Abstract
We have used atomic-force microscopy (AFM) to probe the effect of peptidoglycan crosslinking reduction on the elasticity of the Staphylococcus aureus cell wall, which is of particular interest as a target for antimicrobial chemotherapy. Penicillin-binding protein 4 (PBP4) is a nonessential transpeptidase, required for the high levels of peptidoglycan crosslinking characteristic of S. aureus. Importantly, this protein is essential for β-lactam resistance in community-acquired, methicillin-resistant S. aureus (MRSA) strains but not in hospital-acquired MRSA strains. Using AFM in a new mode for recording force/distance curves, we observed that the absence of PBP4, and the concomitant reduction of the peptidoglycan crosslinking, resulted in a reduction in stiffness of the S. aureus cell wall. Importantly, the reduction in cell wall stiffness in the absence of PBP4 was observed both in community-acquired and hospital-acquired MRSA strains, indicating that high levels of peptidoglycan crosslinking modulate the overall structure and mechanical properties of the S. aureus cell envelope in both types of clinically relevant strains. Additionally, we were able to show that the applied method enables the separation of cell wall properties and turgor pressure.
Introduction
The cell wall is critical for cell survival in most bacteria; it functions as a protection against mechanical and osmotic lysis in addition to maintaining cell shape (1). Furthermore, the cell wall controls the tactile response of bacteria, influencing a wide range of behaviors such as cell adhesion, environmental sensing, or host defense evasion (2, 3, 4). The major component of the cell wall is peptidoglycan, a complex polymer composed of long glycan chains of alternating β-1,4-linked n-acetylglucosamine and n-acetylmuramic acid subunits, which are cross-linked via peptide bridges to form a strong but flexible structure (5, 6). The last stages of peptidoglycan biosynthesis are catalyzed by a group of proteins called penicillin-binding proteins (PBPs), which have both transglycosylase and transpeptidase activities, required for the elongation of the glycan chains and the formation of peptide bonds, respectively (7). As the name suggests, PBPs are the target of β-lactam antibiotics, molecules that block the transpeptidase active site. Bacterial pathogens have developed different mechanisms to resist the action of β-lactams, mainly by destroying the antibiotic molecule through the action of β-lactamases, or by modifying its target, i.e., the PBPs.
Staphylococcus aureus is a Gram-positive clinical pathogen that has developed a remarkable ability to resist the action of virtually all β-lactam antibiotics. Methicillin-resistant S. aureus (MRSA) strains are currently one of the major causes of antibiotic-resistant, hospital-acquired infections, and can also cause infections among healthy individuals in the community. Therefore, the study of the S. aureus cell envelope is of particular importance for the development of new strategies for antimicrobial chemotherapy (8). The cell wall of S. aureus contains not only a thick layer of peptidoglycan (∼20 nm), but also other polymers like the anionic wall teichoic acids and lipoteichoic acids, secondary modifications, and several proteins attached to the peptidoglycan, resulting in a structure with a total width of ∼35 nm (3, 9, 10). Studies over the last decades have focused on the biochemistry of peptidoglycan biosynthesis and most steps in this pathway are now well characterized (6, 11). However, much less is known about the three-dimensional architecture and mechanical properties of this complex polymer.
We are interested in studying the mechanical properties of the peptidoglycan of live S. aureus cells and in identifying key enzymes essential for the final structure of this polymer. One of the characteristics of the staphylococcal peptidoglycan is its very high degree of crosslinking, inasmuch as up to 90% of its muropeptides are linked to adjacent glycan chains in the peptidoglycan mesh. This crosslinking can be classified into one of the two following types:
-
1.
Primary crosslinking, which is responsible for the first level of cross-links between different glycan chains. Such links are necessary in most bacteria to preserve cell integrity, and include muropeptide species with a polymerization degree lower than or equal to pentamers (6).
-
2.
Secondary crosslinking, which is the result of the same transpeptidase chemical reaction as in the primary crosslinking, but leads to higher levels of linkage of the peptidoglycan layers. It includes muropeptide species with a polymerization degree higher than pentamers (6).
According to the literature, secondary crosslinking is mainly the result of the action of S. aureus PBP4, a nonessential transpeptidase (12, 13). We therefore hypothesized that PBP4 could have a major role in defining the local mechanical properties of S. aureus peptidoglycan, a hypothesis that is backed by results on global mechanical properties of the S. aureus cell wall (14). Interestingly, although it is not essential for normal cell growth, PBP4 has been associated with resistance mechanisms against two major classes of antibiotics:
-
1.
Glycopeptides, where the absence of PBP4 was associated with low-level resistance in vancomycin intermediate S. aureus strains (15); and
-
2.
β-lactams, with PBP4 being required for expression of high level β-lactam resistance in community-acquired MRSA (CA-MRSA) strains but not in hospital-acquired (HA-MRSA) strains (16).
In recent years, atomic-force microscopy (AFM) has provided valuable information on the structural, adhesive, and mechanical properties of the cell wall in numerous biological samples (17, 18, 19, 20, 21, 22, 23, 24). AFM can be used not only to obtain topological information on the cell surface and the organization of the peptidoglycan (20, 22, 24, 25, 26), but it is also a powerful tool for quantitative studies of physical properties of the surface, such as the elasticity of the bacterial cell wall (27, 28, 29, 30, 31, 32, 33, 34). To characterize the mechanical properties of the bacterial cell wall, either purified peptidoglycan or live, intact bacteria can be employed. Both approaches have different limitations but should yield to similar results. Classical AFM modes, such as tapping or force volume mode, are limited in terms of either spatial resolution, imaging time, or quantitative analysis. The recently introduced PeakForce Tapping (Bruker, Santa Barbara, CA) mode with its quantitative nanomechanical property mapping (PeakForce QNM; Bruker), however, allows for simultaneous mapping of topography and multiple mechanical properties, featuring the typical spatial resolution and scan speed of the tapping mode (35, 36, 37).
