Significance
Narcolepsy is a chronic sleep disease with autoimmune origin. We explored occurrence of autoantibodies in narcolepsy and other sleep-related disorders (OSRDs) by screening human sera with immunohistochemistry on rat brains. Hypocretin/orexinergic neurons were not stained, but a prominent immunostaining pattern of hypothalamic melanin-concentrating hormone (MCH) and proopiomelanocortin (POMC) neurons was overrepresented in cases of narcolepsy and OSRD patients. The autoantigen was identified as the common C-terminal epitope of neuropeptide glutamic acid-isoleucine/α–melanocyte-stimulating hormone (NEI/αMSH). Purified IgGs from a patient with MCH/POMC staining injected intracerebroventricularly to rats caused disturbed sleep patterns. Also, GABAergic cortical interneurons were stained with other narcolepsy and OSRD sera. Thus, autoantibodies are frequent in patients with sleep disorders, and NEI/αMSH may be a previously unidentified autoantigen involved in pathomechanism(s). These findings indicate possible diagnostic/therapeutic targets.
Keywords: H1N1 vaccination, POMC neurons, autoantigen, neurotransmitter
Abstract
Narcolepsy is a chronic sleep disorder, likely with an autoimmune component. During 2009 and 2010, a link between A(H1N1)pdm09 Pandemrix vaccination and onset of narcolepsy was suggested in Scandinavia. In this study, we searched for autoantibodies related to narcolepsy using a neuroanatomical array: rat brain sections were processed for immunohistochemistry/double labeling using patient sera/cerebrospinal fluid as primary antibodies. Sera from 89 narcoleptic patients, 52 patients with other sleep-related disorders (OSRDs), and 137 healthy controls were examined. Three distinct patterns of immunoreactivity were of particular interest: pattern A, hypothalamic melanin-concentrating hormone and proopiomelanocortin but not hypocretin/orexin neurons; pattern B, GABAergic cortical interneurons; and pattern C, mainly globus pallidus neurons. Altogether, 24 of 89 (27%) narcoleptics exhibited pattern A or B or C. None of the patterns were exclusive for narcolepsy but were also detected in the OSRD group at significantly lower numbers. Also, some healthy controls exhibited these patterns. The antigen of pattern A autoantibodies was identified as the common C-terminal epitope of neuropeptide glutamic acid-isoleucine/α–melanocyte-stimulating hormone (NEI/αMSH) peptides. Passive transfer experiments on rat showed significant effects of pattern A human IgGs on rapid eye movement and slow-wave sleep time parameters in the inactive phase and EEG θ-power in the active phase. We suggest that NEI/αMSH autoantibodies may interfere with the fine regulation of sleep, contributing to the complex pathogenesis of narcolepsy and OSRDs. Also, patterns B and C are potentially interesting, because recent data suggest a relevance of those brain regions/neuron populations in the regulation of sleep/arousal.
Narcolepsy is a chronic neurological disease characterized by irresistible daytime sleepiness (hypersomnia) and disturbed nocturnal sleep. Narcolepsy can be divided into two types: narcolepsy with cataplexy (NC) and narcolepsy without cataplexy. A typical feature of NC is sudden loss of muscle tone triggered by emotions (cataplexy). Other symptoms of narcolepsy are, for example, hypnagogic or hypnopompic hallucinations and sleep paralyses (1). The age of onset is usually around 12–16 y of age, but the disease is often diagnosed several years later (1). It affects ∼25–50 per 100,000 individuals, and the yearly incidence rate has been estimated to be around 1 per 100,000 person-y (2). Narcolepsy has a major negative impact on the quality of life, afflicting both physical and mental parameters (3). A major hallmark of narcolepsy (mostly NC) is the loss of hypocretin-1/orexin-A (Hcrt/Orx), a neuropeptide hormone initially discovered independently by two groups (4, 5), by either a destruction of the orexinergic neurons or a selective down-regulation of Hcrt/Orx expression (6, 7). Consequently, a typical feature of NC is low levels (<110 pg/mL) of Hcrt/Orx peptide in the cerebrospinal fluid (CSF) (1).
The causes of the loss of Hcrt/Orx and narcolepsy are unknown. However, many researchers consider narcolepsy an autoimmune disease based on a strong association with HLA DQB1*06:02 allele (8). In addition, polymorphisms in loci for T-cell receptor-α (9) and P2RY11 (10) have been associated with narcolepsy. However, twin studies are mostly discordant, and environmental triggers likely play a role (1).
Notably, attempts to identify specific autoantibodies against Hcrt/Orx in serum from narcoleptic patients have mainly given negative results (11, 12). Streptococcal infections may be a triggering factor, and antibodies against streptolysin O are increased among narcolepsy patients (13).
Recently, TRIB-2 protein was identified as a putative target for autoantibodies in narcolepsy. It was detected in 14% of narcoleptic patients compared with 5% in healthy controls (14).
During 2009, 6 million individuals in Sweden and 2.8 million individuals in Finland were vaccinated with the influenza vaccine Pandemrix (Glaxo Smith Kline) to limit the spread of the influenza A(H1N1)pdm09 pandemic. In general, few adverse events and good tolerance were noted (15). However, several cases of narcolepsy in vaccinated individuals were encountered. In February of 2011, a 12.7-fold and 7.5-fold increased risk of narcolepsy in children and adolescents after Pandemrix vaccination was reported in Finland and Sweden, respectively (16). In Sweden the risk was recently adjusted to a threefold increase (17). In Finnish children and adolescents below the age of 17 y, a 17-fold incidence was observed (18). Notably, no link between other neurological diseases and vaccination has been found (17). In contrast, a study from China (where no vaccination against influenza occurred) showed a clear seasonal variation in narcolepsy incidence, which increased after the 2009 H1N1 pandemic (19). Thus, it is possible that not only vaccination but also, the influenza virus infection per se and other factors could trigger narcolepsy. There was, however, no serological evidence of H1N1 virus infection in Finnish children with H1N1 vaccine-associated narcolepsy (20).
Although vaccines rarely trigger autoimmunity, it has been reported to occur in susceptible individuals (21). Thus, we, like others, hypothesized that Pandemrix vaccination could induce autoantibodies against the Hcrt/Orx-producing neurons residing in the lateral hypothalamus. To test this hypothesis, sera from vaccinated and unvaccinated Finnish narcoleptic patients, patients diagnosed with other sleep-related disorders (OSRDs) by the Helsinki Sleep Clinic, VitalMed Research Centre (Helsinki), and healthy controls from the same center (HCs), as well 60 MCs (Finnish military recruits) were used to screen for autoantibodies using a previously developed immunohistochemical protocol (22). Several staining patterns were detected, and the potentially most interesting immunopositive structures were phenotyped using double-labeling procedures. Also, the autoantigen of one staining pattern was identified. Immunohistochemical results were compared with the clinical data of the patients as well as their H1N1 infection or Pandemrix vaccination status. Finally, purified IgGs derived from narcoleptic sera were injected intracerebroventricularly (icv.) to rats, and a detailed analysis of sleep architecture and EEG power spectrum was performed.
Results
Baseline Data.
The cohort (screening) consisted of 92 narcoleptic patients, 55 patients diagnosed with OSRD, 85 HCs (Helsinki Sleep Clinic) and 60 MCs (military) (Table S1). The OSRD group included patients with idiopathic hypersomnia (n = 4), other hypersomnia caused by, for example, delayed sleep-phase syndrome (n = 4), obstructive sleep apnea (n = 4), Kleine–Levin syndrome (n = 2), depressive disorder (n = 7), parasomnia (n = 7), postviral fatigue syndrome (n = 2), or others/unknown (n = 22; restless legs syndrome, behaviorally induced insufficient sleep, developmental disorders, or hypersomnia of unknown cause). Sera from 89 narcoleptic patients (81 patients with NC and Hcrt/Orx deficiency), 52 OSRD patients, 77 HCs, and all MCs (n = 60) were available for inclusion in the study. The OSRD patients differed from the narcoleptic cases mainly by lack of cataplexy; Hcrt/Orx levels > 110 pg/mL and longer sleep latency in the Multiple Sleep Latency Test (MSLT) test (Table S1). Data on vaccination status and the link between the vaccination and onset of narcolepsy are provided in Table S1. Notably, the medical history and serology revealed that very few of the included patients had experienced clinical infection with H1N1 virus or influenza-like illness during 2009–2010 (4–7% across the groups). Narcoleptic patients were diagnosed according to International Classification of Sleep Disorders (ICSD)-2 criteria (23).
Immunohistochemical Analyses.
Many sera resulted in discernible but variable staining patterns, often including glia, although some sera, in fact, did not give rise to any signal at all. However, we were able to select three distinct patterns, which were neuronal, robust, reproducible, and apparently interesting.
Neuropeptide-Glutamic Acid Isoleucine/α–Melanocyte-Stimulating Hormone Pattern (Pattern/Group A).
