Abstract
Intestinal dendritic cells are continually exposed to ingested microorganisms and high concentrations of endogenous bacterial flora. These cells can be activated by infectious agents and other stimuli to induce T-cell responses and to produce chemokines which recruit other cells to the local environment. Bacterial probiotics are of increasing use against intestinal disorders such as inflammatory bowel disease. They act as nonpathogenic stimuli within the gut to regain immunologic quiescence. This study was designed to determine the ability of a bacterial probiotic cocktail VSL#3 to alter cell surface antigen expression and cytokine production in bone marrow-derived dendritic cell-enriched populations. Cell surface phenotype was monitored by monoclonal fluorescent antibody staining, and cytokine levels were quantitated by enzyme-linked immunosorbent assay. High-dose probiotic upregulated the expression of C80, CD86, CD40, and major histocompatibility complex class II I-Ad. Neither B7-DC or B7RP-1 was augmented after low-dose probiotic or Lactobacillus casei treatment, but B7RP-1 showed increased expression on dendritic cells stimulated with the gram-negative bacterium Escherichia coli. Functional studies showed that probiotic did not enhance the ability of dendritic cells to induce allogeneic T-cell proliferation, as was observed for E. coli. Substantial enhancement of interleukin-10 release was observed in dendritic cell-enriched culture supernatants after 3 days of probiotic stimulation. These results demonstrate that probiotics possess the ability to modulate dendritic cell surface phenotype and cytokine release in granulocyte-macrophage colony-stimulating factor-stimulated bone marrow-derived dendritic cells. Regulation of dendritic cell cytokines by probiotics may contribute to the benefit of these molecules in treatment of intestinal diseases.
Dendritic cells (DC) are potent antigen-presenting cells which can effectively induce primary immune responses against microbial infection and other stimuli. DC activation can be induced by infectious agents and inflammatory products (5, 10, 21, 35, 37). These organisms induce DC maturation characterized by upregulation of costimulatory molecules, especially those of the B7 family, by cytokine production and by the ability to activate T cells (5, 20, 35, 36, 44). Recognition of microbes by DC is made possible by Toll-like receptors interacting with pathogen-associated molecular patterns such as peptidoglycan or lipopolysaccharide on the microorganism (27, 28, 38).
Several experimental models of inflammatory bowel disease (IBD) indicate that enteric bacteria are very likely involved in the pathogenesis of this disease (11, 47, 52, 54, 62). Probiotics are live microbial food supplements which improve the health of the host (18). Most probiotics belong to the lactic acid group of bacteria (3, 16). These agents are currently being used to prevent or treat a diverse group of clinical conditions including inflammatory bowel disease (12, 19, 23, 24, 30, 42, 53). Probiotics may inhibit the growth of various gram-positive and gram-negative organisms by producing substances which are active against certain pathogens or by preventing the attachment of pathogens, thus facilitating their removal from the intestinal tract (1-3, 41, 51). Few reports address the action of probiotics on cells of the immune system.
DC in the gut may be modulated by microorganisms including the normal flora and those administered orally such as probiotics. The response elicited by these cells will depend on the organism and the type of infection. A recent report demonstrated that single probiotics of the Lactobacillus group can regulate DC surface expression and cytokine production (8). Our study was designed to determine the cytokine responses and phenotypes of DC after treatment with the probiotic mixture VSL#3 and to examine the functional capacity of these cells as determined by induction of allogeneic T-cell proliferation. This probiotic has been used in the treatment of gastrointestinal conditions such as pouchitis, colitis, and irritable bowel syndrome with some success (19, 30, 42, 55, 64). Bone marrow (BM)-derived DC were generated from progenitor cells in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), yielding CD11c-positive cells of similar morphology and phenotypes to those previously described for immature DC (13, 39). The generation of these cells in the presence of high doses of probiotic augmented the surface expression of the costimulatory molecules CD80 (B7-1), CD86 (B7-2), and CD40 and of major histocompatibility complex (MHC) class II I-Ad. These studies also investigated cytokines released in DC-enriched culture supernatants after 3 days of probiotic stimulation. Furthermore, we demonstrated that after exposure of DC to VSL there is no increase in allogeneic T-cell stimulation compared to DC exposed to phosphate-buffered saline (PBS), whereas DC pretreated with E. coli resulted in enhanced T-cell-proliferative activity. The implications of our findings for the improvement of IBD through DC modulation is discussed.
MATERIALS AND METHODS
Animals.
Adult (8 to 12 weeks old) female BALB/c (H-2d) and C57BL/6J (H-2b) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and maintained in a specific-pathogen-free environment in the animal facility at the Case Western Reserve University School of Medicine.
Probiotic and bacteria.
The probiotic VSL#3 (VSL Pharmaceuticals, Fort Lauderdale, Fla.) was obtained as a lyophilized mixture consisting of 8 different gram-positive organisms (Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus plantarum, and Streptococcus salivarius subsp. thermophilus). Each packet contained 450 billion bacteria. Contents were reconstituted in PBS (Life Technologies, Grand Island, N.Y.) without additives, and serial 1:10 dilutions were made in PBS for addition to cultures. Probiotic (10 μl) was added to 1 ml of growing DC cultures in 24-well plates. Studies were also conducted with L. casei (American Type Culture Collection, Manassas, Va.), a gram-positive organism, and with a nonpathogenic strain of Escherichia coli (ATCC 25922). E. coli was grown in Luria broth (Difco, Detroit, Mich.), and L. casei was grown in MRS broth at 37°C in a shaking incubator. Dilutions for these bacteria were also made in PBS. All organisms were added to 1-ml cultures in a 10-μl volume as described. As determined by bacterial plate counts, probiotic and other bacteria were viable at the time of addition to cultures, but growth was inhibited by the presence of antibiotic in the medium, yielding no growth of organisms at the end of a 3-day culture period.
Generation of BM-derived DC.
BM cells were prepared from mice by conventional means. Red blood cells were lysed, and the remaining leukocytes were plated (24-well plates; Costar) at a concentration of 2 × 106 per ml in a 1-ml volume of RPMI 1640 (GIBCO-BRL, Life Technologies), supplemented with 10% (vol/vol) fetal bovine serum (FBS; GIBCO), 100 U of penicillin/ml, 100 μg streptomycin (GIBCO)/ml, and 2 × 10−6 M 2-mercaptoethanol (Sigma), with the addition of 7 ng of recombinant mouse GM-CSF (R&D systems, Minneapolis, Minn.)/ml. Cultures were placed at 37°C in a 5% CO2 humidified incubator. After 2 days, the medium was removed without disturbing the clusters of developing DC and centrifuged and 0.5 ml of this conditioned medium was added back to the wells plus 0.5 ml of freshly made GM-CSF-containing medium, restoring the final volume in each well to 1 ml (13, 22, 39). At day 5, the nonadherent cells released from the clusters were harvested by gentle pipetting for characterization and study. In some experiments, DC were further enriched by using beads for the DC marker CD11c and MACS columns (miniMACS; Miltenyi Biotec, Auburn, Calif.) according to manufacturers' instructions. Recovered cells were routinely greater than 95% CD11c positive.
