Abstract
Citrobacter rodentium, a murine model pathogen for human enteropathogenic Escherichia coli, predominantly colonizes the lumen and mucosal surface of the colon and cecum and causes crypt hyperplasia and mucosal inflammation. Mice infected with C. rodentium develop a secretory immunoglobulin A (IgA) response, but the role of B cells or secretory antibodies in host defense is unknown. To address this question, we conducted oral C. rodentium infections in mice lacking B cells, IgA, secreted IgM, polymeric Ig receptor (pIgR), or J chain. Normal mice showed peak bacterial numbers in colon and feces at 1 week and bacterial eradication after 3 to 4 weeks. B-cell-deficient mice were equally susceptible initially but could not control infection subsequently. Tissue responses showed marked differences, as infection of normal mice was accompanied by transient crypt hyperplasia and mucosal inflammation in the colon and cecum at 2 but not 6 weeks, whereas B-cell-deficient mice had few mucosal changes at 2 weeks but severe epithelial hyperplasia with ulcerations and mucosal inflammation at 6 weeks. The functions of B cells were not mediated by secretory antibodies, since mice lacking IgA or secreted IgM or proteins required for their transport into the lumen, pIgR or J chain, cleared C. rodentium normally. Nonetheless, systemic administration of immune sera reduced bacterial numbers significantly in normal and pIgR-deficient mice, and depletion of IgG abrogated this effect. These results indicate that host defense against C. rodentium depends on B cells and IgG antibodies but does not require production or transepithelial transport of IgA or secreted IgM.
Enteropathogenic Escherichia coli (EPEC) strains are an important cause of diarrheal disease, particularly in young children in developing countries (26). EPEC strains colonize the intestinal mucosa and, by subverting intestinal epithelial cell function, produce a characteristic histopathological feature known as the attaching and effacing (A/E) lesion (26). The A/E lesion is characterized by localized destruction (effacement) of brush border microvilli, intimate attachment of the bacterium to the apical host cell membrane, and formation of an underlying pedestal-like structure in the host cell (39). EPEC strains typically do not invade deeper layers of the mucosa or spread systemically.
Citrobacter rodentium (initially termed Citrobacter freundii biotype 4280) is a murine bacterial pathogen that shares important functional and structural similarities with clinical EPEC isolates (5, 20, 21, 31, 32). C. rodentium produces A/E lesions in the colon indistinguishable from those of clinical EPEC strains (15), and the gene coding for the outer membrane protein responsible for intimate attachment, intimin, is functionally homologous in C. rodentium and clinical EPEC strains (5). Furthermore, the murine and human infections with these pathogens are characterized by similar antibody responses to the bacteria (5, 26).
Infection of mice with C. rodentium causes crypt hyperplasia, loss of goblet cells, and mucosal infiltration with macrophages, lymphocytes, and neutrophils (1, 2, 13, 31). Normal mice clear infection spontaneously within 3 to 6 weeks and acquire effective immunity against secondary challenge (6). Bacterial colonization is limited to the intestinal mucosa, with only a few bacteria reaching systemic sites or the bloodstream (1, 22). The infection is normally accompanied by minimal morbidity and mortality in adult mice, although significant morbidity, such as retarded growth and high mortality, can occur in suckling mice (2).
The lymphocytic host response to C. rodentium is characterized by mucosal infiltration with CD3+ T cells, particularly the CD4+ subset (13). In addition, the cytokines interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha are upregulated in the colons of infected mice, indicating a bias towards a T helper cell type 1 immune response (13). T cells are important for clearance of C. rodentium, since mice with deficient T-cell functions due to transgenic expression of a single αβ T-cell receptor could not control infection and exhibited high morbidity and mortality (22). However, T cells are not likely to be directly responsible for eradicating the bacteria, which are mostly located extracellularly in the intestinal lumen.
B cells are also involved in antibacterial host defense, since infected mice develop IgG and IgA antibody responses to several bacterial proteins involved in virulence, including intimin, EspA, EspB, and Tir (5, 6). Furthermore, subcutaneous immunization with an intimin derivative, which elicited a specific antibody response, conferred protection against oral challenge with live bacteria (6). However, despite the development of specific antibodies against C. rodentium, little is known about the physiologic importance of B cells in host defense against this bacterium. Given that the bacteria reside mostly in the intestinal lumen and at the epithelial surface, we hypothesized that secretory IgA and IgM antibodies are important for eradicating C. rodentium. This hypothesis was tested in different murine models of secretory antibody deficiency.
MATERIALS AND METHODS
Mice.
The following mutant mice were used: C57BL/6-Igh-6tm1Cgn mice (B-cell-deficient mice) have an insertional mutation in the membrane exons of the Ig μ heavy chain gene, which renders them deficient in membrane IgM expression and, therefore, all mature B cells (16). B-cell-deficient mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). IgA-deficient mice (obtained from J. Nedrud, Case Western Reserve University, Cleveland, Ohio) have a deletion in the Iα exon, the Sα switch region, exon 1, and part of exon 2 of the Ig α chain gene (8). These mice are completely deficient in IgA production but have modestly increased levels of IgM and IgG in serum (8, 18). Mice deficient in secreted IgM (sIgM), obtained from J. Chen (Massachusetts Institute of Technology, Boston, Mass.), were generated by deleting the μs exon and its three downstream poly(A) sites in the Ig μ chain gene and replacing it with a cDNA fragment encoding the μm exons already spliced to the Cμ4 exon (3). These mice do not secrete IgM but still express surface IgM and IgD and undergo class switching to express other isotypes (3). Mice deficient for the polymeric Ig receptor (pIgR; obtained from F.-E. Johansen, Institute of Pathology, University of Oslo, Rikshospitalet, Oslo, Norway) have an insertional mutation in exon 3 of the pIgR gene, which disrupts the binding site for polymeric IgA and IgM (14). pIgR-deficient mice have normal, high-level mucosal production of polymeric IgA and IgM but cannot actively transport these isotypes across the intestinal epithelium, so that their levels in serum are >20-fold increased (14). J chain-deficient mice have an insertional mutation in the J chain gene, which disrupts production of functional J chains in B cells and thus efficient formation of polymeric antibodies (11). IgA and IgM are produced at normal levels in the mucosa, but the lack of associated J chain prevents their active transport into the intestinal lumen by pIgR (11), which makes these mice phenotypically similar to pIgR-deficient mice. IgG3-deficient mice have an insertional mutation/deletion in the γ3 heavy-chain constant region locus, such that 54 bp of γ3 CH1 domain is deleted, leaving the switch region intact (34). These mice have no detectable serum IgG3, and their splenocytes do not produce IgG3 after lipopolysaccharide stimulation (34). The genotype and/or phenotype of the different mutant mice was confirmed by PCR analysis of genomic DNA and/or enzyme-linked immunosorbent assay (ELISA) of serum Ig levels.
