Significance
Biochemical signals triggered by the T-cell receptor (TCR) are required for stimulating T cells and can be initiated within seconds. However, a hallmark of T-cell activation, cell division, occurs hours after TCR signaling has begun, implying that T cells require a minimum duration and/or accumulate TCR signaling events to drive proliferation. To visualize the accumulated signaling experienced by T cells, we used a fluorescent reporter gene that is activated by TCR stimulation. This technique showed a threshold between dividing and nondividing T cells for TCR signaling that does not change with stronger or weaker TCR signaling or the presence of growth factor. Together these data may have implications for the development of T-cell–targeted therapies for autoimmunity.
Abstract
T-cell antigen receptor (TCR) signaling is essential for activation, proliferation, and effector function of T cells. Modulation of both intensity and duration of TCR signaling can regulate these events. However, it remains unclear how individual T cells integrate such signals over time to make critical cell-fate decisions. We have previously developed an engineered mutant allele of the critical T-cell kinase zeta-chain-associated protein kinase 70 kDa (Zap70) that is catalytically inhibited by a small molecule inhibitor, thereby blocking TCR signaling specifically and efficiently. We have also characterized a fluorescent reporter Nur77–eGFP transgenic mouse line in which T cells up-regulate GFP uniquely in response to TCR stimulation. The combination of these technologies unmasked a sharp TCR signaling threshold for commitment to cell division both in vitro and in vivo. Further, we demonstrate that this threshold is independent of both the magnitude of the TCR stimulus and Interleukin 2. Similarly, we identify a temporal threshold of TCR signaling that is required for commitment to proliferation, after which T cells are able to proliferate in a Zap70 kinase-independent manner. Taken together, our studies reveal a sharp threshold for the magnitude and duration of TCR signaling required for commitment of T cells to proliferation. These results have important implications for understanding T-cell responses to infection and optimizing strategies for immunomodulatory drug delivery.
Stimulation of the T-cell receptor (TCR) drives the activation, proliferation, and differentiation of naïve T cells and elicits effector functions by antigen-experienced T cells. One phenomenon that remains incompletely understood is the disparate kinetics for TCR-dependent signal transduction versus hallmarks of naïve CD4+ T-cell activation, such as production of IL-2 and clonal expansion. Whereas TCR signal transduction is detectable within seconds of TCR engagement, IL-2 production and cell division are detectable only several hours later (1–3). These observations suggest that integration of biochemical signals over time must occur to drive commitment of naïve T cells to a proliferative response. To this end, much attention has been focused on determining the minimum signaling requirements for TCR-driven proliferation. It has been shown that even a single peptide (p)/MHC ligand can trigger detectable increases in intracellular Ca2+ concentration and is sufficient to drive IL-2 production (2, 4). However, it has been estimated that a threshold of 400 peptide/MHC ligands, or 8,000 TCRs must be engaged to commit a T cell to proliferate (5, 6). These results imply that a quantitative threshold for TCR signal magnitude exists for commitment to the CD4+ T-cell proliferative response. It has been demonstrated in vitro that CD8+ T cells require as little as 2 h of TCR stimulation for commitment to clonal expansion and differentiation, demonstrating the existence of a temporal threshold for T-cell proliferation as well (7–9). Similarly, naive CD4+ T cells require TCR stimulation for at least 18–24 h (1, 10, 11) for commitment to multiple rounds of cell division. However, further in vitro and in vivo studies of both CD4+ and CD8+ T-cell responses to antigenic stimulation have yielded conflicting data and also suggest that prolonged stimulation beyond this minimal temporal threshold is necessary for maximal proliferative responses (10, 12–22). Whether a bona fide “autopilot” model truly applies to both CD4+ and CD8+ T-cell proliferation therefore remains controversial. Resolution of this controversy has important implications for the design of drug dosing protocols for treatment of T-cell–mediated diseases such as transplant rejection and autoimmunity. It is therefore of interest to “visualize” the TCR signaling activation threshold for proliferation, determine whether this threshold is perturbed by modulations of TCR signal magnitude and duration, and determine whether ongoing TCR signaling is required or has a role after the threshold has been reached.
To address these questions, we sought to take advantage of two recently characterized experimental tools. The orphan nuclear hormone receptor Nur77(Nr4a1) is rapidly transcribed in response to antigen receptor signaling in vitro and during negative selection of thymocytes in vivo (23, 24). We and others recently reported two independently generated bacterial artificial chromosome transgenic (BAC Tg) mice in which eGFP expression is under the control of Nr4a1 transcriptional regulatory elements. These Nur77–eGFP transgenes served as faithful reporters of antigen receptor signal strength in vitro and during lymphocyte development in vivo (25, 26). Importantly, Nur77–eGFP reporter expression in thymocytes and peripheral T cells is dependent on MHC expression (as a TCR ligand) and does not respond to IL-2 or inflammatory stimuli in vivo (25). Additionally, we have generated a catalytically active zeta-chain-associated protein kinase 70 kDa (Zap70) mutant that can be selectively inhibited by an analog of the small molecule kinase inhibitor PP1 in a dose-dependent, extremely rapid, and reversible manner (27, 28). Proximal TCR signal transduction is absolutely dependent on Zap70 (29). Titration of the catalytic activity of the “analog-sensitive” Zap70 mutant [hereafter referred to as Zap70(AS)] correspondingly titrates the magnitude of the TCR-induced downstream signals that contribute to T-cell activation and proliferation. We therefore reasoned that combining the Zap70(AS) inhibitor system with the Nur77–GFP reporter would enable us to precisely manipulate and sensitively detect TCR signal strength, respectively.
In this study, we took advantage of our Nur77–GFP reporter to unmask a sharp TCR signaling threshold for commitment to proliferation. Further, we demonstrate that this threshold is unperturbed by titration of TCR stimulus, Zap70 kinase activity, or by IL-2. Similarly, we identify a temporal threshold of TCR signaling that is required for commitment to proliferation, after which T cells are able to proliferate in a Zap70 kinase-independent manner. Taken together, our studies reveal a sharp threshold for the magnitude and duration of TCR signaling required for commitment of T cells to proliferation.
Results
A System for Titration and Integration of TCR Signaling.