Stimulation of DC with probiotic.
Cultures were divided into different groups. To examine the effects of probiotics on DC generation, VSL#3 was added to BM cells at different dilutions (10-μl quantities to each 1-ml well) on day 2 after initiation of cultures, at the time when the nonadherent cells were removed and fresh medium was added as described above. Supernatants from these cultures were collected for analysis at day 5 when cells were harvested. Parallel cultures were treated with either PBS, L. casei, or E. coli. Alternatively, some cultures were left untreated until harvest on day 5. These cells were further enriched on a CD11c MACS column and then plated at 1 × 106 to 2 × 106 cells/ml in 24-well plates, and probiotic or other bacteria were added as before for overnight culture incubation. Cells were harvested the next day for phenotypic characterization.
Cell surface phenotype expression.
Cell surface staining was performed by using a panel of monoclonal antibody (MAb) directed against surface antigens expressed by DC and the appropriate species-specific immunoglobulin G isotype controls. Briefly, cells were preincubated with FCγIII/IIR antibody (CD16/CD32; PharMingen, San Diego, Calif.) for 10 min to block nonspecific antibody staining. Cells were resuspended at 2 × 105 to 5 × 105 cells in 100 μl of staining buffer (PBS, 1% FBS, 0.1% sodium azide) in flow tubes. They were stained directly with MAbs directed against CD11c phycoerythrin (PE) and CD80, CD86, CD40, or anti-MHC class II I-Ad (all fluorescein isothiocyanate [FITC] conjugated; PharMingen). To determine the levels of expression of some of the more recently identified costimulatory markers on DC, cells were stained with B7-DC and B7RP-1 (PE conjugated; Biosource, San Diego, Calif.) and FITC-labeled CD11c (PharMingen). Staining was performed for 30 min at 4°C, and cells were washed twice in staining buffer as before and resuspended in PBS. Events (at least 10,000) were acquired on a FACScan (Becton Dickinson) and analyzed with Cell Quest software.
Allogeneic stimulation of T cells in the presence of probiotic-treated DC.
BM-derived DC from BALB/c mice were harvested at day 5, purified on MACS columns to further enrich for CD11c-positive cells, and stimulated in 24-well plates at a concentration of 2 × 106/well for an overnight period. T cells were purified from the spleens of C57BL/6J mice with CD4+ MACS beads and columns. They were adjusted to a concentration of 7.5 × 106/ml, and carboxy fluorescein diacetate succinimidyl ester (CFSE) was added at a final concentration of 0.5 μM in 5% FBS-PBS for 7 min. Cells were washed 3 times in 5% FBS-PBS, counted, and added to DC as indicated for a period of 3 or 4 days to allow the incorporation of this dye into dividing daughter cells. Cultures were harvested and washed twice in flow staining buffer, and cells were MAb stained with CD4 peridinin chlorophyll protein (PerCP). Dead cells were gated out, and 50,000 events were collected in the live gate and analyzed with Cell Quest software. Histograms represent the double-positive CD4 PerCP-stained CFSE-labeled T cells.
Cytokine determination.
Cell culture supernatants were centrifuged for the removal of cells and stored at −70°C. Cytokine detection was done by enzyme-linked immunosorbent assay (ELISA) for interleukin-10 (IL-10) and gamma interferon (IFN-γ) with paired antibodies (PharMingen) and for bioactive IL-12 (p70) with the Duoset DY 419 kit (R&D Systems).
Statistical analysis.
Analysis of data was performed by Student's t test. A P value of <0.05 was taken as the level of significance.
RESULTS
Probiotic upregulation of DC costimulatory molecules.
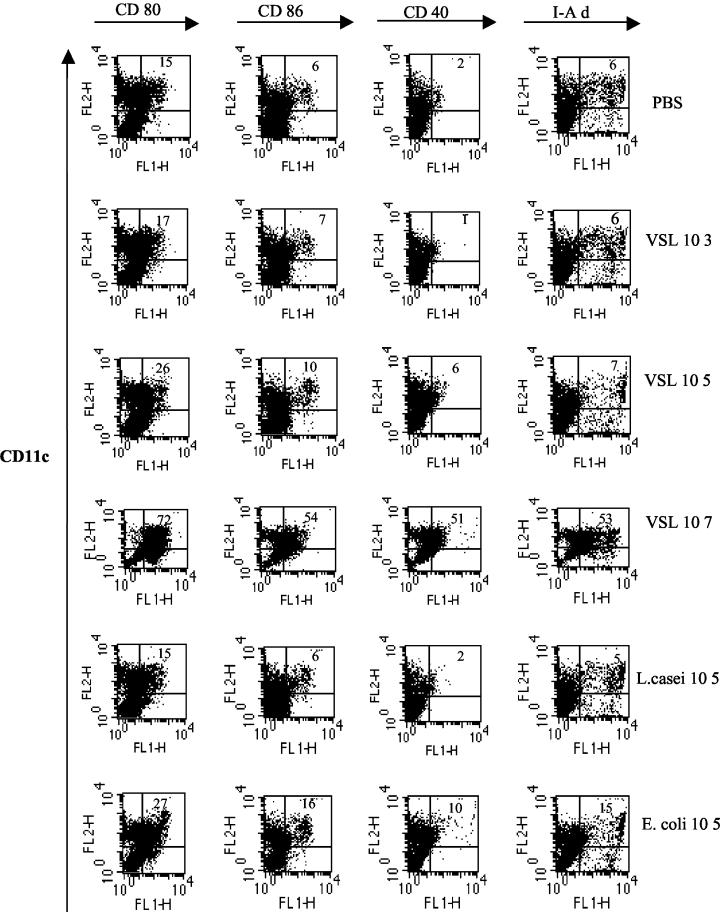
Changes in DC cell surface phenotypes may result in altered DC function or cytokine production (10, 13, 39). To determine the action of the probiotic mixture VSL#3 on DC surface phenotypes, probiotic was added to cultures for the last 3 days (DC generation phase) before harvesting of cells at day 5. By MAb staining and analysis of acquired events, we observed that the addition of 105 organisms per ml to cultures of developing DC did not significantly alter cell surface expression of CD80, CD86, CD40, or MHC class II I-Ad in comparison to PBS controls (Fig. 1). There was low to moderate expression of CD80, CD86, CD40, and MHC class II on CD11c-positive cells. However, the addition of 107 organisms of the probiotic mixture/ml to DC cultures during the generation phase resulted in striking augmentation of costimulatory molecules (Fig. 1). We conducted similar studies with L. casei, one of the component organisms of the probiotic mixture, and a nonpathogenic strain of the gram-negative bacterium E. coli. A similar pattern was observed for expression of the cell surface markers for DC generated in the presence of L. casei as for probiotic. That is, there was no significant change in costimulatory molecule expression with treatment of 105 organisms/ml (Fig. 1), but with treatment with 107 organisms/ml, an increased percentage of CD11c-positive cells expressed CD80, CD86, CD40, and I-Ad as shown for the probiotic (data not shown). In each experiment, treatment with 107 organisms/ml resulted in more cells expressing costimulatory molecules than those negative for these markers after probiotic, L. casei, or E. coli treatment. The increase in DC cell surface markers is consistent with a more mature DC.