As controls for B-cell-deficient mice, C57BL/6J mice were used (Jackson Laboratory). As controls for IgA-deficient, sIgM-deficient, and pIgR-deficient mice, we used wild-type littermates with a similar B6x129 genetic background as the deficient mice, as well as (B6129F1/J)F2 mice (Jackson Laboratory), which are F2 hybrids of B6129F1/J mice derived by mating C57BL/6J and 129/J mice. No significant differences in clearing C. rodentium infection were observed between the two groups of mice, and the data from these mice are reported together (under the designation B6129 mice). J chain-deficient and IgG3-deficient mice were backcrossed to a BALB/c background for >6 generations, so we used BALB/c mice as controls (from Taconic or Jackson Laboratories). Mice were bred and maintained at the University of California, San Diego, animal facilities under specific pathogen-free conditions. All animal studies were approved by the University of California, San Diego, Animal Subjects Committee.
Infections.
C. rodentium was grown overnight in Luria-Bertani broth at 37°C, harvested by centrifugation, and resuspended in fresh Luria-Bertani broth at a concentration of 2.5 × 109/ml. Adult (>10 weeks) mice were infected with 200 μl of the bacterial suspension (5 × 108 bacteria) by oral gavage. Results from males and females were combined, since no significant gender difference in bacterial clearance was observed. For secondary challenge experiments, naïve mice were first infected orally with 5 × 108 C. rodentium cells and then reinfected orally with the same dose after 6 to 13 weeks.
To determine bacterial numbers in the stool, fecal pellets were collected from individual mice over a 2- to 3-h period, weighed, and homogenized in 5 ml of phosphate-buffered saline (PBS). For determining bacterial numbers in the spleen, colon, and cecum, each organ was removed in its entirety (with luminal contents) and homogenized in 2 ml of sterile PBS. Serial dilutions of the homogenates were plated onto MacConkey agar, and the numbers of CFU were determined after overnight incubation at 37°C. The detection limit of the CFU assay was 103 colonies per g of feces or per organ for bacterial counts in the stool and the colon and cecum, respectively, and <101 colonies/spleen. The identity of representative colonies was verified by PCR analysis. Individual colonies were picked with a sterile pipette tip and resuspended in 50 μl of water, of which 5 μl was added directly to a standard PCR, containing the primers 5′-AAGTCTGTCAATACCGCCTC-3′ (sense) and 5′-AATGTGCCAACTGTCTCATC-3′ (antisense). These primers amplify a 95-bp PCR product of the C. rodentium espB gene (27). The amplification profile was 35 cycles of 1 min of denaturation at 95°C and 2.5 min of annealing and extension at 53°C.
Histological analysis.
Organs were removed and fixed in Bouin's solution for 24 h. Colons were opened longitudinally, cleaned, and processed as “Swiss rolls” before fixation. Ceca were fixed in toto without removal of the intestinal contents. Fixed tissues were embedded in paraffin, and 5-μm sections were prepared and stained with hematoxylin and eosin. Crypt depths in colon and cecum were determined microscopically with a calibrated eyepiece reticle. Multiple sites were measured throughout each organ, and the three highest values obtained from different sites at least 10 crypts apart were used to calculate the maximal crypt depth for each sample.
Passive immunization.
To generate immune sera, pIgR-deficient or C57BL/6 mice were infected orally with 5 × 108 C. rodentium cells and then bled from the tail vein 6 to 10 weeks later. Uninfected mice were used to obtain preimmune sera. IgG-depleted immune serum was generated by diluting immune serum twofold with binding buffer (20 mM sodium phosphate, pH 8.0), incubation with protein A/G agarose (Pierce Biotechnology, Rockford, Ill.) for 2 h at room temperature, and subsequent centrifugation to remove the agarose-bound IgG. An isotype-specific ELISA was used to confirm specific depletion of IgG. Immune serum diluted with binding buffer was used as a control.
For passive immunization experiments, naïve C57BL/6 or pIgR-deficient mice were infected orally with C. rodentium and treated by daily intraperitoneal injection on days 4 to 7 and 11 to 14 with 40 μl of immune or preimmune serum, 80 μl of IgG-depleted or twofold-diluted immune serum, or the same volumes of PBS. Numbers of C. rodentium cells in the feces were determined on days 7 and 14 to 18, as described above.
Determination of antibacterial antibody titers.
Antibody titers against C. rodentium were determined by ELISA, using a modification of a published protocol (24). Fifty microliters (per well) of a C. rodentium suspension (2 × 109 cells/ml in water) was added to 96-well polystyrene plates and air dried overnight at room temperature. A fixation solution (200-μl/well 0.15% glutaraldehyde in 0.15 M phosphate, pH 7.0) was added, and plates were incubated for 5 min at room temperature, after which the solution was replaced with a 200-μl/well mixture of 0.15 M glycine and 15 mM phosphate buffer (pH 7.0) to block unreacted aldehyde groups. Plates were then blocked overnight at 4°C with PBS containing 5% nonfat dry milk and 0.5% Tween 20. After washing, serial dilutions of serum samples were added to the wells, and plates were incubated for 2 h at room temperature, followed by three washes with PBS-0.1% Tween 20. Optimal dilutions of peroxidase-conjugated goat antibodies against mouse IgG, IgA, or IgM were added (100 μl/well), followed by a 1-h incubation period at room temperature and three additional washes. Bound peroxidase was visualized with tetramethyl benzidine-H2O2 in acetate buffer, and reactions were stopped with sulfuric acid and read at 450 nm.
