Abstract
Legionella pneumophila colonizes freshwater amoebae and can also replicate within alveolar macrophages. When their nutrient supply is exhausted, replicating bacteria become cytotoxic, motile, and infectious, which is thought to promote transmission to a new amoeba. The differentiation of L. pneumophila is coordinated by the sigma factors RpoS and FliA and the two-component regulator LetA/LetS and is enhanced by the letE locus. Here we demonstrate that letE promotes motility by increasing expression of the flagellin gene flaA but has little impact on the transcription of fliA, the flagellar sigma factor gene. In addition to promoting motility, letE induces the characteristic shape, pigment, and heat resistance of stationary-phase L. pneumophila. To gain insight into how letE promotes the expression of the transmission phenotype, we designed molecular genetic experiments to discriminate between the following three models: letE mutations are polar on milX; letE encodes a small novel protein; or, by analogy to csrB, letE encodes a regulatory RNA that sequesters CsrA to relieve repression. We report that letE encodes an activator protein, as it does not complement an Escherichia coli csrB mutant, it directs the synthesis of an ∼12-kDa polypeptide, and a letE nonsense mutation eliminates function. A monocistronic letE RNA is abundant during the exponential phase, and its decay during the stationary phase requires RpoS and LetA/LetS. We also discuss how the LetE protein may interact with LetA/LetS and CsrA to enhance L. pneumophila differentiation to a transmissible form.
Normally found in fresh water as a parasite of amoebae, Legionella pneumophila can also infect human alveolar macrophages and cause a severe pneumonia, Legionnaires' disease. As one approach to identifying virulence factors that are required for phagocyte infection, analyses of the regulatory circuit that controls the differentiation of replicating L. pneumophila to a transmissible form have been conducted. From genetic screens, isogenic mutant analyses, and overexpression studies of L. pneumophila cultured in broth or in macrophages, the following model for the genetic control of its life cycle has been constructed. By a stringent response-like mechanism, replicating L. pneumophila organisms respond to low amino acid levels by synthesizing the second messenger (p)ppGpp (17). In response to this or some other signal (40), the sigma factors RpoS and FliA induce the transcription of genes of the transmission regulon (4, 5, 14, 23), while the two-component regulator LetA/LetS and the letE locus cooperate to overcome posttranscriptional repression by CsrA (14, 18, 29, 31). As a result, replicating bacteria within phagocyte vacuoles can respond to stress, including amino acid starvation, by rapidly converting to a resilient cytotoxic, motile, and infectious form.
Genetic and biochemical studies of a variety of gram-negative bacteria support a paradigm in which the LetA/LetS family of two-component regulators activates gene expression indirectly by counteracting the repressor of translation known as CsrA or RsmA (reviewed in references 21 and 32). In Escherichia coli, CsrA binds to particular mRNAs at a consensus sequence encompassing the ribosome binding site (RBS), destabilizing the mRNAs and preventing their translation (6). CsrA is antagonized by an untranslated regulatory RNA, known as csrB or rsmB, which contains multiple repeats of a ribosome binding site-like sequence that presumably bind CsrA and sequester it from mRNAs (32). LetA/LetS orthologues activate csrB expression, thereby promoting the translation of transcripts bound by CsrA (1, 20). For example, the overproduction of csrB by Salmonella enterica serovar Typhimurium bypasses its requirement for the LetA orthologue BarA, as judged by its more efficient invasion of cultured epithelial cells (2).
This broadly conserved mechanism of posttranscriptional regulation also appears to govern the cellular differentiation of L. pneumophila. When conditions are favorable for replication, a CsrA homologue of L. pneumophila represses transmission traits (14, 31). When conditions deteriorate, LetA/LetS functions to relieve CsrA repression, since the expression by letA mutants of an entire panel of transmission phenotypes is restored when CsrA is genetically inactivated (31). Accordingly, L. pneumophila LetA/LetS is predicted to induce the expression of a csrB regulatory RNA that alleviates CsrA binding to mRNAs, but the putative csrB homologue has not been identified.
The magnitude of the LetA/LetS induction of L. pneumophila macrophage infection, flagellar motility, and cytotoxicity is enhanced ∼50% by the letE locus by a mechanism that has not been defined (18). Defined by four transposon insertions, the ∼0.4-kb letE locus (GenBank accession no. AY135376) is not predicted to encode a protein according to the GLIMMER algorithm (Columbia Genome Center Legionella Genome Project [http://genome3.cpmc.columbia.edu/∼legion]). The Lasergene Map Draw program identifies a 372-bp open reading frame (ORF), but its predicted 123-amino-acid polypeptide lacks significant homology to known proteins (18). Thus, letE mutant phenotypes may be caused by a disruption of this coding sequence, as the letE-81, -108, and -80 mutations map within the predicted ORF and the fourth insertion mutation (letE-121) lies upstream, perhaps in its promoter. Alternatively, letE transposon insertions may be polar on milX, which is a locus ∼1 kb downstream that is required for intracellular replication (J. D. Sauer and M. Swanson, unpublished data) and is predicted to encode a membrane transporter protein similar to MilA (32% amino acid identity and 51% similarity), a factor that also contributes in some way to the intracellular replication of L. pneumophila (18, 19). The available phenotypic data are also consistent with a third model stating that letE encodes a regulatory RNA analogous to csrB (18).
To ascertain whether the letE locus enhances L. pneumophila post-exponential-phase (PE-phase) gene expression by encoding a regulatory RNA, by acting in cis on milX, or by encoding an activator protein, we performed a series of genetic and molecular tests. The transcription of two genes of the flagellar cascade by wild-type and letE mutant cells was also analyzed. The data demonstrate that the LetE protein enhances multiple traits that are characteristic of stationary-phase L. pneumophila, including a coccoid shape, pigmentation, motility, heat resistance, and the efficient infection of macrophages. We will also discuss how LetE and LetA/LetS may cooperate to counteract posttranscriptional repression by CsrA.
MATERIALS AND METHODS
Bacterial strains, media, and plasmids.
L. pneumophila Lp02, a virulent thymine auxotroph (7), and MB419 (18), a letE-121 mutant, were cultured in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES; Sigma)-buffered yeast extract broth (AYE) supplemented with 100 μg of thymidine per ml at 37°C to the exponential (E; optical density at 600 nm [OD600] of 0.8 to 1.2) or the PE (OD600 of 3.1 to 3.6) phase. Similar results were obtained for at least one other letE allele in assays of motility, cytotoxicity, infectivity, and growth in macrophages (data not shown). Bacteria were plated on ACES-buffered charcoal-yeast extract-agar supplemented with 100 μg of thymidine per ml (CYET). When indicated, gentamicin was added to a final concentration of 10 μg per ml. E. coli strains MG1655, TR1-5 MG1655 (csrA::kanR), and RG1-B MG1655 (csrB::cam) were grown on Kornberg medium (15).
Plasmids encoding inducible letE and milX genes were constructed as follows. The 3.6-kb letE-milX locus was amplified by a PCR using primers LetE1 and LetE2 (18). The PCR fragment was purified (Qiaquick PCR purification kit; Qiagen) and ligated into pGEM-T Easy (Promega). The resulting plasmid, pMB436, was digested with EcoRI, producing a 1-kb fragment containing letE and a 2.6-kb fragment containing milX. These fragments were gel purified (Qiaquick gel purification kit; Qiagen) and ligated into pMMB-GentΔmob digested with EcoRI and treated with shrimp alkaline phosphatase (Roche). The resulting plasmids were screened for the presence and orientation of the insert. The plasmids pLetE and pMilX contained the genes of interest and any upstream, native promoter sequences colinear with the pTac promoter of pMMB-GentΔmobA.
Bacterial cell morphology.