In this work, we employed this AFM mode with viable, genetically defined prototypes of HA- and CA-MRSA and methicillin-susceptible S. aureus (MSSA) strains and their pbp4 mutants to investigate whether the absence of PBP4 and the concomitant decrease in peptidoglycan crosslinking has an effect on the overall structure and mechanical properties of the S. aureus cell wall. Because pbp4 deletion has different effects on the β-lactam resistance of HA- and CA-MRSA, we studied two S. aureus strains, COL (HA-MRSA strain) and MW2 (CA-MRSA strain), as well as their respective pbp4 deletion mutants.
Methods
S. aureus strains and growth conditions
S. aureus strains used in this study are listed in Table 1. S. aureus strains were grown on tryptic soy agar (TSA; Difco Laboratories, Franklin Lakes, NJ) at 37°C or in tryptic soy broth (TSB; Difco Laboratories) at 37° C with aeration. The medium was supplemented, when necessary, with 50 μg/mL of Kanamycin and 50 μg/mL of Neomycin (Sigma, St. Louis, MO).
Table 1.
S. aureus strains used in this study
| S. aureus strain | Relevant characteristics | MIC (16) | Origin |
|---|---|---|---|
| COL | HA-MRSA strain, wt | 256 mg/L | (51) |
| COLΔpbp4 | HA-MRSA strain; pbp4 null mutant | 256 mg/L | (16) |
| MW2 | CA-MRSA strain, wt | 64 mg/L | (52) |
| MW2Δpbp4 | CA-MRSA strain; pbp4 null mutant | 4 mg/L | (16) |
| 8325-4 | MSSA strain, wt | — | R. P. Novick |
| 8325-4Δpbp4 | MSSA strain; pbp4 null mutant | — | (38) |
| RNPBP4YFP | RN4220 expressing PBP4-YFP C-terminal fusion; Kanr | — | (38) |
| MW2pPBP4-YFP | MW2 expressing PBP4-YFP C-terminal fusion; Kanr | — | This study |
| COLpPBP4-YFP | COL expressing PBP4-YFP C-terminal fusion; Kanr | — | This study |
Construction of S. aureus strains
For localization studies of PBP4 in HA-MRSA and CA-MRSA backgrounds, the gene encoding a PBP4-YFP fusion was transduced, using phage 80α, from strain RNPBP4YFP (38) into MW2 and COL strains, as previously described in Veiga and Pinho (39).
Fluorescence microscopy
S. aureus strains were grown to midexponential phase, placed on a thin layer of 1% agarose in phosphate-buffered saline (PBS; 137 mM NaCl, 10 mM phosphate, and 2.7 mM KCl, pH 7.4) and analyzed by fluorescence microscopy. Images were acquired using a Carl Zeiss Z1 microscope with Axio Observer (Carl Zeiss, Oberkochen, Germany) and a PhotoMetrics CoolSNAP HQ2 camera (Roper Scientific; Planegg, Germany), and the software METAMORPH (Ver. 7.5; Molecular Devices, Sunnyvale, CA).
Peptidoglycan purification and HPLC analysis
Peptidoglycan was prepared from exponentially growing cultures of COL and MW2 parental strains and corresponding pbp4 deletion mutants, as previously described in Filipe et al. (40). Muropeptides were obtained from purified peptidoglycan digested with the muramidase mutanolysin M1 (Sigma), an n-acetylmuramidase that cuts glycan strands between the n-acetylmuramic and n-acetylglucosamine residues of both O-acetylated and unmodified peptidoglycan, as previously described in Filipe et al. (40). The resulting muropeptides were reduced with sodium borohydride (Sigma) and analyzed by reversed-phase HPLC using a HypersilODS column (Thermo Fisher Scientific, Waltham, MA). The eluted muropeptides were detected and quantified by determination of their UV absorption at 206 nm, using the software LC SOLUTION (Shimadzu, Kyoto, Japan).
AFM elasticity mapping
For AFM experiments, S. aureus strains were grown in 12 mL of TSB at 37° C with aeration until midexponential phase (optical density OD600nm = 0.6). Cells were harvested and concentrated in one-third of the initial volume in fresh media. After concentration, the cell suspension was gently filtered, so that cells were immobilized by mechanical trapping into porous polycarbonate membranes with a pore size of 1.2 μm (Millipore, Billerica, MA) (41). The filter was gently rinsed with PBS, the excess of cells was removed by gently cleaning with powder free tissue, and the filter was inverted and attached to a glass slide with double-face adhesive tape. A silicone cover was used to create a hydrophobic area around the filter, which was then filled with 1:10 TSB/PBS solution. AFM measurements were performed in the TSB/PBS solution at room temperature using a Bioscope Catalyst (Bruker) in PeakForce QNM (Bruker) mode (36, 37). The bacterial samples were freshly prepared for each series of measurements and characterized usually within 30 min and never later than 2 h after harvesting. The viability of the bacteria was confirmed by live/dead staining, stamping of filters on agar plates, and by the observation of cell division of the trapped bacteria.