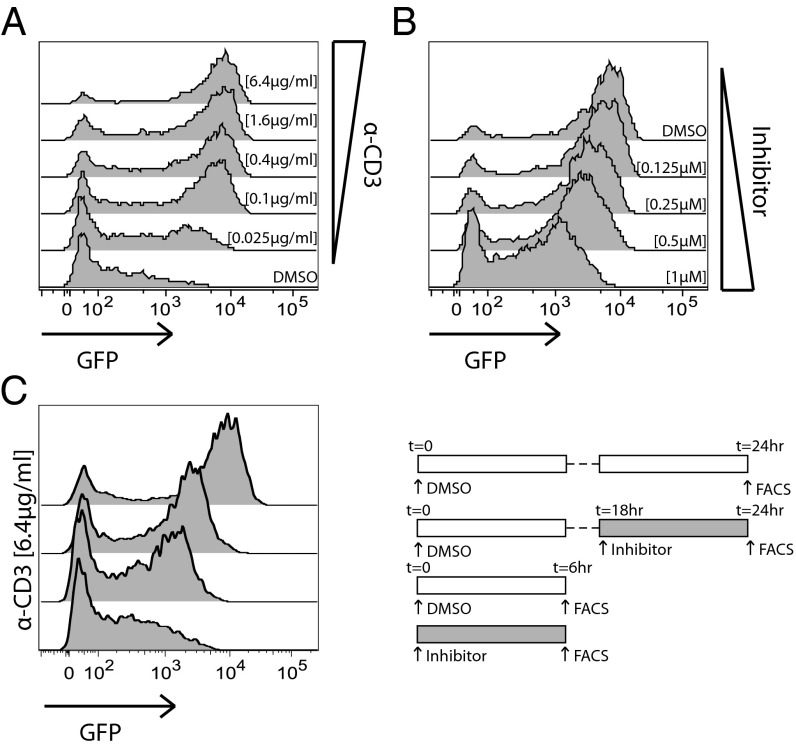
To create T cells in which Zap70 kinase activity was rapidly titratable and in which integrated TCR signaling could be detected and quantified by a fluorescent reporter, we generated mice harboring a Nur77–eGFP BAC transgenic construct and a Zap70(AS) allele on a Zap70-null genetic background, each previously described independently (26, 27, 30). We anticipated that treating T cells harvested from these so-called “Zap70(AS)–GFP” mice with analog inhibitor would block TCR signaling at a proximal node, resulting in reduced GFP expression. Importantly, we reasoned that the Nur77–GFP reporter would reflect not only strength of signaling, but integrated signaling over time because the half-life of reporter eGFP protein is estimated to be as long as 20–24 h, in contrast to endogenous Nur77 (25, 31). To validate this chemical-genetic tool, we stimulated CD4+ and CD8+ Zap70(AS)–GFP T cells ex vivo with varying doses of plate-bound anti-CD3, or with a single high concentration of anti-CD3, and treated with varying doses of HXJ42 (32), a specific PP1 analog small molecule inhibitor of Zap70(AS), for 16 h (33). Anti-CD3 stimulation resulted in a robust enhancement of GFP fluorescence intensity (Fig. 1A and Fig. S1A). Titration of the anti-CD3 stimulus diminished the percentage of responding cells, but did not markedly affect the GFP fluorescence intensity of the responding cells. In contrast, titration of HXJ42 in cells stimulated with a high concentration of anti-CD3 had less effect on the frequency of responding cells, particularly of the CD4+ T cells, but did alter GFP fluorescence intensity of responding cells in an analog, dose-dependent manner (Fig. 1B and Fig. S1 B and C). The all-or-none response to titration of anti-CD3 stimulus likely reflects the digital nature of TCR triggering under limiting TCR ligand conditions (4). In contrast, titration of Zap70 catalytic activity results in modulation of integrated downstream TCR signal strength in an analog fashion, as reflected by reporter GFP expression. To determine whether Nur77–GFP reporter expression was also sensitive to duration of signaling, Zap70(AS)–GFP T cells were stimulated for varying periods of time, ranging from 6 to 24 h. GFP expression was sensitive to the duration of stimulation, varying in a manner that appears to integrate intensity of TCR signal strength over time (Fig. 1C and Fig. S1D). Importantly, GFP expression in mice harboring the wild-type Zap70 allele is unaffected by HXJ42 inhibitor, showing no off-target effects that might influence reporter expression at the maximal dose used in this study (Fig. S1E).
Fig. 1.
A system for titration and detection of TCR signaling. CD4+ T cells from Zap-deficient mice harboring the Zap70(AS) allele and the Nur77–GFP reporter (Zap70(AS)–GFP mice) were stimulated overnight with (A) the indicated concentrations of plate-bound anti-CD3 or with (B) a fixed dose of anti-CD3 (6.4 μg/mL) and the indicated concentrations of analog inhibitor, HXJ42. (C) Zap70(AS) CD4+ cells were stimulated with 6.4 μg/mL anti-CD3 and a high concentration (1 μM) of HXJ42 or vehicle alone was added at 0 h for flow cytometric analysis of GFP expression at 6 h, or cells were stimulated for 18 h, and vehicle or HXJ42 was added for 6 h before analysis of GFP expression at 24 h. Data are representative of at least three independent experiments.
A Sharp, Invariant TCR Signaling Threshold for T-Cell Proliferation.
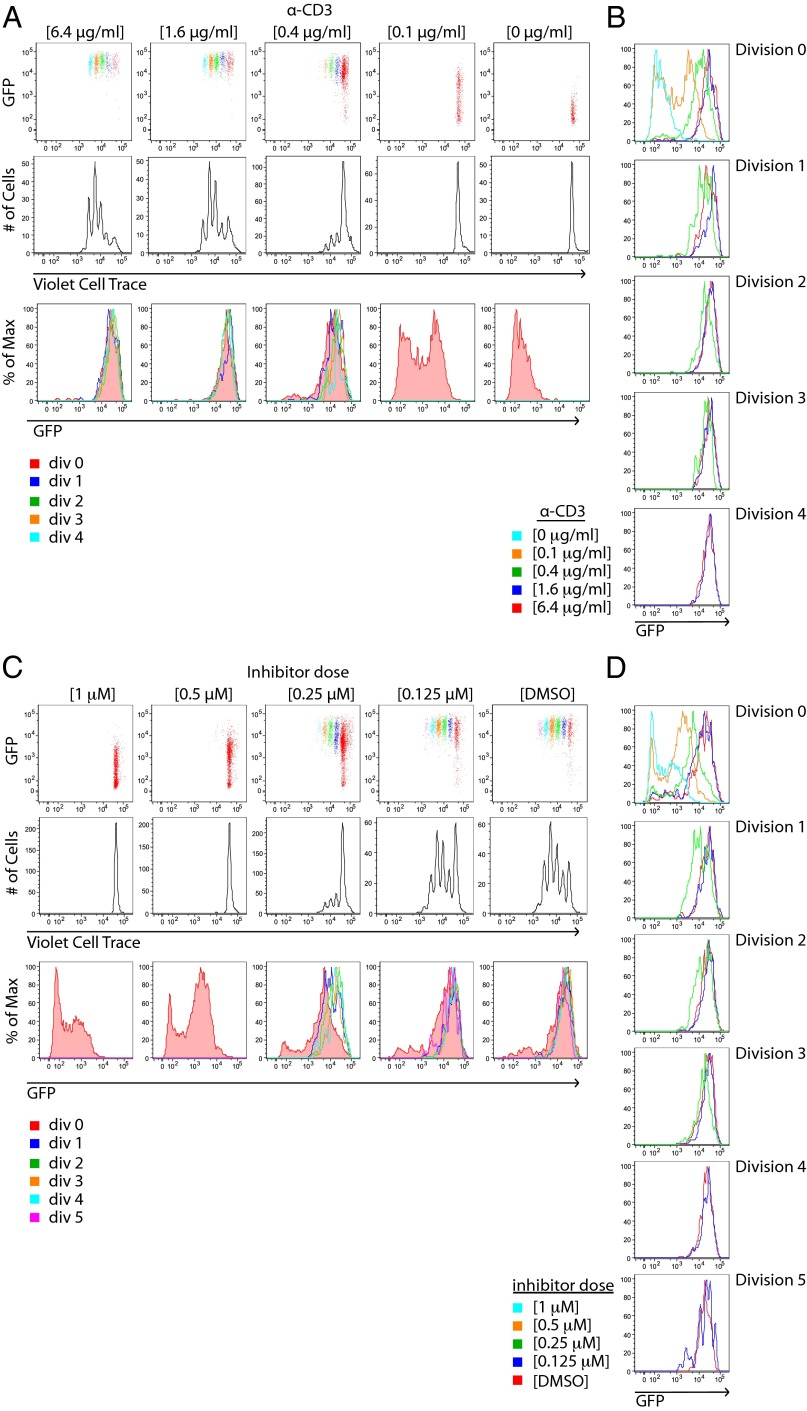
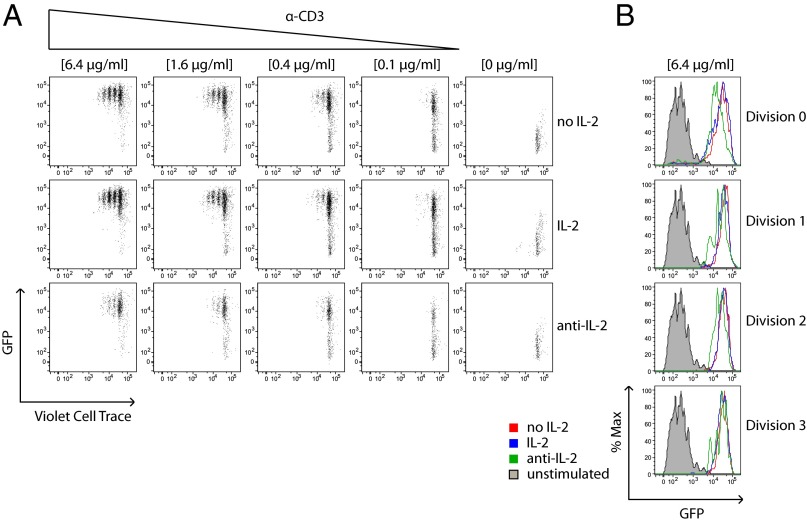
We took advantage of this experimental platform to explore the relationship between TCR signal integration and commitment to proliferation. We treated Nur77–GFP reporter T cells with varying doses of plate-bound anti-CD3 and after 4 d, simultaneously assessed GFP expression and proliferation via fluorescent dye dilution. This approach enabled us to correlate the extent of TCR signaling “perceived” by individual T cells undergoing distinct rounds of cell division. As expected, increases in anti-CD3 dose resulted in more proliferating cells and greater numbers of cell divisions. (Fig. 2A). To our surprise, although GFP expression in undivided T cells varied with stimulus as expected, GFP fluorescence in dividing cells was high and largely independent of anti-CD3 dose in both CD4+ and CD8+ T cells (Fig. 2B and Fig. S2B). Importantly, similar results were obtained in Zap70(AS)–GFP T cells treated with a fixed high concentration TCR stimulus and varying doses of the HXJ42 (Fig. 2C and Fig. S2C). Interestingly, CD4+ T-cell samples treated with a weak stimulus that was barely sufficient to drive proliferation exhibited a subtly lower but still very elevated GFP mean fluorescence intensity (MFI) relative to unstimulated cells (Fig. 2A). These results suggest that a distinct quantitative threshold of TCR signaling is required for T cells to proliferate. Titrating strength of stimulus alters the fraction of cells that cross this threshold, but does not substantially affect the integrated TCR signal perceived by individual proliferating cells (GFP expression) (Fig. 2D and Fig. S2D).
