The phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway is a highly conserved signaling pathway stretching from worms to humans (1). This signaling pathway regulates numerous cellular processes encompassing growth, survival, differentiation, and metabolism and is critical for the survival and growth of cancer (2). Aberrant activation of the PI3K/AKT pathway in cancer can occur through mechanisms such as pathway activating mutations, engagement of receptor tyrosine kinases (RTKs), and loss of tumor suppressors such as phosphatase and tensin homolog (PTEN). The many mechanisms by which this pathway is activated in cancer makes PI3K, AKT, and other components of this pathway intriguing targets for therapeutic intervention. In PNAS, Semenas et al. (3) discover a new chemical compound (ISA-2011B) that inhibits the upstream lipid kinase, PI-4-phosphate 5 kinase α (PIP5K1α), extending the arsenal of promising agents targeting the PI3K pathway that may be used therapeutically in advanced prostate cancer.
Prostate cancer results in nearly 30,000 deaths in men in the United States every year. Initially, prostate cancer relies on androgens for growth and will respond to conventional androgen deprivation therapies. However, this response is temporary, and recurrence eventually develops, leading to castration-resistant prostate cancer (CRPC) and ultimately the death of the patient. Currently, there are few effective treatment options for CRPC, and many mechanisms have been proposed to explain its occurrence, including persistent androgen receptor (AR) signaling and PI3K pathway activation. Recently, two newly approved drugs, abiraterone acetate and enzalutamide, which inhibit intratumoral androgen synthesis and potently inhibit AR activation, respectively, have resulted in survival benefits for men with CRPC, although lethality in these patients still occurs (4, 5). Therefore, additional therapies must be developed to target alternative pathways in advanced prostate cancer (6, 7).
One of the pathways highly implicated in prostate cancer is the PI3K/AKT pathway. In prostate cancer, the PI3K pathway is activated primarily via the loss of the tumor suppressors PTEN or inositol polyphosphate 4-phosphatase-II (INPP4B) and is aberrantly altered in nearly 100% of metastatic prostate cancer (8). The common signaling molecules studied in this pathway include several downstream players such as PI3K, AKT, and mTOR, whereas little is known about how the most upstream kinase, PIP5K1α, contributes to development, growth, or survival of prostate cancer.
PIP5K1α is part of a family of lipid kinases, and the α isoform predominantly generates PI-4,5-P2 (PIP2) from PI-4-phosphate (PI4P). PIP2 is phosphorylated by PI3K to generate PI-3,4,5-P3 (PIP3), which recruits the serine/threonine kinase AKT to the cell membrane, where it becomes activated, initiating signaling cascades. In this study, Semenas et al. (3) find that PIP5K1α is overexpressed in high-grade prostate cancer, and its expression correlates with PIP2 and AR expression in these tissues. The authors further demonstrate that PIP5K1α contributed to malignant transformation in prostate cancer cell lines through increased invasion, enhanced stability or expression of AR, and expression of cellular proliferation markers such as cyclin-dependent kinase 1 (CDK1). Using a high-throughput kinase profiling assay, the authors discovered an inhibitor of PIP5K1α, ISA-2011B. ISA-2011B significantly reversed the protumorigenic effects of PIP5K1α by decreasing prostate cancer cell invasion (via reduced focal adhesion kinase activation), reducing AR and CDK1 expression, inhibiting AKT activation, and increasing apoptosis in vitro. Further, administration of ISA-2011B to mice harboring aggressive PC-3 tumors considerably inhibited growth over the course of 20 d without any measurable toxicity.
The development of PI3K pathway inhibitors has been an intense area of focus for researchers and drug companies over the last decade, and the discovery of ISA-2011B may add to the repertoire of existing agents. Early phase clinical trials of pan PI3K and AKT inhibitors, such as perifosine, have shown reasonable toxicity profiles, with hyperglycemia being the most pronounced mechanism-based toxicity (9). However, these inhibitors, when applied as single agents to patients with prostate cancer, have not clinically performed well (10). In this study, ISA-2011B displayed no adverse events after administration into mice, and it will be important to determine whether ISA-2011B has a different toxicity profile compared with PI3K or AKT inhibitors. Additionally, although the authors did not report the pharmacokinetics and pharmacodynamics of ISA-2011B in vivo, this information plus the toxicity profile will help to determine the future clinical utility of this compound as a therapeutic agent in cancer.
More recently, focus has turned to inhibition of more downstream effector targets such as mTOR. Due to a wide array of signaling inputs to mTOR, mTOR inhibitors can more broadly be applied to cancers not dependent on the PI3K/AKT pathway, increasing the utility of these inhibitors over PI3K and AKT agents. Allosteric mTOR inhibitors, such as everolimus, have not performed well in clinical trials for prostate cancer (11), but mTOR active site inhibitors, such as MLN0128 (INK128), outperformed everolimus in preclinical models of prostate cancer (12). The increased efficacy of mTOR active site inhibitors is attributed to targeting everolimus-resistant substrates such as 4EBP1, as well as completely blocking both mTOR complexes (mTORC1 and C2) and eliminating AKT activation via feedback from S6 kinase (S6K) (13).