Phase-contrast images of L. pneumophila were captured at a ×1,000 magnification with a Zeiss Axioplan 2 microscope and a Spot digital camera. To measure the cells, we analyzed the images with the NIH Image software program (http://rsb.info.nih.gov/nih-image/). Images were processed for a reduction of the background, converted to threshold mode, and measured with the Wand auto-measure function. For each image, all single bacteria were selected manually and the major axis of the bacterial rods was calculated by the software. To minimize errors in calculating bacterial lengths, we omitted clusters of two or more cells from the analysis.
Pigment production.
Pigment accumulation was measured as described previously (40). The density of overnight E-phase broth cultures was quantified by measuring the OD600, and then aliquots were diluted to an OD of ∼0.1 and cultured overnight to an OD of ∼2.5. At 4-h intervals, samples were removed and centrifuged for 10 min at 16,000 × g; supernatants were transferred to cuvettes, and cell pellets were resuspended in the same volume of phosphate-buffered saline (PBS). Pigment accumulation in the supernatants was quantified by measuring the OD550, and the bacterial density was determined by measuring the OD600 of a 1:10 dilution of the cell suspension. To determine viability at the last time point, we diluted the bacterial suspensions in PBS and plated them on CYET with gentamicin.
Stress resistance.
The bacterial viability after a 1-h incubation under either low-pH (citric acid, pH 3), hyperosmolar (5 M NaCl), oxidative (10 mM H2O2), or high-heat (57°C) conditions was measured as previously described (16, 17).
Infectivity.
Infectivity, a measure of the number of viable adherent and intracellular bacteria, was measured as previously described (10), with slight modifications. Briefly, PE-phase bacteria were diluted in RPMI plus 10% fetal bovine serum (Invitrogen), and samples of each inoculum were plated for counts of CFU. For each bacterial strain, triplicate monolayers of 2.5 × 105 murine bone marrow-derived macrophages were infected with ∼5 × 104 bacteria for 2 h at 37°C. For the removal of the majority of the extracellular bacteria, the monolayers were then washed with RPMI prior to being lysed by trituration with cold PBS, diluted in AYE, and plated on CYET for counts of bacterial CFU.
Complementation of E. coli glycogen accumulation.
E. coli strains MG1655, TR1-5 MG1655 (csrA::kanR), and RG1-B MG1655 (csrB::cam) were transformed by electroporation with pLetE or the control vector pMMB-GentΔmob. Transformants were isolated, colony purified, and cultured overnight on Kornberg medium containing 200 μM IPTG (isopropyl-β-d-thiogalactopyranoside). Intracellular glycogen accumulation was visualized with iodine vapor by inverting plates over iodine crystals (15).
Nucleotide sequence analysis.
The RNA secondary structure was predicted with both Lasergene Genequest, using Vienna RNA folding parameters with default settings, and mfold (http://bioinfo.rpi.edu/applications/mfold/), using the following parameters: window, 7; maximum folds, 50; maximum base pairs, 20 (30, 39). Protein folding was predicted by using the predict protein server (34). The prediction of transmembrane domains was done by using the dense alignment surface method (11) and a Kyte-Doolittle hydrophobicity plot (25).
Site-directed mutagenesis.
Single nucleotide substitutions were introduced into the letE ORF in pLetE by use of a QuikChange XL site-directed mutagenesis kit (Stratagene). The plasmid pLetESTOP was constructed by switching codon 7 from a leucine-encoding codon to a stop codon by changing TTA to TAA with primers LetE UAA21A and LetE UAA21B. The mutagenesis control plasmid pLetESIL was made by altering codon 7 from TTA to CTA, a trinucleotide that also encodes leucine, with primers LetE SilA and LetE SilB (Table 1). Plasmid DNA synthesis, the digestion of template DNA, and transformation were performed as directed by the manufacturer. The fidelity of mutagenesis was confirmed by DNA sequencing of the letE locus with primers LetETn1 and LetETn2 (University of Michigan Sequencing Core Facility).
TABLE 1.
Strains, primers, and plasmids used for this study
| Strain, primer, or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| MG1655 | 32 | |
| TR1-5 MG1655 | csrA::kanR | 15 |
| RG1-B MG1655 | csrB::cam | 5 |
| JM109 | F′ traD36 proA+ proB+ lacIqlacZΔM15/recA1 endA1 gyrA96 (Nalr) thi hsdR17 supE44 relA1 Δ(lac-proAB) mcrA | Lab collection |
| L. pneumophila | ||
| Lp02 | Strr Thy− HsdR− | 7 |
| MB 379 | Lp02 rpoS::kan | 5 |
| MB 414 | Lp02 letA-22 | 18 |
| MB 417 | Lp02 letS-36 | 18 |
| MB 419 | Lp02 letE-121 | 18 |
| MB 447 | Lp02 letE-121 containing pMMB-GentΔmob | This work |
| MB 448 | Lp02 letE-121 containing pLetE | This work |
| MB 449 | Lp02 letE-121 containing pLetESTOP | This work |
| MB 450 | Lp02 letE-121 containing pLetESIL | This work |
| MB 451 | Lp02 containing pMMB-GentΔmob | This work |
| MB 452 | Lp02 containing pLetE | This work |
| Primers | ||
| LetE1 | 5′-ATG GAA GGT TGG TTA ATG GTT GAA-3′ | 18 |
| LetE2 | 5′-TTC CCA TGC CAT AAT ATC CAC CTA-3′ | 18 |
| LetE UAA21A | 5′-CAT CAT GAA CGA TAC AAC ATC ATA ATT ATC ACA TCT TAG ATT ACG-3′ | This work |
| LetE UAA21B | 5′-CGT AAT CTA AGA TGT GAT AAT TAT GAT GTT GTA TCG TTC ATG ATG-3′ | This work |
| LetE SilA | 5′-CAT CAT GAA CGA TAC AAC ATC ACT ATT ATC ACA TCT TAG ATT ACG-3′ | This work |
| LetE SilB | 5′-CGT AAT CTA AGA TGT GAT AAT AGT GAT GTT GTA TCG TTC ATG ATG-3′ | This work |
| LetETn1 | 5′-TAC ATG CAC TAA AAA GCG GTT CTG-3′ | This work |
| LetETn2a | 5′-TGA TCA TGC TAC GAG CTC AAG TAA-3′ | This work |
| MilX ORF1 | 5′-AGG CCC CTG TTT CAT TTT TAG TGA-3′ | This work |
| MilX ORF2a | 5′-ATA TGC CGG ACC TAT TGC CTC TC-3′ | This work |
| Plasmids | ||
| pGEMT-Easy | Multiple cloning site within coding region of β-lactamase α-fragment linearized with single-T overhangs, Ampr | Promega |
| pMB436 | pGEMT-Easy containing 3.6-kb letE-milX locus PCR fragment | This work |
| pMMB-GentΔmob | pMMB67EH with fragment containing Gmr, digested with AgeI and ligated to remove mobA ATG site | 17 |
| pLetE | pMMB-GentΔmob containing the letE locus in a 1 kb EcoRI fragment | This work |
| pMilX | pMMB-GentΔmob containing the milX locus in a 2.6-kb EcoRI fragment | This work |
| pLetESTOP | pLetE with a TTA→TAA point mutation in letE codon 7, creating a stop codon | This work |
| pLetESIL | pLetE with a TTA→CTA point mutation in letE codon 7, creating a silent mutation (both encode leucine) | This work |
Used for synthesis of single-stranded DNA probe.
Northern blot analysis.