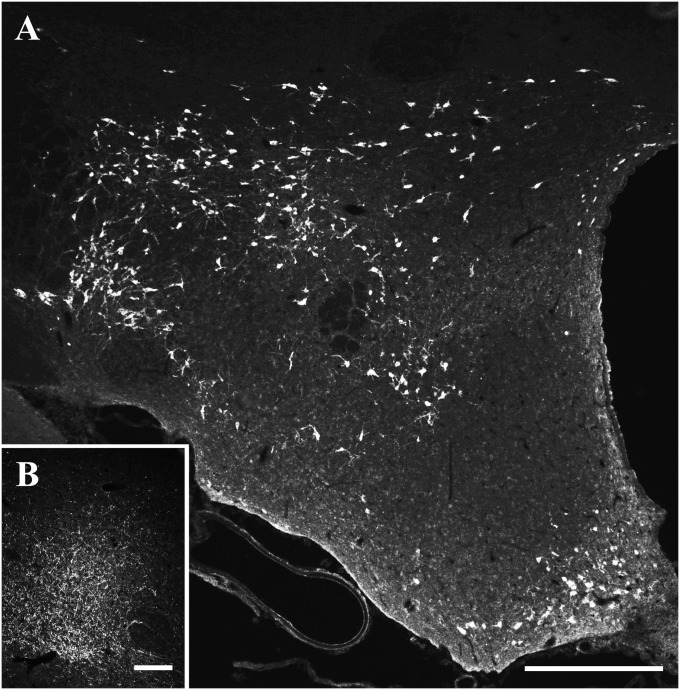
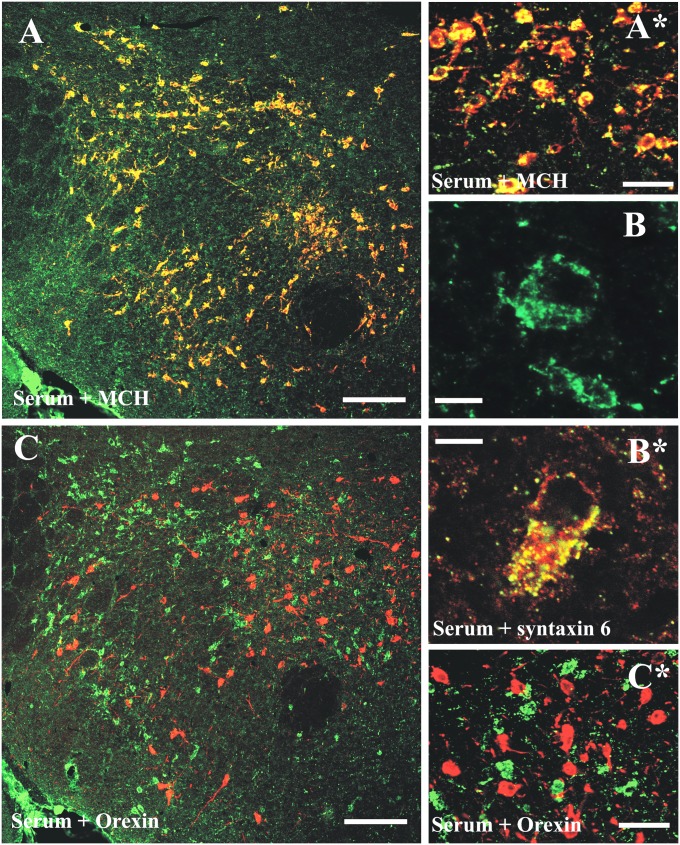
Altogether, 13 sera (6 sera from narcoleptic patients, 3 sera from OSRD cases, 2 HCs, and 2 MCs) (Table 1) displayed a highly selective and distinct immunostaining of cell bodies in the zona incerta-lateral hypothalamic region (ZI-LH) and the arcuate nucleus (Arc) exclusively after colchicine treatment in both rat (Fig. 1A) and mouse (Fig. S1D). The ZI-LH and Arc patterns always appeared together. High-power magnification confocal analysis revealed a dot-like subcellular staining pattern in the cell soma and occasionally, the dendrites of both ZI-LH and Arc neurons, which were highly colocalized with syntaxin-6, a Golgi apparatus subcellular marker (Fig. 2 B and B*). A second serum sample from the same group A patient obtained 28 mo after the first sample showed an identical pattern and staining intensity. A purified IgG preparation from the total serum (diluted 1:5,000 or 1:10,000; compare below) or a CSF sample (diluted 1:50) from the same patient exhibited the same pattern as the serum (Fig. 6 Q–S).
Table 1.
Staining patterns in relation to diagnosis
| Diagnosis | n | A | B | C | A + B + C |
| Narcolepsy | 89 | 6 (6.7%) | 15 (16.9%) | 3 (3.4%) | 24/89 (27.0%) |
| OSRD | 52 | 3* (5.8%) | 7† (13.5%) | 2‡ (3.8%) | 12/52 (22.5%) |
| HCs | 77 | 2 (2.6%) | 4 (5.2%) | 2 (2.6%) | 8/77 (10.4%) |
| MCs | 60 | 2 (3.3%) | 3 (5%) | 1 (1.7%) | 6/60 (10.0%) |
Four different groups were studied: narcolepsy (NCL) patients, patients with OSRD, HCs (Helsinki Sleep Clinic), and MCs (military). There was a significant increase of staining patterns A, B, and C in the narcolepsy group vs. the OSRD group combined with the two controls groups (NCL vs. OSRD + HC + MC; P = 0.015, Fisher test) and either group alone (NCL vs. OSRD vs. HC + MC; P = 0.0034, χ2 test). Likewise, staining patterns A and B were significantly more common in the narcolepsy group vs. the OSRD and control groups (NCL vs. OSRD vs. HC + MC; P = 0.0040, χ2 test). For each individual pattern, there was a significant increase for pattern B only (NCL vs. OSRD vs. HC + MC; P = 0.0136, χ2 test).
Group A: depressive disorder (n = 2) and hypersomnia (n = 1).
Group B: postviral fatigue, depression, parasomnia, hypersomnia, mild mental retardation, and two cases with unknown diagnosis (n = 7).
Group C: delayed sleep-phase disorder and parasomnia/depression (n = 2).
Fig. 1.
Demonstration of the NEI/αMSH staining pattern. (A) Low-power overview from a glycosidase predigested section of a colchicine-treated rat brain incubated with group A serum: a distinct neuron population is present in the ZI-LH region and Arc. (B) A dense fiber network but no cell bodies are seen in rats not treated with colchicine. (Scale bars: A, 500 µm; B, 200 µm.)
Fig. 2.
Characterization of the NEI/αMSH staining pattern in the ZI-LH region (group A). (A and A*) Fluorescent cells are identified as the MCH+ population. (C and C*) These cells are never immunoreactive for Hcrt/Orx. (B) High-power magnification reveals a dot-like subcellular pattern (100× objective, 1.5 digital zoom; merge of six Z-stack 0.5-µm-thick optical-layer micrographs). (B*) Serum staining was distinctly colocalized with syntaxin-6, a Golgi apparatus marker. Green channel, serum staining; red channel, MCH/Hcrt/syntaxin-6. (Scale bars: A and C, 200 µm; B and B*, 10 µm; A* and C*, 30 µm.)
Fig. 6.
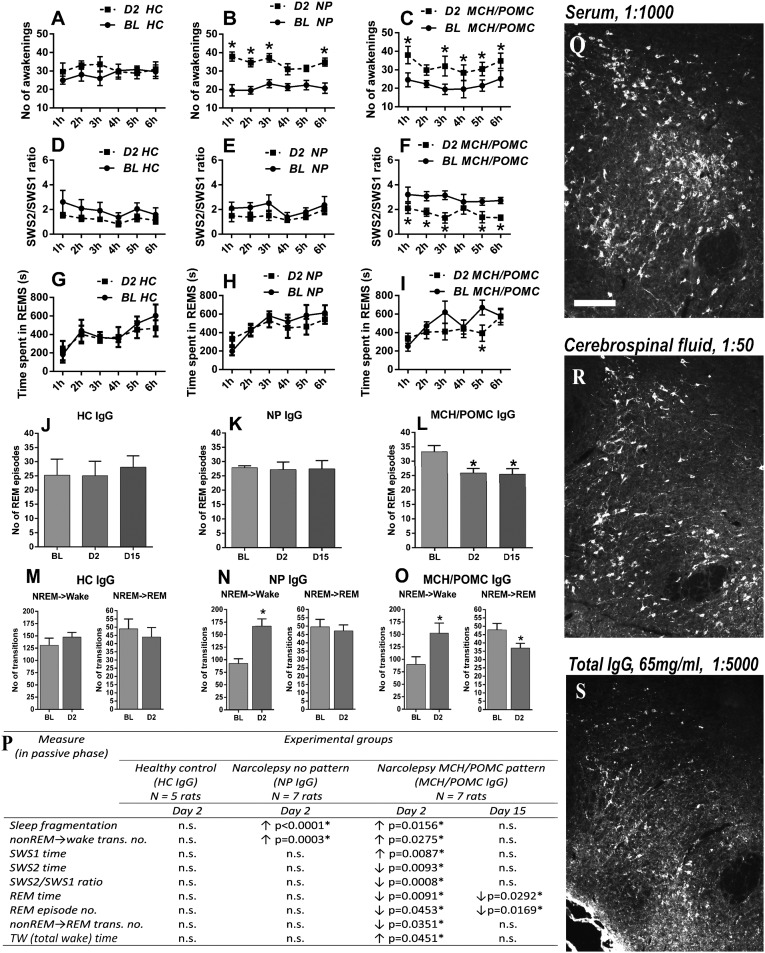
(A–I) Physiological effects of pattern A IgG on the sleep architecture. Graphical representation of different vigilance stages and sleep fragmentation [first 6 h, inactive (light) phase of D2] after icv. injection of IgG preparations (65 mg/mL) from (A, D, and G) an HC case (HC-IgG), (B, E, and H) a narcoleptic case with no staining pattern (NP-IgG), and (C, F, and I) a narcoleptic case with an staining pattern (NEI/αMSH-IgG) compared with the BL recordings. Sleep fragmentation is increased in the cases of both narcoleptic IgG preparations (B and C vs. A). The SWS2/SWS1 ratio and the time spent in REM are selectively decreased only in the case of the NEI/αMSH-IgG (F vs. D and E and I vs. G and H; SWS2/SWS1 and time spent in REM, respectively). Statistical analysis: two-way repeated measure ANOVA matched by pairs (repeated factor: hours 1–6); each group was compared with its own BL. *P < 0.05, Bonferroni multiple comparisons posthoc test for every 1 h. (J–L) Graphical representation of the number of REM episodes during the first 6 h of the inactive phase (D2 and D15). The number of REM episodes significantly decreased only in the NEI/αMSH-IgG group at both time points (L vs. J and K). Statistical analysis: one-way repeated measure ANOVA. *P < 0.05, Tukey posthoc test. (M–O) Graphical representation of the numbers of NREM → wake and NREM → REM transitions (first 6 h, inactive phase, and D2) after the injection (N and O vs. M). The number of NREM → wake transitions increased with both narcoleptic sera, whereas the number of NREM → REM decreased selectively with the NEI/αMSH pattern IgG (O vs. M and N). Statistical analysis: one-way repeated measure ANOVA. *P < 0.05, Tukey posthoc test. (P) Summary of the statistically significant alterations in sleep architecture compared with BL recordings. *Significant results of two-way repeated measure ANOVA or one-way repeated measure ANOVA. In the HC and NP groups, no significant effects were observed at D15 (not shown). n.s., not significant. (Q–S) Representative micrographs of the LH (colchicine-treated rat) stained with (Q) serum, (R) CSF, or (S) IgG preparation from the same group A patient all showing the same NEI/αMSH. (Scale bar: Q–S, 200 µm.)