Fig. 2.
Nur77–GFP reporter reveals a dose-independent TCR signaling threshold for T-cell proliferation in vitro. (A) CD4+ T cells from Nur77–GFP reporter mice were loaded with violet cell trace dye and stimulated with the indicated concentrations of plate-bound anti-CD3 for 4 d. Cells were subsequently stained for T-cell markers and assessed for dye dilution and GFP fluorescence by flow cytometry. Individual cell divisions are color coded (Top Row). (B) Histograms show GFP expression of cells within each cell division that were stimulated with varying concentrations of anti-CD3. (C) CD4+ T cells from Zap70(AS)–GFP mice were loaded with violet cell trace dye and stimulated with a fixed dose of anti-CD3 (6.4 μg/mL) and the indicated concentrations of HXJ42 for 4 d. Cells were subsequently stained for T-cell markers and assessed for dye dilution and GFP fluorescence by flow cytometry. (D) Histograms show GFP expression of cells within each cell division that were treated with varying concentrations of HXJ42. Data are representative of at least three independent experiments.
We sought to determine whether our Nur77–GFP reporter faithfully reflected activity of endogenous Nur77 protein in vitro. To do so, we costained stimulated Nur77–GFP T cells for intracellular Nur77 protein and assessed both GFP fluorescence and dye dilution. We observed that, similar to GFP fluorescence, endogenous Nur77 protein varied in a dose-dependent manner among CD4+ T cells that had not undergone proliferation, but among proliferating T cells, endogenous Nur77 protein levels were invariant with respect to stimulus (Fig. S3 A and B).
To determine whether these observations could be recapitulated in the context of bona fide antigen presenting cell (APC)–T-cell interactions, we generated Nur77–GFP reporter mice harboring the ovalbumin (OVA)323–339 peptide-specific/MHC-II–restricted TCR transgene (referred to as “OT2–GFP” mice) (34). OT2–GFP T cells were cultured in vitro with splenocytes and varying doses of OVA peptide for a similar analysis of proliferation. Again, the frequency of the cells that entered the proliferative response was sensitive to OVA peptide dose, but GFP fluorescence in proliferating T cells reflected a relatively invariant threshold that was independent of OVA dose (Fig. S4 A and B).
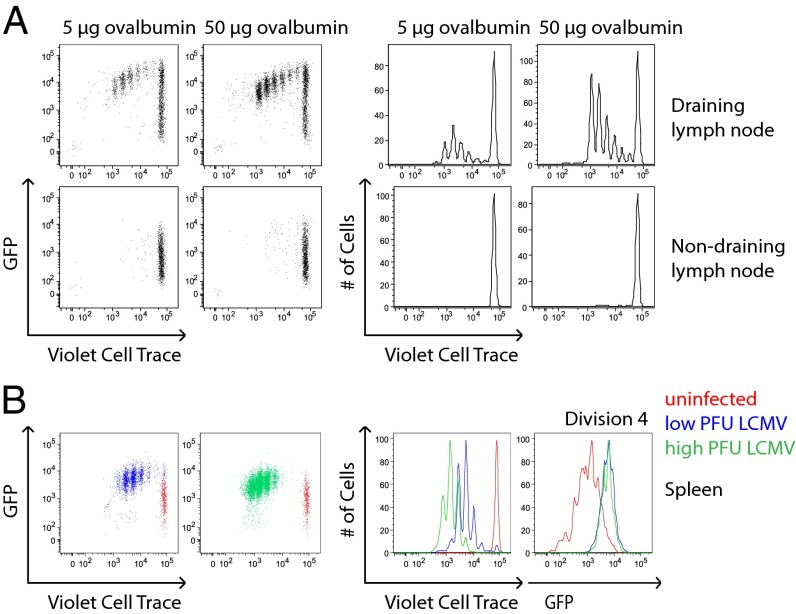
We determined whether this TCR signaling threshold was also enforced in the context of T-cell activation in vivo. OT2–GFP T cells, loaded with a fluorescent dye, were adoptively transferred into congenic hosts, which were subsequently immunized in the footpad with 10-fold different doses of OVA protein in complete Freund’s adjuvant. Both draining and nondraining lymph nodes were harvested after 3 d to assess GFP fluorescence and proliferation via dye dilution in transferred T cells. This revealed robust OT2 T-cell proliferation in the draining lymph node in an OVA dose-dependent manner, but GFP fluorescence of proliferating cells that was independent of OVA dose (Fig. 3A).
Fig. 3.
Nur77–GFP reporter reveals a dose-independent TCR signaling threshold for T-cell proliferation in vivo. (A) CD4+ T cells from OT2 transgenic mice harboring the Nur77–GFP reporter (OT2–GFP mice) were loaded with violet cell trace dye and adoptively transferred into CD45.1+ congenic hosts. The following day, host mice were immunized via s.c. footpad injection with 5 μg or 50 μg ovalbumin protein in complete Freund's adjuvant. At day 3 postimmunization, draining and nondraining contralateral popliteal lymph node cells were harvested, stained for T-cell markers, and assessed for dye dilution and GFP fluorescence by flow cytometry. (B) CD4+ T cells from SMARTA transgenic mice harboring the Nur77–GFP reporter (SMARTA–GFP mice) were loaded with violet cell trace dye and adoptively transferred into congenic CD45.1+ hosts. The following day, host mice were infected with low (2 × 104 cfu) or high (2 × 106 cfu) infectious doses of LCMV. On day 3 of infection, splenocytes were harvested, treated with collagenase, stained for T-cell markers, and assessed for dye dilution and GFP fluorescence by flow cytometry. Data are representative of at least three biological replicates.
To determine whether an analogous threshold for TCR signaling is relevant in the context of a physiologic host response to infection, we took advantage of SMARTA TCR transgenic CD4+ T cells bearing the Nur77–GFP reporter transgene (referred to as “SMARTA–GFP”) (35). SMARTA T cells recognize the lymphocytic choriomeningitis virus (LCMV)-derived peptide glycoprotein61–80. SMARTA–GFP T cells loaded with a fluorescent dye were adoptively transferred into wild-type congenic host mice, which were subsequently infected with two different infectious doses of LCMV. We harvested splenocytes after 3 d to assess GFP fluorescence and proliferation via dye dilution of transferred T cells. For reference, we simultaneously harvested splenocytes from a host mouse subjected to adoptive transfer but not infection. We were able to appreciate a marked difference in the proliferative response of transferred SMARTA–GFP T cells depending on viral infectious dose. Nevertheless, we observed an invariant high level of GFP fluorescence in transferred SMARTA T cells across specific cell divisions, irrespective of viral dose (Fig. 3B). Taken together, these data suggest that T cells in vivo receive a discrete amount of TCR stimulation and then proceed to divide independently of ongoing TCR signaling.