The expression of letE RNA by wild-type strain Lp02 was compared to that of the following mutants: letA-22, letS-36, letE-121 (18), and rpoS null (5). For each strain, the densities of overnight cultures were quantified by measuring the OD600, and then aliquots were diluted to an OD600 of 0.3 or 0.03 and incubated for an additional 16 h to produce PE- and E-phase cultures, respectively. Bacteria from 4.5 ml of E-phase cultures and 1.5 ml of PE-phase cultures were collected by centrifugation and resuspended in 1 ml of Trizol reagent, and then the RNAs were purified according to the manufacturer's instructions (Invitrogen). RNA samples were quantified by measuring the OD260 and then samples of 10 μg of total RNA were electrophoresed in a 1.2% formaldehyde gel. The gel was prepared, electrophoresed, and transferred to BrightStar BioDetect nylon membranes as described for NorthernMax kits (Ambion, Inc.). Biotin-labeled, single-stranded DNA probes were synthesized with the Strip EZ PCR kit (Ambion, Inc.). The probe template was synthesized by a PCR using primers LetETn1 and LetETn2, which are specific for the region of letE transposon insertions (Table 1). Ten nanograms of the resulting fragment was used as a template for a linear PCR using LetETn2 and 200 nM biotin 14-ATP (Invitrogen). Membranes were hybridized by using NorthernMax Ultrahyb and the letE probe at a final concentration of ∼0.7 ng/ml. Probes were quantified and the letE mRNA was subsequently detected by using a BrightStar BioDetect kit (Ambion, Inc.) and Kodak ML chemiluminescence film. The size of the letE transcript was determined by comparing the migration of the major letE band with a gel mobility plot generated from RNA molecular weight markers (RNA molecular weight marker III; Roche Molecular Biochemicals). Probes were stripped by incubating the blot in probe degradation buffer for 10 min at room temperature and then for 10 min in a 68°C water bath, and finally they were washed in blot reconstitution buffer for 10 min at 68°C (Strip EZ PCR kit; Ambion, Inc.). The analysis of fliA and flaA expression in wild-type and letE-121 strains was performed as previously described (4). Samples for time course experiments were collected as previously described (4) and processed as described above.
Protein analysis.
To analyze letE-dependent protein synthesis, we prepared total cell extracts from three L. pneumophila strains: wild-type Lp02 transformed with vector pMMB-GentΔmobA (MB451), the letE-121 mutant transformed with vector pMMB-GentΔmobA (MB447), and the letE-121 mutant transformed with the complementing plasmid pLetE (MB448). E-phase broth cultures were diluted into AYE containing 2 mM IPTG and then were cultured to the PE phase (OD600 of 3.6 to 3.8). Aliquots of 3 × 109 bacteria were collected by centrifugation and then stored at −20°C until they were used. For the preparation of protein extracts, samples were resuspended in 30 μl of water, diluted with Tricine sample buffer (Bio-Rad) (final concentrations of 200 mM Tris-HCl [pH 6.8], 40% glycerol, 2% sodium dodecyl sulfate [SDS], 0.04% Coomassie blue G-250, and 2% β-mercaptoethanol), heated for 3 min in a boiling water bath, and then cleared by centrifugation. The equivalent of 3 × 107 bacteria per lane was separated in a precast Tris-Tricine-16.5% acrylamide gel (Bio-Rad). The molecular weight of the letE product was estimated by comparing its migration to that of the Benchmark prestained protein size standard (Bio-Rad). After fixation for 30 min in 40% methanol and 10% acetic acid, the proteins were stained with a Coomassie blue G-250 solution (Bio-Rad).
RESULTS
letE enhances flaA expression in PE phase.
To construct a flagellum as it enters the PE phase, L. pneumophila expresses fliA, encoding the flagellar sigma factor, and then flaA, encoding flagellin (14, 22). Consistent with their reciprocal control of the transmission phenotype, LetA/LetS and RpoS induce the expression of both fliA and flaA RNAs (4, 5, 18, 29), whereas CsrA represses L. pneumophila motility by decreasing the quantities of both transcripts (14, 31). The letE locus was originally identified in a genetic screen as a positive regulator of flaA: letE mutants carrying a flaA-gfp transcriptional fusion formed colonies that were a lighter green than those of the corresponding wild-type strain (18).
To investigate the mechanism of regulation by letE, we evaluated the expression levels of fliA and flaA in wild-type and letE mutant bacteria by Northern blot analysis. In wild-type L. pneumophila, the fliA transcript of ∼750 nucleotides was in good agreement with the predicted 716-bp ORF (GenBank accession no. X98892); the origin of the smaller ∼200-nucleotide species is not known. Wild-type cells expressed a flaA RNA of ∼1,500 nucleotides, of sufficient length to encode the 1,428-bp ORF (GenBank accession no. X83232) (Fig. 1). Relative to PE-phase wild-type cultures, letE mutants contained slightly less fliA RNA. In comparison, the effect of letE mutations on flaA expression was more severe: flaA transcripts were abundant in wild-type PE-phase cultures but scarce in letE mutant cells (Fig. 1). Therefore, the primary effect of letE on L. pneumophila motility appears to be to enhance either the production or stability of flaA RNA.
FIG. 1.
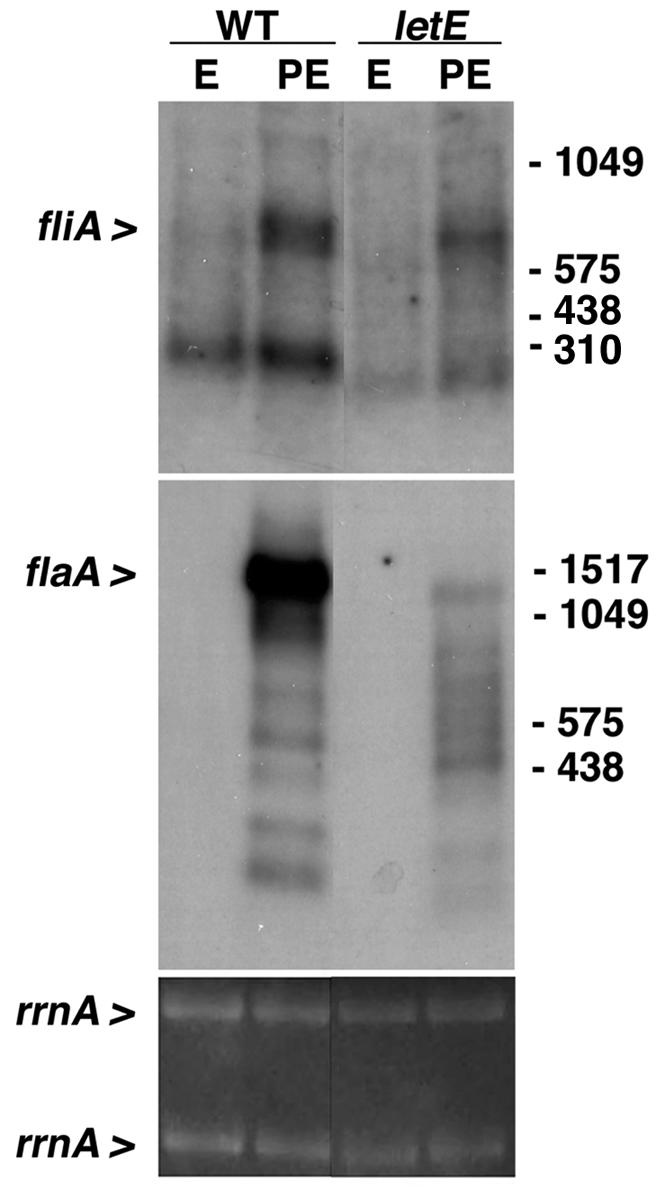
LetE enhances flaA expression in PE phase. A Northern blot analysis was performed with 10 μg each of total RNAs collected from wild-type strain Lp02 and the letE-121 mutant strain MB419 cultured to the E (OD600 of 0.8 to 1.2) and PE (OD600 of 3.1 to 3.6) phase. The hybridization pattern shown is representative of results obtained from two independent sets of RNA samples. Equal loading of the samples is demonstrated by ethidium bromide staining of rrnA in the agarose gel prior to transfer. An expanded version of this figure appears in a separate article (4).
A letE mutant is defective for coccoid cell shape, motility, and pigmentation.
