Significance
Our understanding of life on exoplanets and exomoons must be based on what we know about life on Earth. Liquid water is the common ecological requirement for Earth life. Temperature on an exoplanet is the first parameter to consider both because of its influence on liquid water and because it can be directly estimated from orbital and climate models of exoplanetary systems. Life needs some water, but deserts show that even a little can be enough. Only a small amount of light from the central star is required to provide for photosynthesis. Some nitrogen must be present for life and the presence of oxygen would be a good indicator of photosynthesis and possibly complex life.
Keywords: extremophiles, Mars, astrobiology
Abstract
The requirements for life on Earth, its elemental composition, and its environmental limits provide a way to assess the habitability of exoplanets. Temperature is key both because of its influence on liquid water and because it can be directly estimated from orbital and climate models of exoplanetary systems. Life can grow and reproduce at temperatures as low as −15 °C, and as high as 122 °C. Studies of life in extreme deserts show that on a dry world, even a small amount of rain, fog, snow, and even atmospheric humidity can be adequate for photosynthetic production producing a small but detectable microbial community. Life is able to use light at levels less than 10−5 of the solar flux at Earth. UV or ionizing radiation can be tolerated by many microorganisms at very high levels and is unlikely to be life limiting on an exoplanet. Biologically available nitrogen may limit habitability. Levels of O2 over a few percent on an exoplanet would be consistent with the presence of multicellular organisms and high levels of O2 on Earth-like worlds indicate oxygenic photosynthesis. Other factors such as pH and salinity are likely to vary and not limit life over an entire planet or moon.
The list of exoplanets is increasing rapidly with a diversity of masses, orbital distances, and star types. The long list motivates us to consider which of these worlds could support life and what type of life could live there. The only approach to answering these questions is based on observations of life on Earth. Compared with astronomical targets, life on Earth is easily studied and our knowledge of it is extensive––but it is not complete. The most important area in which we lack knowledge about life on Earth is its origin. We have no consensus theory for the origin of life nor do we know the timing or location (1). What we do know about life on Earth is what it is made of, and we know its ecological requirements and limits. Thus, it is not surprising that most of the discussions related to life on exoplanets focus on the requirements for life rather than its origin. In this paper we follow this same approach but later return briefly to the question of the origin of life.
Limits to Life
There are two somewhat different approaches to the question of the limits of life. The first approach is to determine the requirements for life. The second approach is to determine the extreme environments in which adapted organisms—often referred to as extremophiles—can survive. Both perspectives are relevant to the question of life on exoplanets.
It is useful to categorize the requirements for life on Earth as four items: energy, carbon, liquid water, and various other elements. These are listed in Table 1 along with the occurrence of these factors in the Solar System (2). In our Solar System it is the occurrence of liquid water that appears to limit the occurrence of habitable environments and this appears to be the case for exoplanetary systems as well.
Table 1.
Ecological requirements for life
| Requirement | Occurrence in the Solar System |
| Energy | Common |
| Predominately light | Photosynthesis at 100 AU light levels |
| Chemical energy | e.g., H2 + CO2 → CH4 + H2O |
| Carbon | Common as CO2 and CH4 |
| Liquid water | Rare, only on Earth for certain |
| N,P, S, Na, and other elements | Likely to be common |
From ref. 2.
From basic thermodynamic considerations it is clear that life requires a source of energy. To power metabolism and growth, life on Earth uses only one energy source: that associated with the transfer of electrons by chemical reactions of reduction and oxidation. For example, methane-producing microbes use the reaction of CO2 with H2 to produce CH4. Photosynthetic organisms use a light-absorbing protein, such as chlorophyll, bacteriochlorophylls, and bacteriorhodopsin, to convert photon energy to the energy of an electron which then completes a redox reaction. The electrons from the redox reaction are used to create an electrochemical gradient across cell membranes (3). This occurs in the mitochondria in of most eukaryotes and in the cell membrane of prokaryotic cells. It has recently been shown that electrons provided directly as electrical current can also drive microbial metabolism (4). Although life can detect and generate other energy sources including magnetic, kinetic, gravitational, thermal gradient, and electrostatic, none of these is used for metabolic energy.
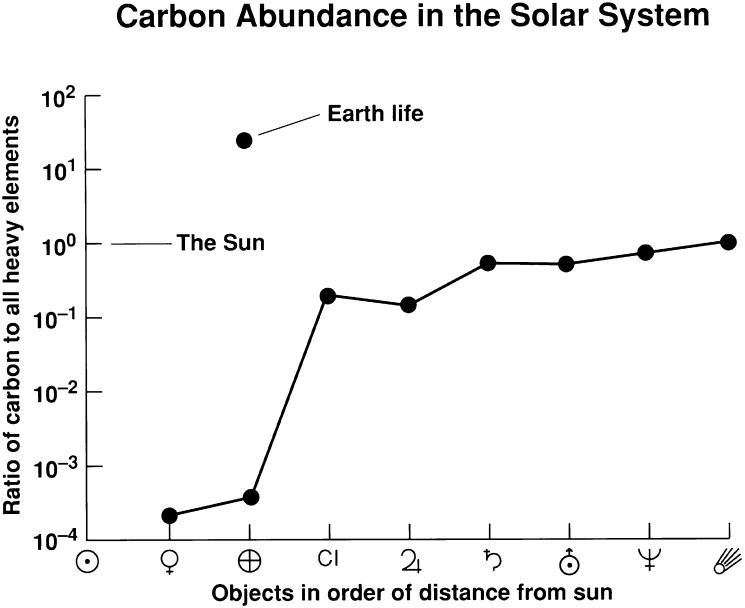
Carbon has the dominant role as the backbone molecule of biochemistry for Earth life and is widespread in the Solar System. However, the abundance of carbon may not be a useful indication of the habitability of an exoplanet. This is illustrated in Fig. 1, which shows that the Earth is significantly depleted in carbon compared with the outer Solar System. The vast majority of the carbon on Earth is stored in sedimentary rocks within the crust. However, because light carbon-containing molecules are volatile––CO2, CO, and CH4––adequate carbon is present at the surface of the Earth, as well as Mars and Venus.