Data analysis.
Colony counts were log10 transformed, and means and standard errors of the mean were calculated from the log values. Samples without detectable C. rodentium colonies were assigned a log10 value equivalent to half of the detection limit of the CFU assay. Differences between groups of mice were evaluated with the Mann-Whitney rank sum test or t test, as appropriate. Differences with a P value of < 0.05 were considered significant.
RESULTS
Crucial role of B cells in eradication of C. rodentium.
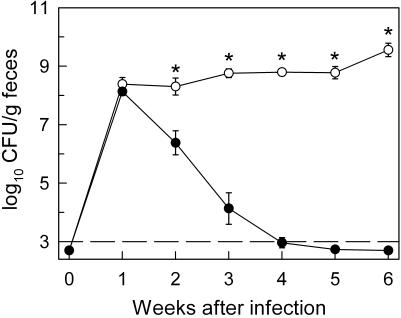
Oral infection of normal C57BL/6 mice with C. rodentium led to a high level of bacterial colonization of the colon and cecum within 1 week, with >107 CFU in the entire colon at that time. In parallel, bacterial numbers in the feces, which showed a good correlation with total bacterial load in colon and cecum, peaked 1 week after infection at >108 CFU/g of feces (Fig. 1) (data not shown). Subsequently, fecal C. rodentium numbers declined and were close to or below the detection limit of the CFU assay by week 4, which represented a >105-fold reduction in bacterial numbers compared to peak levels. No deaths were observed during the infections. Furthermore, secondary oral C. rodentium challenge of C57BL/6 mice that were infected 6 weeks before and had cleared infection revealed no detectable bacterial colonization (<103 CFU/g of feces) at 1 to 3 weeks after infection. These data, in conjunction with prior studies (21), demonstrate that normal mice are highly susceptible to C. rodentium infection but eradicate the infection within 4 weeks and develop effective acquired immunity.
FIG. 1.
C. rodentium infection of B-cell-deficient mice. B-cell-deficient mice (○) and C57BL/6 controls (•) were infected orally with 5 × 108 C. rodentium cells, and bacterial numbers (CFU) in the stool were determined weekly. Data are means ± standard errors (n = 7 to 11 mice per time point). *, P < 0.01 (by rank sum test) relative to C57BL/6 controls at the same time point. The dashed line represents the detection limit of the CFU assay.
To begin to define the key host effector mechanisms against C. rodentium, we focused on the role of B cells and antibodies, since the bacteria reside mostly in an extracellular location in the lumen and at the epithelial surface of the colon and cecum (15). As a first approach, we employed B-cell-deficient mice generated by targeted deletion of the membrane exons of the μ heavy chain gene (16). Oral infection of these mice with C. rodentium led to similar bacterial numbers in the feces at week 1 compared to those in C57BL/6 controls, indicating that both mouse strains were equally susceptible to initial bacterial colonization (Fig. 1). However, B-cell-deficient mice failed to control C. rodentium infection at later times, with the number of bacteria in the feces at week 6 postinfection exceeding the levels at week 1 by 10-fold (Fig. 1). Furthermore, bacteria were recovered at 6 weeks from the spleen of B-cell-deficient mice (log10 CFU per spleen, 3.0 ± 0.2; n = 7) but not C57BL/6 controls (log10 CFU per spleen, <1; n = 5). No bacteria were found in the spleens of B-cell-deficient or C57BL/6 mice at week 1. Together, these findings demonstrate that B cells are crucial for control and eradication of C. rodentium.
Different tissue responses in B-cell-deficient and normal mice infected with C. rodentium.
To characterize further the role of B cells in host defense against C. rodentium, we next examined the tissue responses of B-cell-deficient and C57BL/6 control mice at different times after infection. C57BL/6 mice showed marked crypt hyperplasia, increased mitotic activity in the epithelium, and loss of goblet cells in the colon and cecum at 2 weeks postinfection (Fig. 2B and L and Fig. 3). This was accompanied by mucosal and submucosal infiltration with mostly mononuclear cells, although neutrophils were also present (Fig. 2E and O). Ulcerations or severe transmural inflammation were not observed. The changes had returned to normal by week 6 (Fig. 2C and M).
FIG. 2.
Histological analysis of colon and cecum in C. rodentium-infected mice. C57BL/6 control mice (A to E and K to O) and B-cell-deficient mice (B cell KO) (F to J and P to T) were left uninfected (week 0; A, F, K, and P) or were infected orally with C. rodentium and sacrificed after 2 weeks (B, D, E, G, L, N, O, and Q) or 6 weeks (C, H, I, J, M, R, S, and T). Paraffin sections of colon (A to J) and cecum (K to T) were prepared and stained with hematoxylin and eosin. The colons and ceca of C57BL/6 mice show crypt hyperplasia, increased mitotic activity in the epithelium, and a loss of goblet cells at 2 weeks after infection (B and L). The submucosa is edematous and infiltrated with mostly mononuclear cells, while mucosal infiltration with inflammatory cells is modest (B, E, L, and O). The epithelial surface is intact with a normal-appearing brush border (D and N). In B-cell-deficient mice, extensive crypt hyperplasia in the colon and cecum is seen at 6 weeks, which is accompanied by submucosal inflammation and edema (H, J, R, and T). In parallel, the epithelial surface exhibits erosions and apoptotic/necrotic epithelial cells (I and S; arrows) and is covered by a pseudomembrane of coagulated proteinaceous material and dead epithelial cells (H, I, and S; asterisks). The magnifications are identical for the following groups of panels (with the applicable size bar given in parentheses): A to C and F to H (size bar shown in panel A); D, E, I, and J (size bar in panel D); K to M and P to R (size bar in panel K); N, O, S, and T (size bar in panel N).
FIG. 3.