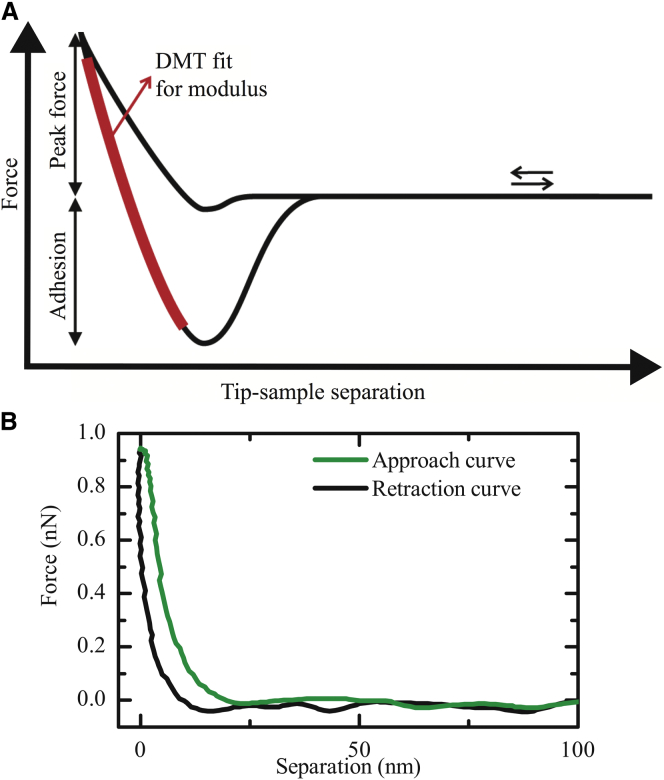
Immediately before each experiment, the AFM probes (Scanasyst-Fluid+; Bruker) were calibrated. Single AFM measurements were carried out with a line scan rate of 0.5 Hz, amplitude of 100–200 nm, a gain of 0.1, a force-curve frequency of 1 kHz, and a peak force threshold of 1 nN (unless mentioned otherwise). The spring constant of the cantilever was determined by the thermal tune method (42) and the optical lever sensitivity by pushing the cantilever against a hard surface before the experiments. Elastic moduli were obtained by a Derjaguin-Muller-Toporov (DMT) fit (43, 44) of the retract part of each single force/distance curve (Fig. 1) described by
where F − FAdh is the determined force relative to the adhesion force, ν is the Poisson ratio (for bacteria usually 0.5 (29, 33)), R is the tip-radius (≈10 nm), d – d0 is the deformation of the sample, and E is the elastic modulus.
Figure 1.
Example of a force/distance curve, which allows insight into local adhesion and elasticity of the sample. (A) Schematic force/distance curve: The maximum adhesion force between the tip and the sample can be extracted as the step height between the base line and the pull-off point. The peak force is defined as the vertical distance between the base line and the point of maximum indentation. By analyzing the retraction phase of each single force/distance curve, the elastic modulus can be determined using a DMT fit (43, 44) (adapted from Pittenger et al. (53)). (B) Approach and retraction part of a typical experimental force/distance curve taken on top of a bacterium. To see this figure in color, go online.
The determination of absolute values for elastic moduli of soft materials at a microscopic scale is not straightforward (45). This holds especially true for biological systems. Thus, the DMT model also does not perfectly represent the complex bacterial cell wall. It is, however, a valid assumption as discussed below, specifically because we are focusing on relative differences between different bacteria with the same experimental setups. The DMT approach assumes a spherical object indenting in the flat surface of an elastic half-space, which is a safe approximation due to the difference of more than two magnitudes in curvatures of a bacterium compared to the AFM tip as well as due to the small indentations. To avoid artifacts due to topography and different angles of indentation, only the topmost part (∼300 × 300 nm2) of each bacterium was taken into account. Effects of local small-scale curvature and inhomogeneities in cell wall composition lead to variability in the acquired data. To overcome these effects, we characterized the entire upper part of the bacteria and collected a large set of datapoints. A total of 50,000–150,000 single elasticity values were obtained from measurements on 10–25 single bacteria per strain. Multiple experiments using different bacterial cultures and AFM probes were performed for each strain.
Results
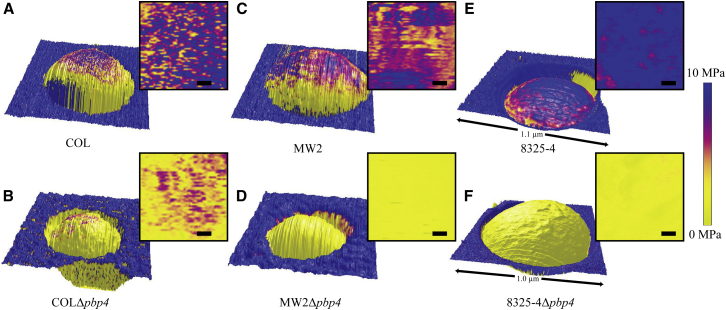
Although the S. aureus cell wall is a complex heterogeneous structure composed of several polymers and proteins (3, 10), it is its main component, the peptidoglycan, that is thought to provide rigidity to bacterial cells, crucial for the cell to withstand the high internal osmotic pressure (20–30 bar) (6, 9). As stated above, PBP4 is responsible for the secondary crosslinking of the S. aureus peptidoglycan (6). Thus, we used PBP4 mutants to test whether highly cross-linked peptidoglycan was required for increased mechanical resistance of live S. aureus cells. For this purpose, we used AFM in PeakForce QNM, a method that detects material variations (such as elasticity or adhesion) at high resolution across a simultaneously acquired topographic image (35, 36, 37). To access primarily the cell wall properties, we used experimental conditions in which the cantilever indentation was less than the proposed thickness of the cell wall, which is in the range of 35 nm (9). Using this methodology, we mapped the elasticity profiles of the cell envelope of two MRSA strains, the HA-MRSA COL and the CA-MRSA MW2, as well as of the respective pbp4 mutants COLΔpbp4 and MW2Δpbp4. Live cells of these strains were immobilized in porous membranes (Fig. 2), and elastic moduli were then determined on individual cells and mapped onto the height image. Only the top of each bacterium was analyzed to avoid additional influence of a change in topography.
Figure 2.
Height images overlaid with the distribution map of the elastic modulus values of (A) COL, (B) COLΔpbp4, (C) MW2, (D) MW2Δpbp4, (E) 8325-4, and (F) 8325-4Δpbp4 single cells trapped in a membrane pore, and respective two-dimensional, top-down representations of the analyzed part of the elastic moduli (scale bars 50 nm). To avoid artifacts due to topography, only the topmost part (∼300 × 300 nm2) of each bacterium was analyzed. The stiffness of the cell wall of the Δpbp4 mutant is significantly decreased in comparison with the wt strain. To see this figure in color, go online.