Fig. 1.
Carbon in the Solar System as ratio by number to total heavy elements (>He) for various Solar System objects. Carbon is depleted in the inner Solar System. The x axis is not a true distance scale but the objects are ordered by increasing distance from the Sun. Data are from the following compilations: comets and Type I carbonaceous chondrites, CI (72); life (73); Earth and Venus (74); Sun, Jupiter, Saturn, Uranus, and Neptune (75). Mars is not shown because the size of its carbon reservoir is unknown. Figure from ref. 76.
Life on Earth uses a vast array of the elements available on the surface in addition to carbon. However, this does not prove that these elements are absolute requirements for carbon-based life in general. Other than H2O and C, the elements N, S, and P are probably the leading candidates for the status of required elements. Table 2, adapted from Davies and Koch (5), lists the distribution of elements in the cosmos and on the Earth and compares these with the common elements in life––represented by humans and the bacterium Escherichia coli. If liquid water and biologically available nitrogen are present, then phosphorous, potassium, sodium, sulfur, and calcium might come next on a requirements list, as these are the next most abundant elements in bacteria. However, there is no definitive list and any list would depend on the organism considered; for example habitability for methanogens requires high nickel levels (6). In a strict sense habitability can only be confirmed by showing inhabitation; no list is conclusive. Of these secondary elements N is probably the one most likely to be in question on an exoplanet, as is discussed below. Sulfur and phosphorous and virtually all of the rest of the elements listed by Davies and Koch (5) as used in life have major refractory phases at the temperatures of liquid water and should be available if water and rock interact.
Table 2.
Elemental abundances by mass
| 1 | Cosmic, % | Earth’s crust, % | Humans, % | Bacteria (E. coli), % | ||||
| 1 | H | 70.7 | O | 46.6 | O | 64 | O | 68 |
| 2 | He | 27.4 | Si | 27.7 | C | 19 | C | 15 |
| 3 | O | 0.958 | Al | 8.13 | H | 9 | H | 10.2 |
| 4 | C | 0.304 | Fe | 5.00 | N | 5 | N | 4.2 |
| 5 | Ne | 0.174 | Ca | 3.63 | Ca | 1.5 | P | 0.83 |
| 6 | Fe | 0.126 | Na | 2.83 | P | 0.8 | K | 0.45 |
| 7 | N | 0.110 | K | 2.59 | S | 0.6 | Na | 0.40 |
| 8 | Si | 0.0706 | Mg | 2.09 | K | 0.3 | S | 0.30 |
| 9 | Mg | 0.0656 | Ti | 0.44 | Na | 0.15 | Ca | 0.25 |
| 10 | S | 0.0414 | H | 0.14 | Cl | 0.15 | Cl | 0.12 |
From ref. 5.
The second approach to the requirements for life is that based on the abilities of extremophiles in a range of environmental factors. Table 3 lists the limits of life under extreme conditions. Our understanding of the requirements for life listed in Table 1 has not changed for many years. In contrast, the limits of life listed in Table 3 have changed in several significant ways over the past few decades. If one compares a list of the limits of life from a few decades ago (7) with Table 3, the most notable change is in the high-temperature limit. This has been raised from 80 °C to 122 °C (8). There has been considerable discussion on the limits of life and their application to the search for life on other worlds (9–11) and it has been realized that the limits vary when organisms face multiple extreme conditions at the same time (12).
Table 3.
Ecological limits to life
| Parameter | Limit | Note |
| Lower temperature | ∼ −15 °C | Limited by liquid water associated with thin films or saline solutions |
| Upper temperature | 122 °C | Solubility of lipids in water, protein stability |
| Maximum pressure | 1,100 atm | Ref. 10 |
| Low light | ∼0.01 μmol m−2⋅s−1 = 5 × 10−6 direct sunlight | Algae under ice and deep sea |
| pH | 0–12.5 | |
| Salinity | Saturated NaCl | Depends on the salt and temperature |
| Water activity | 0.6 | Yeasts and molds |
| 0.8 | Bacteria | |
| UV | ≥1,000 J m−2 | D. radiodurans |
| Radiation | 50 Gy/h | D. radiodurans growth with continuous dose |
| 12,000 Gy | Acute dose, higher for dry or frozen state |
Whereas the limits of life have changed in some ways over the past few decades, there has been a more radical change in our appreciation of where microbial ecosystems can be found. Notable examples of the discovery of unexpected microbial ecosystems include endolithic microorganisms in the Antarctic cold desert (13), hot deep-sea vents (14), cool deep-sea vents (15), deep in basalt (16), deep below the subsurface (17), and in an ice-covered Antarctic lake that has been sealed for thousands of years (18). Several aspects of these recently discovered ecosystems are worth comment: first, the organisms found are not alien and map in expected areas of the tree of life; second, with the exception of the high-temperature vents, the organisms do not greatly extend the limits of life derived from more mundane and accessible ecosystems; third, the organisms themselves do not find these unusual environments extreme and typically are well adapted to the conditions under which they live; and fourth, the organisms in these environments do not in general control the physical environment (temperature and pressure) with their own metabolic activity but rather live in locations where the local physical conditions are suitable even when these environments are nestled within larger inhospitable areas. The lesson to be learned from these discoveries is that microbial life is extremely adept at locating places to live, and we have not been adept at anticipating how small environments can be habitable in otherwise barren locations: microbial life is more clever than we are. This is a factor that should inform our consideration of habitability of exoplanets.
