Significance
Because of their promotional effects on plant growth and development, rare earth elements (REEs) have been widely used in agriculture as plant growth stimulants. However, little is known about the cellular basis of REE actions in plants, and the biological safety of farm REE application in agriculture has not yet attracted enough attention. Here, we show that two types of REEs entered plant cells by endocytosis, and that they both had an activating effect on endocytosis. Moreover, we found that a portion of REEs was finally deposited in plant cells. Our data thus provide novel insights into the cellular mechanisms of REE actions in plants, and may also serve as valuable documentation for evaluating the biological safety of REE application in agriculture.
Keywords: activation, endocytic vesicle
Abstract
It has long been observed that rare earth elements (REEs) regulate multiple facets of plant growth and development. However, the underlying mechanisms remain largely unclear. Here, using electron microscopic autoradiography, we show the life cycle of a light REE (lanthanum) and a heavy REE (terbium) in horseradish leaf cells. Our data indicate that REEs were first anchored on the plasma membrane in the form of nanoscale particles, and then entered the cells by endocytosis. Consistently, REEs activated endocytosis in plant cells, which may be the cellular basis of REE actions in plants. Moreover, we discovered that a portion of REEs was successively released into the cytoplasm, self-assembled to form nanoscale clusters, and finally deposited in horseradish leaf cells. Taken together, our data reveal the life cycle of REEs and their cellular behaviors in plant cells, which shed light on the cellular mechanisms of REE actions in living organisms.
Some trace elements, such as rare earth elements (REEs), have been observed for a long time to be beneficial to plant growth (1, 2). REEs are f-block elements in the periodic table, including 15 lanthanides, plus scandium and yttrium (2). The beneficial effect of REEs on plants was first reported in 1917, when it was found that cerium, an REE, improved physiological activities of water-floss (Spirogyra) (3). Since the 1970s, REEs have been widely used in agriculture as plant growth stimulants (4). Currently, more than 100 of crop species have been applied with REEs, which has increased crop yields by 5–15% (4). In addition, REEs also serve to protect plants against certain plant diseases and some stresses, such as drought, cold, acid rain, or heavy metals (2). The wide use of REEs has caused an overaccumulation of REEs in the ecosystem, including soil (5), atmosphere (6), and water (7). Moreover, it was shown that REEs have dual effects on plant growth: low concentrations of REEs exert positive effects on growth and yield of crops, whereas high concentrations of REEs seem to be harmful for plants, indicating that REEs are not absolutely good for plants (2). Therefore, it is imperative to elucidate how REEs act on plant cells to ensure food safety.
Despite of many advances achieved in recent years, the action mechanisms of REEs on plant cells still remain poorly understood (2). Several explanations, which are mainly focused on the physiological roles of REEs in plant cells, have been proposed (2). These mechanisms include the possible changes of photosynthesis, mineral nutrient uptake and metabolism, catalytic activities of some enzymes, hormonal balance, and so forth (2). However, these hypotheses are not connected to any cellular or molecular principles and are therefore far from explaining the action mechanisms of REEs in plants (2, 8). In addition, little consensus has yet been reached regarding the life cycle of REEs in plant cells. Some studies report that REEs cannot enter the plant cells but only be localized in apoplast (8–14); other studies, however, argue that REEs cannot only enter the plant cells, but also be deposited inside the cells, such as in the membranes of chloroplast, vacuole, cytoplasm, and nucleus (15–21). The discrepancy may be because of different experimental methods and different concentrations of REE ions used in separate studies. Therefore, it is evident that unraveling the complete life cycle of REEs in plant cells would contribute to a better understanding of the action mechanisms of REEs in plants.
Here, we report the life cycle of a light REE, lanthanum [La(III)], and a heavy REE, terbium [Tb(III)], and their cellular behaviors in horseradish leaf cells, using interdisciplinary methods. We show that these two different types of REEs were first anchored on the plasma membrane of horseradish cells in the form of nanoscale particles. Interestingly, the endocytosis of horseradish cells was then activated by these REE ions. In addition, we observed that these REEs subsequently entered the horseradish leaf cells by endocytosis, and that a portion of REEs were released into the cytoplasm, self-assembled, and formed into nanoscale clusters. The endocytosis gradually ceased with the migration of REEs from leaf cells to leaf stalk, root, and soil. Moreover, some of these REE-containing clusters were finally deposited in horseradish cells. Taking these data together, our study reveals that REEs may affect plant growth by activating endocytosis in plant cells.
Results and Discussion
To investigate how REEs affect the growth and development of plants, we determined the yield, growth, and physiological and biochemical responses of horseradish grown in the field and treated it with 13 different concentrations of LaCl3. Interestingly, we observed a maximum improvement of horseradish growth when 30–35 µM LaCl3 was applied (SI Appendix, Fig. S1 and Table S1). The improvements include increased net photosynthetic rate, chlorophyll content, horseradish peroxidase activity, yield, and decreased malondialdehyde content (SI Appendix, Table S1). However, when 80 µM or higher concentrations of LaCl3 were applied, horseradish growth was greatly inhibited (SI Appendix, Fig. S1 and Table S1). This pattern was consistent with those reported in other plants (2). Thus, 30 and 80 µM of LaCl3 were used hereafter in this study as low and high concentrations, respectively, to investigate how different doses of La(III) affect the cellular activities of horseradish leaf cells.