Morphometric analysis of crypt depths after C. rodentium infection. Paraffin sections from uninfected (week 0) and C. rodentium-infected (weeks 2 and 6 postinfection [p.i.]) control (C57BL/6) and B-cell-deficient mice (B cell KO) were stained with hematoxylin and eosin. Crypt depths in colon and cecum were determined microscopically. Data are means ± standard errors (n ≥ 3 mice per time point). *, P < 0.05 (by rank sum test) relative to uninfected mice of the same strain.
In contrast, B-cell-deficient mice displayed only minor alterations in the colon and cecum at 2 weeks, with no significant increase in crypt depth at that time and only a minor infiltration with inflammatory cells (Fig. 2G and Q and Fig. 3). However, by 6 weeks postinfection, severe colonic and cecal inflammation had developed in the mice, as indicated by extensive crypt hyperplasia, increased mitotic activity in the epithelium, loss of goblet cells, and infiltration with mononuclear cells and neutrophils (Fig. 2H, J, R, and T). In addition, we observed focal erosions and ulcerations, often covered by pseudomembranes (Fig. 2I and S), extended crypts filled with mucus, and focal transmural inflammation in the colons of infected B-cell-deficient mice (Fig. 2H and R).
B-cell-deficient and normal mice differed not only in their intestinal but also systemic responses to C. rodentium infection. Thus, the spleens of normal mice at 2 weeks after infection showed a marked broadening of the marginal zone with numerous enlarged mononuclear cells (Fig. 4B), many of which were macrophages as they stained positive for the mouse F4/80 antigen, a lineage marker for macrophages (data not shown). Neutrophil numbers were not notably increased. The splenic morphology had returned to normal by 6 weeks (Fig. 4C). In contrast, B-cell-deficient mice showed few characteristic changes (besides the expected lack of B-cell areas) by 2 weeks after infection (Fig. 4E) but signs of severe acute inflammation by 6 weeks, with large numbers of neutrophils and neutrophilic microabscesses (Fig. 4F). The livers of normal and B-cell-deficient mice also showed a distinct difference in the response to C. rodentium infection. In normal but not B-cell-deficient mice, small foci of mononuclear inflammatory cells were seen mostly in the portal and periportal areas 2 weeks after infection (Fig. 4H and K). By 6 weeks, the livers of normal mice had returned to a normal appearance (Fig. 4I), while numerous inflammatory cell infiltrates were now observed in B-cell-deficient mice (Fig. 4L).
FIG. 4.
Histological analysis of spleen and liver after oral C. rodentium infection. C57BL/6 control mice (A to C and G to I) and B-cell-deficient mice (B cell KO) (D to F and J to L) were left uninfected (week 0; A, D, G, and J) or were infected orally with C. rodentium and sacrificed after 2 weeks (B, E, H, and K) or 6 weeks (C, F, I, and L). Paraffin sections of spleen (A to F) and liver (G to L) were prepared and stained with hematoxylin and eosin. Each spleen section shows areas of the white pulp (WP) and red pulp (RP). In panel B, the normally narrow marginal zone (MZ) between white and red pulp is widened, with numerous enlarged, irregular-shaped mononuclear cells (arrows). Representative examples of these cells (enclosed by the rectangle) are shown at higher magnification in the inset. In panel F, numerous neutrophils are present in clusters and microabscesses within the marginal zone and red pulp (arrows). Examples of these cells (enclosed by the rectangle) are shown at higher magnification in the inset (mouse neutrophils have ring-shaped, nonsegmented nuclei). For the liver, each section is centered around a portal triad containing a portal venule (PV). In panels H and L, mononuclear cell infiltrates are seen in a mostly periportal location (arrows). The magnifications are identical for panels A to F and G to L.
Together, the histological data suggest that normal mice responded relatively rapidly but transiently to infection, as evidenced by characteristic changes in colon, cecum, spleen, and liver by 2 but not 6 weeks. In contrast, B-cell-deficient mice failed to mount early inflammatory responses at 2 weeks but showed delayed and, in some organs, morphologically different responses at later times.
Production and transport of secretory IgA or IgM antibodies are not required for clearing infection.
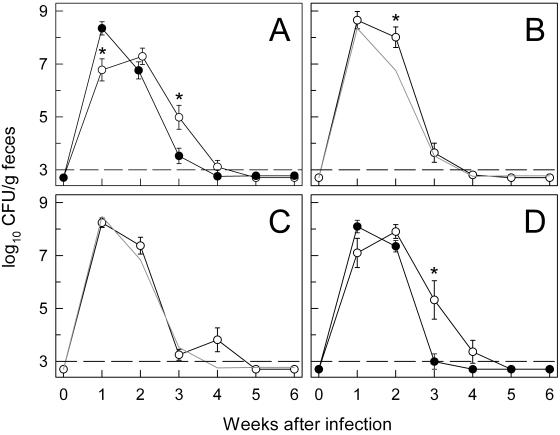
The major effector function of B cells is the production of specific antibodies. C. rodentium resides mostly in the intestinal lumen and at the epithelial surface, suggesting that antibodies that reach those sites might be important in the clearance of the bacteria. IgA is the most abundant isotype in mucosal secretions, and IgA antibodies specific for C. rodentium develop during the course of the infection (5, 6). Thus, we evaluated the importance of IgA in host defense against C. rodentium using IgA-deficient mice generated by disruption of key segments of the Ig α heavy chain gene (8). Oral infection of IgA-deficient mice with C. rodentium led to delayed colonization with 50-fold-lower bacterial numbers than those of controls at week 1 but higher numbers at weeks 2 and 3 (Fig. 5A). However, the cumulative bacterial loads in the first 3 weeks (the area under the curve) were comparable in IgA-deficient and control mice, and IgA-deficient mice had cleared the infection by week 4. Furthermore, IgA-deficient mice and their littermate controls showed similar histopathological lesions 2 weeks after infection, with marked crypt hyperplasia, and a mixed mucosal and submucosal inflammatory cell infiltrate in the colon. These lesions were indistinguishable from those in C57BL/6 mice. The histological changes had returned to normal by 6 weeks. IgA-deficient mice developed effective acquired immunity against a secondary challenge with C. rodentium, since no bacteria could be detected in the stool (<103 CFU/g of feces) 1 to 2 weeks after oral reinfection of mice that had been infected 9 weeks before and had eradicated the bacteria. These results demonstrate that IgA is not required for eradication of C. rodentium, although it might play a limited, indirect role in controlling initial susceptibility to infection.
