Significance
Group 3 innate lymphoid cells (ILC3s) play decisive roles in mammalian physiology including tissue repair, lymphoid tissue development, and immune regulation. So far, the functions of ILC3s in the adult immune system have been mainly linked to their capacity to release cytokines in response to microbial or inflammatory signals. The results presented here show that activated ILC3s can alter the outcome of adaptive immune responses by directly stimulating CD4+ T cells. Indeed, IL-1β–activated ILC3s express costimulatory molecules and induce cognate CD4+ T-cell responses. More importantly, antigen-driven T- and B-cell responses are impaired in vivo when this cellular interaction is disrupted. Overall, our data show that peripheral ILC3s play a yet unappreciated role in T-cell–mediated immunity.
Keywords: T-cell activation, antigen presentation
Abstract
Group 3 innate lymphoid cells (ILC3s) have emerged as important cellular players in tissue repair and innate immunity. Whether these cells meaningfully regulate adaptive immune responses upon activation has yet to be explored. Here we show that upon IL-1β stimulation, peripheral ILC3s become activated, secrete cytokines, up-regulate surface MHC class II molecules, and express costimulatory molecules. ILC3s can take up latex beads, process protein antigen, and consequently prime CD4+ T-cell responses in vitro. The cognate interaction of ILC3s and CD4+ T cells leads to T-cell proliferation both in vitro and in vivo, whereas its disruption impairs specific T-cell and T-dependent B-cell responses in vivo. In addition, the ILC3–CD4+ T-cell interaction is bidirectional and leads to the activation of ILC3s. Taken together, our data reveal a novel activation-dependent function of peripheral ILC3s in eliciting cognate CD4+ T-cell immune responses.
Innate lymphoid cells (ILCs) are a group of lymphocytes that play a critical role in immediate immune host defense as well as mucosal and lymphoid tissue homeostasis. Although they lack somatically rearranged antigen (Ag) receptors, they exhibit a transcription factor and cytokine profile similar to T helper (Th) cells. Therefore, ILCs are classified into three major families (1). Reminiscent of Th1 cells, ILC1s are characterized by Interferon (IFN)γ production and developmental regulation by T-bet. ILC2s secrete interleukin (IL)-5, IL-9, and IL-13 and, analogous to Th2 cells, depend on Gata3. ILC3s produce IL-22 and IL-17A and together with Th17 cells express the retinoic acid receptor-related orphan receptor RORγt (2, 3). ILC3s can be fractioned into NKp46+ and NKp46− subsets including lymphoid tissue inducer (LTi) cells. Here, lin−NKp46−CD4+/−RORγt+ ILCs are referred to as natural cytotoxicity receptor (NCR)−ILC3s. ILCs express Toll-like receptors (TLRs) (4) and IL-1R, indicating that they can directly sense microbial products and inflammatory signals (5–7). The ability to rapidly release cytokines upon microbial challenge fostered the idea that ILCs may bias the outcome of T-cell responses. In addition, both ILC2 and ILC3s were recently shown to modulate CD4+ T-cell responses through Ag-peptide presentation by MHC class II (8, 9). In line with this, it was proposed a decade ago that ILC3s interact with T cells in secondary lymphoid organs and thereby promote CD4+ T-cell memory responses (10, 11). Whether peripheral ILC3s can mature into Ag-presenting cells (APCs) providing costimulation for T-cell–mediated immunity has never been explored. In the present study, we show that in mice where MHC class II is specifically deleted in ILC3s, Ag-specific T-cell and T-dependent (TD) B-cell responses are impaired, demonstrating that ILC3s present Ag to CD4+ T cells in vivo. IL-1β strongly activates fetal liver (FL)-derived and splenic NCR−ILC3s and induces both the expression of costimulatory molecules and the up-regulation of MHC class II molecules, thereby enhancing their T-cell priming potential. Finally, we show that in the presence of Ag, the cognate interaction between CD4+ T cells and NCR−ILC3s leads to the activation of the latter, suggesting an unexpected crosstalk between these two cell types. Altogether, we show that peripheral ILC3s can mature upon activation into bona fide APCs shaping T-cell–mediated immune responses.