FIG. 1.
Regulation of DC surface phenotypes by probiotic. BM cells were grown in GM-CSF for 2 days. Stimulation as indicated was added on day 2 for a further 3 days. Cells were harvested and MAb double stained for CD11c PE (FL2)- and FITC (FL1)-labeled costimulatory molecules. Gating was done to exclude dead cells. The number shown in the top right hand corner of each dot plot quadrant represents the percentage of CD11c-positive cells expressing each marker as indicated. Probiotic VSL#3 is indicated as VSL in the figures. Results are representative of two separate experiments.
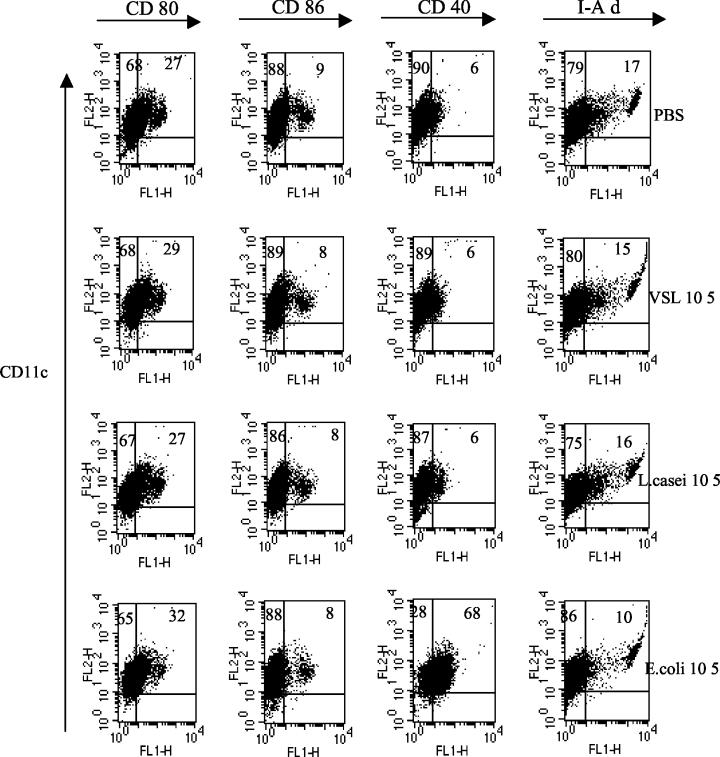
Changes in DC phenotypes after overnight probiotic stimulation.
Next, we examined the ability of probiotic to alter DC cell surface markers over a shorter time period. DC were harvested at day 5, further enriched over a MACS column with CD11c-labeled beads, and stimulated with probiotic, L. casei, or E. coli as previously described. In this series of studies, we primarily used 105 organisms/ml to maintain optimal viability of cells for the overnight subculture period because we observed that in some experiments after harvest, purification, and subculture of DC, treatment of DC with 107 organisms/ml could induce DC maturation and sometimes death of the more mature cells, a phenomenon consistent with mature DC in culture in the absence of a growth factor. There were no significant changes in costimulatory molecules in response to overnight probiotic or L. casei stimulation (Fig. 2). However, E. coli (105 organisms/ml) stimulation led to a resultant increase in costimulatory molecule expression, especially for CD40 (Fig. 2). These findings suggest that treatment of DC with E. coli stimulates DC to a higher level of maturation than treatment with probiotic or L. casei. Even though there was limited recovery of DC after purification on CD11c columns, which was further reduced after stimulation with 107 organisms/ml, in some experiments, where possible, we also examined the influence of 107 organisms of probiotic, L. casei, or E. coli/ml to alter DC surface phenotype after overnight incubation. As before (Fig. 1), probiotic, L. casei, and E. coli at this concentration increased the percentage of CD11c cells expressing costimulatory molecules.
FIG. 2.
Low-dose probiotic maintains immature DC cell surface phenotype. DC cultures were harvested on day 5. Cells were treated with probiotic or bacteria overnight, and cell surface phenotypes were characterized by staining for CD11c PE- and FITC-labeled costimulatory molecules. Dead cells were gated out, and 10,000 events were collected on a FACScan. The percentage of double-positive cells is shown in the upper right quadrant, and the percentage of CD11c single-positive cells is shown in the upper left quadrant. The results shown are representative of three separate experiments.
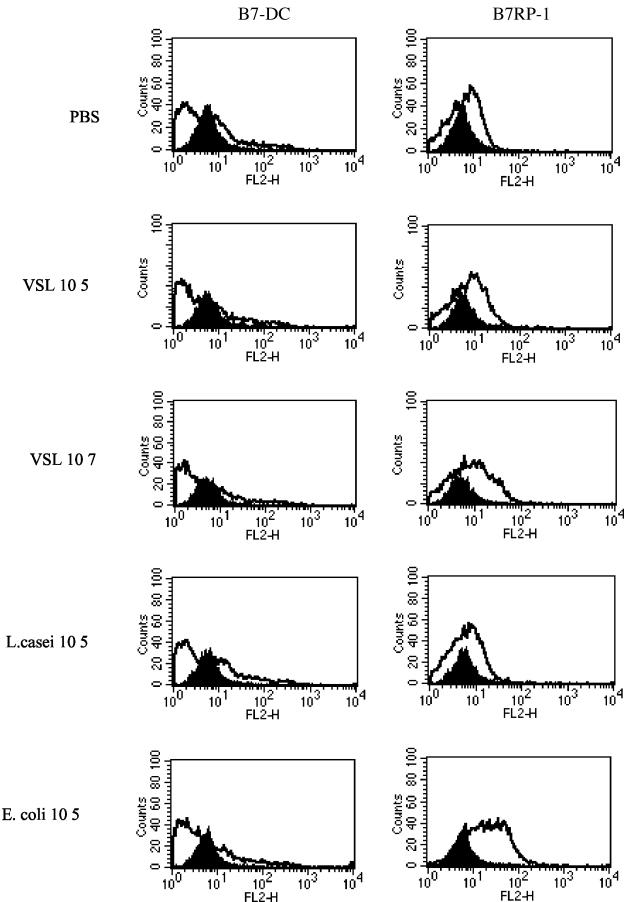
Differential expression of DC B7-DC and B7RP-1 after bacterial stimulation.