FIG. 5.
C. rodentium infection of mice deficient in secretory antibody production or transport into the intestinal lumen. Mice deficient for IgA (A; ○), secreted IgM (B; ○), pIgR (C; ○), or J chain (D; ○) and the appropriate controls—B6129 mice (A; •) for IgA-deficient, secreted IgM-deficient, and pIgR-deficient mice and BALB/c (D; •) for J chain-deficient mice—were infected orally with C. rodentium, and bacterial numbers in the feces were determined weekly. Data are means ± standard errors (n ≥ 6 mice per time point). *, P < 0.05 (by rank sum test) relative to control mice at the same time point. The gray lines in panels B and C represent the means of the data from B6129 mice in panel A and are only shown for comparison. The dashed lines represent the detection limit of the CFU assay.
IgM is the second-most-abundant isotype in mucosal secretions and might compensate for IgA under conditions of IgA deficiency. To determine whether secreted IgM played a role in clearance of C. rodentium, we used mice with a selective deficiency for secreted IgM but not other isotypes (3). These mice were equally susceptible to initial bacterial infection but showed a significant, albeit slight, delay in controlling infection at 2 weeks (Fig. 5B). The secreted IgM-deficient mice had cleared infection normally by 4 weeks (Fig. 5B) and were immune to a secondary challenge with C. rodentium (<103 CFU/g of feces at 1 and 2 weeks after oral reinfection of previously infected mice). Secreted IgM-deficient mice also exhibited similar colonic crypt hyperplasia and inflammatory cell infiltration as their littermate controls at 2 weeks after infection. Thus, secreted IgM, like IgA, plays a minor role in controlling C. rodentium.
The data on IgA- and secreted IgM-deficient mice suggested that these isotypes, by themselves, are not important for eradication of C. rodentium but could not rule out the possibility that they compensate for each other. To test this possibility, we employed two murine models, pIgR- and J chain-deficient mice, in which neither IgA nor IgM can be actively transported into the intestinal lumen, rendering the mice functionally double deficient for luminal IgA and IgM (11, 14). The pIgR is expressed by intestinal epithelial cells and required for active transport of polymeric IgA and IgM into the lumen, while J chain is expressed in B cells and plasma cells and needed for the efficient formation of polymeric IgA and IgM and their transport into the lumen by pIgR. Mice deficient for pIgR showed similar bacterial loads early after C. rodentium inoculation and no significant differences in the subsequent clearance of the infection, relative to their littermate controls (Fig. 5C). They were also immune to secondary challenge with C. rodentium (data not shown). J chain-deficient mice displayed a slight delay in initial bacterial colonization and subsequent clearance compared to normal controls, with a 200-fold difference in bacterial numbers at week 3 (Fig. 5D). However, the mice had eradicated the bacteria normally by week 5. Mice deficient for pIgR showed marked colonic crypt hyperplasia and mixed inflammatory cell infiltrates in mucosa and submucosa by 2 weeks after infection, which had returned to normal by 6 weeks. The changes were comparable to those in infected littermate B6129 controls and in C57BL/6 mice. J chain-deficient mice and their BALB/c controls also exhibited similar, albeit less pronounced, histopathological alterations.
Taken together, the data from the different genetic models of secretory antibody deficiency indicate that production and/or epithelial transport of secretory IgA and IgM is not needed for eradication of C. rodentium, although these isotypes might contribute, in a limited manner, to antibacterial host defense in the early stages of infection.
Deficiency in IgG3 antibodies has no effect on C. rodentium eradication.
Given that B cells were important for C. rodentium clearance but not secretory antibodies, we began to evaluate the role of IgG in antibacterial host defense. IgG3 antibodies are the predominant subclass of IgG antibodies that develop against bacterial cell wall polysaccharides after infections in mice, and this subclass shows the highest functional affinity over other isotypes for binding these bacterial structures (29, 33). To test the role of IgG3 in host defense against C. rodentium, we used mutant mice that lack a key segment of the Ig γ3 heavy chain locus and cannot produce membrane or secreted IgG3 (23, 34). Oral infection of IgG3-deficient mice with C. rodentium led to efficient early bacterial colonization, followed by eradication within 3 weeks, which was similar to the findings in BALB/c controls (Fig. 6). Although some (60%) IgG3-deficient, but not control, mice showed a small second peak of infection at 4 weeks, this did not reach statistical significance. IgG3-deficient mice and BALB/c controls showed a similar degree of crypt hyperplasia and mucosal and submucosal infiltration with mononuclear cells and some neutrophils at 2 weeks after infection, although these changes were less prominent than those observed in C57BL/6 or B6129 mice. The colons of IgG3-deficient and BALB/c mice were histologically normal 6 weeks after infection. Thus, IgG3 has no unique functions in the eradication of C. rodentium.
FIG. 6.
Infection of IgG3-deficient mice with C. rodentium. Mice lacking IgG3 (○) and BALB/c controls (•) were infected orally with C. rodentium, and fecal bacterial numbers were determined weekly. Data are means ± standard error (n = 5 to 6 mice per time point). The dashed line represents the detection limit of the CFU assay.
Passive immune protection against C. rodentium.