Results
ILC3s Elicit Ag-Specific T-Cell Proliferation and TD B-Cell Responses in Vivo.
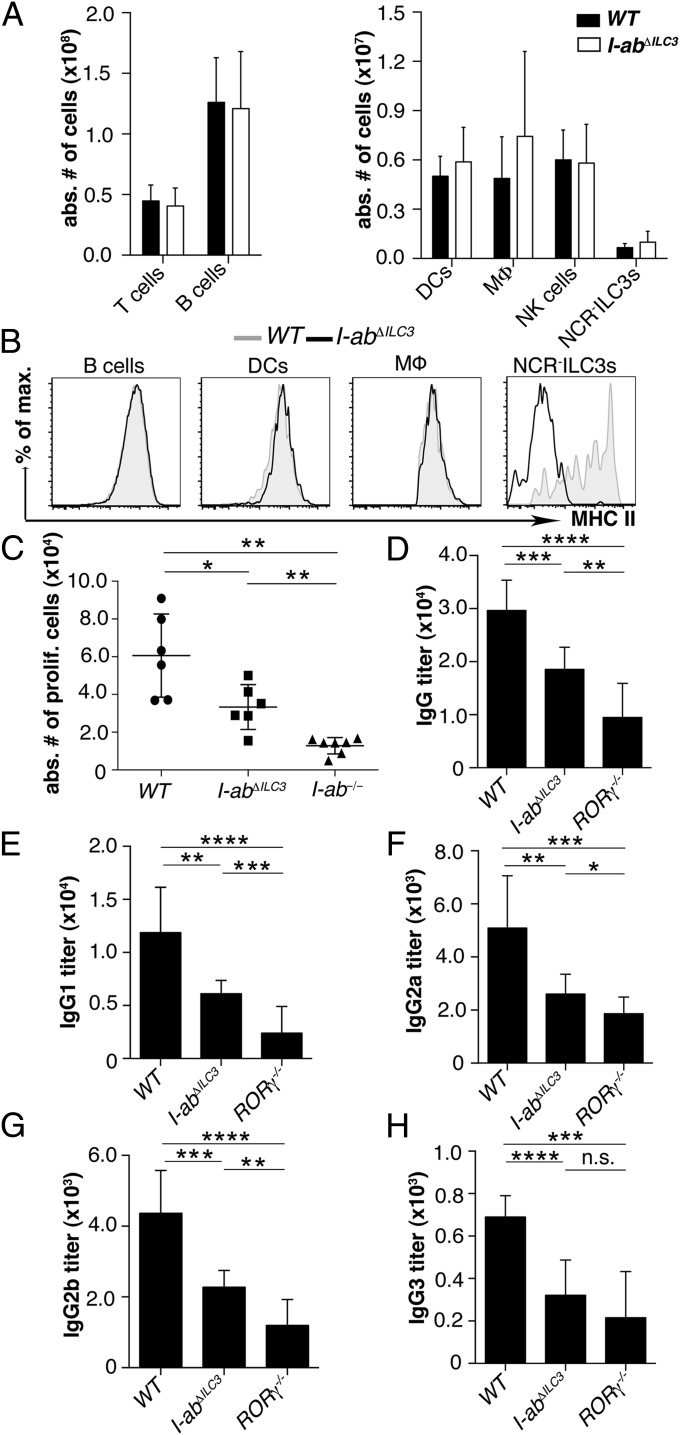
To investigate whether ILC3s can initiate CD4+ T-cell responses in vivo, we generated mice with a deletion of Iab exclusively in ILC3s by crossing mice expressing Cre recombinase under the control of the RORc promoter [RORc(γt)-Cretg] (12) to mice with a floxed H2-Ab1 allele (I-abneo) (13). Mice homozygous for the floxed H2-Ab1 allele and carrying one copy of the Cre transgene, here referred to as I-abΔILC3 mice, were healthy, did not show signs of spontaneous inflammation, and had a normal distribution of lymphocytes, macrophages (MΦ), and dendritic cells (DCs) (Fig. 1A). Numbers of ILC3s were also similar in WT and I-abΔILC3 mice (Fig. 1A). MHC class II expression was normal on splenic B cells, DCs, and MΦ, whereas NCR−ILC3s completely lacked MHC class II (Fig. 1B). To examine Ag-specific CD4+ T-cell proliferation in vivo, 3 × 106 carboxyfluorescein succinimidyl ester (CFSE)-labeled CD4+ T cells from OT-II (H-2b) T-cell receptor transgenic mice (OT-IItg) were adoptively transferred into WT, I-abΔILC3, or I-ab−/− mice. Following immunization with ovalbumin (OVA) peptide323–339 and OVA protein plus CpG, labeled OT-IItg T cells proliferated in WT, but not in I-ab−/− mice (Fig. 1C). In I-abΔILC3 mice, OT-IItg T-cell proliferation was significantly reduced, demonstrating that ILC3s were able to present Ag and to meaningfully alter OVA-specific T-cell responses in vivo. To study the role of ILC3s in TD B-cell responses, we immunized WT, I-abΔILC3, and RORγ−/− mice intraperitoneally (i.p.) with a single dose of Alum-precipitated nitrophenylated-OVA (100 μg) and adoptively transferred 2 × 106 OT-IItg CD4+ T cells plus CpG. The loss of MHC class II on ILC3s (I-abΔILC3) resulted in a significant reduction of 4-hydroxy-3-nitrophenyl-acetyl (NP)-OVA–specific IgG 14 d after immunization (Fig. 1D). Additionally, a more detailed analysis of IgG subtypes revealed that specific IgG1, IgG2a, IgG2b, and IgG3 levels were significantly reduced (Fig. 1 E–H). In RORγ−/− mice, where ILC3s, Th17 cells, lymph nodes, and Peyer’s patches were completely absent, NP-OVA–specific IgG titers were even more reduced. Collectively, these data unambiguously show that Ag presentation by ILC3s contributes to T-cell priming in vivo and that CD4+ T-cell proliferation and TD B-cell responses were impaired when Ag presentation was abolished in ILC3s.
Fig. 1.
ILC3s elicit Ag-specific T-cell proliferation and TD B-cell responses in vivo. (A) Absolute numbers of T cells (CD3+), B cells (CD19+), DCs (CD11c+), MΦ (CD11b+F4/80+), natural killer (NK) cells (NK1.1+NKp46+), and NCR−ILC3s (lin−RORγt+CD117+NCR−) in the spleen of WT and I-abΔILC3 mice. Data are shown as mean ± SD (n = 9; three independent experiments). (B) Representative histograms of MHC class II expression on splenic B cells, DCs, MΦ, and CD4+NCR−ILC3s of WT and I-abΔILC3 mice (three independent experiments). (C) Sort-purified CFSE-labeled OT-IItg CD4+ T cells were injected i.v. into WT (filled circle), I-abΔILC3 (filled square), and I-ab−/− (filled triangle) mice immunized with OVA peptide, OVA protein, and CpG. Absolute numbers of proliferating OT-IItg CD4+ T cells recovered from the spleen 2 d later (mean values ± SD; four independent experiments; n = 6–7; *P ≤ 0.05; **P ≤ 0.01). (D–H) WT, I-abΔILC3, and RORγ−/− mice were i.p. immunized with 100 μg alum-precipitated NP-OVA after i.v. injection of OT-IItg CD4+ T cells plus CpG. NP-OVA–specific IgG (D), IgG1 (E), IgG2a (F), IgG2b (G), and IgG3 (H) levels 14 d after immunization. Data are presented as mean values ± SD (n = 9–10) from three independent experiments (n.s., not significant; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001).
NCR−ILC3s Can Internalize Latex Beads.