The serum+ neurons in the ZI-LH were identified as the MCH+ cell population (Fig. 2 A and A*), and quantitative confocal microscopic analysis showed that all MCH+ cells were serum+ and vice versa: of 536 neurons in the ZI, perifornical, and LH subregions from different rostrocaudal levels, 100% of serum+ cells were MCH+. In contrast, Hcrt/Orx-immunoreactive neurons were never serum+ (Fig. 2 C and C*). In agreement with the MCH colocalization pattern, many but not all serum+ cells were also cocaine- and amphetamine-regulated transcript (CART) -immunoreactive. Most of the nesfatin+ and glutamic acid decarboxylase 67+ (GAD67+) neurons were serum+ in the ZI-LH region. A subset of serum-immunoreactive neurons was also positive for acetylcholine esterase, but no colocalization was found with dynorphin (Fig. S2).
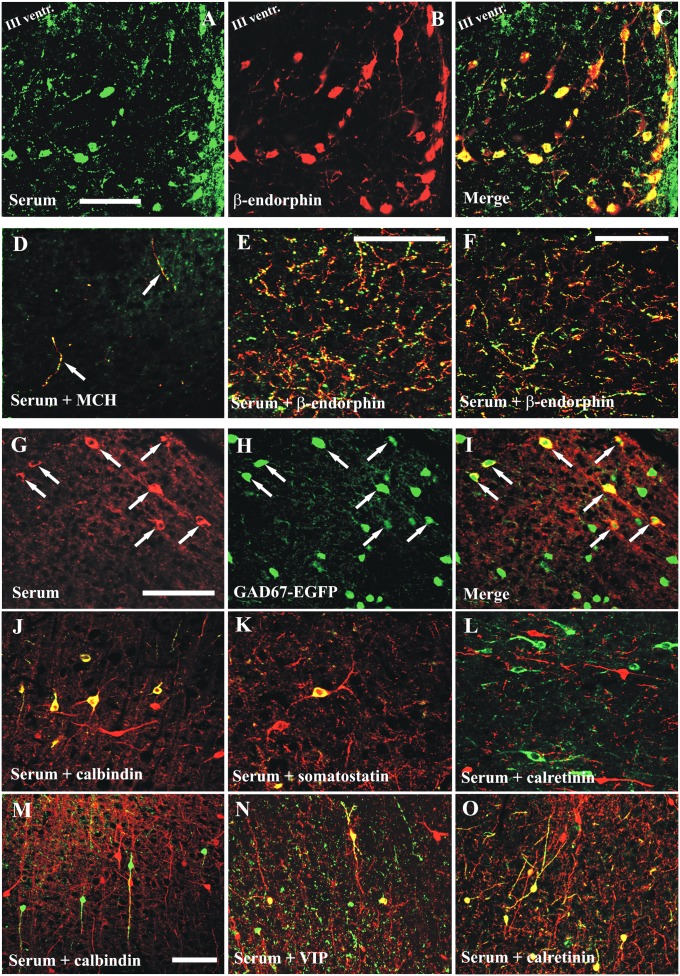
The serum+ neurons in Arc were identified as the proopiomelanocortin (POMC) (β-endorphin+) cell population (Fig. 3 A–C). A quantitative confocal analysis showed that the vast majority of serum+ Arc neurons was also β-endorphin–immunoreactive. We examined 285 neurons at different rostrocaudal levels of Arc: 93% were labeled for β-endorphin and serum, 6% were double-labeled only for β-endorphin, and 1% was labeled only for serum+. A subset of CART-, acetylcholinesterase-, and nesfatin-immunoreactive Arc neurons colocalized with the pattern A sera. However, no colocalization was found with neuropeptide tyrosine (NPY), somatostatin, tyrosine hydroxylase, choline-acetyltransferase (ChAT), or galanin (Fig. S3).
Fig. 3.
Characterization of the NEI/αMSH staining pattern in the Arc (group A) and the cortical interneuron staining pattern (group B). (A–C) Serum+ cells are identical to the POMC-immunoreactive Arc neurons. (D) Only a few serum+ cortical fibers are double stained for MCH (insular cortex). The dense serum+ fiber plexus in the (E) paraventricular thalamic nucleus and (F) ventrolateral/lateral periaqueductal gray strongly colocalizes β-endorphin. (G–I) All pattern B serum+ interneurons express GAD67-EGFP; (J–L) most but not all pattern B interneurons (parietal cortex) are positive for (J) calbindin but never (L) calretinin, and (K) a few are somatostatin+. (M–O) In one group B case (insular cortex), however, nearly all serum+ interneurons are (O) calretinin+ and (N) occasionally, vasoactive intestinal polypeptide+ (VIP+) but never (M) calbindin+. Green channel: (A, C, D–F, and J–O) serum, (H and I) GAD67-EGFP; red channel: (G and I) serum, (B, C, E, and F) β-endorphin, (D) MCH, (J and M) calbindin, (K) somatostatin, (L and O) calretinin, or (N) VIP. (Scale bars: 100 μm.)
In many regions, pattern A serum+ nerve terminals were observed, mostly of the varicose type. These plexuses were seen both with and without colchicine, although it was often more prominent without this treatment (Fig. 3 D–F and Figs. S4 and S5).
To test the possibility that pattern A sera stained other than LH MCH and Arc POMC cell populations/fibers in the rodent brain, we performed immunostaining at numerous rostrocaudal levels of one colchicine-treated brain and one control brain from the olfactory bulb to the spinal cord. No stained cell bodies were found.
Interneuron Pattern (Pattern/Group B).
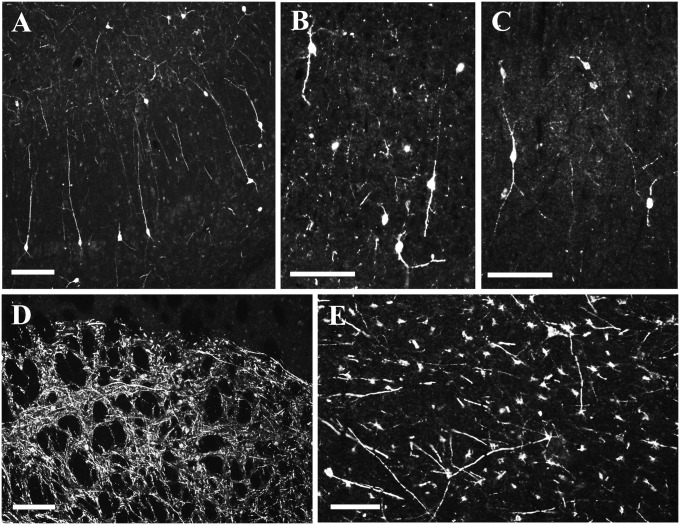
In total, 29 sera (15 sera from narcoleptic patients, 7 sera from OSRD cases, 4 sera from HCs, and 3 sera from MCs) exhibited bipolar and multipolar interneurons in the hippocampus and neocortex. These neurons were surrounded by varicose processes and found throughout the hippocampus (Fig. 4A) and neocortex (Fig. 4B) at all examined rostrocaudal levels. They also appeared without colchicine treatment, although the number of serum+ neurons was higher after the drug. CSF from the same group B patient (1:100 dilution) exhibited the same staining as the serum (Fig. 4C). These neurons were all GAD67-EGFP+ (Fig. 3 G–I), and in most group B cases showed a partial overlap with the calbindin+ cortical interneuron population (Fig. 3J and Fig. S6). A few serum+ cortical neurons were also somatostatin-immunoreactive (Fig. 3K and Fig. S6). The pattern B neurons were always negative for parvalbumin (PV) (Fig. S7A), brain NOS (Fig. S7B), NPY, and cholecystokinin in the examined cortical areas. In many but not all cases, cortical astroglial cells were moderately serum+, especially in the entorhinal/piriform region (colocalized with GFAP but not Iba1) (Fig. S7 F–H). In one exceptional group B case, however, nearly all serum+ cortical interneurons were positive for calretinin (Fig. 3O and Fig. S8 A–C), and they were occasionally VIP+ (Fig. 3N and Fig. S8 D–F); some were positive for GAD67 (Fig. S8 G–I) and some for ChAT (Fig. S8 J–L), but calbindin+ neurons were not detected (Fig. 3M).