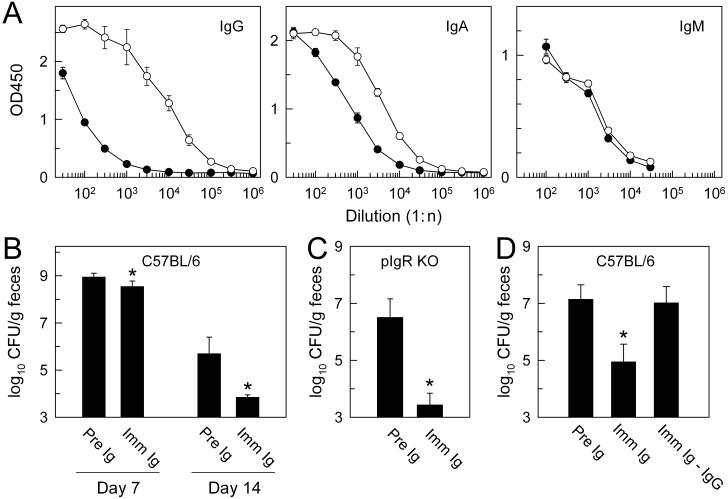
The data from the different isotype-deficient mice did not reveal a role of those isotypes in clearance of C. rodentium, suggesting that the functions of B cells might be independent of antibody production in this context or that other isotypes are involved. To evaluate whether antibodies play any role in controlling C. rodentium, we conducted passive immunization experiments. As a source of immune antibodies, we initially used pIgR-deficient mice previously infected with C. rodentium, as they have ∼50-fold-elevated levels of circulating polymeric IgA and IgM, as well as moderately increased levels of IgG (14). High concentrations of polymeric IgA and IgM in the serum are needed to achieve significant levels of luminal IgA and IgM upon intraperitoneal injection (data not shown). Infected mice had high titers of antibacterial IgG and IgA but not IgM antibodies in serum at 6 weeks postinfection (Fig. 7A). The increases in antibacterial titers in pIgR-deficient mice were comparable to those after infection of C57BL/6 mice (data not shown).
FIG. 7.
Passive immune protection against C. rodentium. Mice deficient for pIgR were infected orally with C. rodentium (○) or left uninfected (•), and sera were collected after 6 weeks and tested for anti-C. rodentium antibodies by isotype-specific ELISA (A). Naive C57BL/6 mice (B and D) or pIgR-deficient mice (C) were infected orally with C. rodentium and injected intraperitoneally on days 4 to 7 and 11 to 14 with identical volumes of immune (Imm Ig), preimmune (Pre Ig), or IgG-depleted immune sera (Imm Ig-IgG). Bacterial numbers (CFU) in the feces were determined on days 7 (B) and 14 to 18 (B to D). Results are the mean ± standard deviation of quadruplicate samples (A) or mean ± standard error of six or more mice (B to D). OD450, optical density at 450 nm. *, P < 0.05 (by t test) relative to preimmune Ig-injected mice at the same time point.
For passive immunization experiments, naïve C57BL/6 mice were infected orally with C. rodentium and subsequently treated with immune or preimmune sera by repeated intraperitoneal injections. Bacterial numbers in the feces were assessed 7 and 14 to 18 days after infection. Administration of immune sera from pIgR-deficient mice significantly decreased bacterial numbers at both time points compared to preimmune sera, although the effects were more pronounced at the later time point (Fig. 7B). The protective effects of the immune sera were also observed after injection into naïve pIgR-deficient mice infected with C. rodentium (Fig. 7C), indicating that antibacterial antibodies did not require active secretion into the intestinal lumen to be effective. Furthermore, injection of immune sera from C57BL/6 mice, which have only low levels of polymeric IgA in the serum, caused a similar decrease in bacterial numbers (log10 CFU per gram of feces at 2 weeks: 6.1 ± 0.4 versus 2.7 ± 0.5 after injection with preimmune and immune sera, respectively; P < 0.01, n = 3). Together, these results suggest that secretory IgA or IgM antibodies are not involved in passive immune protection against C. rodentium, whereas IgG antibodies may be important.
To test directly the importance of IgG in passive immune protection, we depleted IgG from immune sera through protein A/G chromatography. Isotype-specific ELISA showed that this procedure reduced the levels of total IgG and anti-C. rodentium IgG to <2% of those in the nondepleted sera, while total and anti-C. rodentium IgA levels were comparable in depleted and nondepleted sera. Passive immunization of naïve C57BL/6 mice with IgG-depleted immune serum provided no protection against oral C. rodentium infection, with fecal bacterial counts similar to those in mice treated with preimmune serum, whereas injection of nondepleted immune serum caused a significant reduction in fecal bacterial numbers under the same conditions (Fig. 7D). Therefore, IgG antibodies are important for mediating passive immune protection against C. rodentium.
DISCUSSION
Our studies, together with a recently published report (35), show that B cells are of crucial importance for the eradication of C. rodentium, since mice lacking B cells could not clear the bacteria or decrease the bacterial burden over an extended period. These findings resemble the observations in RAG1-deficient mice, which lack B and T cells, although there were differences in the accompanying tissue responses (38). RAG1-deficient mice developed transient colitis and crypt hyperplasia early after infection but few changes at later times despite a high bacterial burden (38), while we found that B-cell-deficient mice displayed few histological changes early after infection but exhibited delayed severe colitis and crypt hyperplasia. These differences suggest that B and T cells mediate different functions in the host response to infection. Early after infection (<2 weeks), T cells probably play a limited role in mediating histological changes (38), whereas B cells contribute to them. At later stages of the infection (>2 weeks), B cells appear to be primarily involved in controlling bacterial numbers as the underlying driving force for the histopathologic changes rather than directly causing these events. In contrast, T cells are responsible for mediating many of those tissue responses. Such a role of T cells is also suggested by the observation that IFN-γ produced by these cells appeared to be important for the development of the histopathology after infection (12). Moreover, differential production of T-cell cytokines such as IFN-γ may also be responsible for the difference in mucosal inflammation we observed in C57BL/6 compared to BALB/c mice (10).
Our data demonstrate that production and transport into the lumen of secretory antibodies are not required for clearance of C. rodentium. This was surprising given that a strong antibacterial mucosal IgA response is elicited by the infection (5) and the vast majority (>95%) of bacteria are located in the lumen and at the epithelial surface, where they might be expected to be prime targets of the actions of secretory antibodies. The apparent independence of bacterial clearance from secretory antibodies does not preclude that other luminal host defenses might be active against the bacteria and important for eradication. For example, inducible nitric oxide synthase (iNOS) is upregulated in the colonic epithelium during C. rodentium infection and plays a role in controlling infection (37). The majority of NO produced by iNOS in polarized colon epithelial cells is released at the apical pole and where it can target microbes located in close proximity (4). Induction of antimicrobial peptides produced by intestinal epithelial cells could also control bacterial infection in the lumen (28, 41). In addition, it is possible that killing of the bacteria not only takes place at the mucosal surface but also within the mucosa, where a small but significant number of bacteria reside (22, 36, 38).