The first conclusion is that there are no patches of specifically high or low elasticity on a single bacterium. This finding is a consequence of our experimental setting, which is designed to analyze solely the mature cell wall and not the septal cell wall undergoing division.
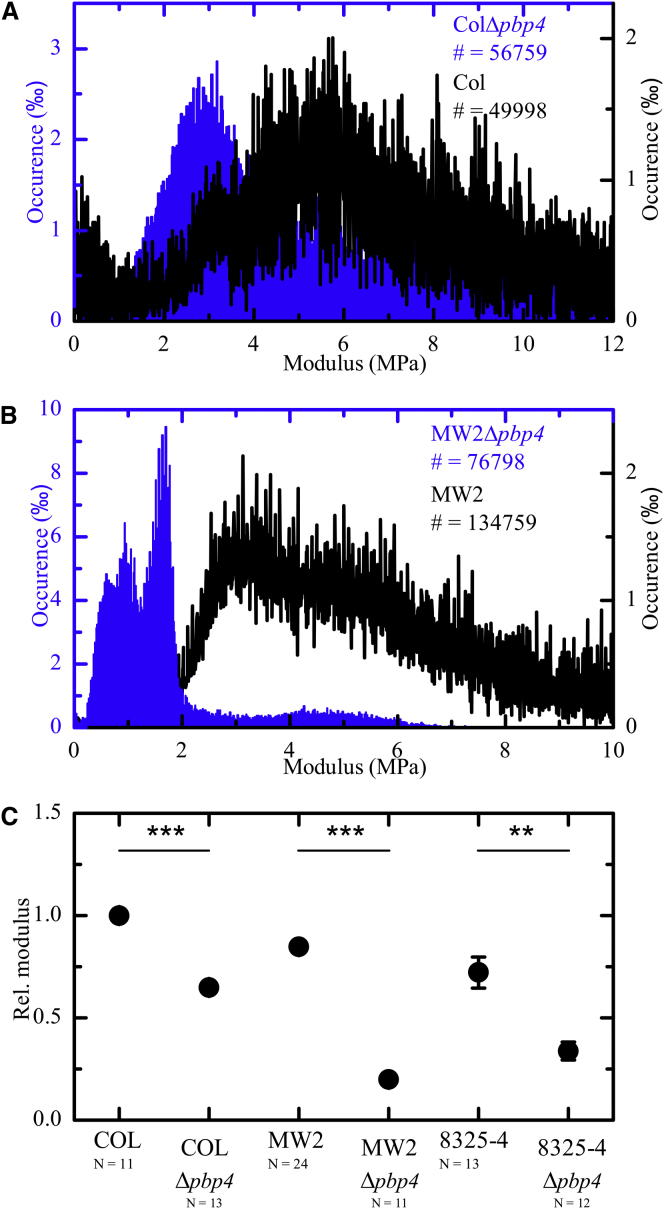
The second and main conclusion of the AFM measurements relates to the significant reduction in the elastic modulus of the cell wall in both CA-MRSA and HA-MRSA strains lacking PBP4, compared to the parental strains (Fig. 3). Because elastic modulus is lower for material with greater elasticity, this data suggests that a decrease in secondary crosslinking results in increased cell wall elasticity (or, in other words, reduced cell wall stiffness) both in COL and in MW2 backgrounds. This is consistent with the hypothesis that a reduction of the number of bonds linking adjacent glycan chains results in a more pliable cell-wall/peptidoglycan structure. Accordingly, when Francius et al. (30) used AFM to study the effect of lysostaphin in live S. aureus cells, they observed a 9.3-fold decrease in cell wall stiffness of lysostaphin-treated cells. Lysostaphin cleaves all pentaglycine bridges that cross-link S. aureus peptidoglycan, effectively destroying both primary and secondary peptidoglycan crosslinking (30). As seen in Fig. 4 C, the pbp4 deletion mutants show a reduction in the levels of secondary crosslinking, but maintain peptidoglycan primary crosslinking, due to the action of the other PBPs present in the cell, which justifies the fact that we observed a smaller decrease in cell wall stiffness (4.4- and 1.5-fold for MW2 and COL, respectively) compared to the action of lysostaphin. However, we cannot formally exclude the possibility that the alterations in cell wall stiffness are due to additional changes in the cell wall surface (in addition to secondary crosslinking reduction) that might result from the absence of PBP4.
Figure 3.
Elastic modulus is reduced upon a reduction of secondary crosslinking. (A and B) Histograms with all the single values from each force curve obtained from all bacteria analyzed. (A) This shows that the surface of the COLΔpbp4 mutant is more elastic than that of the COL wt strain. Approximately 50,000 single elasticity values were obtained from measurements on 11–13 single cells per strain. (B) Similarly, the MW2 wt strain is less elastic than the MW2Δpbp4 strain. Approximately 70,000–130,000 single elasticity values were obtained from measurements on 11–24 single bacteria per strain. (C) Average elastic moduli show that the cell wall of mutants lacking PBP4 is significantly more elastic than the cell wall of the parental strains (values are normalized to the average value of the COL wt strain). To see this figure in color, go online.
Figure 4.