To gain insight into the full scope of traits regulated by letE, we compared the morphologies of a letE mutant and wild-type L. pneumophila. The letE-121 mutant strain MB419 (18) and the wild-type strain Lp02 were cultured in broth to PE phase, and then wet mounts of each culture were observed by phase-contrast microscopy. Unlike wild-type cells, the mutant cells retained the elongated shape that is more typical of the replicative form of L. pneumophila (14, 17, 31), often appearing as doublets (Fig. 2A versus B). Furthermore, <10% of the letE-121 cells were motile, and these bacteria tumbled in a manner unlike the directed movement of wild-type cells (Table 2; data not shown). The failure of PE-phase letE mutants to differentiate into a coccoid, motile form is characteristic of L. pneumophila cells that either lack the LetA activator or carry multiple copies of the wild-type locus encoding the CsrA repressor (14, 31).
FIG. 2.
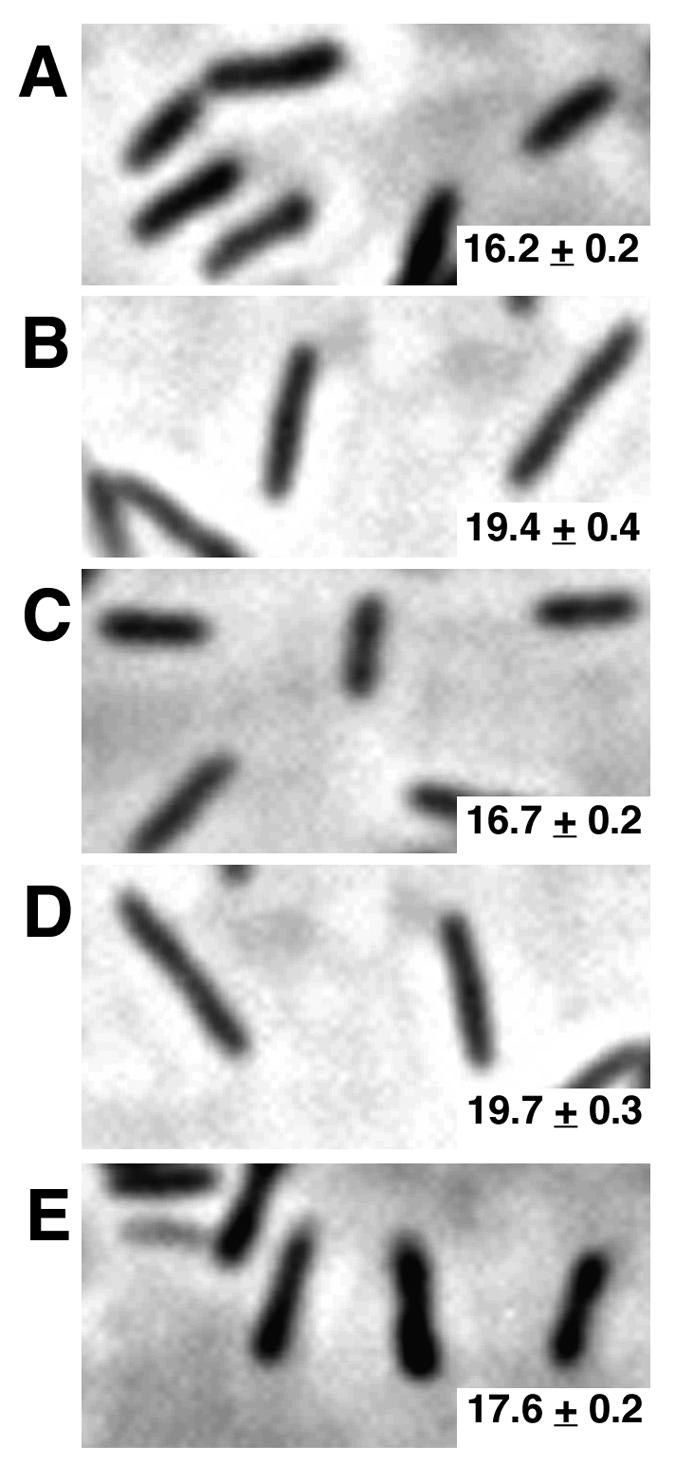
letE is required for coccoid cell morphology in PE phase. Phase-contrast images of PE-phase L. pneumophila cells magnified by a factor of 1,000 are shown. (A) Coccoid wild-type Lp02 containing pMMB-GentΔmob (MB451); (B) elongated letE mutants containing pMMB-GentΔmob (MB447); (C) letE mutants containing pLetE (MB448) and resembling the wild type; (D) letE mutants containing pLetESTOP (MB449) (elongated, resembling the letE mutants in panel B); (E) letE mutants containing pLetESIL (MB450) and resembling the wild type. Cell lengths are shown as mean numbers of pixels ± standard errors. For each strain, at least 275 bacteria were analyzed. The lengths shown are representative of two independent experiments.
TABLE 2.
Summary of complementation studies of PE-phase letE mutantsa
| Strain | Presence of phenotype
|
|||
|---|---|---|---|---|
| Motilityb | Coccoidc | Pigmentd | Sodium sensitivitye | |
| WT/vector | + | + | + | + |
| letE/vector | − | − | − | − |
| letE/pLetE | + | + | + | − |
| letE/pLetESIL | + | + | + | ND |
| letE/pLetESTOP | − | − | − | ND |
| letE/pMilX | − | − | ND | − |
| WT/p-CsrAf | − | − | − | + |
Phenotypes were scored relative to a wild-type PE-phase positive control (+) and a letE PE-phase negative control (−). All assays were performed at least twice, with similar results.
Cultures in which > 50% of the bacteria exhibited directed movement when observed by phase-contrast microscopy were scored as positive; negative cultures were typically 5 to 10% motile.
For the scoring of morphology, phase-contrast images of samples were compared to the positive (Fig. 2A) and negative (Fig. 2B) reference strains.
Cultures whose supernatants had an OD550 of >0.4 were scored as positive (Fig. 3B); negative cultures typically had an OD550 of ∼0.2. ND, not done.
Calculated by the formula (CFU/ml on CYET plus 100 mM NaCI)/(CFU/ml on CYET) × 100. The plating efficiency of positive control samples was <0.3% and that of negative control cultures was >30%. ND, not done.
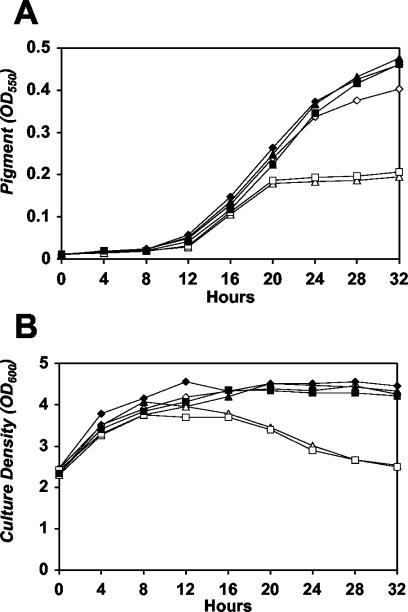
L. pneumophila produces a brown pigment that accumulates in the broth of PE-phase cultures (13); this pathway is also induced by LetA and repressed by CsrA (14, 31). To investigate whether letE also regulates pigment production, we collected samples of broth cultures at the late E phase and at 4-h intervals thereafter and then assayed them for pigment accumulation (OD550) and optical density (OD600) (Fig. 3). Pigment accumulation in both wild-type and letE cultures began when the cultures reached an OD600 of ∼4. However, letE cells produced 50% less pigment than wild-type cells (Fig. 3A; compare open diamonds with open triangles). During the course of the experiment, we also observed that the OD600 of letE mutants dropped to ∼2.5 (Fig. 3B). The decreased OD600 of letE cultures did not indicate cell lysis, as no corresponding change in CFU occurred (data not shown). Consistently, the decline in OD600 coincided with the onset of pigment production, but whether this reflects a defect in either pigment production or secretion is not known. The phenotypic data indicate that letE induces the characteristic shape, motility, and pigmentation of PE-phase L. pneumophila, all of which are induced by LetA and repressed by CsrA (14, 18, 31).