Next, we measured the distribution of La(III) in horseradish leaf cells by performing electron microscopic autoradiography (EMARG), in which 140La(III), a radioactive isotope of La(III), was used. We observed that 12 h after the horseradish leaves were treated with 30 μM La(III), 140La(III), in the form of La(III)-containing nanoscale particles, was anchored on the plasma membranes of the leaf cells (SI Appendix, Fig. S2B). Interestingly, the same amount of time (12 h) after the horseradish leaves were treated with 80 μM La(III), we observed a large number of La(III)-containing nanoscale particles along the plasma membrane, and unexpectedly, we observed the invaginations of the plasma membrane (indicated by arrows, SI Appendix, Fig. S2C). These invaginations were reminiscent of what was observed in the first stage of endocytosis proposed in animals or plants (22–24). Because the endocytic activities in plant cells remain relatively low (24, 25), we speculate that high concentration (80 μM) of La(III) treatment might change the endocytosis of horseradish leaf cells.
La(III) Treatments Activated Endocytosis in Plant Cells.
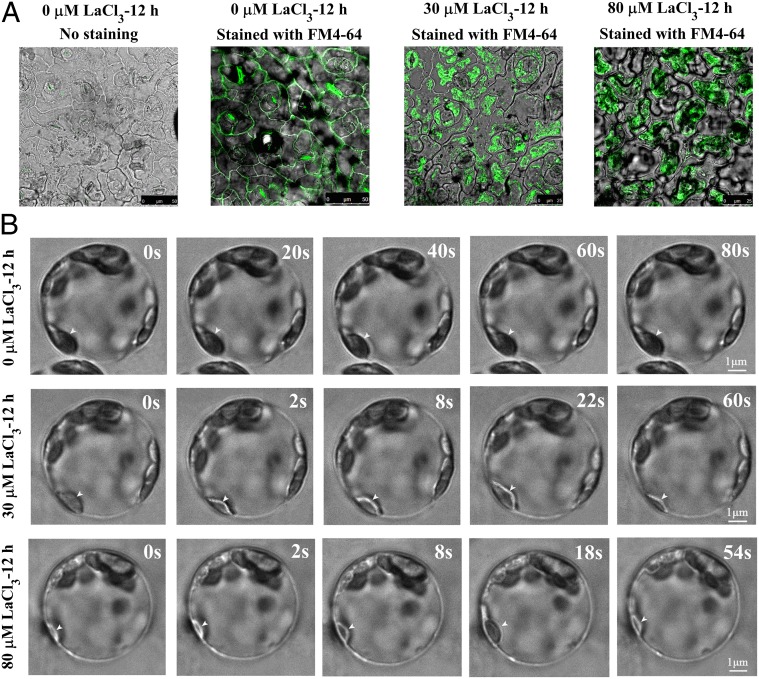
We used the FM4-64 fluorescent dye to visualize the endocytosis in horseradish leaf cells 12 h after the LaCl3 treatments. It was first found that FM4-64 was mainly distributed on the plasma membrane of the control cells (Fig. 1A). In contrast, after the treatment of 80 μM LaCl3, FM4-64 was internalized into the treated cells (Fig. 1A), indicating that high concentration of LaCl3 treatment may activate endocytosis. Unexpectedly, we observed that FM4-64 was also internalized into the cells treated with 30 μM LaCl3 (Fig. 1A). We repeated these assays in Arabidopsis, and made the same observations (SI Appendix, Fig. S3). Taken together, our data imply that both low and high concentrations of LaCl3 may activate endocytosis in plant cells.
Fig. 1.
LaCl3 treatment activated endocytosis. (A) Confocal laser scanning microscopy images of the control horseradish leaf (no staining), the control horseradish leaf stained with FM4-64, and the horseradish leaves 12 h after the treatment of 30 or 80 μM LaCl3 and then stained with FM4-64. (B) Microscopic images of horseradish protoplasts 12 h after the treatment of 0, 30, or 80 μM LaCl3. The images were taken from Movie S1.
Next, we used a new method established recently (26, 27) to visualize the dynamic processes of endocytosis 12 h after the horseradish leaves were treated with 30 or 80 μM LaCl3. Movies S1–S5 were then made after the bright-field images of horseradish and Arabidopsis protoplasts were collected at one frame every 2 s using a total internal reflection fluorescence microscope. The dark chloroplasts shown in the images were used as the background allowing the visibility of endocytic vesicles (the color of which was lighter) formed above the chloroplasts. As shown in Fig. 1B and Movie S1, we failed to observe the formation of any endocytic vesicles in the control protoplasts with prolonged incubation time, suggesting that endocytosis was relatively slow without LaCl3 treatment. Surprisingly, we repeatedly observed the formation of endocytic vesicles in horseradish protoplasts treated with 30 μM LaCl3, and a typical endocytic process lasted ∼60 s (Fig. 1B, SI Appendix, Table S2, and Movie S1). The activation of endocytosis was even more evident with 80 μM LaCl3 treatment, under which a typical endocytic process lasted ∼54 s (Fig. 1B, SI Appendix, Table S2, and Movie S1). We repeated the assays using the Arabidopsis protoplasts and found similar stimulating effect of 80 μM LaCl3 treatment on endocytosis in Arabidopsis (SI Appendix, Fig. S4 and Movie S2). Taken together, our results clearly showed that LaCl3 treatment indeed activated endocytosis in plant cells, and that higher concentration of LaCl3 treatment usually had a stronger effect.