B7-DC is primarily expressed by DC and monocyte/macrophage in the mouse and may be involved in costimulation or suppression of T cells depending on the state of cellular activation (45, 63). B7RP-1 is expressed on B cells, monocytes, and DC and plays an important role in T-cell costimulation. These molecules are maximally expressed at hour 24 after stimulation and decline thereafter (67, 68). To further understand the action of probiotics on CD11c-positive DC surface phenotype modulation, we examined the levels of these B7 family molecules after overnight treatment of purified subcultured DC. B7-DC and B7RP-1 were moderately expressed on DC treated with PBS. Neither probiotic or L. casei (105 organisms/ml) treatment of DC had a significant effect on B7RP-1 surface expression (Fig. 3). However, this molecule was markedly upregulated after overnight treatment of DC in response to E. coli stimulation. We further observed, however, that higher-dose probiotic (107 organisms/ml) had the ability to induce a modest increase in B7RP-1 expression. B7-DC expression remained unchanged by probiotic or bacterial treatments (Fig. 3). Elevated levels of B7RP-1 on E. coli-treated DC may reflect a higher maturation state of these cells with this treatment and may be of importance in the ability of these cells to stimulate T-cell responses and cytokine production.
FIG. 3.
Bacteria differentially modulate B7RP-1 expression on DC. (A) DC cultures were harvested on day 5, further enriched on a CD11c MACS column, and cocultured with probiotic, L. casei, or E. coli at 105 organisms/ml for an overnight period. Cells were double stained for B7-DC or B7RP-1 PE and CD11c FITC. The solid histogram shows results for immunoglobulin G controls, and the unshaded histogram area shows the level of expression of costimulatory molecule as a result of treatment. Results for upregulation of B7RP-1 by E. coli are representative of three separate experiments.
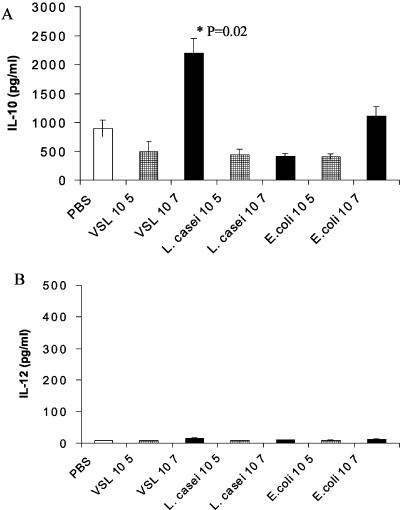
DC Th2 cytokine production in response to probiotic stimulation.
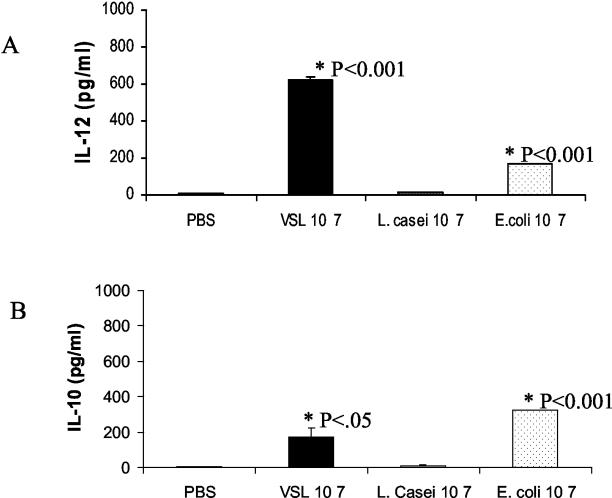
The activation of DC in response to bacterial stimuli may result in changes in cytokine patterns (8, 49). Supernatants collected from probiotic-, L. casei-, or E. coli-stimulated DC cultures at the end of the generation phase were assayed to quantitate the release of Th1 and Th2 cytokines in response to stimulation. After 3 days of stimulation of DC-enriched BM cells, we observed moderate levels of IL-10 released in cultures to which PBS was added as a control (Fig. 4A). Significantly higher levels of IL-10 were observed in supernatants of these cultures in which DC were stimulated with probiotic (107 organisms/ml) than in PBS controls. Increased IL-10 was not observed upon L. casei stimulation, whereas in the case of E. coli, higher, though not significant, levels of IL-10 were released in DC culture supernatants than with PBS. Lower doses of probiotic, L. casei, or E. coli (105 organisms/ml) were not sufficient to induce the release of significant levels of IL-10 (Fig. 4A).
FIG. 4.
Probiotic stimulates IL-10 release in DC cultures. BM cells were grown in GM-CSF. Various concentrations of probiotic or bacteria were added on day 2 for a further 3 days. DC culture supernatants were harvested on day 5, and cytokine levels were measured by ELISA. Results are shown for IL-10 (A) and IL-12 (p70) (B). Error bars represent standard deviations from duplicate wells. P values indicate levels of significance above control PBS. Results are representative of three separate experiments.
Differential release of Th1 cytokines after overnight probiotic stimulation.
Gram-negative and gram-positive bacteria can induce DC to release both Th1 and Th2 cytokines (8, 49). To further evaluate cytokine changes in DC upon exposure to probiotic, we measured levels of IFN-γ and IL-12 (p70) in stimulated DC supernatants in the generation phase of DC. Significant levels of IFN-γ were not detected in any DC culture supernatants.
There was an interesting pattern of release for IL-12 in supernatants tested. IL-12 was not detected in response to PBS, L. casei, or E. coli treatment in any of the 3-day stimulated DC supernatants (Fig. 4B). For probiotic stimulation under these conditions, the highest level of IL-12 release was 84 pg/ml, as obtained in 1 of 3 experiments. However, with the addition of probiotic or E. coli at day 4 of the DC generation phase for an overnight period until day 5 of harvest, there was moderate release of IL-12 (Fig. 5A). Much lower levels of IL-10 were released after overnight probiotic stimulation than after 3-day probiotic stimulation (Fig. 5B). Modulation of DC cytokines by probiotics suggests these bacterial compounds as candidates in disease prevention.
FIG. 5.
Probiotic stimulation of IL-12 (p70). Cells at day 4 of BM DC culture were stimulated for an overnight period by the addition of 107 organisms of probiotic or bacteria/ml until the next day. Supernatants were collected, and IL-12 levels (A) or IL-10 release (B) in supernatants were determined by ELISA. Standard deviation is shown for duplicate wells. The level of significance is indicated by the P value.
Cell division of T cells in the presence of probiotic-treated allogeneic DC.