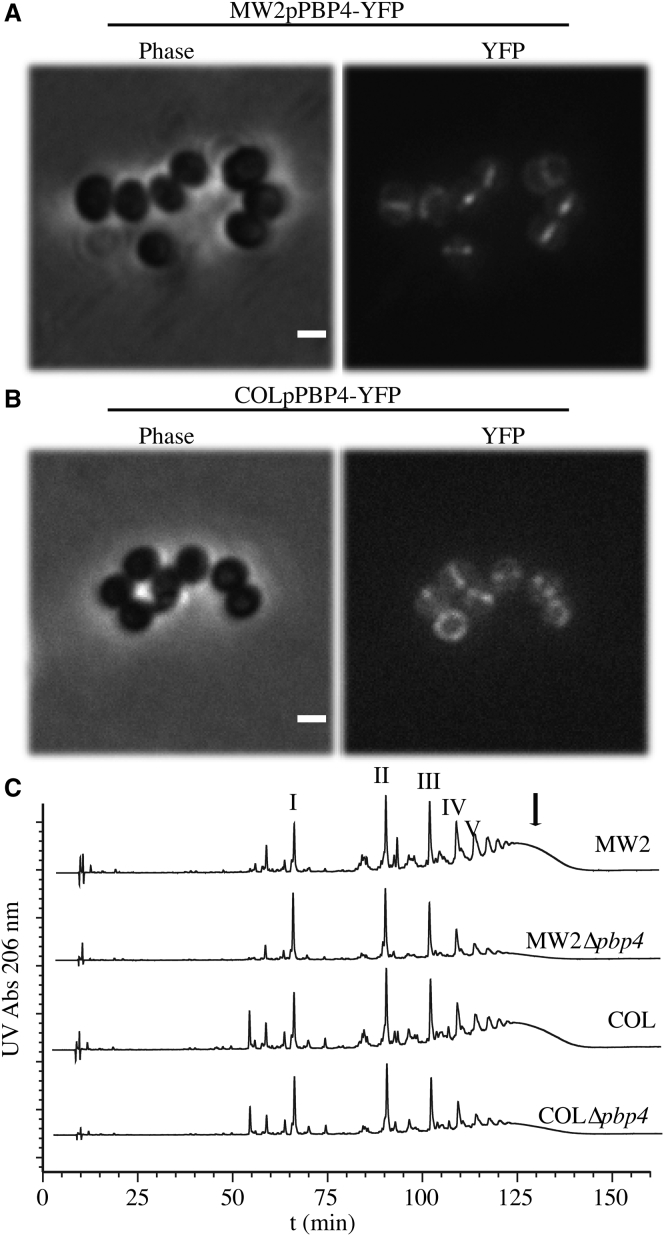
Localization and function of PBP4 are conserved in CA-MRSA and HA-MRSA strains. PBP4 is recruited to the division septa of COL and MW2 strains. Microscopy images of (A) MW2pPBP4-YFP and (B) COLpPBP4-YFP strains, expressing a C-terminal YFP fusion to PBP4 from its native chromosomal locus and under the control of its native promoter, show that PBP4 is recruited to the division septa in these MRSA strains. The protein can be seen as a line corresponding to a septum perpendicular to the plane of the slide, or as a ring when the septum is forming at different angles relative to the plane of the slide (scale bar: 1 μm). (C) Chromatogram of HPLC of the muropeptide composition of peptidoglycan in MW2, MW2Δpbp4, COL, and COLΔpbp4, showing that deletion of pbp4 reduces the secondary crosslinking of the peptidoglycan. (Pointing arrow) Highly cross-linked muropeptide species present in MW2 and COL strains but reduced in pbp4 mutants (MW2Δpbp4 and COLΔpbp4, respectively); I–V muropeptide species from monomers to pentamers. A more detailed characterization was previously published by Memmi et al. (16).
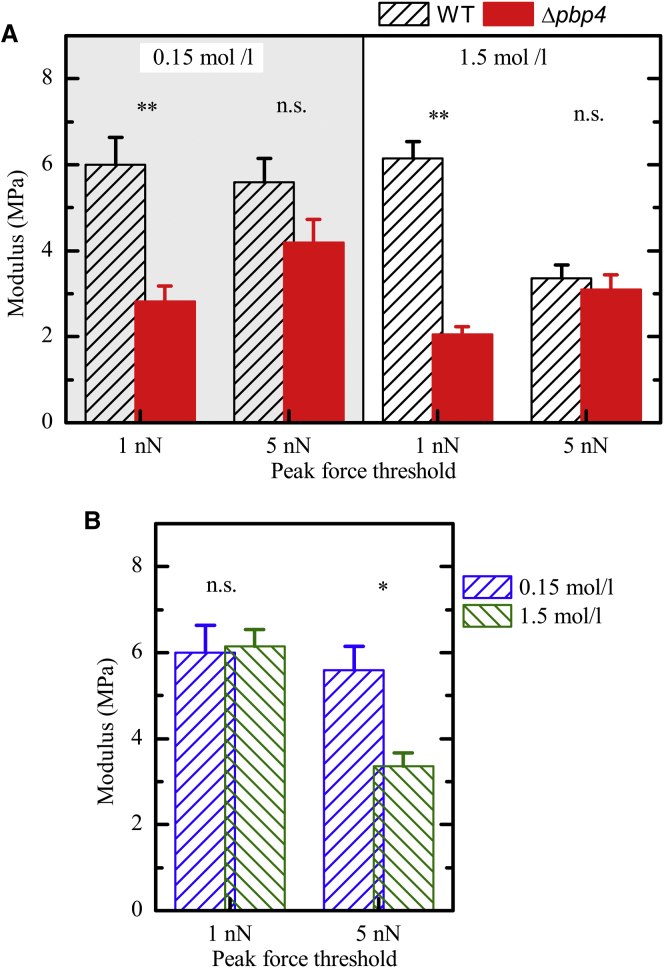
Yet, we can exclude the possibility that the observed reduction in the elastic modulus is due to changes in turgor pressure. To confirm this, we additionally performed experiments using a higher peak force threshold of 5 nN resulting in larger indentations. As shown in Fig. 5 A, the reduction in elastic modulus observed at low indentations diminishes at larger indentations. On the other hand, increasing the NaCl concentration from 0.15 to 1.5 mol/L has no effect on the measured modulus at small indentations (Fig. 5 B). The experiments with larger indentations, however, reveal the induced decrease in cell turgor due to the higher osmotic concentration. Together, these results highlight the potential of the used method to probe different cell properties by choosing different peak force thresholds.
Figure 5.
By separately changing the peak force threshold and the salt concentration, different cell properties can be probed. (A) Elastic moduli of 8325-4 wt and 8325-4Δpbp4 measured using a peak force threshold of 1 and 5 nN (at different salt concentrations), respectively. The reduction in elastic modulus observed for 1 nN diminishes for 5 nN. (B) Elastic moduli of 8325-4 wt from panel A, rearranged to directly compare the influence of different salt concentrations, revealing an effect only for an increased peak force threshold. To see this figure in color, go online.