Fig. 4.
Characterization of patterns B and C neurons. Cortical bi- and multipolar interneurons are seen in (A) the hippocampus and (B) parietal cortex after incubation with group B serum, and (C) in the parietal cortex after incubation with group B CSF. Multipolar neurons with dendritic processes and axons are stained in the (D) globus pallidus and (E) piriform cortex after incubation with group C serum. (Scale bars: A–C and E, 100 µm; D, 200 µm.)
Globus Pallidus Pattern (Pattern/Group C).
The third group of sera (from three narcoleptic cases, two OSRD cases, two HCs, and one MC) stained cell bodies, dendritic processes, and probable axons, particularly in the globus pallidus (Fig. 4D), amygdala, and piriform cortex (Fig. 4E). No nerve terminals were encountered. This pattern also appeared without colchicine treatment.
Other Patterns.
Only sera with clear, distinct, and reproducible staining patterns, defined above, were accepted as group A (Fig. S1 A and E–H), B (Fig. S7 C–E), or C (Fig. 4 D and E) cases. Importantly, no cases with a mix of two or three of these patterns were found. In addition to A, B, and C patterns, staining of apical dendrites of cortical pyramidal neurons, ependymal cells, tanycytes, nuclei (ranging from weak staining in some regions to a strong fluorescence in virtually all cells in the brain), glial cells, or sparse fibers was also noted with several sera, irrespective of their source (narcoleptic/vaccinated or nonnarcoleptic). These staining patterns will not be further dealt with here.
Clinical Data Vs. Staining Patterns.
Next, the immunostaining patterns were compared to the clinical presentation of patients and controls in the study. Nine clinical parameters were studied [body mass index, vaccination status, Ullanlinna Narcolepsy Scale (24), Epworth Sleepiness Scale, numbers of cataplectic attacks per week, number of sleep-onset rapid eye movement (REM) periods (SOREMP), MSLT, Hcrt/Orx levels in CSF, and aggressive behavior near the disease onset]. Regarding most parameters, individuals whose sera produced A, B, or C patterns were not different from narcolepsy cases without these patterns. However, notably, the two cases with the highest numbers of cataplectic attacks were found in group A (Fig. S9E).
Staining Among Nonnarcoleptic Subjects.
Pattern A was found in one patient with depression and aggressive behavior (mean sleep latency of 10.7 min MSLT and three SOREMPs), one patient with hypnagogic hallucinations, sleep paralyses, fragmented sleep, and depression for over 7 y, and one patient with depression. Also, pattern A was found in two healthy sisters of narcoleptic patients and two apparently healthy MCs. Pattern B was found in a parent of a narcoleptic child and some additional controls (n = 4 HCs and n = 3 MCs), as well as in one subject with depression, one subject with developmental disorder associated with aggressive behavior (no ataxia and normal hearing), one subject with parasomnia caused by bruxism, one subject with postviral fatigue syndrome (now in remission), and one individual with hypersomnia caused by insufficient sleep. Pattern C was found in two OSRD patients (delayed sleep-phase disorder and parasomnia/depression), two HCs, and one MC (Table 1).
Identification of the Antigen(s).
The pattern A (staining of MCH and POMC neurons in the hypothalamus) was initially considered to be potentially the most interesting one, and attempts were made to identify its target antigen(s)/epitope(s).
First, we aimed to characterize the biochemical nature of the putative autoantigen. Thus, sections were enzymatically treated with a glycosidase mixture or proteinase K. The immunostaining pattern was not abolished by a 6- or 24-h glycosidase treatment. In fact, the staining was even more distinct after this digestion (compare Fig. S1A with Fig. S1C). In contrast, a 20-min proteinase K treatment (1 or 2 µg/mL at 37 °C) clearly reduced the staining intensity (compare Fig. S1A with Fig. S1B).
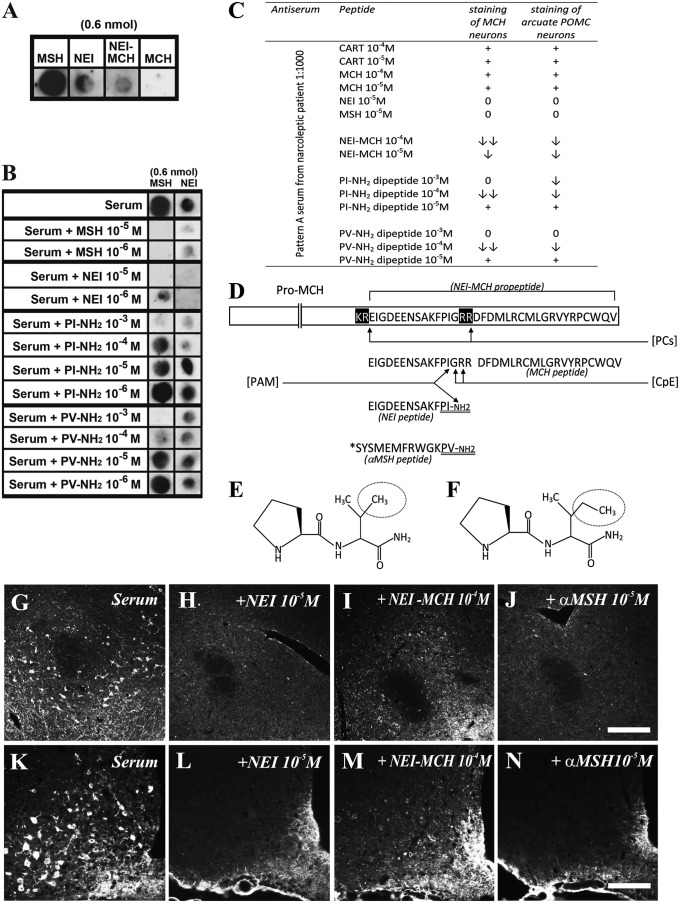
Second, we performed multiple absorption experiments on parallel immunohistochemical slides and dot blot analysis in parallel. Pattern A sera (1:5,000) did not recognize the MCH peptide in a dot blot analysis, and preincubation of the diluted serum (1:1,000) with MCH (or CART) peptides (at 10−5 and 10−4 M concentrations) did not alter the serum immunostaining intensities (Fig. 5 A and C). Pattern A sera, however, recognized α–melanocyte-stimulating hormone (αMSH), neuropeptide glutamic acid-isoleucine (NEI), and less intensely, also NEI-MCH (preproMCH [131–165]) in the dot blot analysis (Fig. 5A). Importantly, preincubation of the diluted serum with αMSH and NEI peptides (10−5 M) completely abolished the serum immunostaining in both the arcuate POMC neurons and the MCH neurons in LH and ZI (Fig. 5 J and N and H and L, respectively). In accordance, preincubation of serum with NEI or αMSH peptides concentration-dependently reduced or abolished the recognition of 0.6 nmol NEI or αMSH peptides in the dot blot analysis (Fig. 5B). Likewise, preincubation of serum with NEI-MCH propeptide substantially reduced (but did not completely abolish) the immunohistochemical staining at 10−4 M in both MCH and POMC neurons (Fig. 5 I and M).
Fig. 5.
Characterization of the immunogenic peptides. Pattern A IgGs recognize a common epitope in the C terminus of αMSH and NEI. (A) Pattern A serum (1:5,000) recognizes αMSH- and NEI-matured peptides (0.6 nmol) and less strongly, NEI-MCH peptide (0.6 nmol; preproMCH [131–165]) but not the matured MCH peptide in the dot blot test. (B) Preincubation of pattern A serum (1:5,000) with 10−5 or 10−6 M αMSH or NEI peptides distinctly attenuates or completely abolishes the binding of serum to NEI or αMSH peptide (0.6 nmol). Preincubation of the same pattern A serum with 10−6–10−3M PV-NH2 or PI-NH2 dipeptides concentration-dependently decreases the binding of serum to NEI or αMSH peptide (0.6 nmol). (C) Summary of adsorption experiments: +, staining intensity does not change; ↓, staining intensity is decreased; ↓↓, staining intensity is highly decreased; 0, staining is completely abolished. (D) Schematic presentation of proMCH and the enzymatic processes leading to formation of matured NEI and MCH peptide. Prohormone-convertase (PC) first cleaves on the C-terminal side of the KR and RR sequences. Carboxypeptidase-C (CpE) removes the RR dibasic extensions. Then, peptidylglycine-α-amidating-monooxygenase (PAM) generates an amide group of G and the C terminus of I in the NEI peptide. (Amidated NEI peptide is the active form in the brain.) Note that the C terminus of αMSH peptide contains an amidated PV C-terminal motif (double underlined). *Acetylation site on the αMSH peptide. Modified from ref. 74. (E and F) Note the high structural similarities between (E) PV-NH2 and (F) PI-NH2 dipeptides (the difference is only a methylene group; circled). (G–N) Representative micrographs of adsorption experiments showing (G–J) the perifornical region (MCH neurons) and (K–N) Arc (POMC neurons). Note that NEI and αMSH peptides at 10−5 M completely abolish the immunostaining, whereas NEI-MCH peptide at 10−4 M concentration distinctly decreases the staining. (Scale bars: G–J, 200 µm; K–N, 100 µm.)