Strategy for Exoplanets
Given the general requirements for life (Table 1), the elemental composition of life (Table 2), and the environmental limits for life (Table 3), we can consider how to assess the habitability of the environment on an exoplanet. It may seem logical to focus on primary production because without that there cannot be an ecosystem. However, it is possible that photochemical processes in an exoplanet atmosphere play the role of primary production as has been suggested for Titan (19). Many of the limits to life in Table 3 such as pH and salinity are unlikely to be extreme over an entire world. As on Earth they would shape the distribution of life on a world but not its possible occurrence and are therefore not considered further. The key parameters that could be extreme over an entire world and the order in which they may limit any life on an exoplanet are listed in Table 4.
Table 4.
Checklist for habitability of an extrasolar planet
| Requirement | Note |
| 1. Temperature and state of water | T between -15 °C and 122 °C, P > ∼0.01 atmospheres |
| 2. Water availability | Few days per y of rain, fog, snow, or RH > 80% |
| 3. Light and redox energy sources | |
| 4. UV and Ionizing radiation | Limits exemplified by D. radiodurans (Table 3) |
| 5. Nitrogen | Enough N2 for fixation or fixed nitrogen present |
| 6. O2 | Over 0.01 atmospheres need to support complex life |
The most important parameter for Earth-like life is the presence of liquid water, which directly depends on pressure and temperature. Temperature is key both because of its influence on liquid water and because it can be directly estimated from orbital and climate models of exoplanetary systems. We can consider the cold and hot limits.
Temperature, Cold Limit.
Many organisms can grow and reproduce at temperatures well below the freezing point of pure water because their intracellular material contains salts and other solutes that lower the freezing point of the solution. Recently, Mykytczuk et al. (20) reported an isolate from Arctic permafrost that grows and divides at −15 °C, the lowest temperature demonstrated to date, and is metabolically active at −25 °C in frozen soils. Thin films of water at the interface between ice and soil grains, augmented by any solutes, provide adequate water for life at these low temperatures (20, 21). The snow algae Chlamydomonas nivalis thrives in liquid water associated with snow, coloring it red, but the algae are the beneficiaries of external processes that melt the snow (22, 23). Microbial activity can generate sufficient heat in permafrost soils (and landfills and composts) such that it is a major contributor to melting (24, 25), but there is no known occurrence of an organism using metabolic energy coupled directly, e.g., through enzymes, to overcome the latent heat of fusion of ice thereby liquefying it.
Temperature, Hot Limit.
Many of the exoplanets discovered to date have high surface temperatures and hence the high-temperature limit of life is of particular interest. Takai et al. (8) showed growth, survival, and methane production by a methanogen at 122 °C where the high pressure (20 MPa, ∼200 atmospheres) stabilized the liquid water. At higher pressure water can be liquid at even higher temperatures. However, as water is heated and maintained as a liquid under pressure, the dielectric constant and the polarity of the liquid decreases sharply, thus significantly changing its characteristics as a solvent and its interaction with dissolved biomolecules, in particular lipids, but also proteins and nucleic acids. At 200 °C the dielectric constant is about half of the room temperature value (26). It is likely that the destabilization of lipid bilayers as they become soluble in the lower dielectric constant water is what sets the high-temperature limit on life. It is therefore perhaps not surprising that the organisms that can survive the highest temperatures are archaea (8, 27), as their membrane lipids are held together with ether bonds, which are chemically more resistant than ester bonds, which are used in the membranes of nonarchaea. Denaturing of proteins with temperature appears also to play a role (28). Hot water in contact with rocks can be efficient in generating or recycling redox couples––this has been suggested for the interior of Enceladus (29). Such ecosystems provide a compelling example of possible life below the ocean of an exoplanet or exomoon and can even be productive enough to support multicellular life––in the presence of an O2-rich environment. Fig. 2 shows a crab at the Lost City hydrothermal vent.
Fig. 2.

High-temperature limit of life in submarine vents can support complex life. Crab at the Lost City hydrothermal site. Image courtesy of D. Kelley, University of Washington, Institute for Exploration, University of Rhode Island Institute for Archeological Oceanography Lost City Science party, and the National Oceanic and Atmospheric Administration.
Water, Dry Limit.
On worlds where the temperature is within the range discussed above, life may be limited by the availability of water; Mars is an example of this. Thus, the dry limit of life is of interest. In dry environments phototrophs seek shelter and water retained in, and below, rocks. Fig. 3 shows photosynthetic cyanobacteria and lichens from several dry deserts. Fig. 3A shows endolithic cyanobacteria which live just below the surface of halite rocks in the dry core of the Atacama Desert (30). The water to support their growth comes from absorption of atmospheric moisture by the deliquescence of the salt (31). Fig. 3B shows the green biofilm of cyanobacteria that live beneath translucent rocks in many deserts surviving on as little as a few days of rain or fog each year (32–34). The example shown is from an unusual carbonate rock from the Mojave Desert that is clear inside but covered with a red coating (35, 36). Fig. 3C shows lichen forming a green and black layer inside sandstone from the Dry Valleys of Antarctica, which obtain water from melting of occasional snow (37, 38). These examples show that a small amount of rain, fog, or snow and even atmospheric humidity can be adequate for photosynthetic production producing a small but detectable microbial community.