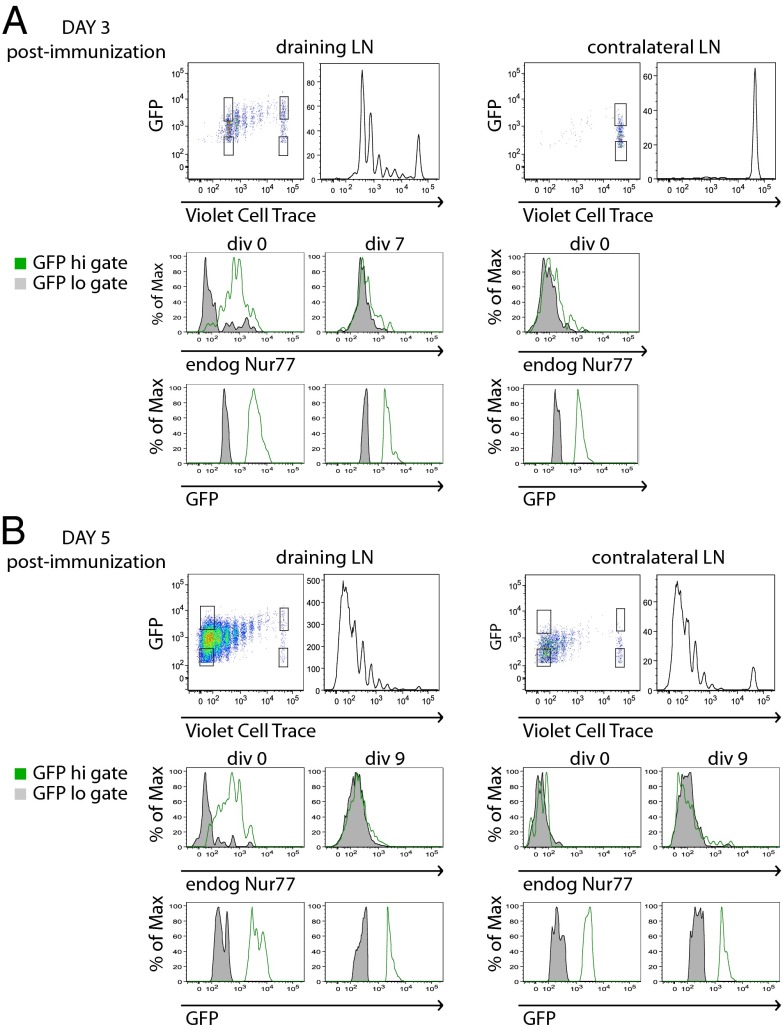
To determine how reporter activity tracked with endogenous Nur77 expression in vivo, we costained OT2–GFP lymphocytes harvested after OVA immunization (as in Fig. 3A) for intracellular endogenous Nur77 protein. In contrast to the longer half-life of reporter eGFP protein (estimated to be 20–24 h), endogenous Nur77 protein has a significantly shorter half-life (25, 31). We observed that endogenous Nur77 strongly correlated with reporter GFP expression but only among undivided lymph node T cells (Fig. 4 A and B). Both proliferating and nonproliferating cells that have migrated to contralateral lymph nodes (which lack antigen drainage) also fail to express endogenous Nur77 but retain GFP expression. Among dividing T cells in the draining lymph node, endogenous Nur77 expression is no longer detectable, presumably because TCR signaling has ceased in these cells. Consistent with this finding, eGFP expression is detectable, but exhibits a progressive decline with each additional division, presumably due to dilution and degradation without new compensatory protein synthesis as a result of cessation of TCR signaling. This suggests that daughter cells of T cells initially stimulated by peptide APC may not continue to integrate TCR signals due to loss of APC contact by competition with other T-cell clones for peptide/MHC or migration away from APCs. By contrast, in our in vitro studies, eGFP and endogenous Nur77 expression remain stably elevated in proliferating T cells plated on anti–CD3-coated wells where TCR stimulation is continuous (Fig. 2 and Figs. S2 and S3).
Fig. 4.
Endogenous Nur77 expression reveals loss of active TCR signaling in proliferating T cells in vivo. CD4+ T cells from OT2–GFP mice were loaded with violet cell trace dye, transferred into congenic CD45.1+ hosts, and immunized as described in Fig. 3A. On (A) day 3 and (B) day 5 postimmunization, draining and nondraining contralateral popliteal lymph node cells were harvested, fixed, permeabilized, and stained for both endogenous Nur77 and T-cell markers. Cells were subsequently assessed for GFP fluorescence, dye dilution, and endogenous Nur77 expression by flow cytometry. Top panels represent gating scheme to identify cells that have undergone no divisions or many divisions, with either low or high GFP expression. Middle panels represent endogenous Nur77 and Bottom panels represent GFP fluorescence in these gates. Data are representative of at least three biological replicates.
TCR Signaling Threshold Is Not Enforced by IL-2.
T cells normally become activated to up-regulate CD25, the IL-2 receptor alpha chain (IL-2Rα), and also secrete IL-2 several hours after TCR stimulation is initiated. IL-2 in turn further acts in an autocrine or paracrine fashion to promote expression of its high-affinity receptor by driving up-regulation of CD25, which also promotes proliferation. We hypothesized that the sharp TCR signaling threshold we observed in proliferating T cells might in turn be enforced by the requirement to produce IL-2 and up-regulate CD25. To test this hypothesis, we treated stimulated WT Nur77–GFP T cells with exogenous IL-2 in excess or, alternatively, with anti–IL-2 neutralizing antibody, and assessed GFP fluorescence and proliferation in response to plate-bound anti-CD3. We reasoned that by supplying excess IL-2 or depleting it entirely, we would perturb the TCR signaling threshold for proliferation as marked by reduced or increased GFP fluorescence in proliferating T cells. Importantly, GFP expression in these reporter cells is not directly up-regulated by IL-2 signaling (25). As expected, anti–IL-2 treatment reduced the proliferative response of T cells across varying doses of TCR stimulation (Fig. 5A). As a further validation of the bioactivity of this anti–IL-2 reagent, we observed down-regulation of CD25 in treated cells (Fig. S5 A and B). However, in contrast to our prediction, GFP expression in proliferating CD4+ T cells was not affected by IL-2 supply (Fig. 5B).
Fig. 5.
TCR signaling threshold is not enforced by IL-2. (A) CD4+ T cells from Nur77–GFP reporter mice were loaded with violet cell trace dye and stimulated with varying doses of plate-bound anti-CD3 without additional reagents (Top), in the presence of exogenous IL-2 (Middle), or presence of neutralizing IL-2 mAb (Bottom) for 4 d. Cells were subsequently stained for T-cell markers and assessed for dye dilution and GFP fluorescence by flow cytometry. (B) Histograms show GFP expression within each cell division for the highest concentration of anti-CD3 used (6.4 μg/mL). Data are representative of at least three independent experiments.
Continuous TCR Signaling Is Dispensible After ∼24 h for Commitment to Proliferation.