To further confirm that the activating effect of endocytosis was caused by La3+ but not Cl−, we repeated the experiments by using 80 μM La(NO3)3, FeCl3, and AlCl3. It was shown that La(NO3)3 treatment had an effect similar to LaCl3 in activating endocytosis of horseradish leaf cells (SI Appendix, Fig. S5A and Table S2, and Movie S3). In contrast, the treatments of 80 μM FeCl3 and AlCl3 had no obvious effects on the formation of endocytic vesicles (SI Appendix, Fig. S5B and Table S2, and Movie S4). Thus, these observations indicated that La(III) indeed activates endocytosis in horseradish leaf cells, and that this activating effect is unique to La(III) but not to Fe(III) and Al(III), although they are all trivalent ions. Moreover, the activating effect was only seen within a short period (e.g., 12 h) after the LaCl3 treatment, whereas after a long period (e.g., 48 h), no evident endocytosis was observed for both low and high concentrations of LaCl3 treatment (SI Appendix, Fig. S6 and Movie S5).
Life Cycle of La(III) in Horseradish Leaf Cells.
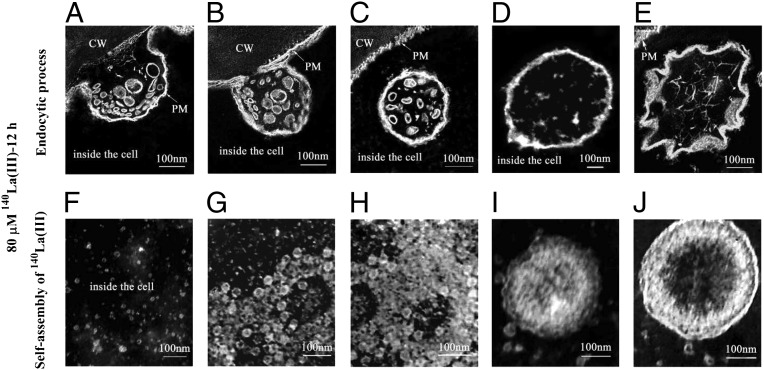
Because our EMARG assays likely captured the initiation of endocytic processes in horseradish leaf cells treated with 80 μM La(III) (SI Appendix, Fig. S2C), we used this technique again to investigate whether La(III) could enter the plant cells and, if so, how it behaves inside the horseradish cells. We thus performed the EMARG assays using the horseradish leaves treated with 80 μM La(III). We repeated the assays ∼20 times, and similar observations were made, as described: (i) 12 h after the La(III) treatment, the plasma membrane was first invaginated and the invaginations encapsulated 140La(III)-containing nanoscale particles, the diameters of which were around 3–60 nm (Fig. 2A); (ii) the invaginations then developed into buds, which contained large numbers of 140La(III)-containing nanoscale particles (Fig. 2B); (iii) the buds were pinched off as 280-nm endocytic vesicles and released into the cytoplasm (Fig. 2C); (iv) the 280-nm endocytic vesicles were possibly merged with lysomes or lytic vacuoles to form the 600-nm vesicles (Fig. 2D) (22–24). These four steps may represent a typical endocytic process observed in animals or plants (22–24). Unexpectedly, a special type of “gear wheel-like” vesicle, the diameter of which was around 300–620 nm (Fig. 2E), was also observed. This unusual type of vesicle accounts for around 10% of all vesicles observed inside a cell. They may also originate from the fusion of 280-nm endocytic vesicles with lysosomes or lytic vacuoles according to their sizes (22–24). However, the mechanisms responsible for the origin of this type of vesicle await further investigation. Taking these data together, we conclude that La(III) could enter the horseradish leaf cells by endocytosis.
Fig. 2.
Images of endocytic processes and self-assembly of 140La(III) in horseradish leaf cells 12 h after the 80-μM 140La(III) treatment. (A) The invagination of the plasma membrane encapsulated the 140La(III)-containing nanoscale particles. (B) The invagination developed further into bud. (C) The 280-nm endocytic vesicle was released into the cytoplasm. (D) The 600-nm vesicle contained 140La(III)-containing nanoscale particles. (E) The 300–620 nm special type of gear wheel-like vesicle. (F) A large amount of 140La(III)-containing nanoscale particles in the cytoplasm. (G–I) The self-assembly of 140La(III)-containing nanoscale particles. (J) The nanoscale clusters in the cytoplasm.
Surprisingly, we observed a large amount of 140La(III)-containing nanoscale particles in the cytoplasm (Fig. 2F), indicating that 140La(III) were released into the cytoplasm from endocytic vesicles. Because it has never been observed that normal (280- or 600- nm) endocytic vesicles could be disintegrated, and because the membranes of the special gear wheel-like vesicles were asymmetric and nonuniform, we propose that this special type of vesicle may finally be disintegrated and release their contents into the cytoplasm. Interestingly, these cytoplasmic 140La(III)-containing particles were likely self-assembled (Fig. 2 G–I) and finally formed into nanoscale clusters (Fig. 2J); this self-assembly process of 140La(III)-containing particles in the cytoplasm of plant cells was similar as that of natural macromolecules in vitro (28–30).