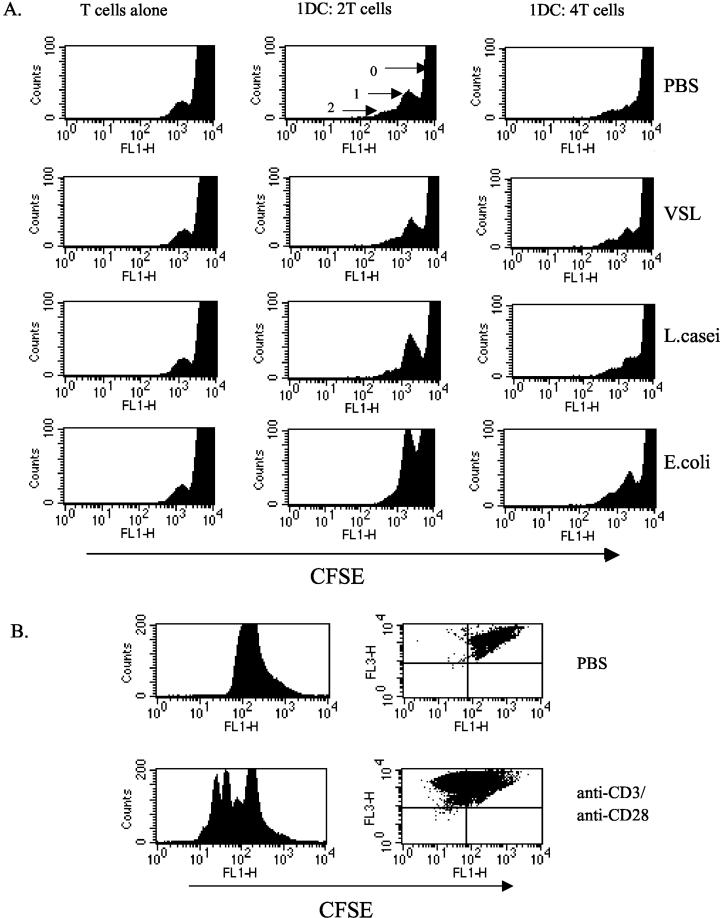
To investigate the functional capacity of stimulated DC, cell division induced in allogeneic T cells was studied. T-cell division was investigated by the use of CFSE staining, a dye which distributes equally to daughter cells upon cell division (40, 61). CFSE-labeled allogeneic T cells were cultured with stimulated DC, and the ability of DC to induce cell division in T cells was measured by flow cytometry. Dot plots of forward scatter versus side scatter showed an increased number of events in the T-cell region and a shift of T cells towards the right in the DC-T cell cocultures, in comparison with T cells alone, indicative of T-cell proliferation (data not shown). Histograms revealed a peak of undivided cells and 1 or 2 peaks of cell division in experiments (Fig. 6A). The area occupied by the cells in the first round of cell division was similar for probiotic- and PBS-treated DC-T cell cocultures, whereas there was an increase in this region for the E. coli-stimulated DC-T cell cocultures at both cell ratios shown (Fig. 6A). The level of T-cell stimulation observed in these experiments is consistent with modest stimulation obtained with immature DC in other systems (13, 39). In 1 of 3 experiments, DC pretreated with L. casei showed modest increase in cells in division cycle one. At higher DC dilutions (1:8 and 1:16) with a fixed number of T cells, no cultures showed increased T-cell division in comparison with T cells alone. This increased T-cell division in the latter case is consistent with the demonstrated phenotype of E. coli-stimulated DC to have higher expression of costimulatory molecules (Fig. 2 and 3) than control PBS- or probiotic-stimulated DC. Syngeneic cocultures did not induce T-cell division above background levels (data not shown). Stimulation of T cells with potent stimuli anti-CD3 and anti-CD28 resulted in several rounds of cell division (Fig. 6B).
FIG. 6.
Probiotic-stimulated DC does not enhance T-cell division in allogeneic T cells. (A) Day 5 BM-derived DC cultures were harvested, and CD11c cells were purified and treated (2 × 106 DC/ml in 24-well plates) with probiotic or bacteria (105 organisms per ml) for an overnight culture period. DC were washed three times. CD4 T cells were purified from the spleens of allogeneic C57BL6/J mice and labeled with 0.5 μM CFSE. Serial dilutions were made of DC which were then added to 96-well flat-bottom plates at the indicated dilution, with 2 × 105 T cells/well. After 4 days, cells were harvested and MAb labeled for CD4 PerCP (FL3). Forward scatter versus side scatter gating was done on live cells, and 50,000 events were collected. Histograms represent the CD4+ PerCP-stained T cells which contained the CFSE dye (FL1). The peak labeled 0 shows the undivided cells, the peak labeled 1 shows the first round of cell division, and the peak labeled 2 shows those cells undergoing a second division. Probiotic-stimulated DC induced minimal T-cell division above control PBS in three separate experiments. (B) Plates were coated with 1-μg/ml anti-CD3 MAb in PBS at 37°C for 4 h. Wells were washed gently with PBS. CFSE-labeled T cells (2 × 106/ml) were added in RPMI medium with supplements, and 2-μg/ml soluble anti-CD28 was added to the cultures. Cells were harvested at day 3 of stimulation and stained with CD4 PerCP, and the division of cells was analyzed as described above. Dot plots show divided cells in the upper left quadrant.
Cytokine production in DC-T cell cocultures.
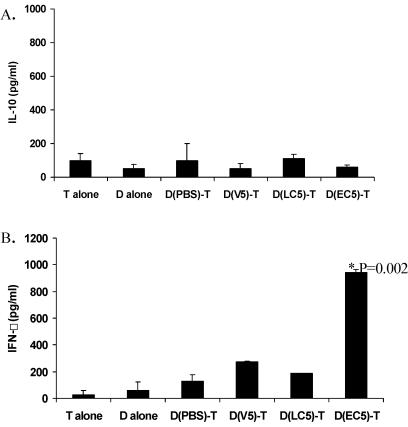
Supernatants from 3-day stimulated DC-T cell cocultures were investigated to measure the ability of this interaction to release cytokines in culture. We were unable to detect elevated levels of IL-10 in any cocultures at the end of the 3-day period assayed, possibly due to the fact that our allogeneic T cells were from C57BL/6, a Th1 responder strain of mouse (Fig. 7A). In these cultures, we observed that probiotic-treated DC did not stimulate the release of significant levels of IFN-γ in DC-T cell cocultures. However, DC pretreated with E. coli resulted in significantly elevated levels of this cytokine in cocultures in comparison with those released in cocultures with PBS-stimulated DC (Fig. 7B). Future studies with DC-T cells from different strains of mice will give further understanding of cytokine production in this allogeneic coculture system. Controls for T cells alone and for DC alone did not show significant cytokine production.
FIG. 7.
Cytokine production in DC and T-cell cocultures. Day 5 BM-derived DC generated from BALB/c mice were harvested, purified for CD11c-positive cells, and treated with probiotic or bacteria (105 organisms/ml). Treated DC-purified T-cell cocultures were set up with 0.5 × 106 DC and 1.5 × 106 T cells in 24-well plates. Supernatants were harvested at day 3 and frozen at −20°C for cytokine analysis by ELISA. D alone represents DC precultured in PBS; similar results were obtained with those precultured in bacteria or probiotic. D(V5), DC pretreated with VSL#3 at 105 organisms/ml; D(LC5), pretreated with L. casei; D(EC5), those pretreated with E. coli. Values represent means ± 1 standard deviation of the results for duplicate wells from two experiments performed. (A) IL-10 production; (B) IFN-γ production in cocultures.
DISCUSSION
The intestinal flora consists of hundreds of bacterial species (66). In the colon, bacterial antigens are present in large amounts, and thus, the mucosal immune balance likely reflects an interaction with these organisms. The involvement of commensal flora in gut disorders and bacteria as a causative agent of IBD is supported by many studies (52, 62). In addition, IL-10-deficient mice fail to develop colitis under germfree conditions but do so when exposed to luminal bacteria, with the extent of the inflammation varying with the amount and composition of the bacteria (32, 54). Furthermore, experimental colitis was induced in this strain of mouse by exposure to Helicobacter hepaticus (33), and infection with Helicobacter bilis caused IBD in severe combined immunodeficient mice (56). In humans, it has also been demonstrated that antibiotics are effective for the improvement of IBD, and probiotics have recently been tried with some success against pouchitis and Crohn's disease (19, 50, 55, 64).