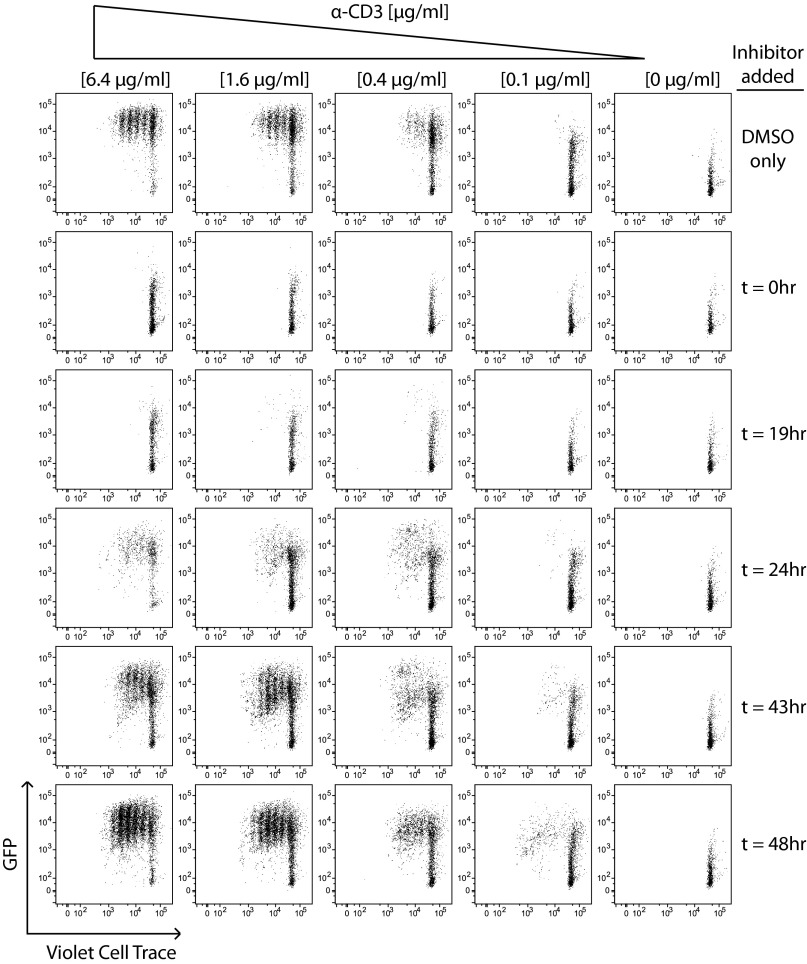
Here we have shown that CD4+ and CD8+ T cells exhibit a sharp quantitative TCR signaling threshold for commitment to proliferation. We next wanted to determine whether T cells exhibit an analogous temporal threshold of TCR signaling duration to commit to proliferation. We therefore stimulated Zap70(AS)–GFP T cells in vitro, added a high concentration of analog inhibitor HXJ42 into culture at various time points after initial stimulation, and assessed GFP fluorescence and proliferation. We found that Zap70 inhibition within the first 24 h of stimulation completely prevented proliferation and largely blocked GFP up-regulation (Fig. 6). However, blocking TCR signaling with analog inhibitor at subsequent time points did not block proliferation. Indeed, beyond 43 h, Zap70 inhibition had no effect on the proliferative response relative to vehicle-treated cells (Fig. 6). Analogous results were obtained with Zap70(AS)–GFP CD8+ T cells in which the so-called autopilot model of T-cell activation and proliferation is widely accepted (Fig. S6A). By extrapolation, we propose that our data establish autopilot commitment of CD4+ T cells to proliferation, although the temporal threshold itself differs between CD4+ and CD8+ T cells.
Fig. 6.
Continuous TCR signaling is dispensable after 24 h for commitment to proliferation. CD4+ T cells from Zap70(AS)–GFP mice were loaded with violet cell trace dye and stimulated with varying doses of plate-bound anti-CD3 along with a fixed dose of anti-CD28 (2 μg/mL). Samples were then treated with a fixed concentration of HXJ42 (1 μM) or vehicle alone at various time points after initial stimulation, and all samples were then harvested at 96 h. Cells were stained for T-cell markers and assessed for GFP fluorescence. Data are representative of at least three independent experiments.
GFP expression in inhibitor-treated proliferating cells remained invariant and independent of stimulus titration, but was lower than in DMSO-treated proliferating cells (Fig. 6 and Fig. S6 B and C). These data suggest that a sharp TCR signaling threshold was still enforced for entry into cell cycle, but subsequent inhibitor treatment dampened TCR signaling after this commitment event occurred. This result suggests that proliferating T cells in this model system stimulated with plate-bound anti-CD3 continue to integrate TCR signals, but that these signals are dispensible for proliferation. Importantly, as shown earlier, adding inhibitor at a range of submaximal doses at the start of culture reduces the proliferative response of T cells (as titrating stimulus might), but does not substantially alter GFP fluorescence (Fig. 2B and Fig. S2B), in contrast with the phenotype of T cells treated with analog inhibitor at later time points (Fig. 6 and Fig. S6C).
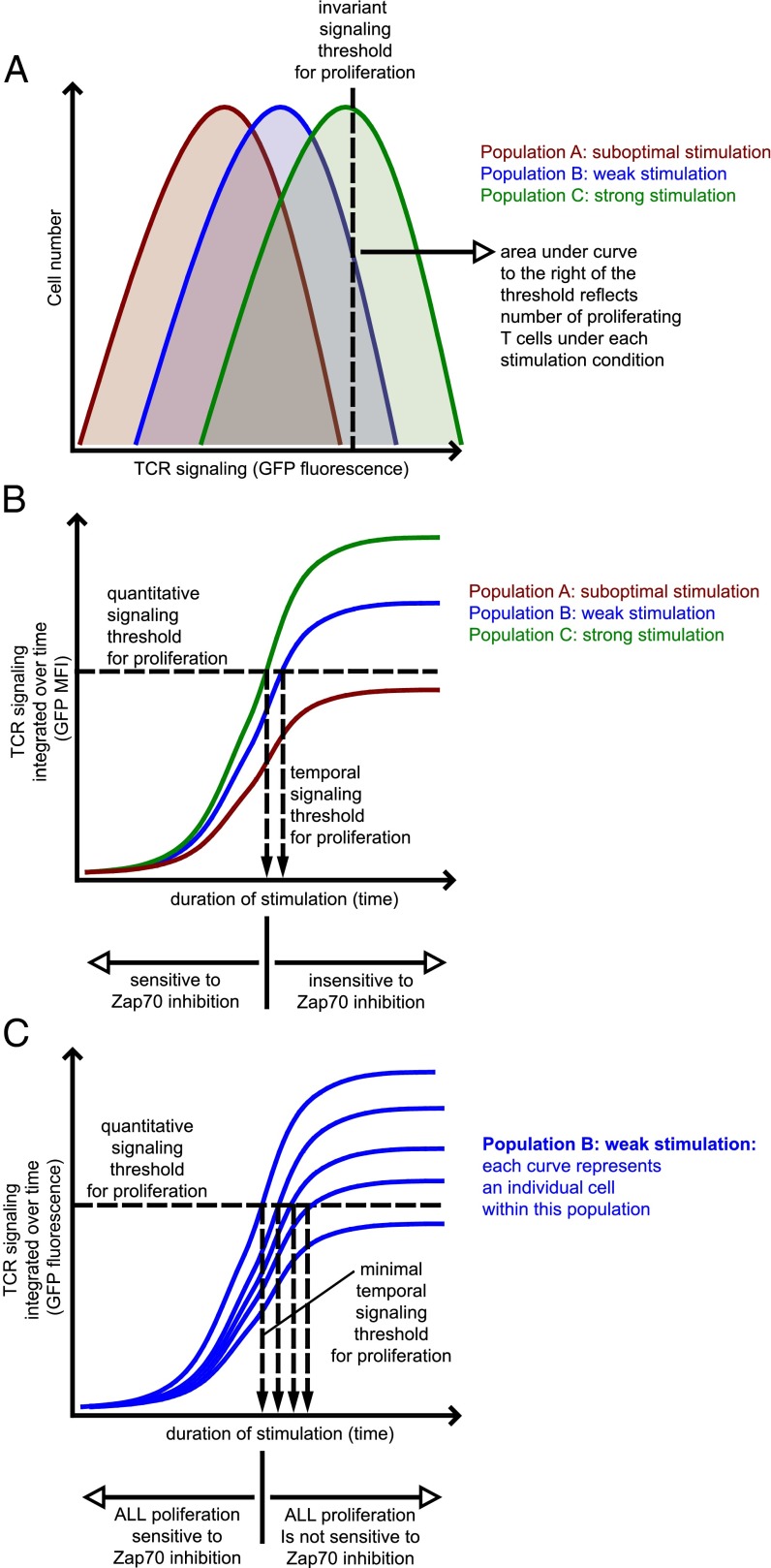
Taken together, our results suggest that the TCR signaling threshold for proliferation is invariant on a single-cell level. Further, these results may provide a link between the quantitative and temporal thresholds identified here such that the quantitative threshold for proliferative responses identified earlier is enforced before late addition of inhibitor (Fig. 7).