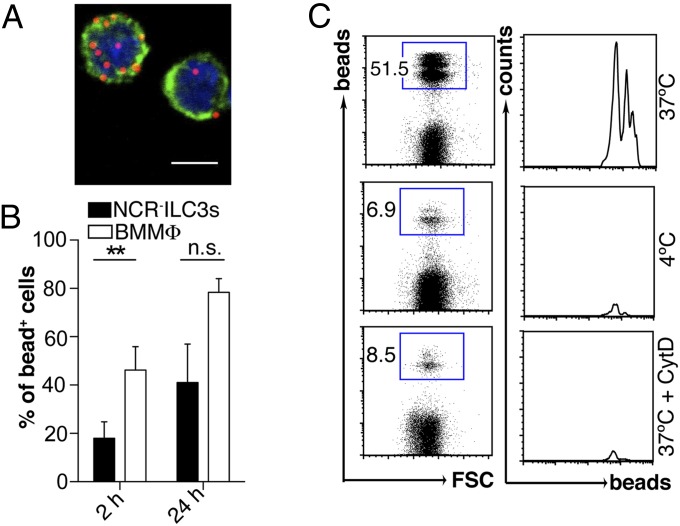
Based on the in vivo finding that the lack of MHC class II molecules by ILC3s impaired T-cell–mediated immune responses, we tested the capacity of NCR−ILC3s generated from α4β7+ FL progenitor cells in vitro (14) or ex vivo isolated from the spleen of adult mice to take up red fluorescent latex beads. We and others have previously shown that FL-derived and adult ILC3s share phenotypic and functional properties such as lymphotoxin β-dependent induction of lymphoid tissue formation (15, 16). Both in vitro-generated and ex vivo-isolated NCR−ILC3s were capable of internalizing red fluorescent latex beads, although with slower kinetics than bone marrow (BM)-derived MΦ (BMMΦ) (Fig. 2 A and B and Fig. S1). Bead uptake was severely inhibited at 4 °C or in the presence of 0.5 µM Cytochalasin D (CytD), an inhibitor of actin polymerization, showing the specificity of internalization (Fig. 2C). In vitro-generated NCR−ILC3s could be subdivided into CD4+ and CD4−NCR−ILC3 subsets (Fig. S2 A and B). Both subsets had a CD69− naïve phenotype and expressed comparable levels of RORγt, LTαβ, common gamma chain (γc), CD117, integrins, and chemokine receptors (Fig. S2C). CD4+ NCR−ILC3s were slightly more efficient in taking up Ag than their CD4− counterpart (Fig. S2D). Thus, NCR−ILC3s can internalize and process exogenous Ags.
Fig. 2.
Naïve NCR−ILC3s can internalize latex beads. (A) Representative immunofluorescence image of red fluorescent latex bead uptake by sort-purified in vitro-generated NCR−ILC3s. (Scale bar, 5 µm.) (B) Bead internalization by NCR−ILC3s and BMMΦ. Percentage of bead+ cells 2 and 24 h after addition of beads (mean values ± SD; n.s., not significant; **P ≤ 0.01). (C) Representative plots of NCR−ILC3s cultured with beads for 6 h at either 37 °C or 4 °C, or at 37 °C in the presence of 0.5 μM CytD. Data are representative of at least three independent experiments (n = 3–5).
Upon Proinflammatory Stimulation NCR−ILC3s Become Activated and Secrete Cytokines.
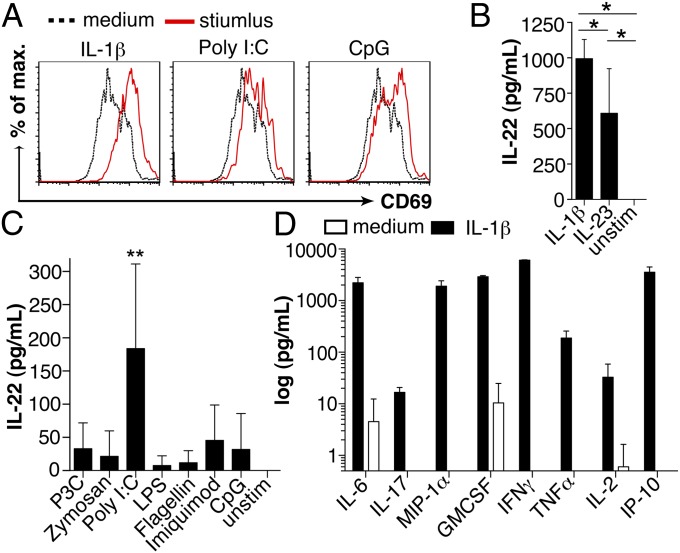
How DCs undergo maturation and activation upon exposure to signals associated with infection and inflammation is well documented (17). We therefore tested proinflammatory cytokines and TLR ligands for their ability to activate NCR−ILC3s. IL-1β, Poly I:C, and CpG up-regulated the expression of surface CD69 on NCR−ILC3 (Fig. 3A). Similar results were obtained from ex vivo-isolated splenic NCR−ILC3s of WT mice 6 h after i.p. injection with CpG (Fig. S3). Upon stimulation with IL-1β, in vitro-generated NCR−ILC3s produced high levels of IL-22, exceeding those induced upon IL-23 stimulation (Fig. 3B). In vitro stimulation with TLR ligands revealed that Poly I:C could induce IL-22 production by NCR−ILC3s (Fig. 3C). Thus, these data show that ligands for TLR 3 and 9 have the ability to directly activate NCR−ILC3s and that IL-1β is a remarkably strong inducer of IL-22 secretion. In addition, IL-1β–exposed NCR−ILC3s secreted IL-2, IL-6, macrophage inflammatory protein 1 (MIP-1)α, IFNγ, and tumor necrosis factor (TNF)α, all known for their capacity to promote T-cell responses (Fig. 3D). IL-1β also induced the secretion of IFN-induced protein of 10 kDa (IP-10), a chemoattractant for mononuclear cells and CXCR3+ effector T cells (18). Collectively, these data demonstrate that IL-1β and TLR ligands can activate NCR−ILC3s, remarkably altering the repertoire of cytokines they produce.
Fig. 3.