FIG. 3.
letE is required for wild-type pigment levels and maintenance of OD600 in PE phase. (A) Pigment production measured first during late E phase (OD600 of 2.5) and at 4-h intervals thereafter. At the times shown, samples of wild-type Lp02 containing pMMB-GentΔmob (MB451; open diamonds), wild-type Lp02 containing pLetE (MB452; closed diamonds), the letE-121 mutant containing pMMB-GentΔmob (MB447; open triangles), the letE-121 mutant containing pLetE (MB448; closed triangles), the letE-121 mutant containing pLetESTOP (MB449; open squares), and the letE-121 mutant containing pLetESIL (MB450; closed squares) were collected by centrifugation at 16,000 × g, and then the pigment accumulation in supernatants was measured as the OD550. The results shown are representative of three independent experiments. (B) OD600 values of the culture samples used for panel A. At the times shown, bacteria collected by centrifugation as described above were resuspended to their original volume and diluted 1:10, and then the OD600 was measured. The results are representative of three independent experiments.
L. pneumophila requires letE to resist heat in PE phase.
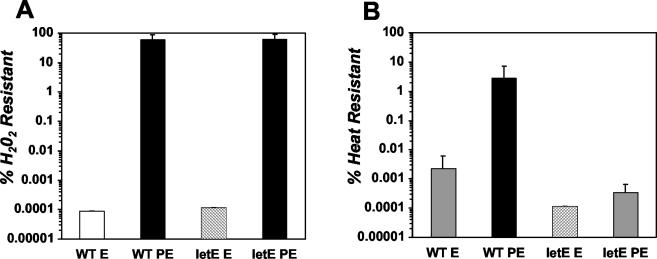
Coincident with the expression of virulence phenotypes, L. pneumophila becomes resistant to multiple stresses, including high levels of osmolarity, heat, acidity, and oxidation (16). Contrary to prevailing models for the regulation of stationary-phase physiology by other gram-negative bacteria (28), PE-phase L. pneumophila does not require RpoS to resist extreme conditions (5, 16). Instead, LetA/LetS induces resistance to oxidative, osmotic, heat, and acid stress in PE-phase L. pneumophila (29, 31). To determine whether the letE product cooperates with LetA/LetS to supplant RpoS as a regulator of PE-phase stress resistance, we exposed letE mutant and wild-type bacteria to acidity (citric acid, pH 3), hyperosmolarity (5 M NaCl), oxidation (10 mM H2O2), or heat (57°C) for 1 h and compared their survival rates. A PE-phase letE mutant was as tolerant as the wild type to a low pH, hyperosmolarity, and oxidative stress (Fig. 4A and data not shown). However, PE-phase letE mutants were exquisitely sensitive to heat, as evidenced by the recovery of ∼6,000-fold fewer mutant CFU of the mutant than of the wild type (Fig. 4B). Therefore, PE-phase L. pneumophila requires letE to tolerate heat, but not other environmental stresses.
FIG. 4.
letE is required for heat resistance, but not H2O2 resistance, in PE phase. (A) Broth cultures of wild-type strain Lp02 (WT) and letE-121 mutant strain MB419 (letE) were grown to E and PE phase, and then duplicate samples of each culture were collected by centrifugation and resuspended in either 1× M63 salts or 10 mM H2O2. Samples were incubated for 1 h at 37°C, washed, diluted, and plated for counts of CFU. The percent H2O2 resistance was calculated by the formula % resistance = (CFUH2O2/CFUM63) × 100. Black bars represent the mean values from three independent experiments; error bars represent standard deviations. For all three experiments, after treatment with 10 mM H2O2, the WT E-phase and letE E-phase CFU were below the limit of detection, a value represented by the white and gray bars, respectively. (B) Duplicate samples of each culture were collected by centrifugation, resuspended in 1× M63 salts, and then incubated for 1 h at 37 or 57°C. Samples were washed in 1× M63 salts, diluted, and plated for counts of CFU. The percent heat resistance was calculated by the formula % resistance = (CFU57°C/CFU37°C) × 100. Black bars represent the mean values calculated from three independent experiments; error bars represent standard deviations. For each of the three letE E-phase samples, the CFU was below the limit of detection, which is represented by the hatched bar. For two of the three WT E-phase samples and one of the three letE PE-phase samples, the CFU was below the limit of detection; for these samples, the maximum possible value was determined and incorporated into the calculated mean, a value represented by the gray bars. Therefore, the calculated means for the wild-type E-phase and letE mutant PE-phase cultures are overestimates of the actual mean heat resistance levels.
The letE locus is transcribed in E phase as an approximately 500-base monocistronic RNA.
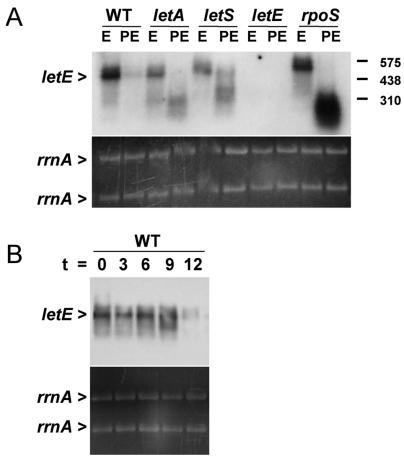
The letE locus, as defined by four insertion mutations (18), is located ∼1 kb upstream from milX, a gene that L. pneumophila requires for its replication in macrophages (J. D. Sauer and M. Swanson, unpublished data) and that bears sequence similarity to the infectivity gene milA (19). To investigate whether transposon insertions in letE are polar on milX, we first performed a Northern blot analysis of RNA samples collected from E- and PE-phase broth cultures. A probe specific for the letE locus hybridized to a transcript that was present in E-phase wild-type bacteria but absent from letE mutants (Fig. 5A). The RNA was approximately 500 bases in length, which is in agreement with the ∼400-bp region defined by the four letE mutations and sufficient to encode the 372-bp putative ORF, but not the 3′ milX locus (GenBank accession no. AY135376) (18). When these and similar RNA samples were assayed with a milX probe, no transcripts were detected for either growth phase (data not shown). Given the ∼500-nucleotide length of the abundant letE transcript, its absence in letE-121 mutant bacteria, the ∼1-kb separation between the letE and milX loci, and the lack of detectable milX transcripts in either wild-type or letE-121 mutant cells, it appears unlikely that letE is cotranscribed with milX or that mariner transposon mutations in letE are polar on milX.
FIG. 5.
A monocistronic letE RNA is expressed by E-phase L. pneumophila. (A) Northern blot analysis performed with a letE-specific probe on 10 μg of total RNA from an E- or PE-phase culture of wild-type Lp02 (WT) or letA-22 (MB414), letS-36 (MB417), letE-121 (MB419), or rpoS null (MB379) mutant bacteria. The hybridization patterns shown are representative of results obtained from three independent sets of RNA samples. Equal loading of the samples is demonstrated as described for Fig. 1. The positions of RNA size standards are indicated to the right. (B) Northern blot analysis of 10-μg samples of total RNA harvested from wild-type L. pneumophila Lp02 at 3-h intervals from E phase (OD600 of ∼0.6) to PE phase (OD600 of ∼4), using a letE probe with the same sequence as that used for panel A. The temporal expression patterns shown are representative of two independent experiments. Equal sample loading is demonstrated as described for Fig. 1.
Expression of letE is dependent on growth phase.