Fig. 7.
A quantitative and temporal threshold model for T-cell proliferation. (A) Integrated TCR signaling among a population of T cells exposed to a uniform stimulus nevertheless exhibits a distribution of GFP expression. This distribution reflects the strength of TCR stimulation, titrated by Zap70 kinase inhibition. The cells within a population in which their integrated TCR signaling (as read out by Nur77–eGFP fluorescence) cross an invariant threshold enter the proliferating pool. This model is compatible with both an invariant signaling threshold at the single-cell level and with a titration of the proliferating cell population by varying TCR signal strength. Importantly, a weak stimulus that barely drives cells over this threshold is predicted to result in slightly lower GFP MFI among the population of proliferating T cells. (B) Each curve here represents the mean perceived TCR signal strength (GFP MFI) of an entire population, including both proliferating and nonproliferating cells. TCR signaling is integrated over time and GFP MFI therefore increases with time. Once cells within a population cross an invariant signaling threshold, further TCR signaling to commit to proliferation is dispensable, as demonstrated by insensitivity of proliferating population to Zap70 inhibition beyond this time point. Cells in population “A” receive subthreshold/suboptimal stimulation and these cells never cross the invariant signaling threshold and never proliferate. (C) As depicted in A, a population of stimulated T cells displays a distribution of GFP fluorescence at any given time point. In this model, each curve represents the perceived TCR signal strength of individual T cells. Only a fraction of cells within this population crosses the invariant signaling threshold at any given time point. There exists a minimum time required for any cells to do so, beyond which those cells committed to proliferation are not sensitive to Zap70 inhibition. However, prolonged duration of stimulation is capable of driving increasing numbers of cells across the threshold. In this manner, prolonged signal duration can recruit more and more cells into the proliferating pool, consistent with prior observations of a temporal threshold and a titration effect on clonal burst size.
Discussion
The combination of the Zap70(AS) chemical-genetic system and the Nur77–GFP reporter enables us, for the first time to our knowledge, to track and modulate TCR signal strength integrated over time and reveals a sharp threshold of signaling required for T-cell proliferation. Our GFP signaling reporter demonstrates that on a single-cell level, individual T cells must cross an invariant threshold of accumulated TCR signaling to undergo proliferation. Importantly, titrating TCR signal strength regulates the number of cells that undergo proliferation but does not alter this threshold. By assessing this threshold in two distinct in vivo model systems of CD4+ T-cell activation, OVA immunization and LCMV infection, we also show that this invariant threshold for T-cell activation is physiologically relevant. Our data imply that individual T cells must accumulate or integrate a certain total “amount” of TCR signaling to proliferate and in turn suggests that commitment to proliferation may be enforced by a transcriptional program.
Although early biochemical events such as calcium entry can be triggered by even a single pMHC complex (2), it has been estimated that many more ligands (400) must be present and many more TCRs engaged (8,000) to commit a T cell to proliferate (5, 6). This implies that biochemical signaling events must be integrated to drive T cells into cell cycle. This apparent threshold of TCR signaling to commit to proliferation is consistent with our observations. Interestingly, other studies have identified distinct thresholds for inducing different T-cell functions. For instance, higher stimulation is required for proliferation than for cytokine production and more still than for cytotoxicity (5, 36–38).
It is well-established for CD8+ T cells that increasing strength of TCR signaling beyond this minimal threshold drives more T-cell proliferation in an apparently continuous manner (12). It is surprising that GFP expression among proliferating reporter T cells in vivo and in vitro in our system remains largely invariant despite titration of stimulation and associated modulation of the proliferative response. We suggest that stronger TCR stimulation does not shift the threshold itself, but rather leads to recruitment of additional cells into cell cycle. We suggest a simple model whereby an invariant threshold is compatible with modulation of the proliferative response by titration of TCR stimulus (Fig. 7A). This model is compatible with work by Bevan and coworkers, who took advantage of a series of OVA-derived altered peptide ligands (APLs) coupled to Listeria to evaluate the clonal burst size of responder OT-1 T cells in vivo. They observed that even the lowest affinity agonist peptide could recruit CD8 T cells to proliferate (e.g., cross the threshold), but importantly, low-affinity peptides resulted in smaller burst size and earlier contraction of the OT-1 population, whereas higher affinity peptides drove greater accumulation of daughter OT-1 T cells. This study is not inconsistent with our model; a truly subthreshold peptide evaluated in this system might be predicted not to trigger proliferation at all, and further evaluation of single-cell dynamics in the Listeria experimental system might be required to truly reconcile results with our predictive model (39). Furthermore, our model does not exclude the possibility that complex in vivo phenotypes such as timing of T-cell contraction may be independently regulated by peptide affinity as well as dose.
All-or-none digital responses on a single-cell level that produce an apparently continuous analog response on a population level are described in the context of other TCR-dependent biological processes, most notably positive selection in the thymus. Titration of signal strength via perturbation of ligand and/or signaling machinery alters the number of positively selecting thymocytes (40, 41). Recently, our laboratory has taken advantage of the Nur77–GFP reporter to demonstrate an analogous invariant threshold for positive selection in the thymus (33); titration of Zap70 catalytic activity in fetal thymic organ culture or thymic slice systems alters the number of positively selected thymocytes, but these selected thymocytes nevertheless exhibit an invariant GFP MFI. Recent work from Davis and coworkers supplies another example of this phenomenon: A single pMHC complex is sufficient to drive IL-2 release in an apparently digital manner, whereas increasing pMHC number recruits additional cells to do so (4). In contrast to titration of Zap70 kinase activity, work from Palmer and coworkers revealed a sharp threshold effect of peptide affinity on CD8+ selection even at the population level (40). We speculate that below a “threshold” of peptide affinity, pMHC–TCR complexes are too short lived to permit accumulation of an unstable signaling intermediate, whereas graded titration of Zap70 kinase activity is less subject to this effect and generates more analog output.
Interestingly, the model we propose in Fig. 7A predicts that even in the setting of an invariant threshold, cell populations exposed to weak or “borderline” stimuli sufficient to drive relatively few cells across this threshold will exhibit a slightly lower GFP MFI among proliferating cells relative to stronger stimuli. Indeed, we observe this to be the case for CD4+ T cells stimulated with anti-CD3 in vitro regardless of whether stimulus or inhibitor is titrated; levels of stimulation barely sufficient to drive cells to proliferate that border subthreshold and robust stimuli (Fig. 2A: 0.4 μg/mL anti-CD3; Fig. 2B: 0.25 μM inhibitor) result in slightly lower GFP expression among proliferating cells than occurs with higher levels of stimulation.
Here we identify an invariant threshold for T-cell proliferation that is independent of strength of TCR stimulation. We also show using the Zap70(AS) system that inhibiting TCR signaling after 24 h does not inhibit proliferation of CD4+ T cells appreciably in vitro. Taken together, these observations suggest that not only do cells need to reach a TCR signaling threshold to proliferate, but once this has been achieved, further signaling is dispensable for proliferation (i.e., we suggest that a TCR signaling threshold is both necessary and sufficient for T-cell proliferation) (Fig. 7A).