The success of bacterial probiotics depends on several criteria (3, 16). The component organisms must be of healthy human origin (14, 51), have tolerance to gastric acidity (14), be able to produce antimicrobial compounds (34), and have the ability to adhere to the gut and prevent the attachment of harmful bacteria (2, 17), and of great importance, they should possess the ability to modulate immune responses.
DC are pivotal cells in the generation of immune responses. These cells are found at different sites in the gastrointestinal tract. They are located proximal to incoming dietary products and bacteria, which can alter costimulatory molecules on these cells, and they direct the cytokine polarization of these cells and subsequently that of responding T cells (29, 48). DC influence the development of Th1 and Th2 responses, either by the cytokines they produce or by providing costimulation for T cells which can then proliferate and elaborate cytokines and chemokines (5, 7, 35-37).
Probiotic VSL#3 stimulation induced the release of significant levels of IL-10 in DC culture supernatants if added for a period of 3 days during the generation of these cells. IL-10 is a critical Th2 cytokine which suppresses IL-12 production and consequently other Th1 cytokines such as IFN-γ and tumor necrosis factor alpha (7). It also prevents activation of antigen-presenting cells, inhibits the maturity of DC, limits T-cell proliferation, and can induce a state of antigen-specific tolerance (6, 15, 60). In the gut, IL-10 is a key molecule for the induction of T regulatory cells and prevention of mouse colitis (4, 46, 65). The suppression of IL-12 in the gut by IL-10 prevents an inflammatory cascade of Th1 cytokines and cellular migration (57).
The probiotic VSL#3 has been used in trials to treat or prevent gastrointestinal conditions such as pouchitis (64), colitis (42, 55), and irritable bowel syndrome (30), many of which are Th1 driven. Patients with pouchitis were reported to have higher IL-10 levels in the mucosal pouch after VSL#3 administration (64). DC in the gut are immature and possess the ability to be influenced by pathogens and other bacteria they encounter. Here, we have demonstrated the ability of this probiotic to induce IL-10 in a population of DC. In vivo, boosting of IL-10 release by this probiotic may be one of the ways in which it can exert its beneficial activity. Consistent with previous reports, we found, too, that E. coli could induce the release of IL-10 (49), whereas L. casei was a low IL-10 stimulator (8). The benefits of IL-10 administered by probiotic bacteria has been investigated by Steidler and coworkers (59). It was shown that administration of IL-10-secreting Lactococcus lactis caused a 50% reduction in colitis in dextran sodium sulfate-treated mice and prevented the onset of colitis in the IL-10-deficient mouse model.
Further examination of probiotic-induced cytokine revealed that, upon stimulation, IL-12 was detected in DC culture supernatants after 1 day of stimulation, but over longer periods (3 days of DC generation), tested levels were low or undetectable. Whether this finding represents an early release of IL-12, which later declines, possibly with the rise of IL-10, will be investigated in future studies. Other investigators showed that lower doses of lethally irradiated freeze-dried Lactobacillus species induced high levels of IL-12 production, whereas higher doses were required to induce high levels of IL-10 (8). IL-12 plays a central role in promoting Th1 responses. It is responsible for the amplification of inflammatory cytokines but is also known to be effective against infectious diseases of viral origin (9, 31, 43). Furthermore, DC in the gastrointestinal tract are prone to be Th2 producers (25, 26), and the presence of IL-12 may be necessary to maintain a Th1/Th2 cytokine balance among cells in the gastrointestinal tract and tolerance to many types of dietary antigens.
E. coli but not L. casei was a stimulus for IL-12 released in DC culture supernatants. There was no release of IFN-γ by probiotic-stimulated DC, as was the case for those treated with the other stimuli. These studies were not intended to directly compare levels of cytokine changes among the different treatments because we are aware that the stock preparations of probiotic and bacterial cultures were available in different forms (lyophilized versus growing bacterial culture, respectively), and this may in itself affect the observed DC response. Rather, we included E. coli and L. casei in our investigations as a measure of the levels of cytokines that DC in our system can produce in response to a gram-negative organism, of which some strains are pathogenic, and a single gram-positive organism found in the probiotic mixture.
Probiotic exposure at 105 organisms/ml did not alter the immature phenotype of DC. The converse was true; however, in the case of a higher concentration, which had the ability to upregulate DC costimulatory molecule expression of CD80, CD86, CD40, and MHC class II I-Ad. The upregulation of CD86 and MHC class II observed with probiotic stimulation is consistent with other studies in which probiotic Lactobacillus species caused a similar enhancement of expression of these molecules (8). We also examined two recently characterized costimulatory molecules of the B7 family. B7-DC binds to programmed death-1, and B7RP-1 exhibits its costimulatory function for T cells through inducible costimulator (45, 58, 63, 67, 68). We did not notice significant upregulation of B7-DC with any of the DC treatments used in our study. The same was true for DC expression of B7RP-1 after probiotic or L. casei treatment; however, E. coli stimulation resulted in an increased percentage of CD11c-positive cells expressing B7RP-1.
Stimulation of T cells by DC plays an important role in antigen presentation, T-cell activation, and cytokine regulation. We conducted studies with allogeneic T cells because of our observation that bacteria induced changes in DC costimulatory molecule expression. Even though low cell division is exhibited because DC used in these allostimulation studies are largely immature, E. coli-stimulated DC had the potential to induce more cells to divide than those stimulated with PBS, probiotic, or L. casei, possibly due to the higher levels of costimulatory molecule expressed on the surface of these cells after E. coli stimulation. Interestingly, we also observed that E. coli DC-T cell cocultures had high levels of the Th1 cytokine IFN-γ, whereas significant levels of this cytokine were not observed in other cocultures. IFN-γ is a proinflammatory cytokine which is found in the intestine at the site of immune damage. It must be stated that the findings observed in our study are most likely caused by the effects of dead bacteria on DC, since in the presence of culture medium, there was reduced viability of organisms when plated after 30 min in culture medium, and there was no bacterial growth after 3 days of incubation of bacteria with DC (data not shown). Future studies will determine the ability of probiotic-treated DC to influence the release of a wider range of cytokines in different allogeneic systems. The area of DC-T cell investigation is of importance because overexpression of costimulatory molecules at sites of immune damage can increase stimulation of T cells and the release of damaging cytokines. This initial work in which probiotic-stimulated DC does not induce significant release of IFN-γ in these cocultures is promising, and future studies will determine whether probiotic may perform a regulatory function in other allogeneic systems.