Cytokine secretion of activated NCR−ILC3s. (A) Representative histograms of CD69 expression on in vitro-generated NCR−ILC3s after 48 h stimulation with IL-1β, Poly I:C, CpG, or in medium alone (four independent experiments). IL-22 secretion by in vitro-generated NCR−ILC3s upon 48 h exposure to IL-1β, IL-23 (B), TLR ligands (C), or medium alone (mean values ± SD, n = 6–7, three independent experiments; *P ≤ 0.05; **P ≤ 0.01). (D) Cytokine production by in vitro-generated NCR−ILC3s after 48 h culture with IL-1β or medium alone. Results are shown as mean values ± SD.
IL-1β Induces the Expression of MHC Class II and Costimulatory Molecules on Peripheral NCR−ILC3s.
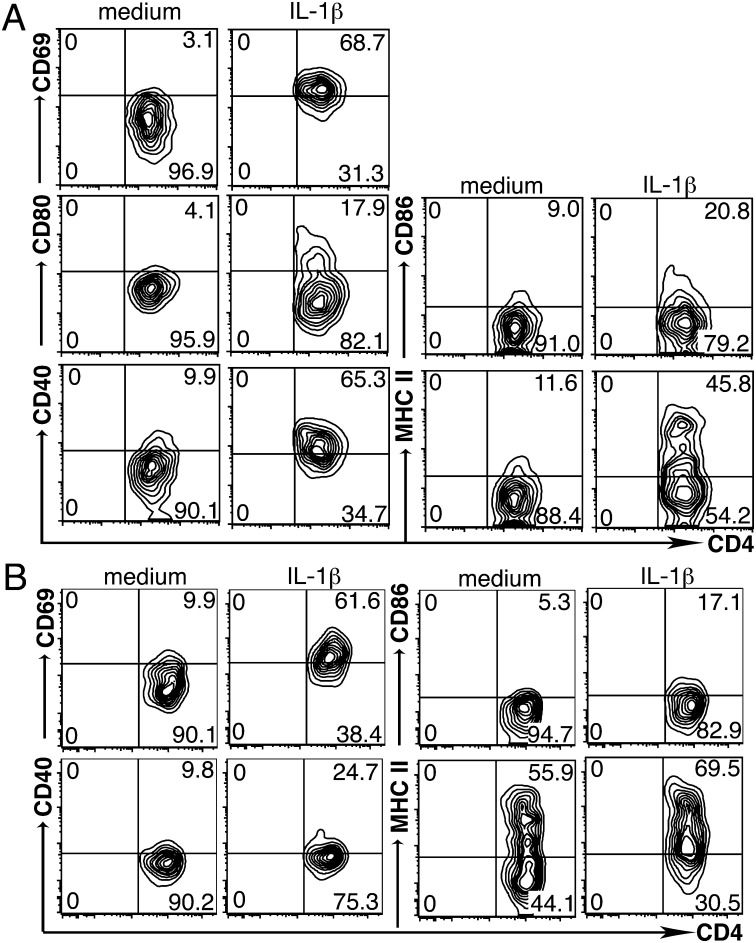
We further asked whether, analogous to DCs, activated NCR−ILC3s express costimulatory molecules and up-regulate MHC class II molecules. Both in vitro-generated and ex vivo-isolated CD4+NCR−ILC3s had a naïve phenotype shown by the absence of CD69 and costimulatory molecules (Fig. 4 A and B). Upon stimulation with IL-1β for 48 h, both sort-purified in vitro-generated CD4+NCR−ILC3s and ex vivo-isolated CD4+NCR−ILC3s expressed the costimulatory molecules CD80 and CD86 and up-regulated the expression of CD40, CD69, and MHC class II (Fig. 4 A and B). Hence, upon IL-1β stimulation, NCR−ILC3s acquire an APC-like phenotype, reminiscent of activated DCs. It has been reported that mucosa-associated ILC3s were unable to express costimulatory molecules (9). Whether these cells could respond to inflammatory stimulation has never been investigated. Therefore, we stimulated ex vivo-isolated small intestinal lamina propria (LP)-derived NCR−ILC3s with IL-1β for 48 h. Interestingly, although IL-1β stimulation resulted in blast formation, even with high concentrations of IL-1β, we were unable to detect up-regulation of MHC class II or expression of CD40 and CD86 (Fig. S4 A and B). These data unambiguously show that peripheral but not LP NCR−ILC3s express costimulatory molecules upon innate activation.
Fig. 4.
IL-1β induces the expression of MHC class II and costimulatory molecules on peripheral NCR−ILC3s. Expression of CD69, CD40, CD80, CD86, and MHC class II on sort-purified in vitro-generated CD4+NCR−ILC3s (A) and ex vivo-isolated splenic CD4+NCR−ILC3s (B) cultured for 48 h with IL-1β or medium alone. Numbers in contour plots show the percentage of cells in each quadrant. Data are representative of three independent experiments.
Activated NCR−ILC3s Can Induce Ag-Specific CD4+ T-Cell Activation and Proliferation.