Schoenberger and coworkers (8), followed by others (7, 9), initially proposed the CD8+ cell autopilot model using an engineered APC system to demonstrate that as little as 2 h of antigenic stimulation by APCs was sufficient to commit responder CD8+ T cells to proliferation. In the present study, we also demonstrate TCR-independent CD8+ T-cell proliferation after addition of Zap70 inhibitor after 48 h. Earlier addition of inhibitor in our assays partially or completely blocked CD8+ T-cell proliferation. We speculate that longer time dependence of our results may reflect use of plate-bound antibody stimulation rather than cognate antigen/APC stimulation. This may in turn relate to unusual dependence of CD8+ T cells upon multiple costimulatory inputs (42). Subsequently, investigators took in vivo approaches to modulate antigen exposure and argued that a somewhat longer duration of stimulation was necessary for optimal expansion and effector function of CD8+ T cells (12, 21). Consistent with this autopilot model, in vivo imaging has revealed a limited duration of T-cell–dendritic cell interactions in vivo despite persistence of antigen (43).
Despite the clear identification of a minimal amount of TCR signal duration required to commit CD8+ T cells to proliferation both in vitro and in vivo, a number of in vivo studies have also demonstrated that proliferative responses of CD8+ T cells are titratable by altering not merely peptide affinity/avidity, but also duration of stimulation beyond the minimal threshold (12, 14, 16, 18–22, 39). Our in vitro data do not demonstrate this phenomenon, but we speculate that either more prolonged or in vivo extension of TCR signaling might serve to recruit additional T cells to cross the quantitative/temporal threshold of signaling required to commit to proliferation (Fig. 7A). In this manner, an invariant and sharp threshold interpreted by individual cells may appear as continuous dependence of proliferation upon magnitude and duration of TCR signaling on a population level. Independently controlling the strength and duration of antigenic stimulus in vivo has been challenging and may also account for discrepant conclusions in various studies. In vivo, T-cell populations contain both naïve and antigen-experienced CD8+ T cells. The rapid kinetics and altered thresholds of antigen-experienced T cells differ from those of pure naïve populations and may also account for discrepant reports from in vivo systems. Alternatively, a strict autopilot model for commitment to proliferation may require further modification to incorporate further decision points such as signaling requirements for activation-induced cell death and persistence in cell cycle.
Although the autopilot model of CD8+ T-cell commitment to proliferation remains well accepted (7–9, 12, 22), it has nevertheless been unclear whether CD4+ T-cell proliferation is analogously regulated. Lanzavecchia and coworkers showed that ∼20 h of continuous TCR signaling was required for peptide-specific transgenic CD4+ T-cell commitment to proliferation (1). Schrum et al. subsequently identified an 18-h temporal threshold in vitro for CD4+ T-cell commitment to proliferation, but also demonstrated that prolonged stimulation leads to greater CD4+ T-cell expansion (10). However, studies from other groups have yielded conflicting results (11, 13, 15–17).
Mathis and Benoist and coworkers developed an in vivo strategy to titrate CD4+ and TCR transgenic T-cell exposure to cognate antigen using a tetracycline (Tet)-inducible promoter system to regulate antigen expression (13). This genetic strategy permitted the investigators to induce antigen decay at various time points relative to adoptive transfer of responder and CD4+ T cells. In this context, the proliferation of CD4+ T cells does appear to depend upon duration of antigen exposure, in apparent contradiction to a strict autopilot model. They conclude that persistent antigenic stimulation is necessary for ongoing CD4+ T-cell proliferation. Our data, however, suggest that beyond ∼24 h of TCR signaling, the proliferative response of CD4+ T cells in vitro is insensitive to Zap70 inhibition. We speculate that the Tet-inducible system used by Mathis and Benoist and coworkers alters the initial dose as well as duration of antigen exposure such that both parameters may influence experimental outcome. Alternatively, use of essentially naïve and T cells by Mathis and Benoit and coworkers may prolong the sensitivity to TCR signal integration required for full commitment to proliferation. Finally, as we speculate earlier, prolonged stimulation may recruit additional T cells into the response, a phenomenon that may be obscured in vitro. Indeed, our assessment of both short half-life endogenous Nur77 and longer half-life eGFP as markers of TCR signaling in vivo suggests either that proliferating OVA-specific CD4+ T cells are no longer receiving TCR stimulation at later cell divisions or that endogenous Nur77 expression is subject to negative feedback at later cell divisions. However, potent inhibition of TCR signals in vitro with the analog inhibitor at late time points suggests that beyond the temporal TCR signaling threshold, ongoing division can occur in the absence of persistent TCR stimulation.
Interestingly, Mathis and Benoist and coworkers also argue that titration of CD4+ TCR stimulation modulates not only recruitment of cells into cell cycle, but also the rate of cell cycling (13). However, assessment of this rate may be confounded by whether truly synchronized or asynchronous T-cell recruitment into cell cycle occurs. We find an invariant TCR signaling threshold in vivo in the context of ovalbumin immunization and LCMV infection, examples of highly asynchronous and extremely synchronous T-cell responses, respectively.
Previous studies of T-cell commitment to proliferation have relied upon inducible genetic expression and/or physical removal of cells from antigen. Particularly in vivo, residual antigen may be retained even after active expression is turned off or cells are adoptively transferred. As a result, such strategies may inadvertently vary both dose and duration of antigen exposure. Our in vitro system relies upon a specific inhibitor of proximal TCR signaling and permits tight and extremely rapid control of both strength and duration of TCR signaling and allows us to vary these parameters independently of one another. We therefore propose that in a simplified, well-controlled model system, CD4+ T-cell commitment to proliferation not only exhibits an invariant TCR signaling threshold, but also conforms to an autopilot model. Importantly, we show that these quantitative and temporal thresholds are imposed at a single-cell level. We suggest that population-level readouts of proliferation obscure digital all-or-none responses on a single-cell level. Population-level analysis has led many investigators to conclude that titration of magnitude or duration of TCR signaling modulates proliferation in an apparently continuous or analog manner (Fig. 7A). An invariant threshold at the level of individual T cells is consistent with apparently continuous population responses if more or longer stimulation recruits additional T cells into cell cycle. There is precedent for such a model; work by Kaech and Ahmed suggests that the number of CD8+ T cells recruited into the proliferative response varies with increasing duration of antigen exposure (12). There is evidence of such an effect in work from Bevan and coworkers with peptide agonists of varying affinity (39).
We show that, surprisingly, IL-2 fails to modulate the proliferative threshold of CD4+ T cells on a single-cell level as marked by GFP expression. How then can this be reconciled with modulation of the proliferative response of an entire population by IL-2 supply? We suggest that perturbing IL-2 supply is analogous to titrating TCR stimulus strength; increasing IL-2 supply may recruit more cells across the invariant threshold. Chakraborty and coworkers have suggested that IL-2 secretion by responding T cells may enforce all-or-none digital immune responses at the level of a population of cells rather than a single cell (44). An antigen that recruits few T cells to respond results in little IL-2 production and produces an immune response that is abortive and decays; conversely, a bona fide infection that recruits a “quorum” of T cells to respond can push additional T cells across the proliferative threshold. IL-2 may thus enforce a digital response on a population level, whereas GFP expression instead marks an IL-2–independent threshold that is enforced at a single-cell level.
Our data identify both a quantitative and a temporal threshold of TCR signaling for commitment to proliferation, and we suggest that the former determines the latter. It is not clear how this threshold is imposed upon the cell, but the time scale involved suggests something other than purely posttranslational “signaling” events may be involved, perhaps changes in gene expression and/or chromatin remodeling. Indeed, titration of TCR immunoreceptor tyrosine-based activation motif (ITAM) multiplicity reveals an apparently continuous and profound reduction in proliferation in the face of relatively preserved proximal signaling and Zap70 phosphorylation (36, 37). Interestingly, the sharp reduction in proliferation with reduced ITAM multiplicity correlates with an abrupt loss of c-Myc expression, and is correspondingly rescued with c-Myc overexpression, suggesting that regulated gene expression of this and perhaps other targets by integrated TCR signaling over time may in turn enforce the sharp signaling threshold we report here (36).