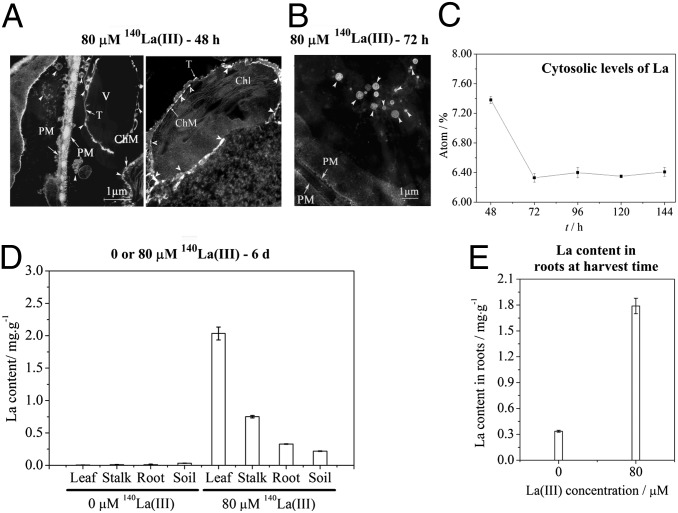
Forty-eight hours after the 80-μM La(III) treatment, we observed that 140La(III) was localized on the cell membranes, including the plasma membrane, vacuole membrane, and chloroplast membrane (Fig. 3A), which was in agreement with our previous observations (18). Moreover, when the incubation time was extended to 72 h, we found that some of 140La(III) nanoscale clusters were eliminated from the cytoplasm and the amount of 140La(III) deposited on the cell membranes and intracellular space was obviously decreased compared with that at 48 h (Fig. 3B). This decrease may be in part because of the weakened radioactivity of 140La(III), the half-life of which was 40.22 h. Therefore, we measured the cytosolic content of La by using scanning electron microscope (SEM) and energy-dispersive X-ray spectroscopy (EDS) techniques so as to accurately determine the content of La inside the cell 48 h after the La(III) treatment. Our results showed that the cytosolic La content was indeed decreased in horseradish leaf cells 72 h after the 80-μM 140La(III) treatment relative to that at 48 h; however, it remained relatively unchanged for longer incubation after 72 h (Fig. 3C). We measured the La content in leaves, leaf stalks, roots, and soil 6 d after the 80-μM 140La(III) treatment. Notably, high levels of La were detected in samples from the treated horseradish compared with those from the control plants (Fig. 3D). We also examined the La content in field-grown horseradish treated with 80 μM La(III), and ∼1.8 mg/g (six-times of the control plants) was detected 6 mo after the La(III) treatment (Fig. 3E). These data indicate: (i) La(III) could be transported from the treated leaves to roots, and finally to soil; and (ii) a portion of La(III) finally remained in treated horseradish a long time after the treatment.
Fig. 3.
Migration of La(III) in horseradish 48 h or longer time after La(III) treatment. (A and B) Images of horseradish leaf cells 48 (A) or 72 h (B) after the treatment of 80 μM 140La(III). (C) Cytosolic levels of La based on EDS spectra after different hours of 80 μM 140La(III) treatment. (D) The contents of La in different organs (leaf, leaf stalk, root) of horseradish and soil 6 d after horseradish was treated with 0 or 80 μM 140La(III). (E) The contents of La in roots of horseradish at harvest time. Values are means ± SD (n = 10). Chl, chloroplast; ChM, chloroplast membrane; PM, plasma membrane; T, tonoplast; V, vacuole.
La(III) Treatment Triggered Complex Physiological Responses in Horseradish Leaf Cells.
To investigate how La(III) treatments induced the cellular responses in horseradish leaf cells, we next compared the levels of several components outside and inside the cells treated with or without LaCl3. Cell-surface carbohydrates (such as mannosyl groups) play important roles in cell growth and development (31, 32). Therefore, we determined the surface mannosyl group levels of horseradish protoplasts using differential pulse voltammetry (DPV), a highly sensitive and convenient technique for evaluating surface mannosyl groups on animal cells (33). We first showed that DPV could also be used to measure the surface mannosyl groups of horseradish protoplasts, despite of the large differences in integrin between animal and plant cells (SI Appendix, Fig. S7A). Then, we showed that 12 h after 30- or 80-μM La(III) treatment, the level of surface mannosyl groups was increased or decreased by 23.28% or 13.65%, respectively, compared with that of the control (curve ii or iii in SI Appendix, Fig. S7 B and D). Moreover, the DPV curve of 80-μM La(III) treatment was obviously left-shifted (curve iii in SI Appendix, Fig. S7B), indicating that the microstructure of the mannosyl groups may be changed (33). Ca2+ and K+ are essential for almost all life activities of plant cells, and can be absorbed through the plasma membrane (34, 35). By using the DPV technique, we showed that 12 h after the 30-µM La(III) treatment, the K+ and Ca2+ levels on the protoplast surface were both decreased relative to the control cells (SI Appendix, Fig. S7 F and I); at the same time after the 80-µM La(III) treatment, the Ca2+ level was still decreased, whereas the K+ level was increased (SI Appendix, Fig. S7 F and I). The biological significance underlying the differential regulations of Ca2+ and K+ on the protoplast surface by La(III) remains to be elucidated.
Next, we determined the chemical compositions of the fatty acids in the plasma membrane of horseradish leaf cells treated with or without LaCl3 by using the gas chromatography technique. As shown in SI Appendix, Fig. S8A and Table S3, 12 h after the 30-μM La(III) treatment, the percentage contents of linoleic acid and linolenic acid, and the index of unsaturated fatty acids (IUFA) in horseradish leaf cells were increased compared with those of the control cells, indicating that 30-μM La(III) treatment may improve the fluidity of the plasma membrane and inhibit membrane lipid peroxidation (36). However, 12 h after the 80-μM La(III) treatment, the total percentage contents of saturated fatty acids (14:0, 16:0, 17:0, 18:0) were increased, whereas those of unsaturated fatty acids (16:1, 18:1, 18:2, 18:3) (especially linolenic acid) and IUFA were decreased, compared with those of the control cells (SI Appendix, Fig. S8A and Table S3), indicating that 80-μM La(III) treatment may lead to the decrease in the fluidity and the damage of the plasma membrane (37), consistent with our observations shown in SI Appendix, Table S1.
