J.H. Determination of tie-line fields for coexisting lipid phases: an ESR study. J. Phys. Chem. B. 2009;113:3957–3971. doi: 10.1021/jp808412x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bezlyepkina N., Gracià R.S., Dimova R. Phase diagram and tie-line determination for the ternary mixture DOPC/eSM/cholesterol. Biophys. J. 2013;104:1456–1464. doi: 10.1016/j.bpj.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsamaloukas A., Szadkowska H., Heerklotz H. Thermodynamic comparison of the interactions of cholesterol with unsaturated phospholipid and sphingomyelins. Biophys. J. 2006;90:4479–4487. doi: 10.1529/biophysj.105.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hess B., Kutzner C., Lindhal E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S., Rosenberg J.M., Kollman P.A. THE weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992;13:1011–1021. [Google Scholar]
- 40.Berger O., Edholm O., Jähnig F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys. J. 1997;72:2002–2013. doi: 10.1016/S0006-3495(97)78845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berendsen H.J.C., Postma J.P.M., Hermans J. Intermolecular Forces. D. Reidel; Dordrecht: 1981. Interaction models for water in relation to protein hydration; pp. 331–342. [Google Scholar]
- 42.Parrinello M., Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 1981;52:7182–7190. [Google Scholar]
- 43.Nose S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984;52:255–268. [Google Scholar]
- 44.Essmann U., Perera L., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 45.Hess B., Bekker H., Fraaije J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997;18:1463–1472. [Google Scholar]
- 46.Hockney R., Goel S., Eastwood J. Quiet high-resolution computer models of a plasma. J. Comput. Phys. 1974;14:148–158. [Google Scholar]
- 47.Schwierz N., Horinek D., Netz R.R. On the relationship between peptide adsorption resistance and surface contact angle: a combined experimental and simulation single-molecule study. J. Am. Chem. Soc. 2012;134:19628–19638. doi: 10.1021/ja304462u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.