Fig. 3.
Photosynthesis in dry environments. In the driest environments on Earth, photosynthesis occurs inside and under rocks. (A) Green layer of cyanobacteria living just below the surface of halite rocks in the dry core of the Atacama Desert (30). (B) Inverted samples of red-coated, carbonate translucent rocks from the Mojave desert showing green biofilm of cyanobacteria that live beneath the rock (32, 35, 36). (C) Lichen forming a green and black layer inside sandstone from the Dry Valleys of Antarctica (37). Scale bar in all images, 1 cm. Images A, B, and C are courtesy of J. Wierzchos, C. McKay, and E.I. Friedmann, respectively.
Energy.
Energy for life can come from chemical redox couples generated by geothermal processes or light from the central star. Geothermal flux can arise from (i) the planet cooling off from its gravitational heat of formation, (ii) decay of long-lived radioactive elements, or (iii) tidal heating for a close-orbiting world or moon. Note that on Earth only a tiny fraction of the geothermal heat is converted into chemical energy, whereas about half the solar flux occurs at wavelengths that are usable for photosynthesis. This is expected as the free energy available in heat flow is much less than that available in low-entropy photons. The example of Earth indicates that a biosphere can have effects on a global scale, and hence be detectable over interstellar distances, only when it is powered by light. Life based on geothermally derived chemical energy would, by dint of energy restrictions, always remain small and globally insignificant. Life is able to use light at very low levels. Littler et al. (39) reported on growth of red macroalgae on deep seamounts at light levels of 0.01 μmol m−2⋅s−1. Raven et al. (40) have reviewed the minimum light levels for photosynthesis and also concluded that 0.01 μmol m−2⋅s−1 is needed (40) or ∼5 × 10−6 of the direct solar flux at Earth (2,000 μmol m−2⋅s−1). Even at the orbit of Pluto, light levels exceed this value by a factor of ∼100. It has been suggested that exoplanets around M stars––a common star type which radiates more in the infrared compared with the Sun––could support photosynthesis using a three- or four-photon mechanism photon instead of the two-photon system used in plants on Earth (41).
UV and Radiation.
Complex life forms (such as humans) are sensitive to radiation but the dose that can be tolerated by many microorganisms is astonishingly high given natural levels of radiation in the environment. Table 3 lists the tolerances and acute dose survival for Deinococcus radiodurans, a well-studied soil heterotroph with high radiation tolerance (42). It has been suggested that the high radiation tolerance of D. radiodurans is due to adaptation to dehydration stress (43). Desert cyanobacteria of the genus Chroococcidiopsis (shown in their characteristic hypolithic growth form in Fig. 3B) are extremely resistant to desiccation, ionizing radiation, and UV (44, 45). An exoplanet would not require a magnetic field to be habitable. Any plausible field would not deflect galactic cosmic rays because these particles are much too energetic. These particles are primarily stopped by the mass of the atmosphere or surface materials. The column mass Earth's atmosphere is equivalent to 1 kg/cm2. The Earth's magnetic field does deflect solar protons channeling these particles to the polar regions creating the aurora. However, even without the magnetic field these particles would not penetrate the Earth's atmosphere and would not reach the surface. Earth occasionally loses its strong dipole field during field reversals. These events are not correlated with extinctions in the fossil record.
Nitrogen.
Life requires a source of nitrogen. After carbon, nitrogen is arguably the most important element needed for life (46). Experiments have shown that aerobic microorganisms require a minimum of 1–5 × 10−3 atmospheres N2 for fixation (47). A variety of energetic processes such as aurorae, lightning, and volcanoes can convert N2 to nitrate even in CO2 atmospheres (48). In the reducing conditions of the outer Solar System N is present as ammonia which is also biologically usable. The biological availability of nitrogen in an important factor in the assignment of habitability for Mars (49, 50).
O2.
Multicellular life on Earth generally relies on oxygen metabolism, and the rise of multicellular life over Earth history tracked the rise of oxygen (51). There are interesting exceptions to the connection between oxygen and multicellular life (52, 53) and the link to O2 may be in need of further scrutiny (54). Nonetheless, levels of O2 over a few percent on an exoplanet would be consistent with, and possibly indicative of, the presence of multicellular organisms. Owen (55) suggested that O2 and O3 would be suitable targets for spectroscopy in the search for evidence of life on exoplanets and exomoons. It is generally agreed that high levels of O2 on Earth-like worlds indicate photosynthesis.
Origin of Life
Discussions of life on an exoplanet should logically begin with a consideration of the possible origin of life on that world. However, our understanding of the origin of life is speculative and so we can only assume that planets that have a diversity of habitable environments are also generative of life (1).