A possible source of variation of peptidoglycan stiffness between cells could result from measurements being taken at locations with recently synthesized peptidoglycan as well as locations with mature peptidoglycan, i.e., peptidoglycan that underwent secondary crosslinking by PBP4. This was unlikely, given that in previously reported S. aureus strains PBP4 localized (and presumably acted) specifically at the division septum (38). To confirm that this was also the case in the strains used in this study, we characterized the localization of PBP4 fused to yellow fluorescent protein (YFP), expressed from its native chromosomal locus, in strains COL and MW2. In both strains, fluorescence microscopy results clearly attribute the YFP signal to the septal ring (Fig. 4, A and B), indicating that immature peptidoglycan is only present at that place, and not in the cell surface that was analyzed by AFM.
Interestingly, although peptidoglycan is not a rigid structure and can withstand severe changes without compromising the overall cell envelope integrity (9, 46), our data also suggest that there is a lack of a compensatory mechanism able to maintain the overall stiffness of the cell wall in the absence of PBP4.
Discussion
The aim of this work was to study the change of the elasticity of the peptidoglycan of live S. aureus cells upon reduction of secondary peptidoglycan crosslinking that results from pbp4 deletion and to compare the effect on HA- and CA-MRSA. By employing AFM PeakForce QNM (Bruker) with viable, genetically defined prototypes of HA- and CA-MRSA strains and with their pbp4 mutants, we showed that the absence of PBP4 and the concomitant decrease in peptidoglycan crosslinking has an effect on the overall structure and local elasticity of the S. aureus cell wall. These findings are in line with previous reports on a further, more global aspect of the mechanical properties of the cell wall of different S. aureus strains, which showed an effect on the global deformability by pressing entire bacteria against flat surfaces (14).
Given that deletion of pbp4 results in a strong decrease of β-lactam resistance in CA-MRSA strains, such as MW2 (Oxacillin minimum inhibitory concentrations (MICs) for wild-type (wt), 64 g/mL, and pbp4 mutant, 4 g/mL), but not in HA-MRSA backgrounds, such as COL (Oxacillin MICs for wt and pbp4 mutant, 256 g/mL), we tested whether a lack of PBP4 has more influence on the structure of the cell surface of MW2 than of COL. The stiffness decreasing effect was observed both in HA- and CA-MRSA. The extent of the effect as well as the absolute values, however, are different for HA- and CA-MRSA, indicating that a correlation might exist between the requirement of PBP4 for β-lactam resistance—as solely observed in CA-MRSA—and the mechanical properties of the peptidoglycan. Because the mechanical properties of the cell wall play an important role in bacteria/surface interactions, a correlation with bacterial adhesion forces is also conceivable (47).
The AFM elasticity measurements can be correlated with the cell wall structure of the S. aureus CA-MRSA and HA-MRSA strains, by comparing them to the HPLC analysis of purified peptidoglycan from parental strains COL and MW2 and their respective pbp4 deletion mutants. As previously shown, pbp4 gene deletion results in significant decrease of the highly cross-linked muropeptide species that typically elute as a broad peak at the end of the HPLC chromatogram (Fig. 4 C, arrow) (16). We therefore used these isogenic pairs of bacterial strains differing in the peptidoglycan crosslinking degree for AFM analysis.
In general, a variation in studies of peptidoglycan elasticity may result from obtaining measurements on newly synthesized cell wall as well as on mature cell wall, which may have different levels of crosslinking. In the rod-shaped, Gram-positive model organism Bacillus subtilis, cell wall synthesis occurs both at the septum, for cell division, and at the lateral wall, for cell elongation (6, 7). Although the septum of live cells is not accessible to the AFM cantilever before separation of daughter cells, in B. subtilis measurements made at the lateral wall (side of the cylindrical cell) would include the mature peptidoglycan as well as helical bands of newly synthesized peptidoglycan (48). The use of S. aureus as a model organism avoids this source of variation in peptidoglycan composition, inasmuch as round S. aureus cells do not elongate and therefore only synthesize cell wall at the division septum. Moreover, we were able to confirm that PBP4 is localized specifically at the division septum. Hence, elasticity measurements in S. aureus cell surfaces reflect the structure of the mature cell wall, distant from the sites of PBPs localization, and therefore the sites of synthesis of new peptidoglycan.
Importantly, PBP4 localization was identical in CA-MRSA and HA-MRSA backgrounds, despite its role in these different backgrounds of β-lactam resistance. After cell separation, the mature septum of S. aureus becomes one hemisphere of each daughter cell (49). Therefore, in this organism, the entire cell surface should correspond to mature peptidoglycan of different ages with the possible exception of a single band around the division site corresponding to the outer edge of the septum, identified on the AFM height images of dividing S. aureus cells (30, 50). Thus, our experimental setup enables us to map the elasticity of mature cell wall to assess the effect of the reduction of secondary crosslinking on the mechanical properties of the S. aureus cell wall.
Conclusion
We have demonstrated that alterations in the secondary peptidoglycan crosslinking, caused by a lack of the nonessential transpeptidase PBP4, trigger changes in the local mechanical properties of the S. aureus cell wall, enhancing the overall elasticity of staphylococcal cells both in HA and CA-MRSA strains. Interestingly, we observed a stronger effect of the reduction of the secondary crosslinking on the mechanical properties of the cell wall in CA-MRSA strain MW2 (which requires PBP4 for β-lactam resistance) than in HA-MRSA strain COL. These results were obtained by imaging live S. aureus cells and mapping the local cell-wall elasticity of single cells with high spatial resolution using AFM in the recently introduced PeakForce Tapping (Bruker) mode. This mode allows to probe cell wall elasticity and turgor separately, depending on the chosen experimental parameters. The differences in the effect in the MRSA strains highlight the necessity to investigate the role that mechanical properties play in the context of β-lactam resistance.