The letE transcripts were abundant in E-phase bacteria but were rare in PE-phase cells (Fig. 5). To test whether either LetA/LetS or RpoS induces the transmission phenotype in part by regulating letE expression, we compared the amounts of letE RNA in E- and PE-phase wild-type, letA, letS, and rpoS cultures (Fig. 5A). In the E phase, the expression of letE was completely independent of RpoS and largely independent of LetA/LetS, as mutations in the two-component regulator genes had a modest effect on letE transcript levels. In contrast, the down-regulation of the ∼500-base letE RNA by PE-phase L. pneumophila was strongly affected by each of the regulatory mutations (Fig. 5A). In the PE phase, letA and letS mutants contained letE RNA molecules that were smaller than the wild-type transcript. More striking was the accumulation by rpoS mutants of a large pool of RNA molecules of a range of low molecular weights that hybridized to the letE probe. The small letE RNA species could result either from initiation at different sites or from posttranscriptional processing; in either case, since most of the small RNAs were <300 bases long, they could not encode the entire 372-bp ORF. The results of the Northern blot analysis indicated that L. pneumophila expresses an ∼500-base monocistronic letE RNA in the E phase; in the PE phase, LetA/LetS and RpoS regulate its decay. Its scarcity in the PE phase is inconsistent with the idea that letE encodes a regulatory RNA that sequesters CsrA to relieve repression of the transmission phenotype (20).
The letE locus is sufficient to restore PE-phase phenotypes to letE mutants.
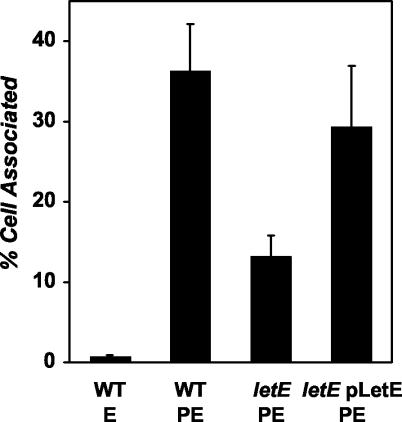
To test whether letE mutant phenotypes are caused by polar effects on milX, we defined the genomic region that confers letE activity by using genetic complementation tests. Either the control vector, pMMB-GentΔmob, or pLetE, which bears the putative letE ORF but not milX, was transformed into letE-121 mutant MB419, and then the ability of each strain to infect bone marrow-derived macrophages was compared to that of the wild type (Fig. 6). After a 2-h incubation, only 13% of the PE-phase letE mutant inoculum was viable and cell associated, whereas 29% of the PE-phase mutants that carried pLetE were infectious, which is similar to the efficiency of 36% of the PE-phase wild type. The complementation of infectivity was statistically significant according to the Student t test (P < 0.05). Furthermore, PE-phase cultures of the mutant strain carrying pLetE developed the shape (Fig. 2) and motility (Table 2) that are characteristic of wild-type L. pneumophila, produced wild-type quantities of pigment (Fig. 3A), and maintained their OD600 value (Fig. 3B). In contrast, the milX locus carried by plasmid pMilX failed to complement the motility defect (Table 2) and exacerbated the cytotoxicity defect of letE mutants, reducing it from ∼50% to only 25% of wild-type levels (data not shown). Neither pMilX nor pLetE restored sodium sensitivity to letE mutant cultures (Table 2). The sodium resistance of letE mutants is unlikely to be caused by a second site mutation, since both the original letE-121 isolate and the backcrossed mutant strain (MB419) are sodium resistant (18). The capacity to complement five of six letE defects in trans demonstrates that the letE locus encodes an activity that enhances the expression of several traits that are characteristic of PE-phase L. pneumophila.
FIG. 6.
L. pneumophila requires the letE ORF to infect macrophages efficiently. The percentage of each L. pneumophila strain that was cell associated after a 2-h infection of 2.5 × 105 macrophages is shown. Macrophages were infected at a multiplicity of infection of 0.2 with the following strains: PE-phase wild-type Lp02 containing pMMB-GentΔmob (MB451), E-phase wild-type Lp02 containing pMMB-GentΔmob (MB451), PE-phase letE-121 mutant containing pMMB-GentΔmob (MB447), and PE-phase letE-121 mutant containing pLetE (MB448). The results shown are the means from triplicate samples of infected macrophages; error bars represent the standard deviations.
letE does not complement an E. coli csrB mutant.
Several of the phenotypes conferred by letE mutations are also caused by multiple copies of the wild-type csrA locus (14). Initially identified by its nucleotide sequence similarity, the L. pneumophila csrA gene was shown to complement an E. coli csrA mutant (14). To investigate whether letE impinges upon the CsrA regulatory pathway, we transformed plasmid pLetE into isogenic wild-type and csrB mutant E. coli cells (kindly provided by Tony Romeo) and then assessed their ability to accumulate glycogen by staining colonies with iodine vapor (27, 33). When letE expression from the Ptac promoter was induced by IPTG, the letE locus did not complement the csrB mutant. Therefore, if letE encodes a regulatory RNA, it cannot replace E. coli csrB.
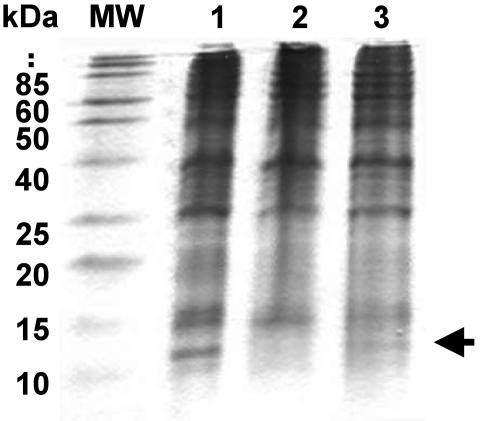
The letE locus directs the synthesis of a 12-kDa protein.
To investigate whether the letE locus directs the synthesis of a protein, we compared cell extracts prepared from L. pneumophila strains that harbored zero, one, or multiple copies of the letE locus after separation by SDS-polyacrylamide gel electrophoresis. Wild-type L. pneumophila produced a protein species of ∼12 kDa that was not apparent in letE-121 mutants but that was restored by the transformation of letE mutant cells with pLetE (Fig. 7). The electrophoretic mobility of the letE-dependent protein was in agreement with the ∼14-kDa size predicted from its nucleotide sequence (GenBank accession no. AY135376); nevertheless, an understanding of whether this species is the LetE protein or a target of letE regulation requires more specific molecular analysis.
FIG. 7.
letE directs the synthesis of an ∼12-kDa protein. Total cell extracts prepared from PE-phase cultures of wild-type L. pneumophila Lp02 transformed with vector pMMB-GentΔmob (MB451; lane 1), the letE-121 mutant strain transformed with vector pMMB-GentΔmob (MB447; lane 2), or the letE-121 mutant strain transformed with complementing plasmid pLetE (MB448; lane 3) were separated by SDS-polyacrylamide gel electrophoresis in a Tris-Tricine-16.5% acrylamide gel and then were stained with Coomassie blue. The arrow indicates the position of the predicted LetE protein. Approximate molecular masses of the size standards are shown; the positions of the 120- and 190-kDa species are indicated with dots. Similar results were obtained in two other independent experiments.
As an independent test of whether letE acts as a regulatory RNA or a protein, we constructed two separate mutations in pLetE and then tested their effects on its complementation activity. Codon 7 of the predicted letE ORF was converted by site-directed mutagenesis from TTA to TAA, a stop codon, creating pLetESTOP. Although the nucleotides of codon 7 were not predicted by either the mfold or Lasergene Genequest algorithm to contribute to the RNA secondary structure of this region, as a control we introduced a different mutation that would alter the sequence of an RNA but not a protein at the same position. Codon 7 was changed from TTA to CTA, which is a silent mutation because both sequences encode leucine. If LetE functions as a protein, the resulting plasmid, pLetESIL, was predicted to complement a letE mutant, whereas pLetESTOP was not.