We finally measured the levels of intracellular Ca2+, main nutrient elements, and DNA to gain more insights into the intracellular activities induced by La(III) treatment in plant cells. Our results showed that 12 h after horseradish was treated with 30 µM La(III), the levels of these tested intracellular components were increased, except that the Cu level was decreased, compared with those of the control cells (SI Appendix, Fig. S9 and Table S4). However, 12 h after the 80-µM La(III) treatment, the intracellular levels of free Ca2+, some of nutrient elements (P, S, Ca, Cu), and DNA were increased (SI Appendix, Fig. S9 and Table S4), whereas the intracellular levels of some other nutrient elements (N, K, Mg, Fe, and Zn) were decreased (SI Appendix, Table S4).
Taken together, our data show that La(III) treatment triggered complex responses outside and inside the cells. However, after a long period (e.g., 6 d), the effects of both low and high concentrations of La(III) treatment on protoplast surface mannosyl groups, extracellular K+, IUFA, and intracellular Ca2+ became weaker (SI Appendix, Figs. S7–S9 and Tables S3 and S4). These observations are consistent with lower levels of cytosolic La (Fig. 3C) and endocytic activities at 6 d (SI Appendix, Fig. S6), possibly because of the transport of La(III) from leaves to soil (Fig. 3D).
La(III) Treatment Affected Cell Expansion in Horseradish.
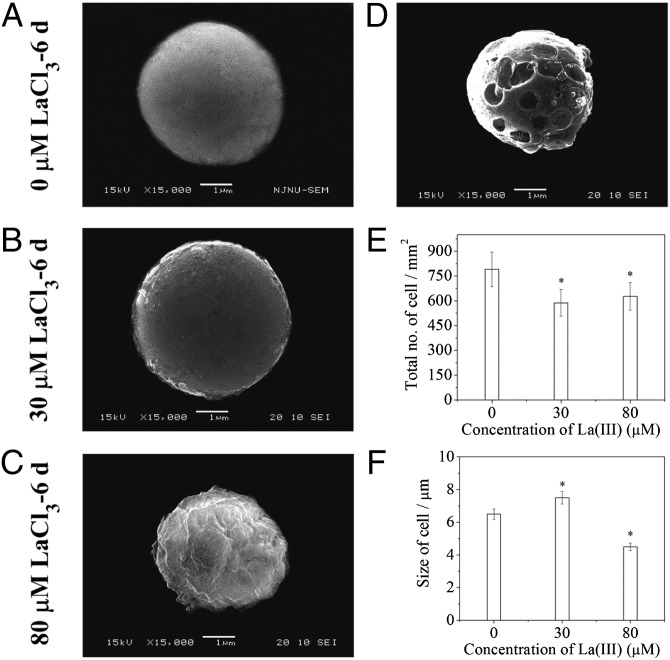
Next, we determined the size and number of horseradish leaf cells to investigate how La(III) affects cell expansion. Our SEM data showed that after the treatment of 30 μM La(III), the average size of cells became larger than that of the control cells (Fig. 4 A, B, and F). Meanwhile, the number of cells per square millimeter area in the treated horseradish leaf became less compared with the control (Fig. 4E), indicating that 30-μM La(III) treatment promoted cell expansion in horseradish. However, after horseradish was treated with 80 μM La(III), the cell size and number were both decreased relative to the control cells (Fig. 4 A, C, E, and F). In addition, we noticed that the surface of horseradish leaf cells became uneven after the 80 μM La(III) treatment (Fig. 4C), and surprisingly, 300 μM La(III) treatment led to the damage of cells (Fig. 4D). Thus, our data revealed the dual effects of La(III) on horseradish cell growth: low concentration of La(III) treatment promoted cell expansion, whereas high concentration of La(III) inhibited cell expansion or even caused cell damage. These findings may partially explain the observations shown in SI Appendix, Fig. S1 and Table S1.
Fig. 4.
Number and size of horseradish leaf cells 6 d after the LaCl3 treatment. (A–C) SEM images of protoplasts treated with 0 (A), 30 (B), or 80 μM (C) LaCl3. (D) SEM image of a protoplast treated with 300 μM LaCl3. (E and F) Number (E) and average size (F) of cells within 1 mm2 of the fully expanded fourth true leaf of horseradish treated with 0, 30, or 80 μM LaCl3. Values are means ± SD (n = 10). *P < 0.05 (Student t test) for the differences between control and LaCl3-treated groups.
Tb(III) Showed Similar Cellular Behaviors and Life Cycle as La(III) in Horseradish Leaf Cells.
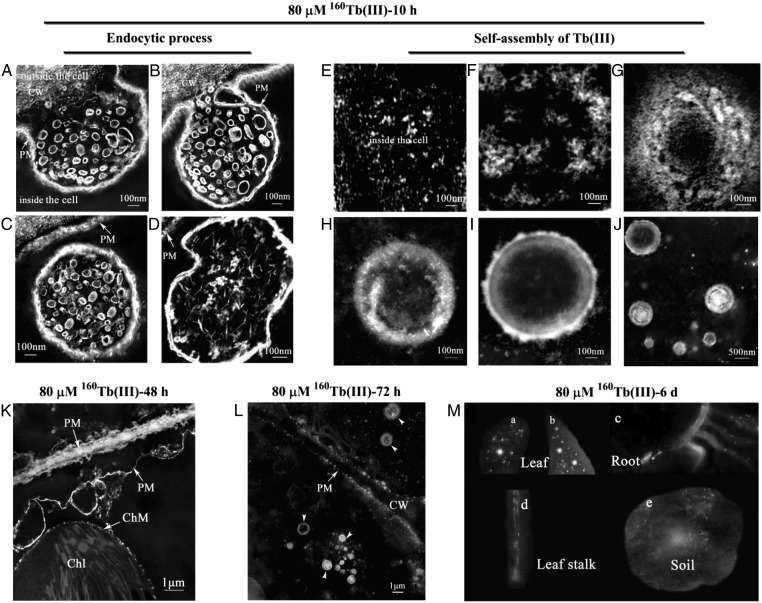
REEs are often divided to light REEs (e.g., La) and heavy REEs (e.g., Tb). To further investigate how REEs affect the growth and development of plant cells, we studied the cellular activities of a type of heavy REE ion, Tb(III), in horseradish leaf cells using the EMARG technique. Interestingly, we observed similar cellular behaviors and life cycle of Tb(III) as those of La(III) in horseradish leaf cells: (i) Tb(III) was first localized on the plasma membrane, then entered the cells by endocytosis in the form of Tb(III)-containing nanoscale particles (Fig. 5 A–C and SI Appendix, Fig. S10); (ii) irregular type of endocytic vesicles was also observed (Fig. 5D); and (iii) a portion of Tb(III)-containing nanoscale particles were released into the cytoplasm, then self-assembled to form clusters, and finally deposited in horseradish cells (Fig. 5 E–M and SI Appendix, Fig. S10). These observations strongly indicate that different types of REEs may act through similar mechanisms in plant cells.