Acknowledgments
The authors thank Ambrose Cheung (Dartmouth Medical School, Hanover, NH 03755, USA) for the generous gift of the pbp4 mutant strain, Richard P. Novick (New York University School of Medicine, New York, NY 10016, USA) for the 8325-4 strains, and Alexandre Berquand (Bruker, 76187 Karlsruhe, Germany) for the help with the PeakForce QNM (Bruker) mode.
This work was supported by grant No. PEst-OE/EQB/LA0004/2011 (from Fundação para a Ciência e Tecnologia), grant No. PTDC/BIA-MIC/099151/2008 (from Fundação para a Ciência e Tecnologia, to M.G.P.), and grant No. ERC-2012-StG-310987 (from the European Research Council, to M.G.P.). It was also supported by fellowships No. SFRH/BD/41119/2007 (to P.M.P.) and No. EMBO ASTF 427-2011 (to P.M.P.), and by the Federal Ministry of Education and Research (Germany) under grant No. 01Kl1301B and by the Deutsche Forschungsgemeinschaft under grants No. SFB 1027, No. GRK 1276, and No. INST 256/305-1 FUGG.
Editor: Jochen Guck.
Footnotes
Peter Loskill, Pedro M. Pereira, Mariana G. Pinho and Karin Jacobs contributed equally to this work.
Peter Loskill’s present address is: Department of Bioengineering and California Institute for Quantitative Biosciences (QB3), University of California at Berkeley, Berkeley, California 94720, USA
Contributor Information
Mariana G. Pinho, Email: mgpinho@itqb.unl.pt.
Karin Jacobs, Email: k.jacobs@physik.uni-saarland.de.
References
- 1.Cabeen M.T., Jacobs-Wagner C. Bacterial cell shape. Nat. Rev. Microbiol. 2005;3:601–610. doi: 10.1038/nrmicro1205. [DOI] [PubMed] [Google Scholar]
- 2.Beveridge T.J., Makin S.A., Li Z. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 1997;20:291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 3.Foster T.J., McDevitt D. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol. Lett. 1994;118:199–205. doi: 10.1111/j.1574-6968.1994.tb06828.x. [DOI] [PubMed] [Google Scholar]
- 4.Chavakis T., Wiechmann K., Herrmann M. Staphylococcus aureus interactions with the endothelium: the role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb. Haemost. 2005;94:278–285. doi: 10.1160/TH05-05-0306. [DOI] [PubMed] [Google Scholar]
- 5.Beeby M., Gumbart J.C., Jensen G.J. Architecture and assembly of the Gram-positive cell wall. Mol. Microbiol. 2013;88:664–672. doi: 10.1111/mmi.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollmer W., Blanot D., de Pedro M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 7.Scheffers D.-J., Pinho M.G. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 2005;69:585–607. doi: 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers H.F., Deleo F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matias V.R.F., Beveridge T.J. Native cell wall organization shown by cryo-electron microscopy confirms the existence of a periplasmic space in Staphylococcus aureus. J. Bacteriol. 2006;188:1011–1021. doi: 10.1128/JB.188.3.1011-1021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidenmaier C., Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 11.Typas A., Banzhaf M., Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 2012;10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyke A.W., Ward J.B., Curtis N.A.C. A role in vivo for penicillin-binding protein-4 of Staphylococcus aureus. Eur. J. Biochem. 1981;119:389–393. doi: 10.1111/j.1432-1033.1981.tb05620.x. [DOI] [PubMed] [Google Scholar]
- 13.Kozarich J.W., Strominger J.L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J. Biol. Chem. 1978;253:1272–1278. [PubMed] [Google Scholar]
- 14.Chen Y., Norde W., Busscher H.J. Bacterial cell surface deformation under external loading. mBio. 2012;3:e00378. doi: 10.1128/mBio.00378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieradzki K., Pinho M.G., Tomasz A. Inactivated pbp4 in highly glycopeptide-resistant laboratory mutants of Staphylococcus aureus. J. Biol. Chem. 1999;274:18942–18946. doi: 10.1074/jbc.274.27.18942. [DOI] [PubMed] [Google Scholar]
- 16.Memmi G., Filipe S.R., Cheung A. Staphylococcus aureus PBP4 is essential for β-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob. Agents Chemother. 2008;52:3955–3966. doi: 10.1128/AAC.00049-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaussart A., El-Kirat-Chatel S., Dufrêne Y.F. Single-cell force spectroscopy of probiotic bacteria. Biophys. J. 2013;104:1886–1892. doi: 10.1016/j.bpj.2013.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picas L., Rico F., Scheuring S. Direct measurement of the mechanical properties of lipid phases in supported bilayers. Biophys. J. 2012;102:L01–L03. doi: 10.1016/j.bpj.2011.11.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre G., Deghorain M., Dufrene Y.F. Fluorescence and atomic force microscopy imaging of wall teichoic acids in Lactobacillus plantarum. ACS Chem. Biol. 2011;6:366–376. doi: 10.1021/cb1003509. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler R., Mesnage S., Foster S.J. Super-resolution microscopy reveals cell wall dynamics and peptidoglycan architecture in ovococcal bacteria. Mol. Microbiol. 2011;82:1096–1109. doi: 10.1111/j.1365-2958.2011.07871.x. [DOI] [PubMed] [Google Scholar]
- 21.Scheuring S., Dufrêne Y.F. Atomic force microscopy: probing the spatial organization, interactions and elasticity of microbial cell envelopes at molecular resolution. Mol. Microbiol. 2010;75:1327–1336. doi: 10.1111/j.1365-2958.2010.07064.x. [DOI] [PubMed] [Google Scholar]
- 22.Andre G., Kulakauskas S., Dufrêne Y.F. Imaging the nanoscale organization of peptidoglycan in living Lactococcus lactis cells. Nat. Commun. 2010;1:27. doi: 10.1038/ncomms1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alsteens D., Dupres V., Dufrêne Y.F. Structure, cell wall elasticity and polysaccharide properties of living yeast cells, as probed by AFM. Nanotechnology. 2008;19:384005. doi: 10.1088/0957-4484/19/38/384005. [DOI] [PubMed] [Google Scholar]