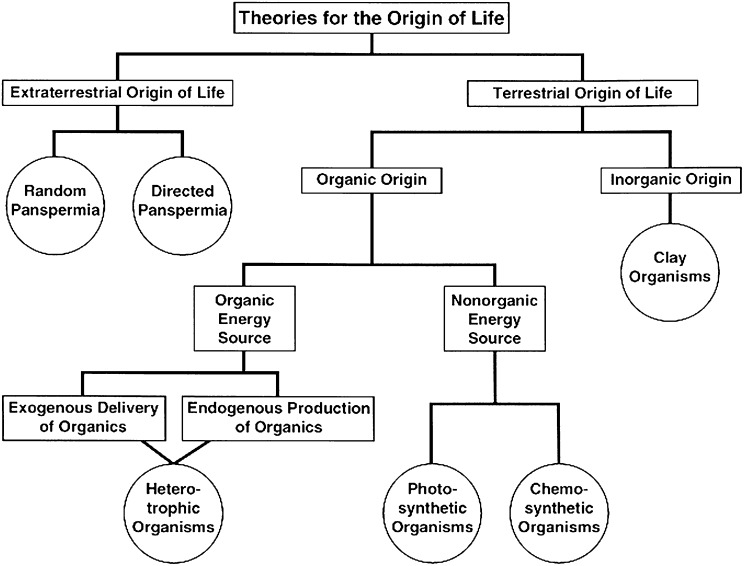
As shown in Fig. 4, it is useful to divide theories for the origin of life on Earth into two main categories, depending on whether life originated independently on a world or was carried to that world from somewhere else (1). The latter category is usually called panspermia, and versions that involve both natural and directed panspermia have been considered (1).
Fig. 4.
Theories for the origin of life on Earth as categorized by Davis and McKay (1) intended for Mars but applicable to the origin of life on exoplanets.
There are possible panspermia schemes that are relevant to exoplanets. Napier (56) has proposed that life could be carried on dust between stars (see also ref. 57), and others have suggested rocks could travel between star systems (58, 59). If such dust grains or rocks were incorporated into the preplanetary nebula, then every planet and moon that formed would be infected with life.
Theories for the origin of life that propose that life on Earth began on Earth are labeled as “Terrestrial” in Fig. 4 and could apply to suitable exoplanets as well. A key question for life on exoplanets is how long the habitable conditions––liquid water––must persist for life to begin. The fossil record on Earth provides only broad constraints on how long it took for life to start on this planet. Simulations of the formation of Earth suggest that habitable conditions were present no sooner than 3.9 billion y ago. The earliest indication of possible life is present in the carbon isotope record at 3.8 billion y ago (60, 61), and convincing evidence of life is present at 3.4 billion y ago (62). Thus, the origin of life occurred within 100–500 million y after the formation of Earth. This is only an upper limit, however, and the process may have been much faster. In a review of this question, Lazcano and Miller (63) suggested that “in spite of the many uncertainties involved in the estimates of time for life to arise and evolve to cyanobacteria, we see no compelling reason to assume that this process, from the beginning of the primitive soup to cyanobacteria, took more than 10 million years.” However, Orgel (64) criticized this conclusion and stated that we do not understand the steps that lead to life; consequently, we cannot estimate the time required: “Attempts to circumvent this essential difficulty are based on misunderstandings of the nature of the problem.” Thus, until new data are obtained the problem of origin of life remains unsolvable.
Titan Life
In the previous sections, the considerations of life on exoplanets have centered on Earth-like life requiring liquid water. This is certainly a reasonable starting point in the search for life. However, it may be that liquids other than water are also suitable media for carbon-based life forms. Benner et al. (65) first suggested that the liquid hydrocarbons on Titan could be the basis for life, playing the role that water does for life on Earth. Those researchers concluded that in many senses, hydrocarbon solvents are better than water for managing complex organic chemical reactivity. There is also suitable redox energy available for life. Organic molecules on the surface of Titan (such as acetylene, ethane, and solid organics) would release energy if they reacted with hydrogen present in the atmosphere forming methane (19, 66). Acetylene yields the most energy. However, all these reactions are kinetically inhibited and thus could be used by biology if suitable catalysts were evolved. Based on this, McKay and Smith (19) predicted that a sign of life on a Titan-like world would be a depletion of hydrogen, acetylene, and ethane. Lunine (67) suggested that Titan-like worlds and moons might be more common in the galaxy than Earth-like worlds. Gilliam and McKay (68) showed how Titan-like worlds orbiting M-type stars could maintain liquid methane and ethane surface reservoirs.
Titan is an example moon that is of interest with respect to astrobiology. In our Solar System Europa and Enceladus are similarly of interest. Indeed, Enceladus seems to have all of the requirements for habitability (69). It has long been recognized that moons of giant planets may be warmed by tidal heating from the primary planet and receive sufficient light from a central star to power photosynthesis (70). This provides a model for possible habitable moons orbiting giant exoplanets (71).
Conclusion
As the number of known exoplanets and exomoons expands we will certainly find worlds that resemble the Earth to varying extent. Based on our understanding of life on Earth we can present a checklist for speculating on the possibilities of life on these distant worlds. (i) Is the temperature between −15 °C and 122 °C, and a total pressure high enough to keep water liquid water stable (P > ∼0.01 atmospheres)? (ii) If the world is arid, are there at last a few days per year of rain, fog, snow, or RH > 80%? (iii) Are there adequate light or geothermal energy sources––light determined by distance from the star, geothermal energy estimated by bulk density? (iv) Are the UV and ionizing radiation below the (very high) limits of microbial tolerance? (v) Is there a biologically available source of nitrogen? (vi) If O2 is present at over 0.01 atmospheres there could be complex life, and the presence of O2 is convincing indicator of photosynthetic life on Earth-like worlds.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.S.B. is a guest editor invited by the Editorial Board.