This is a novel study in which we have demonstrated the immunoregulatory potential of the probiotic VSL#3 and its effect on the release of Th1 and Th2 cytokines in populations of murine BM-derived DC. Findings showed that this probiotic mixture stimulates high quantities of IL-10 and more modest levels of IL-12. These initial studies were conducted on DC generated from BM because we could obtain higher numbers to perform extensive investigations. Future investigations will be conducted with gut DC from normal and diseased mice. From our studies, we believe that the presence of probiotic bacteria during the development of DC will influence the outcome of the immune response. These studies were focused on the probiotic mixture, since this is the form in which the treatment is administered to patients. Studies with altered forms of this mixture or its component organisms will be the subject of future investigations. Probiotics are of increasing use against many diseases, especially those of the gastrointestinal tract. Further in vivo and in vitro studies on the manipulation of DC cytokines by probiotics will aid in the selection of new probiotics with more direct and potent effects against specific classes of disease.
Acknowledgments
We thank Melvin Berger of the Department of Pediatrics, Case Western Reserve University, for the kind use of his flow cytometer.
This work was supported by National Institutes of Health grants T32-A152067-02, DK-57756, and DK-046461.
Editor: F. C. Fang
REFERENCES
- 1.Alakomi, H. L., E. Skytta, M. Saarela, T. Mattila-Sandholm, K. Latva-Kala, and I. M. Helander. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66:2001-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alander, M., R. Satokari, R. Korpela, M. Saxelin, T. Vilpponen-Salmela, T. Mattila-Sandholm, and A. von Wright. 1999. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annuk, H., J. Shchepetova, T. Kullisaar, E. Songisepp, M. Zilmer, and M. Mikelsaar. 2003. Characterization of intestinal lactobacilli as putative probiotic candidates. J. Appl. Microbiol. 94:403-412. [DOI] [PubMed] [Google Scholar]
- 4.Asseman, C., S. Mauze, M. W. Leach, R. L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 6.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavani, A., F. Nasorri, C. Prezzi, S. Sebastiani, C. Albanesi, and G. Girolomoni. 2000. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J. Investig. Dermatol. 114:295-302. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, H. R., H. Frokiaer, and J. J. Pestka. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168:171-178. [DOI] [PubMed] [Google Scholar]
- 9.Dalod, M., T. P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C. A. Biron. 2002. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong, E. C., P. L. Vieira, P. Kalinski, J. H. Schuitemaker, Y. Tanaka, E. A. Wierenga, M. Yazdanbakhsh, and M. L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse th cell-polarizing signals. J. Immunol. 168:1704-1709. [DOI] [PubMed] [Google Scholar]
- 11.Dianda, L., A. M. Hanby, N. A. Wright, A. Sebesteny, A. C. Hayday, and M. J. Owen. 1997. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am. J. Pathol. 150:91-97. [PMC free article] [PubMed] [Google Scholar]
- 12.Dieleman, L. A., M. S. Goerres, A. Arends, D. Sprengers, C. Torrice, F. Hoentjen, W. B. Grenther, and R. B. Sartor. 2003. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut 52:370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drakes, M. L., L. Lu, V. M. Subbotin, and A. W. Thomson. 1997. In vivo administration of flt3 ligand markedly stimulates generation of dendritic cell progenitors from mouse liver. J. Immunol. 159:4268-4278. [PubMed] [Google Scholar]
- 14.Dunne, C., L. O'Mahony, L. Murphy, G. Thornton, D. Morrissey, S. O'Halloran, M. Feeney, S. Flynn, G. Fitzgerald, C. Daly, B. Kiely, G. C. O'Sullivan, F. Shanahan, and J. K. Collins. 2001. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 73:386S-392S. [DOI] [PubMed] [Google Scholar]
- 15.Enk, A. H., V. L. Angeloni, M. C. Udey, and S. I. Katz. 1993. Inhibition of Langerhans cell antigen-presenting function by IL-10. A role for IL-10 in induction of tolerance. J. Immunol. 151:2390-2398. [PubMed] [Google Scholar]
- 16.Fernandez, M. F., S. Boris, and C. Barbes. 2003. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94:449-455. [DOI] [PubMed] [Google Scholar]
- 17.Forestier, C., C. De Champs, C. Vatoux, and B. Joly. 2001. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res. Microbiol. 152:167-173. [DOI] [PubMed] [Google Scholar]
- 18.Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365-378. [PubMed] [Google Scholar]
- 19.Gionchetti, P., F. Rizzello, A. Venturi, P. Brigidi, D. Matteuzzi, G. Bazzocchi, G. Poggioli, M. Miglioli, and M. Campieri. 2000. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119:305-309. [DOI] [PubMed] [Google Scholar]
- 20.Hart, D. N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245-3287. [PubMed] [Google Scholar]
- 21.Huang, Q., D. Liu, P. Majewski, L. C. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 22.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isolauri, E. 2003. Probiotics for infectious diarrhoea. Gut 52:436-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isolauri, E., S. Salminen, and T. Mattila-Sandholm. 1999. New functional foods in the treatment of food allergy. Ann. Med. 31:299-302. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki, A., and B. L. Kelsall. 1999. Mucosal immunity and inflammation. I. Mucosal dendritic cells: their specialized role in initiating T cell responses. Am. J. Physiol. 276:G1074-G1078. [DOI] [PubMed] [Google Scholar]
- 27.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78-83. [DOI] [PubMed] [Google Scholar]
- 28.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 166:5688-5694. [DOI] [PubMed] [Google Scholar]
- 29.Kelsall, B. L., and W. Strober. 1996. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J. Exp. Med. 183:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, H. J., M. Camilleri, S. McKinzie, M. B. Lempke, D. D. Burton, G. M. Thomforde, and A. R. Zinsmeister. 2003. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 17:895-904. [DOI] [PubMed] [Google Scholar]
- 31.Komastu, T., D. D. Ireland, and C. S. Reiss. 1998. IL-12 and viral infections. Cytokine Growth Factor Rev. 9:277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 33.Kullberg, M. C., A. G. Rothfuchs, D. Jankovic, P. Caspar, T. A. Wynn, P. L. Gorelick, A. W. Cheever, and A. Sher. 2001. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect. Immun. 69:4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kullisaar, T., M. Zilmer, M. Mikelsaar, T. Vihalemm, H. Annuk, C. Kairane, and A. Kilk. 2002. Two antioxidative lactobacilli strains as promising probiotics. Int. J. Food Microbiol. 72:215-224. [DOI] [PubMed] [Google Scholar]