A series of complementation tests indicated clearly that LetE functions as an activator protein. Most striking was the failure of pLetESTOP to complement pigment production by the letE mutant (Fig. 3A, open squares), whereas pLetESIL restored its pigment production to wild-type levels (Fig. 3A, closed squares). In addition, pLetESTOP did not prevent the OD600 decline of letE mutant cultures, which caused a decrease in the OD600 from 3.76 to 2.76 over a period of 24 h. In contrast, cultures of letE bacteria carrying pLetESIL maintained an OD600 of ∼4.2 (Fig. 3B). The complementation of cell morphology and motility also required the intact protein coding sequence (Fig. 2; Table 2). Taken together, the molecular, biochemical, and genetic data indicate that the 372-bp letE ORF encodes a protein that cooperates with LetA/LetS to mediate the differentiation of replicating L. pneumophila cells to a form that is suitable for transmission between host phagocytes.
DISCUSSION
In L. pneumophila, the LetE protein amplifies the expression of several stationary-phase traits that are thought to promote bacterial transmission from one amoebae to another (36). In addition to the coccoid morphology, pigment production, and heat tolerance that are characteristic of PE-phase bacteria, letE enhances L. pneumophila cytotoxicity, motility, and infection of macrophages. Each of the traits affected by letE mutation is activated by the LetA/LetS two-component regulatory system (18) and repressed by CsrA (31), a protein that binds particular mRNAs and inhibits their translation (6, 32). Accordingly, the phenotypic data predict that the LetE protein either augments LetA/LetS activity or inhibits the CsrA function directly or indirectly.
The transmission regulon activated by LetA/LetS and LetE is thought to promote the fitness of planktonic L. pneumophila cells during their travel between host amoebae. During stationary phase, many Legionella spp. secrete a brown pigment that confers protection from light (35). Pigment production requires at least two distinct genetic loci, named pig and lly. The lly gene product, legiolysin, catalyzes the formation of homogentisic acid, which likely polymerizes to form a melanin-like pigment (37, 38). Although not required for intracellular growth (35), pigment accumulation and the efficiency of macrophage infection by L. pneumophila are correlated genetically. For both traits, the wild type and rpoS mutants exhibit high levels of activity (16), letE mutant bacteria are intermediate (Fig. 3 and 6), and letA/letS mutants are as defective as E-phase L. pneumophila (31). It remains to be determined whether pigment accumulation contributes to L. pneumophila fitness by scavenging reactive oxygen species or by protecting planktonic bacteria in their natural reservoir from light or if it instead indicates a degradation of the aromatic amino acids that power transmission to a new host. In any case, pigment production can be regarded as a reliable marker of the L. pneumophila transmissive phase.
LetE also potentiates structural changes in PE-phase L. pneumophila, as judged by alterations in the cell shape (Fig. 2) and the maintenance of the OD of broth cultures (Fig. 3). Starved E. coli adopt a coccoid shape through reductive division which requires RpoS-dependent genes such as bolA (9, 26). Wild-type and letE mutant PE-phase cultures retain roughly constant CFU, but wild-type cells differentiate to form short rods while letE mutants continue to elongate in the PE phase, a phenomenon that may affect their OD in culture. LetE, together with LetA/LetS, influences multiple stationary-phase phenotypes, indicating that the expression of the transmission phenotype is tightly coupled to a broad physiological adaptation to starvation.
Studies of broth cultures that model the L. pneumophila life cycle indicate that a stringent response-like mechanism coordinates the transformation of replicating bacteria to a form that can be efficiently transmitted to a new phagocyte (5, 17). The differentiation of intracellular L. pneumophila is postulated to be triggered by (p)ppGpp (17), an alarmone synthesized by RelA and also by SpoT which in some manner activates the LetA/LetS and LetE regulon (18). Although L. pneumophila requires neither RelA nor LetE for its replication in phagocytes, relA and letE mutants do exhibit similar quantitative defects in flaA expression, motility, and pigment production in the PE phase (Fig. 1 and 3) (18, 40). In particular, it appears that only some of the letE mutant cells sense or reach the threshold that triggers differentiation to the transmission phenotype. When cultured in broth, PE-phase letE mutants contain very little flaA RNA (Fig. 1), but they do harbor a small number of motile bacteria (Table 2). Accordingly, in some manner, LetE enhances either the amplitude or the fidelity of the signaling cascade initiated by (p)ppGpp in response to stress.
In numerous species of gram-negative bacteria, homologues of LetA/LetS activate gene expression indirectly by a posttranscriptional mechanism. Members of this family of two-component regulators induce the expression of the csrB regulatory RNA, which sequesters the CsrA repressor, permitting the translation of target mRNAs (20). However, unlike csrB, L. pneumophila LetE appears to function as a protein, not an RNA. The wild-type locus did not complement an E. coli csrB mutant, the cloned gene directed the synthesis of an ∼12-kDa protein (Fig. 7), and a letE nonsense mutation eliminated functioning, whereas a mutation in the same position that maintained the protein sequence did not (Fig. 2 and 3; Table 2). Also, a letE mutation did not affect the flagellar regulon in the manner reported for csrA overexpression: letE mutations greatly reduced the amount of flaA RNA (Fig. 1), whereas multiple copies of csrA repressed both flaA and fliA (14). Therefore, the LetE protein enhances the expression of the transmission phenotype by a LetA/LetS-dependent mechanism that remains to be discovered.
A computer analysis of the LetE amino acid sequence provided few clues to its biochemical activity. The LetE polypeptide is predicted to contain 123 amino acids and to have a molecular mass of 14 kDa and an isoelectric point of 4.1. Two folding algorithms (the PredictProtein and Garnier-Robson algorithms) indicated that LetE assembles into five alpha-helical regions; the fifth helix, encompassing residues 99 to 116, is predicted to contain a transmembrane domain based on its hydrophobicity and a comparison to known membrane proteins (by a Kyte-Doolittle hydrophobicity plot [Lasergene Protean] and a DAS transmembrane prediction profile). Standard BLAST searches found no homology (defined as an E value of <1) to any dynamically translated microbial genomes in GenBank; likewise, a search of all GenBank proteins yielded no entries with an E value of <0.4. A reiterative PSI-BLAST analysis (3) of the LetE predicted protein sequence did reveal a limited similarity to proteins whose biochemical activities differ but which each interact with tRNAs, including some peptidyl-tRNA hydrolases, aminoacyl-tRNA synthetases, and retroviral reverse transcriptases (A. Molofsky, unpublished observations). Whether LetE interacts with tRNA to augment the expression of the transmission phenotype requires further study.
Certain aspects of the regulation of the L. pneumophila transmission phenotype parallel the regulation of the E. coli universal stress protein A, which coordinates tolerance to a wide variety of harsh conditions. During periods of starvation, repression of the uspA promoter by an active FadR protein can be overridden when the alarmone ppGpp accumulates (24). By this “emergency derepression” mechanism, cells can activate critical metabolic pathways despite a paucity of resources. Transcripts of letE are abundant during the E phase in broth, and their production does not require LetA, LetS, or RpoS (Fig. 5A). Therefore, a pool of letE RNA is likely available to intracellularly replicating L. pneumophila. Should conditions suddenly deteriorate, the LetE protein could quickly respond to the ppGpp alarm by overriding CsrA-mediated repression, thereby promoting transmission to a new host.
A number of observations suggest that a posttranscriptional mechanism contributes to the induction of the L. pneumophila transmission phenotype. First, in a variety of other gram-negative bacteria, homologues of LetA/LetS activate gene expression indirectly by counteracting the effects of CsrA, a repressor of translation (8, 12, 20, 21). Secondly, in PE-phase L. pneumophila, LetA/LetS and RpoS may mediate the decay of letE RNA (Fig. 5A). Likewise, short rpoS RNA species also accumulate in PE-phase letA, letS, and letE mutants (4). Differentiation from the replicative to the transmissive form is a comprehensive physiological change that must occur rapidly in the face of declining precursors for gene expression. For a minimization of this energetic challenge, the induction of transmission loci could be coupled to the decay of replicative gene transcripts. According to this model, LetE would amplify the developmental switch by stabilizing LetA/LetS-dependent transmission transcripts and destabilizing LetA/LetS-independent transcripts, including letE itself.