- 24.Hayhurst E.J., Kailas L., Foster S.J. Cell wall peptidoglycan architecture in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2008;105:14603–14608. doi: 10.1073/pnas.0804138105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaussart A., Rolain T., Dufrêne Y.F. Binding mechanism of the peptidoglycan hydrolase Acm2: low affinity, broad specificity. Biophys. J. 2013;105:620–629. doi: 10.1016/j.bpj.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner R.D., Ratcliffe E.C., Foster S.J. Peptidoglycan architecture can specify division planes in Staphylococcus aureus. Nat. Commun. 2010;1:26. doi: 10.1038/ncomms1025. [DOI] [PubMed] [Google Scholar]
- 27.Dokukin M.E., Guz N.V., Sokolov I. Quantitative study of the elastic modulus of loosely attached cells in AFM indentation experiments. Biophys. J. 2013;104:2123–2131. doi: 10.1016/j.bpj.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerf A., Cau J.-C., Dague E. Nanomechanical properties of dead or alive single-patterned bacteria. Langmuir. 2009;25:5731–5736. doi: 10.1021/la9004642. [DOI] [PubMed] [Google Scholar]
- 29.Gaboriaud F., Parcha B.S., Strugnell R.A. Spatially resolved force spectroscopy of bacterial surfaces using force-volume imaging. Colloids Surf. B Biointerfaces. 2008;62:206–213. doi: 10.1016/j.colsurfb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Francius G., Domenech O., Dufrêne Y.F. Direct observation of Staphylococcus aureus cell wall digestion by lysostaphin. J. Bacteriol. 2008;190:7904–7909. doi: 10.1128/JB.01116-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaboriaud F., Dufrêne Y.F. Atomic force microscopy of microbial cells: application to nanomechanical properties, surface forces and molecular recognition forces. Colloids Surf. B Biointerfaces. 2007;54:10–19. doi: 10.1016/j.colsurfb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Dufrêne Y.F. Using nanotechniques to explore microbial surfaces. Nat. Rev. Microbiol. 2004;2:451–460. doi: 10.1038/nrmicro905. [DOI] [PubMed] [Google Scholar]
- 33.Touhami A., Nysten B., Dufrene Y.F. Nanoscale mapping of the elasticity of microbial cells by atomic force microscopy. Langmuir. 2003;19:4539–4543. [Google Scholar]
- 34.Yao X., Jericho M., Beveridge T. Thickness and elasticity of Gram-negative murein sacculi measured by atomic force microscopy. J. Bacteriol. 1999;181:6865–6875. doi: 10.1128/jb.181.22.6865-6875.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamcik J., Berquand A., Mezzenga R. Single-step direct measurement of amyloid fibrils stiffness by peak force quantitative nanomechanical atomic force microscopy. Appl. Phys. Lett. 2011;98:193701. [Google Scholar]
- 36.Berquand, A. 2011. Quantitative imaging of living biological samples by PeakForce QNM atomic force microscopy. Bruker Corporation Application Note. Bruker, Santa Barbara, CA.AN135:1–10.
- 37.Berquand A., Roduit C., Hafner M. Atomic force microscopy imaging of living cells. Micros Today. 2010;18:8–14. [Google Scholar]
- 38.Atilano M.L., Pereira P.M., Filipe S.R. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2010;107:18991–18996. doi: 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veiga H., Pinho M.G. Inactivation of the SauI type I restriction-modification system is not sufficient to generate Staphylococcus aureus strains capable of efficiently accepting foreign DNA. Appl. Environ. Microbiol. 2009;75:3034–3038. doi: 10.1128/AEM.01862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filipe S.R., Tomasz A., Ligoxygakis P. Requirements of peptidoglycan structure that allow detection by the Drosophila Toll pathway. EMBO Rep. 2005;6:327–333. doi: 10.1038/sj.embor.7400371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasas S., Ikai A. A method for anchoring round shaped cells for atomic force microscope imaging. Biophys. J. 1995;68:1678–1680. doi: 10.1016/S0006-3495(95)80344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutter J.L., Bechhoefer J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993;64:1868–1873. [Google Scholar]
- 43.Derjaguin B.V., Muller V.M., Toporov Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975;53:314–326. [Google Scholar]
- 44.Maugis D. 1st Ed. Springer; Berlin, Germany: 1999. Contact, Adhesion and Rupture of Elastic Solids. [Google Scholar]
- 45.Dimitriadis E.K., Horkay F., Chadwick R.S. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys. J. 2002;82:2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou L.-T., Marquis R.E. Electromechanical interactions in cell walls of Gram-positive cocci. J. Bacteriol. 1970;101:92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busscher H.J., van der Mei H.C. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 2012;8:e1002440. doi: 10.1371/journal.ppat.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniel R.A., Errington J. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell. 2003;113:767–776. doi: 10.1016/s0092-8674(03)00421-5. [DOI] [PubMed] [Google Scholar]
- 49.Pinho M.G., Errington J. Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol. Microbiol. 2003;50:871–881. doi: 10.1046/j.1365-2958.2003.03719.x. [DOI] [PubMed] [Google Scholar]
- 50.Touhami A., Jericho M.H., Beveridge T.J. Atomic force microscopy of cell growth and division in Staphylococcus aureus. J. Bacteriol. 2004;186:3286–3295. doi: 10.1128/JB.186.11.3286-3295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gill S.R., Fouts D.E., Fraser C.M. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baba T., Takeuchi F., Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 53.Pittenger, B., N. Erina, and C. Su. 2010. Quantitative mechanical property mapping at the nanoscale with PeakForce QNM. Bruker Corporation Application Note. Bruker, Santa Barbara, CA. AN128:1–12.