Because the mature αMSH and NEI peptides contain a nearly identical (amidated) dipeptide epitope in their C terminal [proline-valine (PV)-NH2 and proline-isoleucine (PI) -NH2, respectively] (Fig. 5 D–F), we tried to block the pattern A staining with these dipeptide motifs. Preincubation of serum with amidated dipeptides concentration-dependently reduced or abolished the immunostaining on slides and the staining of 0.6 nmol NEI or αMSH peptides on dot blot. Remarkably, PV-NH2 dipeptide was more effective at blocking the recognition of αMSH, and PI-NH2 was more effective at blocking the recognition of NEI by the pattern A serum on dot blot (Fig. 5 B and C).
Sleep Experiments: Effects of Centrally Administered Pattern A IgGs.
After a 24-h baseline (BL) recording, Wistar rats were injected icv. with total IgG preparations from a HC individual (HC-IgG, n = 5 rats), a narcoleptic patient without any immunostaining pattern [no pattern (NP); NP-IgG, n = 7 rats], and a narcoleptic patient with pattern A immunostaining (NEI/αMSH-IgG, n = 7 rats). On day 2 (D2) and D15 after injections, sleep architecture and spectral distribution of EEG power were evaluated in the first 6 h of both inactive (light) and active (dark) phases.
Several alterations in the sleep architecture were found in the inactive phase but not in the active phase. We noted that injection with both narcolepsy IgGs (with or without NEI/αMSH pattern) resulted in a strong increase in sleep fragmentation (number of awakenings). Furthermore and in accordance with fragmented sleep, the number of non-REM (NREM) sleep to wake transitions was also increased in both narcolepsy IgG groups but not in the HC-IgG on D2 (sleep fragmentation) (Fig. 6 A–C): F(1,6) = 243.7, P < 0.0001 and F(1,6) = 11.16, P = 0.0156 for NP IgG and NEI/αMSH-IgG, respectively, compared with BL recordings. NREM to wake transitions (Fig. 6 M–O): F(1.42,8.523) = 34.11, P = 0.002 and F(1.091,4.365) = 11.55, P = 0.023 for NP-IgG and NEI/αMSH-IgG, respectively, compared with BL recordings. Notably, several alterations occurred selectively in case of the NEI/αMSH-IgG but not with the NP-IgGs or HC-IgGs. (i) The number of NREM → REM sleep transitions decreased significantly only in the NEI/αMSH-IgG–treated group on D2 (Fig. 6 M–O) [P = 0.0224, F(1.993,11.96) = 5.313]. (ii) Regarding the NREM sleep, the time spent in light slow-wave sleep (SWS1) increased and the time spent in deep SWS (SWS2) decreased significantly only in the NEI/αMSH-IgG group on D2 compared with BL [F(1,6) = 14.64, P = 0.0087 and F(1,6) = 14.22, P = 0.0093, respectively). Consequently, the ratio of SWS2 to SWS1 decreased significantly [F(1,6) = 38.76, P = 0.0008] and selectively on this day in the NEI/αMSH-IgG–treated group (Fig. 6 D–F). These latter effects were not detectable on D15. (iii) The amount of REM selectively diminished in case of the NEI/αMSH-IgG group on D2 compared with BL, and this effect was still significant on D15 (Fig. 6 G–I) [F(1,6) = 14.32, P = 0.0091 and F(1,6) = 8.115, P = 0.0292 on D2 and D15, respectively). (iv) The number of REM episodes (Fig. 6 J–L) showed a significant decrease on both D2 and D15 after the injection (Fig. 6 J–L) [one-way ANOVA: F(1.9,11.4) = 7.75, P = 0.008]. SOREMPs typically occur in narcolepsy but were not observed in any of the sera-treated groups, and the total time spent in SWS was unaffected.
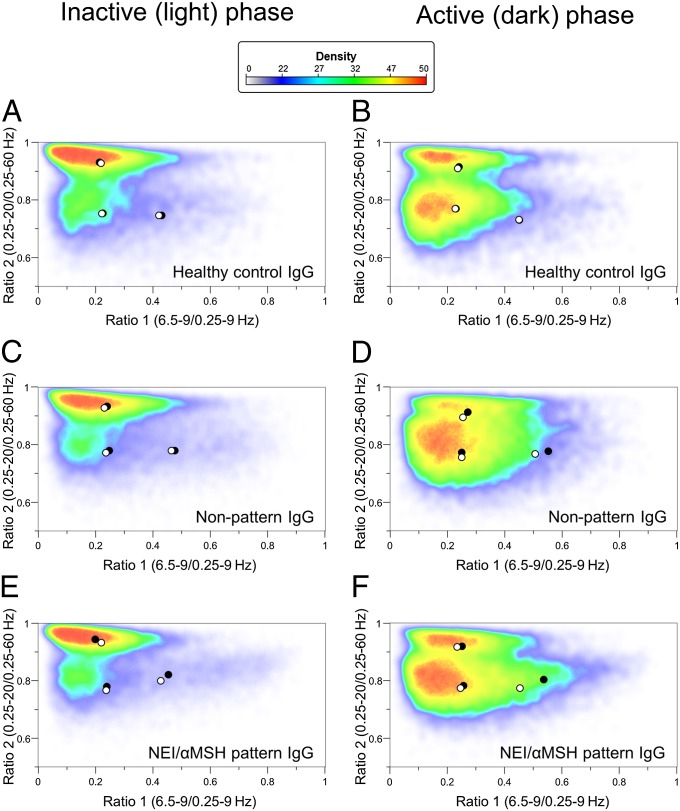
Next, the spectral distribution of EEG power of different vigilance stages in the first 6 h of inactive and active phases was evaluated on D2 by adapting the state space analysis technique (modified from ref. 25). The EEG power values were plotted as ratio 1 (6.5–9/0.25–9 Hz) on the abscissa and ratio 2 (0.25–20/0.25–60 Hz) on the ordinate. This depiction defines 2D spectra with three separate clusters, which correspond to conventionally determined vigilance stages: namely REM, NREM, and wake. To visualize the dispersion but also, the density of power data of clusters, a heat map with centroids was created (Fig. 7). The general positions of state space clusters associated with REM, NREM, and wake stages seemed to be conserved in the BL recordings. In the active phase on D2, the NEI/αMSH-IgG group revealed a significant decrease in the centroid distance of REM to wake by the shift of the REM centroid toward the wake centroid compared with BL (Fig. 7F) (P = 0.0035). This difference did not reach statistical significance in the inactive (light) phase. In the case of HC- and NP-IgG groups, no significant alteration in the distance of centroids, compared with BL, between any of the vigilance stages was detected (Fig. 7 A–D).
Fig. 7.
Effect of icv.-injected IgG from (A and B) an HC, from (C and D) an NC without the NEI/αMSH pattern, and (E and F) an NC with the NEI/αMSH pattern on the distribution and density of EEG power on a 2D-state space heat map in passive and active phases. Plotting the spectral ratios of EEG power data (ratio 1 on the x axis and ratio 2 on the y axis) separated three distinct clusters of EEG power points: right, REM sleep; upper left, NREM sleep; lower left, wake. Each plot represents EEG power data (including all animals per groups) of 6-h recordings on color-coded density maps (digits on the scale show the number of overlapping epochs on a given area). Centroids of different sleep stages are indicated by circles (black, BL; white, day 2). Noteworthy, REM point of NEI/αMSH group shifted toward wake in the active (dark) phase on day 2 vs. BL. The method is modified from ref. 25.
Discussion
The results show that more than one-quarter of narcolepsy patients (27.0%) harbor antibodies, presumably autoantibodies, that, when applied to sections from normal and/or colchicine-treated rat and mouse brains, exhibit characteristic and reproducible staining patterns for subpopulations of neurons. Notably, all of the three described patterns were not exclusive for narcolepsy but also detected, albeit in lower numbers, in nonnarcoleptic patients with a diagnosed sleep disturbance and some control individuals. The pattern A (immunostaining of MCH and POMC neurons) seemed most relevant in relation to narcolepsy, and we, therefore, described its neuroanatomical distribution in detail, identified the antigen targets of the presumptive autoantibodies, and then, explored functional aspects by injecting purified IgGs icv. into rats and monitored sleep behavior.
Pattern A.
A hallmark of narcolepsy is the loss of Hcrt/Orx neurons in the LH and low Hcrt/Orx levels in CSF (6, 7). However, we and others failed to detect any antibody binding to Hcrt/Orx neurons. In contrast, the MCH neurons, partly intermingling with the Hcrt/Orx neurons, were stained with sera from six narcoleptic patients, three OSRD individuals (with bipolar/depressive disorder and sleep problems), two HCs (in fact, they were close relatives to the narcoleptic patients), and two MCs. MCH cells have a structural/functional connection with Hcrt/Orx neurons, and an increasing amount of data emphasizes the role of these neurons in the regulation of sleep processes (26–31). Thus, our findings are potentially important, even if the MCH system seems to be intact in narcoleptic patients (6, 32).