Administration of immune sera significantly reduced the bacterial burden in normal mice, which suggests that antibacterial antibodies can play a role in controlling C. rodentium infection. The protective function was probably not mediated by sIgA or sIgM antibodies, since pIgR-deficient mice, which cannot actively transport such antibodies into the intestinal lumen, were also protected. However, it is possible that passive leakage of either IgA or IgM across the epithelium is sufficient for protection. In contrast to secretory antibodies, IgG antibodies were important in passive immune protection, since their depletion from immune sera abolished protection. A role of IgG antibodies in host defense against C. rodentium is further supported by prior reports that subcutaneous immunization with an intimin derivative and the accompanying induction of specific IgG responses conferred protection against oral challenge with live bacteria (6). The underlying mechanisms of action are not known, but IgG antibodies can either reach the lumen in sufficient quantities to be functionally significant and/or relevant immune defenses take place within the mucosa. In support of the former possibility, we observed ∼15-fold-increased levels of fecal IgG in the acute stage (2 weeks) after C. rodentium infection (data not shown), which might be related to a partial breakdown in epithelial barrier function during acute colitis. It is not clear which IgG subclasses are involved in protection. The normal bacterial eradication in IgG3-deficient mice indicates that this subclass, at least by itself, is not important. This suggests that bacterial polysaccharides such as lipopolysaccharide may not be major targets of the B-cell-dependent immune defense against C. rodentium, since IgG3-deficient mice have a defective ability to mount IgG3-mediated protective responses against other bacterial pathogens such as Streptococcus pneumoniae (23).
The histopathologic analysis revealed that B-cell-deficient mice showed a marked delay in mounting intestinal and systemic tissue responses to infection compared to normal mice, despite comparable bacterial burdens early after infection. These data suggest that B cells are involved in the rapid induction of the cellular inflammatory and immune responses in response to bacterial infection. Similar observations were made with another enteric pathogen, Cryptosporidium parvum, where B cells were required for the development of intestinal inflammatory lesions after infection (40). The underlying mechanisms are not known, but B cells play a developmental role in the formation of the intestinal lymphoid tissue (7), which may be important for the detection of bacterial pathogens and for mounting effective immune defenses, even in the absence of a direct B-cell involvement. B cells are also potent antigen-presenting cells (9, 30) and have been shown to facilitate the development of effective T-cell responses in host defense against microbial infections (19, 42). In addition, B cells can secrete chemotactic cytokines that are important for the recruitment of immune and inflammatory cells (17).
Our results have potential implications for the rational design of immunization strategies against EPEC under the assumption that the murine host defense against C. rodentium reflects key features of human EPEC infections. Thus, the data caution that eliciting antibacterial sIgA responses with suitable mucosal adjuvants may not induce the most effective host defenses against EPEC, although we cannot rule out the possibility that very strong induction of such responses could prevent infection (6, 25). Instead, antigens and adjuvants targeted towards B-cell activation and generation of antibacterial IgG may be more likely to elicit robust protective responses.
Acknowledgments
This work was supported by National Institutes of Health grants AI56075 (L.E.), DK35108 (M.F.K.), and AI32596 (J.R.S.); grants from the Medicine Education and Research Foundation, San Diego (L.E.), and the Crohn's and Colitis Foundation of America (L.E.); and a fellowship from the Deutsche Forschungsgemeinschaft (C.M.).
We thank John Leopard for expert technical support and Finn-Eirik Johansen and Barbara Hendrickson for providing mutant mice.
Editor: A. D. O'Brien
REFERENCES
- 1.Barthold, S. W., G. L. Coleman, P. N. Bhatt, G. W. Osbaldiston, and A. M. Jonas. 1976. The etiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 26:889-894. [PubMed] [Google Scholar]
- 2.Barthold, S. W., G. L. Coleman, R. O. Jacoby, E. M. Livestone, and A. M. Jonas. 1978. Transmissible murine colonic hyperplasia. Vet. Pathol. 15:223-236. [DOI] [PubMed] [Google Scholar]
- 3.Boes, M., C. Esau, M. B. Fischer, T. Schmidt, M. Carroll, and J. Chen. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776-4787. [PubMed] [Google Scholar]
- 4.Eckmann, L., F. Laurent, T. D. Langford, M. L. Hetsko, J. R. Smith, M. F. Kagnoff, and F. D. Gillin. 2000. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defense against the lumen-dwelling pathogen Giardia lamblia. J. Immunol. 164:1478-1487. [DOI] [PubMed] [Google Scholar]
- 5.Frankel, G., A. D. Phillips, M. Novakova, H. Field, D. C. A. Candy, D. B. Schauer, G. Douce, and G. Dougan. 1996. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 64:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghaem-Maghami, M., C. P. Simmons, S. Daniell, M. Pizza, D. Lewis, G. Frankel, and G. Dougan. 2001. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect. Immun. 69:5597-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golovkina, T. V., M. Shlomchik, L. Hannum, and A. Chervonsky. 1999. Organogenic role of B lymphocytes in mucosal immunity. Science 286:1965-1968. [DOI] [PubMed] [Google Scholar]
- 8.Harriman, G. R., M. Bogue, P. Rogers, M. Finegold, S. Pacheco, A. Bradley, Y. Zhang, and I. N. Mbawuike. 1999. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J. Immunol. 162:2521-2529. [PubMed] [Google Scholar]
- 9.Hayglass, K. T., S. J. Naides, C. F. Scott, Jr., B. Benacerraf, and M. S. Sy. 1986. T cell development in B cell-deficient mice. IV. The role of B cells as antigen-presenting cells in vivo. J. Immunol. 136:823-829. [PubMed] [Google Scholar]