References
- 1.Davis WL, McKay CP. Origins of life: A comparison of theories and application to Mars. Orig Life Evol Biosph. 1996;26(1):61–73. doi: 10.1007/BF01808160. [DOI] [PubMed] [Google Scholar]
- 2.McKay CP, Davis WL. Astrobiology. In: McFadden L-A, Weissman PR, Johnson TV, editors. Encyclopedia of the Solar System. 2nd Ed. San Diego: Elsevier Academic; 2006. [Google Scholar]
- 3.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191(4784):144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 4.Rabaey K, Rozendal RA. Microbial electrosynthesis - revisiting the electrical route for microbial production. Nat Rev Microbiol. 2010;8(10):706–716. doi: 10.1038/nrmicro2422. [DOI] [PubMed] [Google Scholar]
- 5.Davies RE, Koch RH. All the observed universe has contributed to life. Philos Trans R Soc Lond B Biol Sci. 1991;334(1271):391–403. doi: 10.1098/rstb.1991.0124. [DOI] [PubMed] [Google Scholar]
- 6.Konhauser KO, et al. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature. 2009;458(7239):750–753. doi: 10.1038/nature07858. [DOI] [PubMed] [Google Scholar]
- 7.Kushner D. Extreme environments: Are there any limits to life? In: Ponnamperuma C, editor. Comets and the Origin of Life. Holland: D. Reidel Publishing Company, Dordrecht; 1981. pp. 241–248. [Google Scholar]
- 8.Takai K, et al. Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA. 2008;105(31):10949–10954. doi: 10.1073/pnas.0712334105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavicchioli R. Extremophiles and the search for extraterrestrial life. Astrobiology. 2002;2(3):281–292. doi: 10.1089/153110702762027862. [DOI] [PubMed] [Google Scholar]
- 10.Stan-Lotter H. 2007. Extremophiles, the physicochemical limits of life (growth and survival). Complete Course in Astrobiology, eds Horneck G, Rettberg P (Wiley-VCH, Weinheim, Germany), pp 121–150.
- 11.Dartnell L. Biological constraints on habitability. Astron Geophys. 2011;52(1):1–25. [Google Scholar]
- 12.Harrison JP, Gheeraert N, Tsigelnitskiy D, Cockell CS. The limits for life under multiple extremes. Trends Microbiol. 2013;21(4) Issue 4:204–212. doi: 10.1016/j.tim.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Friedmann EI, Ocampo R. Endolithic blue-green algae in the dry valleys: Primary producers in the antarctic desert ecosystem. Science. 1976;193(4259):1247–1249. doi: 10.1126/science.193.4259.1247. [DOI] [PubMed] [Google Scholar]
- 14.Corliss JB, et al. Submarine thermal springs on the galapagos rift. Science. 1979;203(4385):1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 15.Kelley DS, et al. AT3-60 Shipboard Party An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30 ° N. Nature. 2001;412(6843):145–149. doi: 10.1038/35084000. [DOI] [PubMed] [Google Scholar]
- 16.Stevens TO, McKinley JP. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science. 1995;270(5235):450–454. [Google Scholar]
- 17.Lin L-H, et al. Long-term sustainability of a high-energy, low-diversity crustal biome. Science. 2006;314(5798):479–482. doi: 10.1126/science.1127376. [DOI] [PubMed] [Google Scholar]
- 18.Murray AE, et al. Microbial life at -13 °C in the brine of an ice-sealed Antarctic lake. Proc Natl Acad Sci USA. 2012;109(50):20626–20631. doi: 10.1073/pnas.1208607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay CP, Smith HD. Possibilities for methanogenic life in liquid methane on the surface of Titan. Icarus. 2005;178:274–276. [Google Scholar]
- 20.Mykytczuk NC, et al. Bacterial growth at -15 °C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 2013;7(6):1211–1226. doi: 10.1038/ismej.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA. Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol. 2000;66(8):3230–3233. doi: 10.1128/aem.66.8.3230-3233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoham RW. Optimum temperatures and temperature ranges for growth of snow algae. Arct Alp Res. 1975;7:13–24. [Google Scholar]
- 23.Dove A, Heldmann J, McKay CP, Toon OB. Physics of a thick seasonal snowpack with possible implications for snow algae. Arct Antarct Alp Res. 2012;44(1):36–49. [Google Scholar]
- 24.Zimov SA, et al. Winter biotic activity and production of CO2 in Siberian soils: A factor in the greenhouse effect. J Geophys Res, D, Atmospheres. 1993;98(D3):5017–5023. [Google Scholar]
- 25.Koven CD, et al. Permafrost carbon-climate feedbacks accelerate global warming. Proc Natl Acad Sci USA. 2011;108(36):14769–14774. doi: 10.1073/pnas.1103910108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr AG, Mammucari R, Forster NR. A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chem Eng J. 2011;172(1):1–17. [Google Scholar]
- 27.Blöchl E, et al. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113 ° C. Extremophiles. 1997;1(1):14–21. doi: 10.1007/s007920050010. [DOI] [PubMed] [Google Scholar]