POMC arcuate cells, which are virtually always serum+ with pattern A sera, are involved in the control of food intake (33). They innervate MCH neurons, forming a functional system for the regulation of energy homeostasis (i.e., mediating leptin’s action in the hypothalamus) (34). Narcolepsy is often associated with metabolic problems, particularly a high risk for obesity (1, 35). Importantly, other than the MCH and POMC neurons and terminals, no additional pattern A cell bodies or nerve terminals were detected in the entire rat brain.
Identification of the Pattern A Autoantigen.
A series of adsorption experiments were performed by using dot blot analysis and immunohistochemical techniques. Both αMSH and NEI (10−5 M) (36) but not the MCH peptide completely blocked the pattern A signal in both MCH neurons and POMC cells. Also, the amidated C-terminal dipeptide sequence (PV-NH2 and PI-NH2), present in αMSH and NEI peptides (37–39), reduced or blocked the NEI/αMSH pattern, albeit at higher concentrations (10−4 and 10−3 M). These results indicate that pattern A IgGs selectively recognize a common (amidated) dipetide motif on the C terminus of αMSH and NEI peptides. The fact that the signal is exclusively visible after colchicine treatment, which inhibits axonal transport and causes accumulation of proteins in the neuron soma, supports the notion that the targets of pattern A serum are axonally transported, perhaps even secreted peptide(s).
Autoantibodies against neuropeptides have been described previously in relation to, for example, depressive disorder (NPY) or anorexia/bulimia [αMSH and luteinizing hormone-releasing hormone (LHRH)] (22, 40). Also, a recent study on invertebrates reported that affinity-purified polyclonal antibodies against conserved amidated C-terminal dipeptide motifs of neuropeptides can selectively label distinct neuronal populations and their projections in a broad range of species (41). In our study, pattern A immunostaining can be strongly reduced also by the MCH-NEI propeptide at a higher concentration (10−4 M). This propeptide contains a P-I-G motif inside the sequence, which suggests that the C-terminal amidation significantly enhances the epitope recognition of pattern A IgGs. However, they can bind, although with less affinity, without amidation of the dipeptides but in the presence of an adjacent G (nonamidated PV/PI dipeptides are completely ineffective to block even at 10−3 M). Removal of sugar residues with glycosidase digestion increased the intensity of the pattern A staining, indicating that glycosylation partly masks the binding epitope. This result is in agreement with previous findings that glycosylation is an important posttranslational modification of neuropeptides, especially with regard to POMC derivatives (42).
Physiological Effects of the NEI/αMSH-IgG Preparation.
To reveal the potential effect of pattern A autoantibodies on sleep, we performed passive transfer experiments in rats and analyzed EEG and behavior. The dose of injected IgGs was determined based on previous publications on passive transfer experiments (43, 44). Although the number of sera that were used for IgG preparation was limited, we found consistent results and some statistically significant alterations.
Purified IgGs from both narcoleptic patients but not from the control induced a strong increase in sleep fragmentation in the inactive phase, an important symptom of narcolepsy. However, no other narcolepsy symptoms were found (i.e., our passive transfer experiment could not induce narcolepsy) (1, 45). It should be noted, however, that the changes were obtained after a single injection. Thus, acute silencing/functional modification of certain neuronal populations induced by experimental manipulations is fundamentally different from chronic pathological conditions (like narcolepsy) or even genetic Hcrt/Orx deletion (46, 47), where substantial reorganization of the complex downstream circuitry controlling sleep and wakefulness may occur (48). It would, therefore, be interesting to administer IgG for a longer period through an osmotic pump.
The IgG prepared from a pattern A serum (NEI/αMSH-IgG), however, induced several selective and significant effects, affecting both NREM and REM in the inactive phase (more superficial SWS, prolonged decrease of REM time and REM episode number, significant even 15 d after injection, and a decreased number of NREM to REM transitions). NEI/αMSH-IgG also induced alterations in the active phase, but here, interestingly, in the EEG θ-power density REM sleep dominates in rodents, and not the time spent in different vigilance stages: it revealed a significant shift from the higher toward lower θ-frequencies on D2. This change in θ-power of REM suggests alteration in REM sleep regulation in the active phase and emphasizes that NEI/αMSH-IgG influences the active and inactive phases of sleep in different ways. Notably, as reported in a recent paper, optogenetical silencing of MCH neurons decreased the peak frequency of θ-power in mice (49).
Despite the moderate magnitude of these effects, they were all statistically significant and found only with the NEI/αMSH-IgG, suggesting a specific effect of pattern A IgG-type autoantibodies. However, the strong sleep fragmentation effect of both narcoleptic IgGs is probably linked to some other IgG-type antibodies that cannot be detected by our technique.
As we discussed earlier, pattern A sera selectively recognize a common dipeptide motif on the C terminus of NEI and αMSH peptides and also recognize, albeit with less efficiency, the NEI-MCH propeptide containing the same motif inside its sequence (there is a 100% homology in the entire mature NEI peptide sequence between human/rat/mouse; the same is found in the case of the αMSH peptide). To our knowledge, there are no data available on the potential effect of the NEI peptide on sleep; i.p.- or icv.-administered (desacetylated) αMSH was found to be mildly hypnogenic (increased SWS) in rats (50, 51), although another study showed a prolonged sleep latency after icv. administration of acetylated αMSH (52). It has been shown that NEI-MCH propeptide (preproMCH [131–165]) represents a functional superagonist of the mature MCH peptide, because it is much more resistant to proteolytic digestion (53). However, NEI-MCH was detected mostly in peripheral organs, whereas the matured MCH and NEI were found to be the main forms in the CNS, at least during resting conditions (54). It is still an open question whether NEI-MCH could be expressed in the brains in response to specific stimuli (53) (e.g., during autoantibody challenge), in which case pattern A autoantibodies indeed could directly antagonize MCH effects. Remarkably, our selective effects of NEI/αMSH-IgGs on sleep pattern highly resemble the result of MCH1 receptor antagonist administration to rats (55). Optogenetic stimulation of MCH cells increases the total time of NREM and REM sleep (56), and optogenetic silencing of Hcrt/Orx cells induces SWS sleep in the inactive phase (48). Moreover, selective pharmacogenic excitation of Hcrt/Orx neurons increased wakefulness and decreased both SWS and REM sleep (57).
A second sample from a group A patient, collected 28 mo after the first sampling, exhibited the NEI/αMSH immunostaining pattern with the same intensity, which suggests that a long-lasting pool of memory B cells is involved. Taken together, it may be speculated that the well-established mutual functional connection of MCH and Hcrt/Orx neurons (31, 58) may be disturbed by the chronic availability of NEI/αMSH autoantibodies in narcoleptic patients. These autoantibodies may derange or destabilize the fine regulation of sleep–wake processes (59) in genetically vulnerable individuals and among other factors, contribute to the disease. Interestingly, MCH plus Hcrt/Orx double KO mice have much more fragmented sleep and more cataplexy attacks than Hcrt/Orx single KOs (60).
Pattern B.
These narcoleptic sera selectively stained a subset of GABAergic hippocampal and neocortical interneurons, a staining only marginally influenced by colchicine. These neurons were mostly calbindin+ but in one case, calretinin+. However, none expressed parvalbumin or NOS. Recently, a subpopulation of sleep-active cortical neurons, positive for NOS and calretinin, was identified in the mouse brains (61). Importantly, Pasumarthi et al. (62) have pointed to a surprising degree of interspecies variation in colocalization of NOS with calcium-binding proteins (62, 63). Pasumarthi et al. (62) also showed that the percentage of Fos+/calbindin but not Fos+/parvalbumin cortical neurons was significantly higher after a 6-h sleep deprivation than after a rebound sleep. Because pattern B sera sometimes also stain astroglial cells, multiple targets probably exist; however, the target antigen of pattern B sera is currently unknown. Importantly, these presumptive autoantibodies also reach the brain tissue, because pattern B CSF shows the same staining (at 1:100 dilution) as the serum itself.
Pattern C.
This distinct colchicine-independent pattern involves neuronal cell bodies with prominent proximal dendrites located in the globus pallidus, ventral cortices, and amygdala nuclei. Because recent reports emphasize a role of basal ganglia and the dorsostriato-pallido-cortical loop in the regulation of sleep–wake behavior and cortical activation (64), it raises the possibility that pattern C might also have physiological effects on sleep. For example, rats with globus pallidus lesions exhibited profound insomnia and pronounced sleep fragmentation (65). In fact, the latter effect is comparable with the functional consequence of the lesion of ventrolateral preoptic nucleus, a well-established sleep-promoting center (66, 67).
Staining Patterns: Clinical Symptoms and Vaccination Status.
Despite a careful analysis of nine clinical parameters relevant to narcolepsy, a general relationship between clinical data and certain autoantibody staining patterns could not be established. Thus, narcolepsy patients with these staining patterns do not exhibit a worse clinical profile than those without it. However, the two narcolepsy cases with the by far highest numbers of cataplexy attacks involved in this study were found in group A.
Another issue is the relation to vaccination with Pandemrix (68). However, narcolepsy patients with staining patterns A–C were not vaccinated more frequently than patients without discernible staining patterns. Thus, there was no apparent link between vaccination and a distinct staining pattern. Of the analyzed narcolepsy patients, about 40% (33 of 86) had an onset before the pandemic 2009/2010 influenza epidemic.
Conclusions
More than one-quarter (27%) of narcoleptic sera stained neurons in the LH-Arc (pattern A), cortex (interneurons; pattern B), or globus pallidus (pattern C), supporting the view that narcolepsy has an autoimmune component.