- 10.Heinzel, F. P., M. D. Sadick, B. J. Holaday, R. L. Coffman, and R. M. Locksley. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J. Exp. Med. 169:59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrickson, B. A., D. A. Conner, D. J. Ladd, D. Kendall, J. E. Casanova, B. Corthesy, E. E. Max, M. R. Neutra, C. E. Seidman, and J. G. Seidman. 1995. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J. Exp. Med. 182:1905-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 13.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald.1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen, F. E., M. Pekna, I. N. Norderhaug, B. Haneberg, M. A. Hietala, P. Krajci, C. Betsholtz, and P. Brandtzaeg. 1999. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 190:915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson, E., and S. W. Barthold. 1979. The ultrastructure of transmissible murine colonic hyperplasia. Am. J. Pathol. 97:291-313. [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 17.Krzysiek, R., E. A. Lefevre, W. Zou, A. Foussat, J. Bernard, A. Portier, P. Galanaud, and Y. Richard. 1999. Antigen receptor engagement selectively induces macrophage inflammatory protein-1 alpha (MIP-1 alpha) and MIP-1 beta chemokine production in human B cells. J. Immunol. 162:4455-4463. [PubMed] [Google Scholar]
- 18.Langford, T. D., M. P. Housley, M. Boes, J. Chen, M. F. Kagnoff, F. D. Gillin, and L. Eckmann. 2002. Central importance of immunoglobulin A in host defense against Giardia spp. Infect. Immun. 70:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langhorne, J., C. Cross, E. Seixas, C. Li, and T. von der Weid. 1998. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc. Natl. Acad. Sci. USA 95:1730-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luperchio, S. A., J. V. Newman, C. A. Dangler, M. D. Schrenzel, D. J. Brenner, A. G. Steigerwalt, and D. B. Schauer. 2000. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of C. rodentium and mouse-pathogenic Escherichia coli. J. Clin. Microbiol. 38:4343-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 22.Maggio-Price, L., K. L. Nicholson, K. M. Kline, T. Birkebak, I. Suzuki, D. L. Wilson, D. Schauer, and P. J. Fink. 1998. Diminished reproduction, failure to thrive, and altered immunologic function in a colony of T-cell receptor transgenic mice: possible role of Citrobacter rodentium. Lab. Anim. Sci. 48:145-155. [PubMed] [Google Scholar]
- 23.McLay, J., E. Leonard, S. Petersen, D. Shapiro, N. S. Greenspan, and J. R. Schreiber. 2002. Gamma 3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides. II. Increased susceptibility to fatal pneumococcal sepsis due to absence of anti-polysaccharide IgG3 is corrected by induction of anti-polysaccharide IgG1. J. Immunol. 168:3437-3443. [DOI] [PubMed] [Google Scholar]
- 24.Metcalf, E. S., and A. D. O'Brien. 1981. Characterization of murine antibody response to Salmonella typhimurium by a class-specific solid-phase radioimmunoassay. Infect. Immun. 31:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michetti, P., M. J. Mahan, J. M. Slauch, J. J. Mekalanos, and M. R. Neutra. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman, J. V., B. A. Zabel, S. S. Jha, and D. B. Schauer. 1999. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect. Immun. 67:6019-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Neil, D. A., E. M. Porter, D. Elewaut, G. M. Anderson, L. Eckmann, T. Ganz, and M. F. Kagnoff. 1999. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163:6718-6724. [PubMed] [Google Scholar]
- 29.Perlmutter, R. M., D. Hansburg, D. E. Briles, R. A. Nicolotti, and J. M. Davie. 1978. Subclass restriction of murine anti-carbohydrate antibodies. J. Immunol. 121:566-572. [PubMed] [Google Scholar]
- 30.Rivera, A., C. C. Chen, N. Ron, J. P. Dougherty, and Y. Ron. 2001. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int. Immunol. 13:1583-1593. [DOI] [PubMed] [Google Scholar]
- 31.Schauer, D. B., and S. Falkow. 1993. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect. Immun. 61:2486-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schauer, D. B., and S. Falkow. 1993. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 61:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schreiber, J. R., L. J. Cooper, S. Diehn, P. A. Dahlhauser, M. F. Tosi, D. D. Glass, M. Patawaran, and N. S. Greenspan. 1993. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J. Infect. Dis. 167:221-226. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro, D. A., D. S. Threadgill, M. J. Copfer, D. A. Corey, T. L. McCool, L. L. McCormick, T. R. Magnuson, N. S. Greenspan, and J. R. Schreiber. 1998. γ3 gene-disrupted mice selectively deficient in the dominant IgG subclass made to bacterial polysaccharides undergo normal isotype switching after immunization with polysaccharide-protein conjugate vaccines. J. Immunol. 161:3393-3399. [PubMed] [Google Scholar]
- 35.Simmons, C. P., S. Clare, M. Ghaem-Maghami, T. K. Uren, J. Rankin, A. Huett, R. Goldin, D. J. Lewis, T. T. MacDonald, R. A. Strugnell, G. Frankel, and G. Dougan. 2003. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons, C. P., N. S. Goncalves, M. Ghaem-Maghami, M. Bajaj-Elliott, S. Clare, B. Neves, G. Frankel, G. Dougan, and T. T. MacDonald. 2002. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-γ. J. Immunol. 168:1804-1812. [DOI] [PubMed] [Google Scholar]
- 37.Vallance, B. A., W. Deng, M. De Grado, C. Chan, K. Jacobson, and B. B. Finlay. 2002. Modulation of inducible nitric oxide synthase expression by the attaching and effacing bacterial pathogen Citrobacter rodentium in infected mice. Infect. Immun. 70:6424-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallance, B. A., W. Deng, L. A. Knodler, and B. B. Finlay. 2002. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 70:2070-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters, W. R., M. V. Palmer, M. J. Wannemuehler, R. E. Sacco, and J. A. Harp. 2000. B cells are required for the induction of intestinal inflammatory lesions in TCRalpha-deficient mice persistently infected with Cryptosporidium parvum. J. Parasitol. 86:1073-1077. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 42.Yang, X., and R. C. Brunham. 1998. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J. Immunol. 161:1439-1446. [PubMed] [Google Scholar]