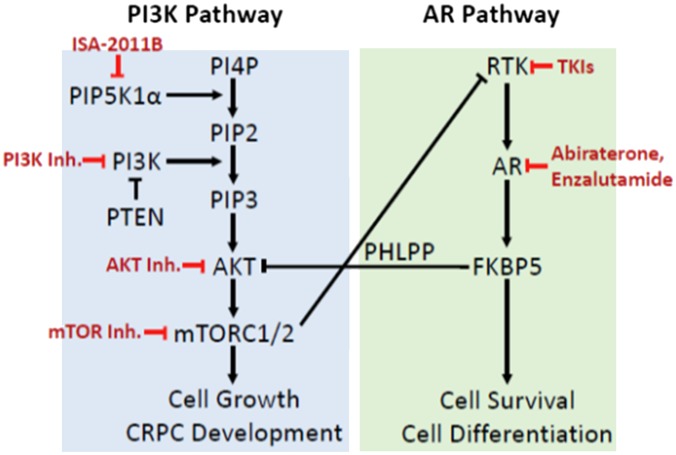
Where are we now in the field of PI3K pathway inhibitors for treatment of prostate cancer? There are several PI3K agents available, but to date, the clinical benefit has been limited if these compounds are used as a single agent. The lack of clinical benefit could be due to the activation of alternative signaling pathways such as AR or other kinase pathways. Indeed, development of CRPC may be due to the activation of the PI3K pathway during AR inhibitor therapy, suggesting cross-talk between these two pathways (14, 15). As such, combination therapies targeting both the PI3K/AKT pathway and AR are beginning to be tested clinically (16). The development of combination strategies will be imperative to achieve the goal of significantly improving survival in patients suffering from cancer. The central dilemma seems to be in choosing the right combinations. In the PI3K pathway, several combination strategies are currently being or will be tested including PI3K/AKT inhibitors combined with mTOR inhibitors (vertical treatment approach) and PI3K/AKT/mTOR inhibitors with AR inhibitors (horizontal treatment approach) (Fig. 1). Hence, it would be worthwhile to investigate the effects of combination therapies using ISA-2011B with an AR inhibitor, or other pathway inhibitors, in
Semenas et al. find that PIP5K1α is overexpressed in high-grade prostate cancer, and its expression correlates with PIP2 and AR expression in these tissues.
preclinical models of prostate cancer and especially CRPC.
Fig. 1.
Cotargeting the PI3K/AKT and AR pathways in CRPC. The administration of AR-targeted agents such as abiraterone acetate and enzalutamide is thought to promote the development of castration resistance due to the loss of negative feedback regulation of the PI3K/AKT pathway. The development of the PIP5K1α inhibitor, ISA-2011B, adds another tool for targeting the PI3K pathway, as well as provide the impetus to cotarget with inhibitors of RTK and AR signaling pathways in CRPC. TKI, tyrosine kinase inhibitor.
Overall, the studies of Semenas et al. uncover the mechanism of an underappreciated lipid kinase in the PI3K pathway, PIP5K1α, and developed a novel inhibitor, ISA-2011B, that targets this kinase. This study will open up new avenues into the investigation of PIP5K1α as a therapeutic target in preclinical models of prostate cancer, in addition to other cancers dependent on the PI3K pathway for growth and survival. It will be important to evaluate this compound further, individually and in combination, in several preclinical models of advanced prostate cancer including genetically engineered mouse models (such as PTEN−/−) (17), mouse and human tissue recombination cancer models (18–20), and CRPC models (21). If verified, this might be a new promising member in the ever-expanding list of PI3K pathway-specific inhibitors.
Supplementary Material
Acknowledgments
J.M.D. is supported by the Department of Defense Prostate Cancer Research Program Grant W81XWH-14-1-0148. J.H. is supported by the Department of Defense Prostate Cancer Research Program Grants W81XWH-11-1-0227 and W81XWH-12-1-0206, University of California, Los Angeles, Specialized Program in Research Excellence in prostate cancer, National Cancer Institute Grants 1R01CA158627 and 1R01CA172603-01A1, West Coast Prostate Cancer Dream Team supported by Stand Up to Cancer/American Association for Cancer Research/Prostate Cancer Foundation, and Prostate Cancer Foundation Honorable A. David Mazzone Special Challenge Award.
Footnotes
The authors declare no conflict of interest.
See companion article on page E3689.
References
- 1.Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290(5489):147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 2.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20(1):87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenas J, et al. The role of PI3K/AKT-related PIP5K1α and the discovery of its selective inhibitor for treatment of advanced prostate cancer. Proc Natl Acad Sci USA. 2014;111:E3689–E3698. doi: 10.1073/pnas.1405801111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bono JS, et al. COU-AA-301 Investigators Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher HI, et al. AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 6.Drake JM, et al. Metastatic castration-resistant prostate cancer reveals intrapatient similarity and interpatient heterogeneity of therapeutic kinase targets. Proc Natl Acad Sci USA. 2013;110(49):E4762–E4769. doi: 10.1073/pnas.1319948110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arora VK, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posadas EM, et al. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol Ther. 2005;4(10):1133–1137. doi: 10.4161/cbt.4.10.2064. [DOI] [PubMed] [Google Scholar]
- 10.Chee KG, et al. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: A phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5(7):433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 11.Nakabayashi M, et al. Phase II trial of RAD001 and bicalutamide for castration-resistant prostate cancer. BJU Int. 2012;110(11):1729–1735. doi: 10.1111/j.1464-410X.2012.11456.x. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh AC, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver BS, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulholland DJ, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19(6):792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. 2014;16(3):378–386. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 18.Drake JM, et al. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci USA. 2012;109(5):1643–1648. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoyanova T, et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc Natl Acad Sci USA. 2013;110(50):20111–20116. doi: 10.1073/pnas.1320565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329(5991):568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin D, et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014;74(4):1272–1283. doi: 10.1158/0008-5472.CAN-13-2921-T. [DOI] [PubMed] [Google Scholar]