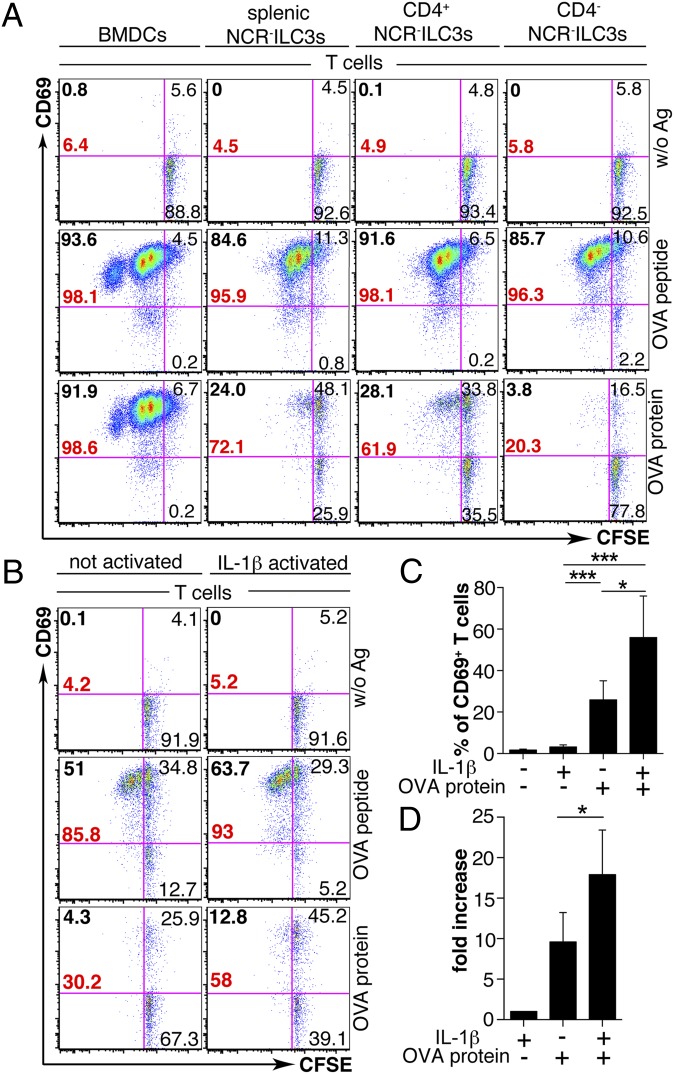
We next examined whether peripheral NCR−ILC3s could trigger naïve T-cell priming in vitro. NCR−ILC3s (H-2b) were in vitro stimulated with IL-1β and incubated with CFSE-labeled OT-IItg CD4+ T cells in the presence of OVA peptide323–339 or OVA protein. Ex vivo-isolated and in vitro-generated CD4+ and CD4− NCR−ILC3s were able to activate the majority of CD4+ T cells in the presence of OVA peptide monitored by CD69 expression (Fig. 5A). In vitro-generated CD4+ and ex vivo-isolated NCR−ILC3s were able to induce several rounds of OVA-specific CD4+ T-cell proliferation when pulsed with OVA peptide or, to a lesser extent, with OVA protein. CD4−NCR−ILC3s were considerably less efficient in inducing protein Ag-specific CD4+ T-cell responses (Fig. 5A). To further examine the effect of activation of NCR−ILC3s on the capacity to elicit T-cell responses, we stimulated ex vivo-isolated splenic NCR−ILC3s with IL-1β or left them untreated and cocultured them with OT-IItg CD4+ T cells and OVA protein (Fig. 5 B–D). A total of 30.2% of the T cells in culture with untreated NCR−ILC3s and OVA protein expressed CD69, and only 4.3% of the T cells proliferated (Fig. 5B). IL-1β–activated NCR−ILC3s increased the percentage of both CD69+ and proliferating T cells (Fig. 5 B–D). Compared with splenic NCR−ILC3s, LP NCR−ILC3s were three times less efficient to induce cognate T-cell activation in vitro (Fig. S5 A and B). Interestingly, we noted that the CD69 surface expression level of NCR−ILC3s increased approximately sevenfold in the presence of OT-IItg CD4+ T cells and OVA protein, compared with cocultures without Ag and without previous IL-1β stimulation. This phenomenon was not further increased by previously activating NCR−ILC3s with IL-1β (Fig. S6A). It is important to note that CD69 expression on NCR−ILC3s peaked early after activation and decreased in cocultures, unless T cells and Ag were added. Ag alone was not able to sustain CD69 expression (Fig. S6B). Together, these results show that IL-1β increased the capacity of NCR−ILC3s to induce CD4+ T-cell activation and that the cognate ILC3–CD4+ T-cell interaction led to the activation of NCR−ILC3s.
Fig. 5.
NCR−ILC3s can induce Ag-specific CD4+ T-cell activation and proliferation. (A) Naïve CFSE-labeled OT-IItg CD4+ T cells were cultured with either BMDCs, IL-1β–activated ex vivo-isolated splenic NCR−ILC3s, or in vitro-generated sorted CD4+ or CD4−NCR− ILC3s in the presence of OVA peptide, OVA protein, or medium alone (without Ag). Representative plots of CD69 and CFSE expression by CD4+ T cells 72 h later. Bold black numbers, percentage of proliferating T cells; bold red numbers, percentage of total CD69+ T cells. (B) Naïve CFSE-labeled OT-IItg CD4+ T cells were cultured with non- or IL-1β–activated splenic NCR−ILC3s in the presence of OVA peptide, OVA protein, or medium alone (without Ag) for 72 h. (C) Percentage of CD69+ CD4+ T cells upon coculture with non- or IL-1β–activated splenic NCR−ILC3s in the presence or absence of Ag. Data are shown as mean values ± SD (n = 3–7; *P ≤ 0.05; ***P ≤ 0.001). (D) Fold increase of percentage of CD69+ CD4+ T cells upon coculture with non- or IL-1β–activated splenic NCR−ILC3s in the presence of OVA protein relative to coculture of IL-1β–activated NCR−ILC3s and T cells in the absence of Ag. Data are shown as mean values ± SD (n = 3–7; *P ≤ 0.05). Data are representative of at least three independent experiments.
Discussion
We show here that upon IL-1β stimulation NCR−ILC3s expressed MHC class II and costimulatory molecules and became bona fide APCs as they were able to promote OVA-specific CD4+ T-cell proliferation in mice. In addition, activated NCR−ILC3s expressed an unexpected repertoire of cytokines known to alter T-cell responses. Ag-specific T-cell proliferation and IgG-mediated humoral immunity were impaired in animals in which Ag presentation was abolished exclusively in ILC3s. Finally, in the presence of Ag, the T-cell priming led to an extended activation of ILC3s. These novel data suggest that upon inflammation the cognate interaction of NCR−ILC3s and CD4+ T cells contributes to adaptive immunity.