Fig. 5.
The life cycle of 160Tb(III) in horseradish leaf cells. (A–D) Images of endocytic process in horseradish leaf cells 10 h after the 80 µM 160Tb(III) treatment: (A) the invagination of the plasma membrane encapsulated the 160Tb(III)-containing nanoscale particles; (B) the invagination developed further into bud; (C) the 1,000-nm endocytic vesicle was released into the cytoplasm; (D) the irregular type of vesicle (1,000–2,000 nm) in the cytoplasm. (E–J) Self-assembly of 160Tb(III)-containing nanoscale particles in the cytoplasm of horseradish 10 h after the 80 µM 160Tb(III) treatment: (E) a large amount of 160Tb(III)-containing nanoscale particles in the cytoplasm; (F–H) the self-assembly of 160Tb(III)-containing nanoscale particles; (I) the nanoscale cluster in the cytoplasm; (J) the nanoscale clusters of different diameters in the cytoplasm. (K and L) Images of horseradish cells 48 (K) or 72 h (L) after the 80 µM 160Tb(III) treatment. (M) Images of leaf, leaf stalk, root and soil 6 d after horseradish was treated with 160Tb(III). Chl, chloroplast; ChM, chloroplast membrane; CW, cell wall; PM, plasma membrane.
It has long been observed that REEs could affect the growth and yield of plants in the field (2). However, little is known about the cellular basis of REE actions in plants. In this study, we used two different types of REEs to investigate their respective effects on cellular activities, but discovered similar life cycles and cellular behaviors in plant cells. These data strongly suggest that our conclusions may represent the general action mechanisms for all REEs in plant cells. Specifically, we found that REE treatments activated the endocytic process in plant cells, which may serve as the cellular basis of REE actions in plants. Moreover, we show that La(III) treatments improved at low concentration—but inhibited at high concentration—the expansion and growth of horseradish cells (Fig. 4), which is consistent with the actual effects of different concentrations of REEs on horseradish growth in the field (SI Appendix, Fig. S1). Therefore, our study provides novel insights into the cellular mechanisms of REEs in plants, which may be extrapolated to other nonplant organisms, as it was shown that certain REE treatments also promoted or inhibited the growth of animal cells (2).
At the same time, it should be pointed out that although REEs have been widely applied in agriculture, no related regulations or standards have been established thus far regarding the limitations or dosage of REE application. In this study, we found that although both low and high concentrations of La(III) treatments activated endocytosis in plant cells, La(III) entered the cells (by endocytosis) only when high concentration of La(III) was applied (Fig. 2 and SI Appendix, Fig. S2). In addition, we showed that after horseradish leaves were treated with high concentration of La(III) or Tb(III), these different types of REEs were partially released into the cytoplasm, and finally deposited in horseradish leaf cells. Our study, therefore, has provided statistical evidence as well as biological basis for establishing the related international standards of REE application, and may also serve as a valuable documentation to evaluate the biological safety of REE application in agriculture.
Materials and Methods
Chemicals.
The 140La2O3 and 160Tb4O7 powders were purchased from Beijing Atom High Tech and used to prepare for 140La(NO3)3 and 160Tb(NO3)3 by dissolving in the concentrated HNO3 and H2O2 under heated conditions. The nuclear emulsion was purchased from the Technical Institute of Physics and Chemistry, China. All other chemicals were purchased from Aldrich Chemical.
Pot Experiment.
Horseradish tubers were obtained from the Planting Base of Exported Horseradish. The treated tubers were soaked in 30 or 80 µM LaCl3 solution, respectively, at 25 °C for 48 h, whereas the control tubers were soaked in deionized water. Subsequently, the tubers were planted in 30 × 30-cm2 pots without any fertilizer, and the soil was similar to that used in the field plot experiment. Horseradish plants were grown in a glasshouse at 20–25 °C under a 16-h photoperiod with 300 µmol⋅m−2⋅s−1 irradiance and 70% relative humidity. At the stage of four leaves, horseradish plants were sprayed once with 30- or 80-µM LaCl3 solutions, respectively, and the control plants were sprayed with deionized water. Twelve hours and 6 d after the spray, the fresh leaves treated with or without LaCl3 were collected for the measurements.
EMARG Measurement of Subcellular Distribution of La(III).
Horseradish leaves were treated with La(III), in which the total concentration of La(III) was 30 or 80 μM, and 140La(III) radioactivity was 75 μCi⋅mL−1. Then, the leaves were cut into ultrathin (∼60 nm thick) 1.5 × 2-mm sections using the Reichert-Jung Ultracut E ultramicrotome. After the nuclear emulsion was painted on the leaf section, followed by the development and fixation, the subcellular distribution of La(III) was observed with transmission electron microscope (H-600-A-2, Hitachi) (38, 39).