- 28.Laczkó-Dobos H, Szalontai B. Lipids, proteins, and their interplay in the dynamics of temperature-stressed membranes of a cyanobacterium, Synechocystis PCC 6803. Biochemistry. 2009;48(42):10120–10128. doi: 10.1021/bi9011034. [DOI] [PubMed] [Google Scholar]
- 29.McKay CP, Porco CC, Altheide T, Davis WL, Kral TA. The possible origin and persistence of life on Enceladus and detection of biomarkers in the plume. Astrobiology. 2008;8(5):909–919. doi: 10.1089/ast.2008.0265. [DOI] [PubMed] [Google Scholar]
- 30.Wierzchos J, Ascaso C, McKay CP. Endolithic cyanobacteria in halite rocks from the hyperarid core of the Atacama Desert. Astrobiology. 2006;6(3):415–422. doi: 10.1089/ast.2006.6.415. [DOI] [PubMed] [Google Scholar]
- 31.Davila AF, Hawes I, Ascaso C, Wierzchos J. Salt deliquescence drives photosynthesis in the hyperarid Atacama Desert. Environ Microbiol Rep. 2013;5(4):583–587. doi: 10.1111/1758-2229.12050. [DOI] [PubMed] [Google Scholar]
- 32.Nienow JA. Extremophiles: Dry Environments (Including Cryptoendoliths). Encyclopedia of Microbiology. Oxford: Elsevier; 2009. pp. 159–173. [Google Scholar]
- 33.Warren-Rhodes KA, et al. Hypolithic cyanobacteria, dry limit of photosynthesis, and microbial ecology in the hyperarid Atacama Desert. Microb Ecol. 2006;52(3):389–398. doi: 10.1007/s00248-006-9055-7. [DOI] [PubMed] [Google Scholar]
- 34.Warren‐Rhodes KA, et al. Physical ecology of hypolithic communities in the Central Namib Desert: The role of fog, rain, rock habitat and light. J Geophys Res: Biogeosci. 2013;118(4):1451–1460. [Google Scholar]
- 35.Bishop JL, et al. Carbonate rocks in the Mojave Desert as an analogue for Martian carbonates. Int J Astrobiol. 2011;10:349–358. [Google Scholar]
- 36.Smith HD, et al. 2014. Comparative analysis of cyanobacteria inhabiting rocks with different light transmittance in the Mojave Desert: a Mars terrestrial analogue. Int J Astrobiol. [DOI]
- 37.Friedmann EI. Endolithic microorganisms in the antarctic cold desert. Science. 1982;215(4536):1045–1053. doi: 10.1126/science.215.4536.1045. [DOI] [PubMed] [Google Scholar]
- 38.Sun HJ. Endolithic microbial life in extreme cold climate: Snow is required, but perhaps less is more. Biology. 2013;2(2):693–701. doi: 10.3390/biology2020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Littler MM, et al. Deep-water plant communities from an uncharted seamount off San Salvador Island, Bahamas: Distribution, abundance, and primary productivity. Deep-Sea Res A, Oceanogr Res Pap. 1986;33(7):881–892. [Google Scholar]
- 40.Raven JA, Kübler JE, Beardall J. Put out the light, and then put out the light. J Mar Biol Assoc U K. 2000;80(1):1–25. [Google Scholar]
- 41.Wolstencroft RD, Raven JA. Photosynthesis: Likelihood of occurrence and possibility of detection on Earth-like planets. Icarus. 2002;157(2):535–548. [Google Scholar]
- 42.Daly MJ. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 2009;7(3):237–245. doi: 10.1038/nrmicro2073. [DOI] [PubMed] [Google Scholar]
- 43.Mattimore V, Battista JR. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol. 1996;178(3):633–637. doi: 10.1128/jb.178.3.633-637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billi D, Friedmann EI, Hofer KG, Caiola MG, Ocampo-Friedmann R. Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl Environ Microbiol. 2000;66(4):1489–1492. doi: 10.1128/aem.66.4.1489-1492.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baqué M, Viaggiu E, Scalzi G, Billi D. Endurance of the endolithic desert cyanobacterium Chroococcidiopsis under UVC radiation. Extremophiles. 2013;17(1):161–169. doi: 10.1007/s00792-012-0505-5. [DOI] [PubMed] [Google Scholar]
- 46.Capone DG, Popa R, Flood B, Nealson KH. Geochemistry. Follow the nitrogen. Science. 2006;312(5774):708–709. doi: 10.1126/science.1111863. [DOI] [PubMed] [Google Scholar]
- 47.Klingler JM, Mancinelli RL, White MR. Biological nitrogen fixation under primordial Martian partial pressures of dinitrogen. Adv Space Res. 1989;9(6):173–176. doi: 10.1016/0273-1177(89)90225-1. [DOI] [PubMed] [Google Scholar]
- 48.Navarro-González R, McKay CP, Mvondo DN. A possible nitrogen crisis for Archaean life due to reduced nitrogen fixation by lightning. Nature. 2001;412(6842):61–64. doi: 10.1038/35083537. [DOI] [PubMed] [Google Scholar]
- 49.Archer PD, et al. Abundances and implications of volatile-bearing species from evolved gas analysis of the Rocknest aeolian deposit, Gale Crater, Mars. J Geophys Res Planets. 2014 doi: 10.1002/2013JE004493. [DOI] [Google Scholar]
- 50.Stern J, et al. Detection and quantification of nitrogen compounds in the first drilled martian solid samples by the Sample Analysis at Mars (SAM) instrument suite on the Mars Science Laboratory (MSL) Lunar and Planetary Institute Science Conference Abstracts. 2014;45:2743. [Google Scholar]