Our in vivo data demonstrate that peripheral NCR−ILC3s process protein Ags and stimulate Ag-specific CD4+ T-cell responses. Whether the cognate interaction between NCR−ILC3s and CD4+ T cell leads directly to the priming of T cells or whether this interaction rather polarizes or enhances the T-cell response has yet to be established. In either case, the cytokines that are secreted by NCR−ILC3s upon activation are likely to decisively impact the outcome of T-cell responses. Because NCR−ILC3s reside at the interface between B- and T-cell zones (10, 19), they are located strategically within the lymphoid microenvironment to efficiently promote immune responses in vivo. Considering their 10 times lower numbers compared with DCs in the spleen of WT mice, our in vivo data emphasize the potential of ILC3s to induce CD4+ T-cell proliferation. The immunization of I-abΔILC3 mice indeed showed that the specific lack of MHC class II on NCR−ILC3s drastically impaired T and TD B-cell responses in the spleen. The overall decrease in Ag-specific Ab isotypes suggests that cognate ILC3–T cell interactions did not significantly affect Th cell polarization. In vitro, NCR−ILC3s were less potent than BM-derived DCs in priming naïve T cells, probably because DCs expressed higher levels of costimulation and MHC class II molecules. Despite this, our data show that ILC3s are crucial for mounting adaptive immune responses in vivo, suggesting that their ability to present Ag is increased in the splenic microenvironment. This could be either due to the splenic cytokine milieu or the crosstalk between ILC3s and other immune cells. In line with the finding that CD4+NCR−ILC3s are more differentiated than their CD4− counterpart (20), CD4+NCR−ILC3s had a greater potential of inducing Ag protein-specific T-cell proliferation.
The I-abΔILC3 mouse strain generated by others has been reported to spontaneously develop signs of systemic inflammation (9). In our I-abΔILC3 mouse colony that we kept under strict specific pathogen-free conditions, we did not observe any pathology or abnormal lymphocyte numbers. The discrepancy between our mice and the mice reported by Hepworth et al. might be due to microbial exposure in different animal facilities. Whereas ILC3s appear to limit T-cell responses to commensal bacteria in the intestine through Ag presentation in the absence of costimulation (9), we show here that splenic NCR−ILC3s have the ability to promote T- and B-cell responses in the periphery. Interestingly, we were unable to detect costimulatory molecules on LP NCR−ILC3s ex vivo or after in vitro stimulation with IL-1β. Hence, it appears that the microenvironments in which ILC3s reside profoundly alter their function, as suggested by the different expression signature profiles displayed by ILC3s isolated from various tissues (21, 22). We observed that ex vivo-isolated splenic ILC3s from naïve mice lacked costimulatory molecules but a substantial fraction expressed MHC class II. Activated NCR−ILC3s, however, expressed both MHC class II and costimulatory molecules and were fully capable of inducing T-cell responses in vitro and in vivo. In the mucosa, the microbiota-driven release of IL-1β promotes the production of granulocyte macrophage colony-stimulating factor (GM-CSF) by ILC3s. This triggers the release of retinoic acid and IL-10 by mucosal DCs and MΦ, leading to the expansion of regulatory T cells (23). These data together with our findings support the idea that IL-1β is a strong activator of ILC3s and that the outcome of ILC3 effector functions depends on additional tissue-specific cells and regulatory cytokines. Hence, splenic ILC3s are fully capable of priming T-cell responses, whereas mucosal ILC3s may prevent T-cell responses through absence of costimulation and induction of tolerogenic cytokine responses by other cells. Because of their remarkable ability to secrete cytokines, ILCs are emerging as important cellular players, actively modulating immune responses. For instance, human splenic ILC3s enhance the survival of marginal zone B cells through the release of B-cell activating factor (BAFF) and stimulate perifollicular neutrophils through GM-CSF (24). In humans, IL-1R signaling was shown to induce NCR−ILC3s to produce IL-17, IL-22, and IFNγ (25, 26). We show here that IL-1β increased the secretion of IL-2, IL-6, MIP-1α, IP-10, IFNγ, TNFα, and GM-CSF in vitro. Although our in vitro data suggest that IL-1β is likely to play an important role in ILC activation and immunity, the actual molecular and cellular events leading to NCR−ILC3 activation in vivo are still unknown. Ag-dependent cognate interaction between ILC3s and T cells also led to NCR−ILC3 activation reflected by the up-regulation of CD69 (Fig. S6A). This result may explain why naïve NCR−ILC3 could trigger T-cell priming in the absence of previous activation by IL-1β (Fig. 5B). A similar TCR-dependent crosstalk occurs during ILC2–T cell interactions (8), illustrating that T cells or T-cell–derived cytokines may reciprocally act on ILC responses. Collectively, our data reveal an important function for group 3 ILCs in promoting peripheral T-cell–mediated responses and improve our understanding of how these cells may link innate and adaptive immune responses.
Materials and Methods
Mice.
C57BL/6 were purchased from Janvier (Saint Berthevin). RORγ−/− (3) and MHCIIΔ/Δ (here referred to as I-ab−/−) (27) were described elsewhere. OT-IItg mice were kindly provided by A. Rolink, Rag2−/− mice by G. Hollaender (University of Basel, Basel, and Jesus College, Oxford), I-abneo (13) mice by E. Palmer (University of Basel, Basel), and RORc(γt)-Cretg mice (12) by A. Diefenbach (University of Freiburg, Freiburg im Breisgau, Germany). All mice were bred and maintained under specific pathogen-free conditions. I-abΔILC3 mice were generated by crossing I-abneo mice, which contain a floxed H2-Ab1 allele, with RORc(γt)-Cretg mice. F1 generations were backcrossed to I-abneo mice. The animal experiments received the approval of the Cantonal Veterinary Office of the city of Basel.
Antibodies.
FITC, Phycoerythrin (PE), PerCP–eFluor 710, PE–Cy7, Allophycocyanin (APC), APC–Cy7, BV421 or biotin-conjugated anti-CD3 (17A2), anti-CD4 (RM4-5), anti-CD11b (M1/70), anti-CD11c (117310, N418), anti-CD19 (6D5), anti-CD29 (HMb1-1), anti-CD40 (1C10), anti-CD45R (RA3-6B2, B220), anti-CD69 (H1.2F3), anti-CD90.2 (30-H12), anti-CD117 (2B8), anti-CD196 (29-2L17), anti-CD197 (4B12), anti–Gr-1 (RB6-8C5, Ly-6G), anti-TCRβ (H57-597), anti-TCRγδ (UC7-13D5), anti-MHC II (M5/114.15.2), anti-α4β7 (DATK32), and anti-NKp46 (29A1.4) antibodies (Abs) were purchased from BioLegend; anti-CD54 (3E2), anti-CD132 (TUGm2, γc chain), anti-CD184 (2B11, CXCR4), anti-CD185 (2G8, CXCR5), and anti-MHC II (25-9-17) Abs from BD Bioscience; and anti-CD3 (145-2C11), anti-CD8α (53-6.7), anti-CD11c (N418), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-F4/80 (BM8), anti-NK1.1 (PK136), NKp46 (29A1.4), and anti-RORγt (AFKJS-9, B2D) Abs from eBioscience. As secondary reagent, fluorochrome-conjugated streptavidin (BioLegend) was used.