Confocal Laser Scanning Microscopy Observation.
For FM4-64 staining, horseradish leaves treated with 0, 30, or 80 μM LaCl3 were transferred to 2.5 µM FM4-64 diluted with deionized water for 3 min. Then these leaves were observed under confocal laser scanning microscopy (Zeiss).
Total Internal Reflection Fluorescence Microscope Observation of Protoplast Endocytosis.
The endocytosis of isolated protoplasts treated with 0, 30, or 80 μM LaCl3 was observed using a total internal reflection fluorescence microscope (Olympus) with high spatial and temporal resolutions, as previously reported (26, 27). Bright-field movies (∼30 images) were collected at one frame every 2 s using an Olympus microscope equipped with a 100× objective, as previously reported (26, 27).
Cell Number and Size Analyses.
The fourth fully expanded true leaves of horseradish were fixed in 100 mM Na-phosphate buffer (pH 7.0) containing 4% (vol/vol) glutaraldehyde. After dehydration with an ethanol series, the samples were infiltrated and embedded in paraffin, and then were cut into sections. The number and size of cells were counted or measured in the same area (70 mm × 90 mm) under a microscope (Olympus).
SEM Observation.
The isolated protoplasts were fixed for 2 h with 1% glutaraldehyde in 50 mL buffer solution (0.8 M mannitol and 50 mM Tris⋅HCl, pH 6.8). The samples were washed several times with the 50 mM buffer solution and then dried supercritically. The dried samples were mounted over the stubs with double-sided conductivity tape, and a thin layer of gold metal was applied over the samples using an automated sputter coater (K500X, Quorum Technologies). The samples were examined under low vacuum with secondary electron detector at 15 kV using SEM (JSM-5600LV, JEOL) at various magnifications.
Supplementary Material
Acknowledgments
We thank Jiemin Zhou (University of British Columbia) for her excellent work in radiation cytology; Fan Jin (University of Science and Technology of China) for providing the measurement technique of dynamic endocytosis; and Erkang Wang and Shaojun Dong (Chinese Academy of Sciences), Min Wang (Nanjing University of Aeronautics and Astronautics), and Chunhui Huang (Peking University) for their valuable suggestions on the project. This work was supported by the Natural Science Foundation of China (21371100, 31170477), PhD Programs Foundation of Ministry of Education of China (20130093120006), the Foundation of State Developing and Reforming Committee (GFZ2071609), the Natural Science Foundation of Jiangsu Province (BK2011160), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413376111/-/DCSupplemental.
References
- 1.Feng R, Wei C, Tu S. The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot. 2013;87(0):58–68. [Google Scholar]
- 2.Redling K. 2006. Rare earth elements in agriculture with emphasis on animal husbandry. PhD dissertation (Ludwig-Maximilians-Universität München, Munich, Germany)
- 3.Chien SQ, Ostenhout WJ. Physiological function of Ba, Sr, and Ce on water-floss (Spirogyra) Baranical Gazette. 1917;63:406–409. [Google Scholar]
- 4.Hu ZY, Richter H, Sparovek G, Schnug E. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: A review. J Plant Nutr. 2004;27(1):183–220. [Google Scholar]
- 5.Biasioli M, Fabietti G, Barberis R, Ajmone-Marsan F. An appraisal of soil diffuse contamination in an industrial district in northern Italy. Chemosphere. 2012;88(10):1241–1249. doi: 10.1016/j.chemosphere.2012.03.081. [DOI] [PubMed] [Google Scholar]
- 6.Moreno T, et al. Lanthanoid geochemistry of urban atmospheric particulate matter. Environ Sci Technol. 2008;42(17):6502–6507. doi: 10.1021/es800786z. [DOI] [PubMed] [Google Scholar]
- 7.Kato Y, et al. Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements. Nat Geosci. 2011;4(8):535–539. [Google Scholar]
- 8.Wyttenbach A, Tobler L, Furrer V. The concentration of rare earth elements in plants and in the adjacent soils. J Radioanal Nucl Chem. 1996;204(2):401–413. [Google Scholar]
- 9.Nagahashi G, Thomson WW, Leonard RT. The casparian strip as a barrier to the movement of lanthanum in corn roots. Science. 1974;183(4125):670–671. doi: 10.1126/science.183.4125.670. [DOI] [PubMed] [Google Scholar]
- 10.Quiquampoix H, Ratcliffe RG, Ratković S, Vučinić Ž. 1H and 31P NMR investigation of gadolinium uptake in maize roots. J Inorg Biochem. 1990;38(4):265–275. [Google Scholar]
- 11.Ma Y, et al. Phytotoxicity and biotransformation of La2O3 nanoparticles in a terrestrial plant cucumber (Cucumis sativus) Nanotoxicology. 2011;5(4):743–753. doi: 10.3109/17435390.2010.545487. [DOI] [PubMed] [Google Scholar]
- 12.Han F, et al. Organic acids promote the uptake of lanthanum by barley roots. New Phytol. 2005;165(2):481–492. doi: 10.1111/j.1469-8137.2004.01256.x. [DOI] [PubMed] [Google Scholar]
- 13.Bingham D, Dobrota M. Binding of lanthanides to cell membranes in the presence of ligands. J Inorg Biochem. 1995;59(1):39–52. doi: 10.1016/0162-0134(94)00053-d. [DOI] [PubMed] [Google Scholar]