L. pneumophila alternately replicates within potentially deadly phagocytes and travels between cells through nutrient-sparse freshwater. Its biphasic life cycle demands rapid and extensive adaptations to stress and starvation through efficient selective gene expression. For example, when nutrients are depleted within the replicative vacuole, a strong, immediate induction of cytotoxicity may mediate the lysis of host membranes and bacterial egress (10). Additional experiments can determine how LetE functions as one member of a multicomponent sensor and response mechanism that coordinates the conversion of L. pneumophila from the replicative to the transmissive form.
Acknowledgments
We gratefully acknowledge the generous contributions of Tony Romeo, who provided E. coli csrA and csrB mutant strains; Amal Amer, who analyzed the letE protein; Ari Molofsky, who analyzed the letE nucleotide sequence by the PSI-BLAST algorithm; and Victor DiRita, N. Cary Engleberg, Philip Hanna, and Alex Ninfa, who critically reviewed the manuscript.
This project was funded by NIH grant AI 44212-01 and the University of Michigan President's Initiative Fund for Graduate Training in Microbial Pathogenesis.
Editor: J. N. Weiser
REFERENCES
- 1.Aarons, S., A. Abbas, C. Adams, A. Fenton, and F. O'Gara. 2000. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J. Bacteriol. 182:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altier, C., M. Suyemoto, A. I. Riuz, K. D. Burnham, and M. Maurer. 2000. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol. Microbiol. 35:635-646. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zheng, W. Miller, and D. J. Lippman. 1977. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachman, M. A., and M. Swanson. 2004. Genetic evidence that Legionella pneumophila RpoS modulates expression of the transmission phenotype in both the exponential and the stationary phase. Infect. Immun. 72:2468-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachman, M. A., and M. S. Swanson. 2001. RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40:1201-1214. [DOI] [PubMed] [Google Scholar]
- 6.Baker, C. S., I. Morozov, K. Suzuki, T. Romeo, and P. Babitzke. 2002. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol. Microbiol. 44:1599-1610. [DOI] [PubMed] [Google Scholar]
- 7.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. [DOI] [PubMed] [Google Scholar]
- 8.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. USA 96:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon, D. E., N. Connell, J. Keener, A. Tormo, M. Espinosa-Urgel, M. M. Zambrano, and R. Kolter. 1991. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of sigma 70. J. Bacteriol. 173:4482-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 12.Cui, Y., A. Chatterjee, and A. K. Chatterjee. 2001. Effects of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and harpinEcc. Mol. Plant-Microbe Interact. 14:516-526. [DOI] [PubMed] [Google Scholar]
- 13.Feeley, J. C., G. W. Gorman, R. E. Weaver, D. C. Mackel, and H. W. Smith. 1978. Primary isolation media for Legionnaires' disease bacterium. J. Clin. Microbiol. 8:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fettes, P. S., V. Forsbach-Birk, D. Lynch, and R. Marre. 2001. Overexpression of the Legionella pneumophila homologue of the E. coli regulator csrA affects cell size, flagellation, and pigmentation. Int. J. Med. Microbiol. 291:353-360. [DOI] [PubMed] [Google Scholar]
- 15.Gudapaty, S., K. Suzuki, X. Wang, P. Babitzke, and T. Romeo. 2001. Regulatory interactions of Csr components: the RNA binding protein CsrA activates csrB transcription in Escherichia coli. J. Bacteriol. 183:6017-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hales, L. M., and H. A. Shuman. 1999. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J. Bacteriol. 181:4879-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 18.Hammer, B. K., E. Tateda, and M. Swanson. 2002. A two-component regulator induces the transmission phenotype of stationary phase Legionella pneumophila. Mol. Microbiol. 44:107-118. [DOI] [PubMed] [Google Scholar]
- 19.Harb, O. S., and Y. Abu Kwaik. 2000. Characterization of a macrophage-specific infectivity locus (milA) of Legionella pneumophila. Infect. Immun. 68:368-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeb, S., and D. Haas. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol. Plant-Microbe Interact. 14:1351-1363. [DOI] [PubMed] [Google Scholar]
- 22.Heuner, K., C. Dietrich, C. Skriwan, M. Steinert, and J. Hacker. 2002. Influence of the alternative sigma(28) factor on virulence and flagellum expression of Legionella pneumophila. Infect. Immun. 70:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heuner, K., J. Hacker, and B. C. Brand. 1997. The alternative sigma factor σ28 of Legionella pneumophila restores flagellation and motility to an Escherichia coli fliA mutant. J. Bacteriol. 179:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kvint, K., C. Hosbond, A. Farewell, O. Nybroe, and T. Nystrom. 2000. Emergency derepression: stringency allows RNA polymerase to override negative control by an active repressor. Mol. Microbiol. 35:435-443. [DOI] [PubMed] [Google Scholar]
- 25.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 26.Lange, R., D. Fischer, and R. Hengge-Aronis. 1995. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the sigma S subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 177:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, M. Y., G. Gui, B. Wei, J. F. Preston III, L. Oakford, U. Yuksel, D. P. Giedroc, and T. Romeo. 1997. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 272:17502-17510. [DOI] [PubMed] [Google Scholar]
- 28.Loewen, P. C., B. Hu, J. Strutinsky, and R. Sparling. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707-717. [DOI] [PubMed] [Google Scholar]
- 29.Lynch, D., N. Rieser, K. Gloggler, V. Forsbach-Brik, and R. Marre. 2003. The response regulator LetA regulates the stationary-phase stress response in Legionella pneumophila and is required for efficient infection of Acanthamoeba castellanii. FEMS Microbiol. Lett. 219:241-248. [DOI] [PubMed] [Google Scholar]
- 30.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 31.Molofsky, A., and M. Swanson. 2003. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50:445-461. [DOI] [PubMed] [Google Scholar]
- 32.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 33.Romeo, T., and M. Gong. 1993. Genetic and physical mapping of the regulatory gene csrA on the Escherichia coli K-12 chromosome. J. Bacteriol. 175:5740-5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rost, B. 1996. PHD: predicting one-dimensional protein structure by profile based neural networks. Methods Enzymol. 266:525-539. [DOI] [PubMed] [Google Scholar]
- 35.Steinert, M., H. Engelhard, M. Flugel, E. Wintermeyer, and J. Hacker. 1995. The Lly-protein protects Legionella pneumophila from light but does not directly influence its intracellular survival in Hartmanella veriformis. Appl. Environ. Microbiol. 61:2428-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 37.Wiater, L. A., A. B. Sadosky, and H. Shuman. 1994. Mutagenesis of Legionella pneumophila using Tn903dIIlacZ: identification of a growth-phase-regulated pigmentation gene. Mol. Microbiol. 11:641-653. [DOI] [PubMed] [Google Scholar]
- 38.Wintermeyer, E., U. Rdest, B. Ludwig, A. Debes, and J. Hacker. 1991. Characterization of legiolysin (lly), responsible for hemolytic activity, colour production, and fluorescence of Legionella pneumophila. Mol. Microbiol. 5:1135-1143. [DOI] [PubMed] [Google Scholar]
- 39.Zuker, M., D. H. Mathews, and D. H. Turner. 1999. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide, p. 11-43. In J. Barciszewski and B. F. C. Clark (ed.), RNA biochemistry and biotechnology. Kluwer Academic Publishers, Amsterdam, The Netherlands.
- 40.Zusman, T., O. Gal-Mor, and G. Segal. 2002. Characterization of a Legionella pneumophila relA insertion mutant and roles of RelA and RpoS in virulence gene expression. J. Bacteriol. 184:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]