Flow Cytometry and Cell Sorting.
Flow cytometry stainings were performed using standard protocols (28). Intracellular RORγt staining was performed using a fixation/permeabilization kit (FoxP3 staining buffer kit, eBioscience). Dead cells were identified using fixable Aqua Live/Dead cell staining kit (Molecular Probes, Life Technologies) or propidium iodide solution (Sigma-Aldrich). Lymphotoxin staining was performed as previously described (29). Data acquisition was performed with a FACSCalibur or FACSCanto II (BD Bioscience), and data were analyzed using FlowJo software (Tree Star). Cell sorting was done using a FACS Aria (BD Bioscience, >98% purity).
Cell Isolation and Culture.
BMDCs and BMMΦ were generated as described elsewhere (30, 31). Cells were harvested after 7 d in culture at 37 °C, 10% CO2. NCR−ILC3s were generated in vitro from α4β7+ precursors as described before (14) and sorted based on CD90.2, CD117, and CD4 expression. Splenic and small intestinal LP NCR−ILC3s were isolated from the spleen and small intestine of adult Rag2−/− mice. The small intestine was opened longitudinally, cut into pieces, incubated in 1× PBS containing 30 mM EDTA (30 min, 4 °C), washed several times in 1× PBS, and then incubated in DMEM containing 0.025 mg/mL DNaseI (Roche) and 1 mg/mL Collagenase D (Roche) for 1 h at 37 °C. Every 15 min, the supernatant was collected, and tissue pieces were washed and reincubated with medium containing DNaseI and Collagenase D. Cell suspension was pelleted, resuspended in 5 mL 40% Percoll (GE Healthcare), underlayed with 3 mL 80% Percoll, and centrifuged at 20 °C and 630 × g for 30 min. Cells of the interphase were collected. Spleens were also cut into pieces, washed in 1× PBS, and digested with DNaseI and Collagenase D as described above. After digestion, spleen cells were washed and erythrolysis was performed. Ex vivo-isolated splenic or LP NCR−ILC3s were sorted based on the expression of CD117 and the lack of the lineage marker CD3, CD8α, CD11c, CD19, B220, Gr-1, TCRβ, TCRγδ, NK1.1, and NKp46. CD4+ T cells from the spleen and lymph nodes of OT-IItg mice were magnetically purified by using CD4 beads (LTR4, Miltenyi Biotec) following the manufacturer’s instruction. MACS-enriched CD4+ T cells were sorted to reach >98% purity and labeled with 7.5 μM CFSE (Molecular Probes) in 1× PBS (8 min, room temperature).
NCR−ILC3 Stimulation and Ag Presentation Assay.
Sort-purified in vitro-generated NCR−ILC3s or ex vivo-isolated splenic or LP NCR−ILC3s were cultured in a 96-well plate (Costar, Corning, Inc.) in the presence of either TLR ligands [100 ng/mL Pam3Cys, 25 μg/mL Poly I:C, 1 μg/mL Flagellin, 1 μg/mL Imiquimod (InvivoGen), 10 μg/mL Zymosan, 1 μg/mL lipopolysaccharide (LPS) (Sigma-Aldrich), 1 μM CpG (Trilink Biotechnologies, ODN1826 sequence InvivoGen)], proinflammatory cytokines [20, 100 ng/mL IL-1β (Biovision, Inc.), 20 ng/mL IL-23 (eBioscience)], or in medium alone for 48 h. To test Ag presentation and T-cell activation, sort-purified stimulated (20 ng/mL IL-1β, 24 h) NCR−ILC3s were cocultured in the presence of OT-IItg CD4+ T cells and either OVA323–339 peptide (5 µg/mL, AnaSpec), OVA protein (100 µg/mL, Imject Ovalbumin, Thermo Fisher Scientific, Inc.), or medium alone (without Ag) for 48–72 h.
Fluorescent Latex Bead Uptake.
Fluorescent latex bead uptake was performed as previously described (32) with some adaptations. Briefly, in vitro-generated or ex vivo-isolated splenic NCR−ILC3s were cultured in a 96-well plate, and latex beads [FluoSpheres carboxylate-modified microspheres, 1 µm, red fluorescent (580/605), Molecular Probes, Life Technologies] were added for 6 h at 37 °C, 4 °C, or 37 °C in the presence of 0.5 µM CytD (Applichem). To compare bead uptake of BMMΦ and NCR−ILC3s, cells were harvested after 2 h or 24 h. Bead internalization was analyzed by flow cytometry. For immunofluorescence microscopy, in vitro-generated NCR−ILC3s were stained with FITC-conjugated anti-CD90.2 (30-H12, 30 min at 4 °C) and HOECHST dye (Hoechst 33342, Invitrogen, 30 min at 37 °C) after incubation with beads. Bead uptake was monitored using a confocal laser-scanning microscope (Zeiss LSM 510 Meta). Images were analyzed with ImageJ (W. Rasband, National Institutes of Health, Bethesda). An adjustment of brightness and contrast was performed.
Adoptive Cell Transfer and Immunization.
We injected 3 × 106 OT-IItg CD4+ T cells (CFSE+) intravenously (i.v.) into WT, I-abΔILC3, and I-ab−/− recipient mice i.v. immunized with OVA323–339 peptide (20 µg/mL), OVA protein (100 μg/mL), and CpG (50 μM). Forty-eight hours later, OT-IItg CD4+ T-cell proliferation was examined in the spleen. We i.v. injected 2 × 106 OT-IItg CD4+ T cells plus CpG (25 μM) into WT, I-abΔILC3, and RORγ−/− mice. Mice were immunized i.p. with 100 μg alum-precipitated NP-OVA [NP (18)-OVAL, Biosearch Technologies, Inc.]. Sera were collected 14 d after NP-OVA immunization.