- 14.Yuan L, Du P, Wang K, Yang XG. Uptake of diterbium transferrin, a potential multi-photon-excited microscopy probe, into human leukemia K562 cells via a transferrin-receptor-mediated process. J Biol Inorg Chem. 2009;14(8):1243–1251. doi: 10.1007/s00775-009-0567-8. [DOI] [PubMed] [Google Scholar]
- 15.Wei ZG, et al. Subcellular and molecular localization of rare earth elements and structural characterization of yttrium bound chlorophyll a in naturally grown fern Dicranopteris dichotoma. Microchem J. 2005;80(1):1–8. [Google Scholar]
- 16.Zhang Z, Wang Y, Li F, Chai Z. Determination of rare earth elements in chloroplasts of Brassia napus by INAA and biochemical separation techniques. J Radioanal Nucl Chem. 2001;247(3):557–560. [Google Scholar]
- 17.Li Z, et al. Direct measurement of lanthanum uptake and distribution in internodal cells of Chara. Plant Sci. 2008;174(5):496–501. [Google Scholar]
- 18.Wang L, Zhou Q, Lu T, Ding X, Huang X. Molecular and cellular mechanism of the effect of La(III) on horseradish peroxidase. J Biol Inorg Chem. 2010;15(7):1063–1069. doi: 10.1007/s00775-010-0665-7. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Zhou Q, Lu T, Fang M, Huang X. Distribution and translocation of 141Ce(III) in horseradish. Ann Bot (Lond) 2007;100(7):1459–1465. doi: 10.1093/aob/mcm244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Zhou Q, Zhao B, Huang X. Toxic effect of heavy metal terbium ion on cell membrane in horseradish. Chemosphere. 2010;80(1):28–34. doi: 10.1016/j.chemosphere.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 21.Guo S, et al. One of the possible mechanisms for the inhibition effect of Tb(III) on peroxidase activity in horseradish (Armoracia rusticana) treated with Tb(III) J Biol Inorg Chem. 2008;13(4):587–597. doi: 10.1007/s00775-008-0347-x. [DOI] [PubMed] [Google Scholar]
- 22.Sorkin A, von Zastrow M. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Šamaj J, Baluška F, Menzel D. Plant endocytosis. In: Robinson DG, editor. Plant Cell Monographs. Vol 1. Springer, Heidelberg; 2006. [Google Scholar]
- 24.Samaj J, Read ND, Volkmann D, Menzel D, Baluska F. The endocytic network in plants. Trends Cell Biol. 2005;15(8):425–433. doi: 10.1016/j.tcb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Buchanan B, Gruissem W, Jones R. Biochemistry and Molecular Biology of Plants. Rockville, MD: John Wiley & Sons; 2000. [Google Scholar]
- 26.Gibiansky ML, et al. Bacteria use type IV pili to walk upright and detach from surfaces. Science. 2010;330(6001):197. doi: 10.1126/science.1194238. [DOI] [PubMed] [Google Scholar]
- 27.Jin F, Conrad JC, Gibiansky ML, Wong GCL. Bacteria use type-IV pili to slingshot on surfaces. Proc Natl Acad Sci USA. 2011;108(31):12617–12622. doi: 10.1073/pnas.1105073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding D, et al. Nanospheres-incorporated implantable hydrogel as a trans-tissue drug delivery system. ACS Nano. 2011;5(4):2520–2534. doi: 10.1021/nn102138u. [DOI] [PubMed] [Google Scholar]
- 29.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277(5330):1232. [Google Scholar]
- 30.Slingsby C, Clark AR. Flexible nanoassembly for sequestering non-native proteins. Structure. 2013;21(2):193–194. doi: 10.1016/j.str.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Velasquez SM, et al. O-glycosylated cell wall proteins are essential in root hair growth. Science. 2011;332(6036):1401–1403. doi: 10.1126/science.1206657. [DOI] [PubMed] [Google Scholar]
- 32.Meier S, Duus J. Carbohydrate dynamics: Antibody glycans wiggle and jiggle. Nat Chem Biol. 2011;7(3):131–132. doi: 10.1038/nchembio.526. [DOI] [PubMed] [Google Scholar]
- 33.Cheng W, Ding L, Lei J, Ding S, Ju H. Effective cell capture with tetrapeptide-functionalized carbon nanotubes and dual signal amplification for cytosensing and evaluation of cell surface carbohydrate. Anal Chem. 2008;80(10):3867–3872. doi: 10.1021/ac800199t. [DOI] [PubMed] [Google Scholar]
- 34.Bauer P, Elbaum R, Weiss IM. Calcium and silicon mineralization in land plants: Transport, structure and function. Plant Sci. 2011;180(6):746–756. doi: 10.1016/j.plantsci.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Szczerba MW, Britto DT, Kronzucker HJ. K+ transport in plants: Physiology and molecular biology. J Plant Physiol. 2009;166(5):447–466. doi: 10.1016/j.jplph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Murata N, Los DA. Membrane fluidity and temperature perception. Plant Physiol. 1997;115(3):875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millar AA, Wrischer M, Kunst L. Accumulation of very-long-chain fatty acids in membrane glycerolipids is associated with dramatic alterations in plant morphology. Plant Cell. 1998;10(11):1889–1902. doi: 10.1105/tpc.10.11.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feig S, Harting JK. Ultrastructural studies of the primate parabigeminal nucleus: electron microscopic autoradiographic analysis of the tectoparabigeminal projection in Galago crassicaudatus. Brain Res. 1992;595(2):334–338. doi: 10.1016/0006-8993(92)91068-p. [DOI] [PubMed] [Google Scholar]
- 39.Chuartzman SG, et al. Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell. 2008;20(4):1029–1039. doi: 10.1105/tpc.107.055830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.