- 51.Knoll AH. The early evolution of eukaryotes: A geological perspective. Science. 1992;256(5057):622–627. doi: 10.1126/science.1585174. [DOI] [PubMed] [Google Scholar]
- 52.Danovaro R, et al. The first metazoa living in permanently anoxic conditions. BMC Biol. 2010;8(1):30. doi: 10.1186/1741-7007-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mills DB, et al. Oxygen requirements of the earliest animals. Proc Natl Acad Sci USA. 2014;111(11):4168–4172. doi: 10.1073/pnas.1400547111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knoll AH, Sperling EA. Oxygen and animals in Earth history. Proc Nat Acad Sci USA. 2014;111(11):3907–3908. doi: 10.1073/pnas.1401745111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owen T. The search for early forms of life in other planetary systems: future possibilities afforded by spectroscopic techniques. In: Papagiannis MD, editor. Strategies for the Search for Life in the Universe. Holland: D. Reidel Publishing Company, Dordrecht; 1980. pp. 177–185. [Google Scholar]
- 56.Napier WM. A mechanism for interstellar panspermia. Mon Not R Astron Soc. 2004;348(1):46–51. [Google Scholar]
- 57.Weber P, Greenberg JM. Can spores survive in interstellar space? Nature. 1985;316:403–407. [Google Scholar]
- 58.Adams FC, Spergel DN. Lithopanspermia in star-forming clusters. Astrobiology. 2005;5(4):497–514. doi: 10.1089/ast.2005.5.497. [DOI] [PubMed] [Google Scholar]
- 59.Belbruno E, Moro-Martín A, Malhotra R, Savransky D. Chaotic exchange of solid material between planetary systems: Implications for lithopanspermia. Astrobiology. 2012;12(8):754–774. doi: 10.1089/ast.2012.0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schidlowski M. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature. 1988;333(6171):313–318. [Google Scholar]
- 61.Mojzsis SJ, et al. Evidence for life on Earth before 3,800 million years ago. Nature. 1996;384(6604):55–59. doi: 10.1038/384055a0. [DOI] [PubMed] [Google Scholar]
- 62.Tice MM, Lowe DR. Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature. 2004;431(7008):549–552. doi: 10.1038/nature02888. [DOI] [PubMed] [Google Scholar]
- 63.Lazcano A, Miller SL. How long did it take for life to begin and evolve to cyanobacteria? J Mol Evol. 1994;39(6):546–554. doi: 10.1007/BF00160399. [DOI] [PubMed] [Google Scholar]
- 64.Orgel LE. The origin of life—how long did it take? Orig Life Evol Biosph. 1998;28(1):91–96. doi: 10.1023/a:1006561308498. [DOI] [PubMed] [Google Scholar]
- 65.Benner SA, Ricardo A, Carrigan MA. Is there a common chemical model for life in the universe? Curr Opin Chem Biol. 2004;8(6):672–689. doi: 10.1016/j.cbpa.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 66.Schulze-Makuch D, Grinspoon DH. Biologically enhanced energy and carbon cycling on Titan? Astrobiology. 2005;5(4):560–567. doi: 10.1089/ast.2005.5.560. [DOI] [PubMed] [Google Scholar]
- 67.Lunine JI. Saturn's Titan: A strict test for life's cosmic ubiquity. Proc Am Philos Soc. 2009;153(4):403–418. [Google Scholar]
- 68.Gilliam AE, McKay CP. Titan under a red dwarf star and as a rogue planet: Requirements for liquid methane. Planet Space Sci. 2011;59(9):835–839. [Google Scholar]
- 69.McKay CP, Anbar AD, Porco C, Tsou P. Follow the plume: The habitability of enceladus. Astrobiology. 2014;14(4):352–355. doi: 10.1089/ast.2014.1158. [DOI] [PubMed] [Google Scholar]
- 70.Reynolds RT, McKay CP, Kasting JF. Europa, tidally heated oceans, and habitable zones around giant planets. Adv Space Res. 1987;7(5):125–132. doi: 10.1016/0273-1177(87)90364-4. [DOI] [PubMed] [Google Scholar]
- 71.Cassidy TA, et al. Massive satellites of close-in gas giant exoplanets. Astrophys J. 2009;704(2):1341–1348. [Google Scholar]
- 72.Jessberger EK, Kissel J, Rahe J. The composition of comets. In: Atreya SK, Pollack JP, Matthews MS, editors. Origin and Evolution of Planetary and Satellite Atmospheres. Tucson, AZ: Univ of Arizona Press; 1989. pp. 167–191. [Google Scholar]
- 73.Chang S. 1981. Organic chemical evolution. Life in the Universe, ed Billingham J, pp 21–46. NASA Conf. Publ. 2156, Washington, DC.
- 74.Pollack JB, Black DC. Implications of the gas compositional measurements of pioneer venus for the origin of planetary atmospheres. Science. 1979;205(4401):56–59. doi: 10.1126/science.205.4401.56. [DOI] [PubMed] [Google Scholar]
- 75.Gautier D, Owen T. The composition of the outer planets' atmospheres. In: Atreya SK, Pollack JB, Matthews MS, editors. Origin and Evolution of Planetary and Satellite Atmospheres. Tucson, AZ: Univ of Arizona Press; 1989. pp. 487–512. [Google Scholar]
- 76.McKay CP. Urey Prize lecture: Planetary evolution and the origin of life. Icarus. 1991;91(1):93–100. doi: 10.1016/0019-1035(91)90128-g. [DOI] [PubMed] [Google Scholar]