Ab and Cytokine Detection by ELISA and Luminex Assay.
To detect NP-OVA–specific Abs in the serum of immunized mice, NUNC immunoplate Maxisorb F96 plates (Thermo Scientifics) were coated with 5 μg/mL NP-OVA (Biosearch Technologies, Inc.) in 1× PBS at 4 °C overnight. Sera were incubated for 1.5 h at room temperature, and after washing [H2O 0.1% Tween-20 (AppliChem)] biotin-conjugated goat anti-mouse IgG, IgG1, IgG2a, IgG2b, or IgG3 (Caltag Laboratories) was added, incubated for 1.5 h at room temperature, and detected by alkaline-phosphatase–conjugated Steptavidin (Roche, 45 min, room temperature). Plates were developed with dinitrophenyl phosphate (1 mg/mL, Sigma) in substrate buffer [0.1 g MgCl2 × 6 H2O (Merck), 10 mM NaN3 (Sigma), and 10% diethanolamine (Sigma) at pH 9.8 filled up to 1 L]. The reaction was stopped with 1 M NaOH (Fluka). OD was determined at 405 nm with an ELISA reader (ASYS Expert plus). IL-22 was determined in the supernatant of NCR−ILC3s cultures by using mouse/rat ELISA MAX Deluxe Set (Biolegend) according to the manufacturer’s instructions. OD was determined at 450 nm with an ELISA reader (ASYS Expert Plus). In addition, cytokines were quantified using a multiplex-bead–based Luminex assay (mouse cytokine 20-plex panel, Invitrogen, Life Technologies) according to the manufacturer’s protocol. Analysis was performed with a Luminex 100 (LX100) analyzer (Invitrogen, Life Technologies).
Statistical Analysis.
Statistical analysis was performed using Mann–Whitney U test, unpaired Student t test, and Wilcoxon test with Prism software (GraphPad Software, Inc.).
Supplementary Material
Acknowledgments
We thank the members of the D.F. laboratory for discussions and comments on the manuscript, A. Peter for technical assistance, T. Barthlott and C. Berkemeier for cell sorting, S. Sawa for helpful discussions and protocols, and S. Eckervogt, E. Terszowska, and R. Recinos for animal work. We also thank R. Ceredig for critical reading of the manuscript. This work was supported by Swiss National Science Foundation Grants 310030_130674/1 and CRSII3_136286/1 (to D.F.), 310030_146187 (to F.T.-C.), and 31003AB_131090 (to J.P.); Sinergia Grant CRS133_124819 (to J.P. and A.R.); and the Optimus Foundation and a European Molecular Biology Organization long term fellowship (S.B.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406908111/-/DCSupplemental.
References
- 1.Spits H, et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 2.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: Implications for intestinal health and disease. Immunity. 2012;37(4):601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coccia M, et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209(9):1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynders A, et al. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt- lymphoid cells. EMBO J. 2011;30(14):2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirchandani AS, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192(5):2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 9.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498(7452):113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MY, et al. CD4(+)CD3(-) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18(5):643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 11.Gaspal FM, et al. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol. 2005;174(7):3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 12.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305(5681):248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto K, Joshi SK, Koni PA. A conditional null allele of the major histocompatibility IA-beta chain gene. Genesis. 2002;32(2):152–153. doi: 10.1002/gene.10056. [DOI] [PubMed] [Google Scholar]
- 14.Chappaz S, Gärtner C, Rodewald HR, Finke D. Kit ligand and Il7 differentially regulate Peyer’s patch and lymph node development. J Immunol. 2010;185(6):3514–3519. doi: 10.4049/jimmunol.1000665. [DOI] [PubMed] [Google Scholar]
- 15.Scandella E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9(6):667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 16.Schmutz S, et al. Cutting edge: IL-7 regulates the peripheral pool of adult ROR gamma+ lymphoid tissue inducer cells. J Immunol. 2009;183(4):2217–2221. doi: 10.4049/jimmunol.0802911. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 18.Khan IA, et al. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12(5):483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim MY, et al. OX40 signals during priming on dendritic cells inhibit CD4 T cell proliferation: IL-4 switches off OX40 signals enabling rapid proliferation of Th2 effectors. J Immunol. 2005;174(3):1433–1437. doi: 10.4049/jimmunol.174.3.1433. [DOI] [PubMed] [Google Scholar]
- 20.van de Pavert SA, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508(7494):123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monticelli LA, Sonnenberg GF, Artis D. Innate lymphoid cells: Critical regulators of allergic inflammation and tissue repair in the lung. Curr Opin Immunol. 2012;24(3):284–289. doi: 10.1016/j.coi.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Han S, Kim MY. Heterogeneity of IL-22-producing lymphoid tissue inducer-like cells in human and mouse. Immune Netw. 2010;10(4):115–119. doi: 10.4110/in.2010.10.4.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343(6178):1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magri G, et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15(4):354–364. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci USA. 2010;107(24):10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes T, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32(6):803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen L, et al. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA. 1999;96(18):10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier D, et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26(5):643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197(9):1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brasel K, De Smedt T, Smith JL, Maliszewski CR. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood. 2000;96(9):3029–3039. [PubMed] [Google Scholar]
- 31.Johnson HM, Torres BA. Mechanism of calcium ionophore A23187-induced priming of bone marrow-derived macrophages for tumor cell killing: Relationship to priming by interferon. Proc Natl Acad Sci USA. 1985;82(17):5959–5962. doi: 10.1073/pnas.82.17.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosedasgupta S, Pieters J. Inflammatory stimuli reprogram macrophage phagocytosis to macropinocytosis for the rapid elimination of pathogens. PLoS Pathog. 2014;10(1):e1003879. doi: 10.1371/journal.ppat.1003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.