- 35.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311-316. [DOI] [PubMed] [Google Scholar]
- 36.Lanzavecchia, A., and F. Sallusto. 2001. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr. Opin. Immunol. 13:291-298. [DOI] [PubMed] [Google Scholar]
- 37.Lanzavecchia, A., and F. Sallusto. 2001. Regulation of T cell immunity by dendritic cells. Cell 106:263-266. [DOI] [PubMed] [Google Scholar]
- 38.Liu, T., T. Matsuguchi, N. Tsuboi, T. Yajima, and Y. Yoshikai. 2002. Differences in expression of toll-like receptors and their reactivities in dendritic cells in BALB/c and C57BL/6 mice. Infect. Immun. 70:6638-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu, L., J. Woo, A. S. Rao, Y. Li, S. C. Watkins, S. Qian, T. E. Starzl, A. J. Demetris, and A. W. Thomson. 1994. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J. Exp. Med. 179:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyons, A. B. 2000. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J. Immunol. Methods. 243:147-154. [DOI] [PubMed] [Google Scholar]
- 41.Mack, D. R., S. Michail, S. Wei, L. McDougall, and M. A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941-G950. [DOI] [PubMed] [Google Scholar]
- 42.Madsen, K., A. Cornish, P. Soper, C. McKaigney, H. Jijon, C. Yachimec, J. Doyle, L. Jewell, and C. De Simone. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580-591. [DOI] [PubMed] [Google Scholar]
- 43.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 44.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen, L. T., S. Radhakrishnan, B. Ciric, K. Tamada, T. Shin, D. M. Pardoll, L. Chen, M. Rodriguez, and L. R. Pease. 2002. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J. Exp. Med. 196:1393-1398. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Powrie, F., M. W. Leach, S. Mauze, L. B. Caddle, and R. L. Coffman. 1993. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5:1461-1471. [DOI] [PubMed] [Google Scholar]
- 47.Rath, H. C., H. H. Herfarth, J. S. Ikeda, W. B. Grenther, T. E. Hamm, Jr., E. Balish, J. D. Taurog, R. E. Hammer, K. H. Wilson, and R. B. Sartor. 1996. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Investig. 98:945-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 49.Rosenzwajg, M., F. Jourquin, L. Tailleux, and J. C. Gluckman. 2002. CD40 ligation and phagocytosis differently affect the differentiation of monocytes into dendritic cells. J. Leukoc. Biol. 72:1180-1189. [PubMed] [Google Scholar]
- 50.Rutgeerts, P., M. Hiele, K. Geboes, M. Peeters, F. Penninckx, R. Aerts, and R. Kerremans. 1995. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology 108:1617-1621. [DOI] [PubMed] [Google Scholar]
- 51.Saarela, M., G. Mogensen, R. Fonden, J. Matto, and T. Mattila-Sandholm. 2000. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol. 84:197-215. [DOI] [PubMed] [Google Scholar]
- 52.Schultz, M., S. L. Tonkonogy, R. K. Sellon, C. Veltkamp, V. L. Godfrey, J. Kwon, W. B. Grenther, E. Balish, I. Horak, and R. B. Sartor. 1999. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am. J. Physiol. 276:G1461-G1472. [DOI] [PubMed] [Google Scholar]
- 53.Schultz, M., C. Veltkamp, L. A. Dieleman, W. B. Grenther, P. B. Wyrick, S. L. Tonkonogy, and R. B. Sartor. 2002. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm. Bowel Dis. 8:71-80. [DOI] [PubMed] [Google Scholar]
- 54.Sellon, R. K., S. Tonkonogy, M. Schultz, L. A. Dieleman, W. Grenther, E. Balish, D. M. Rennick, and R. B. Sartor. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shibolet, O., F. Karmeli, R. Eliakim, E. Swennen, P. Brigidi, P. Gionchetti, M. Campieri, S. Morgenstern, and D. Rachmilewitz. 2002. Variable response to probiotics in two models of experimental colitis in rats. Inflamm. Bowel Dis. 8:399-406. [DOI] [PubMed] [Google Scholar]
- 56.Shomer, N. H., C. A. Dangler, M. D. Schrenzel, and J. G. Fox. 1997. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect. Immun. 65:4858-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson, S. J., S. Shah, M. Comiskey, Y. P. de Jong, B. Wang, E. Mizoguchi, A. K. Bhan, and C. Terhorst. 1998. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J. Exp. Med. 187:1225-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, K. M., J. M. Brewer, P. Webb, A. J. Coyle, C. Gutierrez-Ramos, and P. Garside. 2003. Inducible costimulatory molecule-b7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and th2 T cells. J. Immunol. 170:2310-2315. [DOI] [PubMed] [Google Scholar]
- 59.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 60.Steinbrink, K., H. Jonuleit, G. Muller, G. Schuler, J. Knop, and A. H. Enk. 1999. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood 93:1634-1642. [PubMed] [Google Scholar]
- 61.Suchin, E. J., P. B. Langmuir, E. Palmer, M. H. Sayegh, A. D. Wells, and L. A. Turka. 2001. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J. Immunol. 166:973-981. [DOI] [PubMed] [Google Scholar]
- 62.Taurog, J. D., J. A. Richardson, J. T. Croft, W. A. Simmons, M. Zhou, J. L. Fernandez-Sueiro, E. Balish, and R. E. Hammer. 1994. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180:2359-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tseng, S. Y., M. Otsuji, K. Gorski, X. Huang, J. E. Slansky, S. I. Pai, A. Shalabi, T. Shin, D. M. Pardoll, and H. Tsuchiya. 2001. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 193:839-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ulisse, S., P. Gionchetti, S. D'Alo, F. P. Russo, I. Pesce, G. Ricci, F. Rizzello, U. Helwig, M. G. Cifone, M. Campieri, and C. De Simone. 2001. Expression of cytokines, inducible nitric oxide synthase, and matrix metalloproteinases in pouchitis: effects of probiotic treatment. Am. J. Gastroenterol. 96:2691-2699. [DOI] [PubMed] [Google Scholar]
- 65.Van Montfrans, C., E. Hooijberg, M. S. Rodriguez Pena, E. C. De Jong, H. Spits, A. A. Te Velde, and S. J. Van Deventer. 2002. Generation of regulatory gut-homing human T lymphocytes using ex vivo interleukin 10 gene transfer. Gastroenterology 123:1877-1888. [DOI] [PubMed] [Google Scholar]
- 66.Wilson, K. H., J. S. Ikeda, and R. B. Blitchington. 1997. Phylogenetic placement of community members of human colonic biota. Clin. Infect. Dis. 25(Suppl. 2):S114-S116. [DOI] [PubMed] [Google Scholar]
- 67.Yoshinaga, S. K., J. S. Whoriskey, S. D. Khare, U. Sarmiento, J. Guo, T. Horan, G. Shih, M. Zhang, M. A. Coccia, T. Kohno, A. Tafuri-Bladt, D. Brankow, P. Campbell, D. Chang, L. Chiu, T. Dai, G. Duncan, G. S. Elliott, A. Hui, S. M. McCabe, S. Scully, A. Shahinian, C. L. Shaklee, G. Van, T. W. Mak, et al. 1999. T-cell co-stimulation through B7RP-1 and ICOS. Nature 402:827-832. [DOI] [PubMed] [Google Scholar]
- 68.Yoshinaga, S. K., M. Zhang, J. Pistillo, T. Horan, S. D. Khare, K. Miner, M. Sonnenberg, T. Boone, D. Brankow, T. Dai, J. Delaney, H. Han, A. Hui, T. Kohno, R. Manoukian, J. S. Whoriskey, and M. A. Coccia. 2000. Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int. Immunol. 12:1439-1447. [DOI] [PubMed] [Google Scholar]