In contrast to our hypothesis, only MCH and not Hcrt/Orx neurons were stained in the LH. This finding could be of significance, because the MCH neurons connect to and regulate Hcrt-/Orx-ergic neurons and are involved in sleep regulation. The antigen of pattern A autoantibodies was identified as the common C-terminal epitope of NEI/αMSH peptides, and passive transfer experiments showed moderate but significant effects of pattern A IgG on REM and SWS sleep time parameters in the inactive phase and EEG θ-power in the active phase. Pattern A IgGs seem to be present in the brain, because CSF exhibits the same staining as serum. Thus, circulating autoantibodies can reach secreted molecules in the brain, including neuropeptides. The influx of antibodies into the brain seems to preferentially occur during inflammatory conditions, which have been shown in a model for systemic lupus and autoantibody-mediated psychotic conditions (69).
Additional studies are required to identify the target antigens of the B and C staining patterns, which are also potentially interesting, because the stained brain regions/neuronal populations are relevant in the regulation of sleep and arousal.
In summary, we propose that NEI/αMSH is a previously unidentified sleep-related autoantigen, which in parallel with TRIB-2 (14), is present in a subgroup of narcolepsy patients as well as patients with OSRDs. Our findings may form a platform for future studies on the role of autoantibodies in narcolepsy as well as OSRDs.
Materials and Methods
Clinical Evaluation of Subjects.
Narcolepsy was diagnosed according to ICSD-2 criteria (23) based on full-night polysomnography after MSLT (unmedicated). Available medical history was collected. Subjects went through a clinical interview and thorough neurological examination. HLA typing was done. Lumbar puncture was done to measure CSF Hcrt/Orx levels whenever appropriate and needed for diagnosis. CSF Hcrt/Orx analyses were made at the Rinnekoti Research Laboratory using RIA assay (Phoenix Pharmaceuticals) with Stanford reference sample.
Human Sera/CSF Samples.
Sera from patients with narcolepsy or OSRD and HCs were obtained with ethical permission and written informed consent from subjects and in case of underaged subjects, their parents. The ethical permission was obtained from the coordinating ethical board of Hospital District of Helsinki and Uusimaa. CSF samples from two patients (one patient with pattern A immunostaining and one patient with pattern B immunostaining) were applied.
Animals, Fixation, and Cutting.
The morphological experiments were performed on male Wistar rats and male C57BL/6 mice plus two GAD67-EGFP GAD-GFP knockin mice (70) and two ChAT-(BAC)-EGFP mice (71). For the physiological (EEG) experiments at the Semmelweis University, male Wistar rats were used. All procedures practiced on animals were approved by local ethical committees and followed protocols by the European Community and National Institutes of Health. Some rats and mice received icv. injections of colchicine and were killed after 24 h. All animals were deeply anesthetized and perfused through the ascending aorta with picric acid-formalin solution. After removal, the brains were cryoprotected, snap-frozen, and coronally sectioned at 20 μm in a cryostat.
Serum/CSF Immunostaining and Protease/Glycosidase Pretreatments.
Sections were incubated with serum (1:1,000) or CSF (1:25–1:100) of patients or controls and visualized using a commercial kit based on tyramide signal amplification. For GAD67-EGFP and ChAT-EGFP sections, tetramethylrhodamine-labeled antibodies were used. For double labeling, sections were reincubated with new primary antibody followed by appropriate Cy3-conjugated IgG secondary antibodies (Table S2). Some sections were incubated in a glycosidase enzyme mixture to remove all N-linked oligosaccharides and most O-linked sugars and with proteinase K to remove proteins. Control sections were incubated with buffer without enzymes.
Microscopic Analysis.
Sections were examined using a Nikon Eclipse E600 fluorescence microscope; alternatively, a Zeiss LSM 510 Meta confocal system installed on a Zeiss Axioplan 2 microscope was used. Digital images from the microscopy were slightly modified to optimize for brightness and contrast. The number of MCH/serum- or POMC/serum-immunoreactive cells was counted in double-stained sections from different rostrocaudal levels of the hypothalamus.
Adsorption Experiments in Dot Blot and Immunohistochemistry.
For dot blots, the sample was spotted onto a nitrocellulose membrane, dried, fixed with glutaraldehyde, washed, and incubated overnight with serum (1:5,000) followed by anti-human IgG conjugated with HRP (1:50,000). For visualization, an ECL Western blot detection system was used. For immunohistochemical absorption control experiments, prediluted sera (1:1,000) were incubated overnight with the antigen at 10−6–10−3 M (Table S3).
Preparation of IgG from Patient Sera.
Purified IgG antibodies were prepared from one group A narcoleptic serum with the NEI/αMSH immunostaining pattern (NEI/αMSH-IgG) and one narcoleptic serum without any staining patterns (NP-IgG) as well as an HC (HC-IgG). All three individuals were Pandemrix-vaccinated. For the purification, we used HPLC with a HiTrap Protein G affinity column (1 mL) packed with protein G Sepharose. The eluates were lyophilized and redissolved in artificial CSF, and the final concentration of IgG preparations was 65 mg/mL. The purified NEI/αMSH-IgG preparation (1:10,000 or 1:5,000) exhibited the same pattern A immunostaining as the initial serum.
Physiological Experiments 1: icv. Injection of Purified IgGs, EEG Registration, and Sleep Time Data.
Rats were equipped with electroencephalogram (EEG) and electromyogram (EMG) electrodes, and an icv. cannula was stereotaxically implanted into the right lateral ventricle. At the beginning of inactive phase (light onset) on D1, IgG preparations were injected under halothane anesthesia. EEG, EMG, motility, and a 24-h video were recorded for each animal before icv. injections (BL) and on D2 and D15 after IgG administration. Food intake and body weight were monitored during the entire experiment.
The vigilance states were classified using conventional criteria as described earlier (72, 73). The following parameters were calculated: time spent in wake, REM, SWS1 and SWS2 (per hour), sleep fragmentation, sleep-onset REM, number of transitions (NREM–wake and NREM–REM), and number of REM episodes.
Most statistics were carried out by two-way repeated measure ANOVA matched by pairs (repeated factor: hours from 1 to 6). For posthoc analysis (for every hour from 1 to 6), a Bonferroni multiple comparisons test was used. One-way repeated measure ANOVA and Tukey posthoc test were applied in the evaluation of the number of transitions and the number of REM episodes. Each group was compared with its own BL.
Physiological Experiments 2: Spectral Distribution of EEG Power.
Adopting the state space technique from Diniz Behn et al. (25) with small modifications (SI Materials and Methods), we plotted ratio 1 (6.5–9/0.25–9 Hz) on the x axis and ratio 2 (0.25–20/0.25–60 Hz) on the y axis, defining 2D spectra in any of the sera-treated groups to separate clusters corresponding to REM, NREM, and wake stages; each point represents EEG power data of a 4-s epoch. Ratio 1 was determined to emphasize high θ-activity (6.5–9 Hz; dominates REM in rodents), and ratio 2 was developed to separate NREM and wake clusters. To visualize the density of EEG power data of clusters, we created a heat map (25). Centroids, calculated by averaging EEG power data of rats within each cluster, show the shift from BL (Fig. 7, black circles) to D2 (Fig. 7, white circles). Distances of centroids (D2 vs. BL) were evaluated by paired t tests.
More detailed information on the materials and methods used is SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank our subjects and, especially, the children and their families who made this study possible. We thank Professor Emmanuel Mignot for valuable discussions and advice. The important contribution of Blanca Silva-Lopez and Agnes Ruzsits to this study is gratefully acknowledged. We also thank the following colleagues for generous donation of antisera: Drs. L. de Lecea (hypocretin/orexin), J. Massoulie (acetylcholinesterase), (the late) M. Goldstein (tyrosine hydroxylase), R. Benoit (somatostatin), P. Emson (neuronal nitric oxide synthase), G. Bakalkin (dynorphin), and E. Theodorsson (galanin and neuropeptide tyrosine, NPY). This study was supported by Swedish Research Council Grant VR K2012-99X-21765; 4X-2887, The Swedish Medical Products Agency (www.mpa.se), funds from the Karolinska Institutet, and Medical Research Council of Academy of Finland (NARPANord) Grant 260603.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412189111/-/DCSupplemental.
References
- 1.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369(9560):499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30(1):13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 3.David A, Constantino F, dos Santos JM, Paiva T. Health-related quality of life in Portuguese patients with narcolepsy. Sleep Med. 2012;13(3):273–277. doi: 10.1016/j.sleep.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 4.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 6.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mignot E, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68(3):686–699. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallmayer J, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41(6):708–711. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornum BR, et al. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011;43(1):66–71. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overeem S, et al. Immunohistochemical screening for autoantibodies against lateral hypothalamic neurons in human narcolepsy. J Neuroimmunol. 2006;174(1-2):187–191. doi: 10.1016/j.jneuroim.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Scammell TE. The frustrating and mostly fruitless search for an autoimmune cause of narcolepsy. Sleep. 2006;29(5):601–602. doi: 10.1093/sleep/29.5.601. [DOI] [PubMed] [Google Scholar]
- 13.Aran A, et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32(8):979–983. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cvetkovic-Lopes V, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120(3):713–719. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardage C, et al. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: Population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956. doi: 10.1136/bmj.d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wijnans L, et al. The incidence of narcolepsy in Europe: Before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine. 2013;31(8):1246–1254. doi: 10.1016/j.vaccine.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Persson I, et al. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: A population- and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275(2):172–190. doi: 10.1111/joim.12150. [DOI] [PubMed] [Google Scholar]
- 18.Partinen M, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS ONE. 2012;7(3):e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han F, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70(3):410–417. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 20.Melén K, et al. No serological evidence of influenza A H1N1pdm09 virus infection as a contributing factor in childhood narcolepsy after Pandemrix vaccination campaign in Finland. PLoS ONE. 2013;8(8):e68402. doi: 10.1371/journal.pone.0068402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israeli E, Agmon-Levin N, Blank M, Chapman J, Shoenfeld Y. Guillain-Barré syndrome—a classical autoimmune disease triggered by infection or vaccination. Clin Rev Allergy Immunol. 2012;42(2):121–130. doi: 10.1007/s12016-010-8213-3. [DOI] [PubMed] [Google Scholar]
- 22.Fetissov SO, et al. Autoantibodies against alpha -MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc Natl Acad Sci USA. 2002;99(26):17155–17160. doi: 10.1073/pnas.222658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Academy of Sleep Medicine . International Classification of Sleep Disorders, 2nd Edition: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 24.Hublin C, Kaprio J, Partinen M, Koskenvuo M, Heikkilä K. The Ullanlinna Narcolepsy Scale: Validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res. 1994;3(1):52–59. doi: 10.1111/j.1365-2869.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 25.Diniz Behn CG, Klerman EB, Mochizuki T, Lin SC, Scammell TE. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 2010;33(3):297–306. doi: 10.1093/sleep/33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitka T, et al. Association between the activation of MCH and orexin immunorective neurons and REM sleep architecture during REM rebound after a three day long REM deprivation. Neurochem Int. 2011;59(5):686–694. doi: 10.1016/j.neuint.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Verret L, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci USA. 2009;106(7):2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36(6):1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 30.Xie X, et al. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574(Pt 2):399–414. doi: 10.1113/jphysiol.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42(4):635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 32.Peyron C, et al. Melanin concentrating hormone in central hypersomnia. Sleep Med. 2011;12(8):768–772. doi: 10.1016/j.sleep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Gao Q, Horvath TL. Neuronal control of energy homeostasis. FEBS Lett. 2008;582(1):132–141. doi: 10.1016/j.febslet.2007.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145(6):2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- 35.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 36.Bittencourt JC, et al. The melanin-concentrating hormone system of the rat brain: An immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319(2):218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 37.Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125(4):2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- 38.Watson SJ, Akil H. The presence of two alpha-MSH positive cell groups in rat hypothalamus. Eur J Pharmacol. 1979;58(1):101–103. doi: 10.1016/0014-2999(79)90351-0. [DOI] [PubMed] [Google Scholar]
- 39.Saper CB, Akil H, Watson SJ. Lateral hypothalamic innervation of the cerebral cortex: Immunoreactive staining for a peptide resembling but immunochemically distinct from pituitary/arcuate alpha-melanocyte stimulating hormone. Brain Res Bull. 1986;16(1):107–120. doi: 10.1016/0361-9230(86)90018-3. [DOI] [PubMed] [Google Scholar]
- 40.Garcia FD, et al. Anti-neuropeptide Y plasma immunoglobulins in relation to mood and appetite in depressive disorder. Psychoneuroendocrinology. 2012;37(9):1457–1467. doi: 10.1016/j.psyneuen.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Conzelmann M, Jékely G. Antibodies against conserved amidated neuropeptide epitopes enrich the comparative neurobiology toolbox. Evodevo. 2012;3(1):23. doi: 10.1186/2041-9139-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi A, Mizusawa K. Posttranslational modifications of proopiomelanocortin in vertebrates and their biological significance. Front Endocrinol (Lausanne) 2013;4:143. doi: 10.3389/fendo.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisullo G, et al. A human anti-neuronal autoantibody against GABA B receptor induces experimental autoimmune agrypnia. Exp Neurol. 2007;204(2):808–818. doi: 10.1016/j.expneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Geis C, et al. Human stiff-person syndrome IgG induces anxious behavior in rats. PLoS ONE. 2011;6(2):e16775. doi: 10.1371/journal.pone.0016775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorensen GL, Knudsen S, Jennum P. Sleep transitions in hypocretin-deficient narcolepsy. Sleep. 2013;36(8):1173–1177. doi: 10.5665/sleep.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, et al. The development of hypocretin (orexin) deficiency in hypocretin/ataxin-3 transgenic rats. Neuroscience. 2007;148(1):34–43. doi: 10.1016/j.neuroscience.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beuckmann CT, et al. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci. 2004;24(18):4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsunematsu T, et al. Acute optogenetic silencing of orexin/hypocretin neurons induces slow-wave sleep in mice. J Neurosci. 2011;31(29):10529–10539. doi: 10.1523/JNEUROSCI.0784-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jego S, et al. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16(11):1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chastrette N, Cespuglio R. Influence of proopiomelanocortin-derived peptides on the sleep-waking cycle of the rat. Neurosci Lett. 1985;62(3):365–370. doi: 10.1016/0304-3940(85)90576-2. [DOI] [PubMed] [Google Scholar]
- 51.Panskepp J, et al. Effects of alpha-MSH on motivation, vigilance and brain respiration. Pharmacol Biochem Behav. 1976;5(Suppl 1):59–64. doi: 10.1016/0091-3057(76)90329-4. [DOI] [PubMed] [Google Scholar]
- 52.Koo BB, Feng P, Dostal J, Strohl KP. Alpha-melanocyte stimulating hormone and adrenocorticotropic hormone: An alternative approach when thinking about restless legs syndrome? Mov Disord. 2008;23(9):1234–1242. doi: 10.1002/mds.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maulon-Feraille L, et al. Appetite-boosting property of pro-melanin-concentrating hormone(131-165) (neuropeptide-glutamic acid-isoleucine) is associated with proteolytic resistance. J Pharmacol Exp Ther. 2002;302(2):766–773. doi: 10.1124/jpet.302.2.766. [DOI] [PubMed] [Google Scholar]
- 54.Viale A, et al. The melanin-concentrating hormone gene in human: Flanking region analysis, fine chromosome mapping, and tissue-specific expression. Brain Res Mol Brain Res. 1997;46(1-2):243–255. doi: 10.1016/s0169-328x(97)00018-1. [DOI] [PubMed] [Google Scholar]
- 55.Ahnaou A, et al. Blocking melanin-concentrating hormone MCH1 receptor affects rat sleep-wake architecture. Eur J Pharmacol. 2008;579(1-3):177–188. doi: 10.1016/j.ejphar.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Konadhode RR, et al. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33(25):10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki K, et al. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS ONE. 2011;6(5):e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao Y, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28(37):9101–9110. doi: 10.1523/JNEUROSCI.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adamantidis A, Carter MC, de Lecea L. Optogenetic deconstruction of sleep-wake circuitry in the brain. Front Mol Neurosci. 2010;2(2010):31. doi: 10.3389/neuro.02.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willie JT, Sinton CM, Mieda M, Maratos-Flier E, Yanagisawa M. Orexin and melanin-concentrating hormone (MCH) double knockout mice: Compensatory role for MCH in narcolepsy-cataplexy. Sleep. 2003 26(Abstr Suppl):A50 (abstr) [Google Scholar]
- 61.Gerashchenko D, et al. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci USA. 2008;105(29):10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasumarthi RK, Gerashchenko D, Kilduff TS. Further characterization of sleep-active neuronal nitric oxide synthase neurons in the mouse brain. Neuroscience. 2010;169(1):149–157. doi: 10.1016/j.neuroscience.2010.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González-Albo MC, Elston GN, DeFelipe J. The human temporal cortex: Characterization of neurons expressing nitric oxide synthase, neuropeptides and calcium-binding proteins, and their glutamate receptor subunit profiles. Cereb Cortex. 2001;11(12):1170–1181. doi: 10.1093/cercor/11.12.1170. [DOI] [PubMed] [Google Scholar]
- 64.Lazarus M, Chen JF, Urade Y, Huang ZL. Role of the basal ganglia in the control of sleep and wakefulness. Curr Opin Neurobiol. 2013;23(5):780–785. doi: 10.1016/j.conb.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur J Neurosci. 2010;31(3):499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 68.Thebault S, Vincent A, Gringras P. Narcolepsy and H1N1 vaccination: A link? Curr Opin Pulm Med. 2013;19(6):587–593. doi: 10.1097/MCP.0b013e328365af97. [DOI] [PubMed] [Google Scholar]
- 69.Kowal C, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA. 2006;103(52):19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamamaki N, et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467(1):60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- 71.Tallini YN, et al. BAC transgenic mice express enhanced green fluorescent protein in central and peripheral cholinergic neurons. Physiol Genomics. 2006;27(3):391–397. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- 72.Kantor S, Jakus R, Balogh B, Benko A, Bagdy G. Increased wakefulness, motor activity and decreased theta activity after blockade of the 5-HT2B receptor by the subtype-selective antagonist SB-215505. Br J Pharmacol. 2004;142(8):1332–1342. doi: 10.1038/sj.bjp.0705887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vas S, et al. Nesfatin-1/NUCB2 as a potential new element of sleep regulation in rats. PLoS ONE. 2013;8(4):e59809. doi: 10.1371/journal.pone.0059809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rovere C, Viale A, Nahon J, Kitabgi P. Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obese fat/fat mice. Endocrinology. 1996;137(7):2954–2958. doi: 10.1210/endo.137.7.8770919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.