It has previously been proposed that serial T-cell antigen encounters drive T-cell activation, possibly through accumulation of an unstable signaling intermediate (4, 45–47). An important implication of our data is that integration of TCR signaling within a given T cell is continuous over multiple encounters with pMHC. This model is consistent with recent studies in which we demonstrate the existence of a temporal threshold for positive selection of CD4+CD8+ double positive thymocytes, which correlates with an integration of multiple transient encounters with positively selecting ligands. We further show that interruptions in Zap70-dependent signaling decreased the frequency and duration of these signaling events, impairing the efficiency of positive selection (33).
Here we propose that inhibiting TCR signaling intermittently rather than continuously may be sufficient to limit T-cell responses in the context of autoimmune disease and/or transplant tolerance. A pharmaceutical dosing strategy of this kind might in turn reduce off-target drug toxicity without impairing immunosuppressive potency. What, if any, implications does this threshold model have for physiologic host defense responses to infection? Imposing a relatively high TCR signaling threshold for commitment to proliferation may prevent spurious subthreshold triggering of the adaptive immune response. Conversely, the temporal threshold guarantees that, beyond a minimal threshold, even relatively rapid antigen clearance will not prematurely curtail evolution of a full adaptive immune response complete with appropriate memory precursor T-cell expansion.
Materials and Methods
Mice.
Mice used in these studies were housed in the specific pathogen-free facility at the University of California, San Francisco (UCSF), and were treated according to protocols approved by the Institutional Animal Care and Use Committee in accordance with National Institutes of Health (NIH) guidelines. Zap70+/−, Zap70(AS), and Nur77–eGFP mice were described previously (26, 27, 30). The Nur77–eGFP strain was interbred with Zap70(AS), OT2, and SMARTA strains (34, 35). WT C57BL/6, OT2, and CD45.1 BoyJ mice were from The Jackson Laboratory.
Antibodies and Reagents.
Zap70(AS) inhibitor HXJ42 has been described previously (33). CD4, CD8, CD25, CD90.2, and CD45.1 antibodies conjugated to PE, PerCPCy5.5, PECy7, APC, or Qdot 605 (BD Biosciences, eBioscences, Life Technologies), Nur77 Ab conjugated to PE (eBiosciences; clone 12.14). Antibodies used for in vitro T-cell stimulation and culture include anti-CD3 (clone 145–2C11; Harlan), anti-CD28 (clone 37.51; UCSF monoclonal antibody core facility), and anti–IL-2 (clone JES6-5H4; Biolegend). Recombinant human IL-2 was from the NIH AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH: Maurice Gately (Hoffmann-La Roche). OT2 TCR transgenic T cells were stimulated in vitro with ovalbumin (OVA323–339) peptide (New England Peptide).
Flow Cytometry.
Cells were analyzed on a BD LSR Fortessa flow cytometer and flow cytometry plots were generated using FlowJo (Tree Star) software.
T-Cell Activation and Proliferation.
Lymph node cells in single-cell suspension were applied at a concentration of 0.5–1 × 106 cells per milliliter to 96-well plates. For studies of T-cell proliferation, cells were labeled with Cell Trace Violet according to the manufacturer’s instructions (Life Technologies) before plating. Nur77–eGFP reporter T cells harboring WT Zap70 (Zap70+/−) were stimulated by plate-bound anti-CD3 at the concentrations indicated in the figure legends ± IL-2 or anti–IL-2. Zap70(AS)–GFP T cells were stimulated by plate-bound anti-CD3 at the concentrations indicated in the figure legends with anti-CD28 (2 μg/mL). OT2 TCR transgenic cells were stimulated with APCs and OVA peptide (concentrations indicated in figures/figure legends). Cells were harvested and stained at various time points to assess activation and proliferation by flow cytometry.
T-Cell Purification.
CD4+ and CD8+ T cells were enriched from suspensions of spleen and lymph node cells by using the EasySep CD4+ Enrichment kit (Stem Cell Technologies) according to the manufacturer’s instructions.
Intracellular Staining for Nur77.
Live cells were washed and stained with cell surface markers as indicated. They were then stained with Live Dead Fixable Stain (Life Technologies) in PBS. After washing, cells were fixed for 20 min with 4% (vol/vol) fresh paraformaldehyde at room temperature. Cells were then permeabilized using the Mouse Regulatory T-Cell Staining kit 1 (eBioscience) per manufacturer's instruction. After permeabilization, cells were stained with Nur77-PE.
OVA Immunization.
OT2–GFP T cells were loaded with violet cell trace fluorescent dye and adoptively transferred via tail vein injection (1–2 × 106 cells per mouse) into CD45.1 (BoyJ) recipients, which were subsequently immunized by s.c. footpad injection with an emulsion of complete Freund’s adjuvant (Difco) and ovalbumin protein (5 or 50 μg; Sigma). Both draining and nondraining popliteal lymph nodes were harvested after 3 d to assess GFP fluorescence and proliferation via dye dilution of transferred T cells.
LCMV Infection.
Congenic CD45.1+ SMARTA–GFP T cells were loaded with violet cell trace fluorescent dye and adoptively transferred via tail vein injection (1–2 × 106 cells per mouse) into B6 hosts, which were infected the following day with low (2 × 104) or high (2 × 106) pfu LCMV Armstrong virus via tail vein injection. On day 3 postinfection, spleens were harvested and collagenase treated after 3 d to assess GFP fluorescence and proliferation via dye dilution of transferred SMARTA T cells.
Supplementary Material
Acknowledgments
We thank Al Roque for animal husbandry and Chao Zhang for synthesis of HXJ42. This work was supported by an Arthritis Foundation Postdoctoral Fellowship 5476 (to B.B.A.-Y.); the Rosalind Russell Medical Research Foundation Bechtel Award (to J.Z.); an Arthritis National Research Foundation grant (to J.Z.); National Institutes of Health Grants K08 AR059723 (to J.Z.), AI091580 (to A.W.), and RC2AR058947 (to A.W.); a Rheumatology Research Foundation Scientist Development award (to J.A.); and a Rheumatology Research Foundation Within Our Reach grant (to A.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413726111/-/DCSupplemental.
References
- 1.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8(1):89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 2.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419(6909):845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 3.Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172(10):6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity. 2013;39(5):846–857. doi: 10.1016/j.immuni.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimachi K, Croft M, Grey HM. The minimal number of antigen-major histocompatibility complex class II complexes required for activation of naive and primed T cells. Eur J Immunol. 1997;27(12):3310–3317. doi: 10.1002/eji.1830271230. [DOI] [PubMed] [Google Scholar]
- 6.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273(5271):104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 7.Bevan MJ, Fink PJ. The CD8 response on autopilot. Nat Immunol. 2001;2(5):381–382. doi: 10.1038/87676. [DOI] [PubMed] [Google Scholar]
- 8.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2(5):423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 9.Wong P, Pamer EG. Cutting edge: Antigen-independent CD8 T cell proliferation. J Immunol. 2001;166(10):5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 10.Schrum AG, Palmer E, Turka LA. Distinct temporal programming of naive CD4+ T cells for cell division versus TCR-dependent death susceptibility by antigen-presenting macrophages. Eur J Immunol. 2005;35(2):449–459. doi: 10.1002/eji.200425635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4(8):749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 12.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2(5):415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201(10):1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203(9):2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajénoff M, Wurtz O, Guerder S. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4(+) T cells. J Immunol. 2002;168(4):1723–1729. doi: 10.4049/jimmunol.168.4.1723. [DOI] [PubMed] [Google Scholar]
- 16.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4(4):355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 17.Lee WT, Pasos G, Cecchini L, Mittler JN. Continued antigen stimulation is not required during CD4(+) T cell clonal expansion. J Immunol. 2002;168(4):1682–1689. doi: 10.4049/jimmunol.168.4.1682. [DOI] [PubMed] [Google Scholar]
- 18.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171(10):5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 19.Shaulov A, Murali-Krishna K. CD8 T cell expansion and memory differentiation are facilitated by simultaneous and sustained exposure to antigenic and inflammatory milieu. J Immunol. 2008;180(2):1131–1138. doi: 10.4049/jimmunol.180.2.1131. [DOI] [PubMed] [Google Scholar]
- 20.Stock AT, Mueller SN, van Lint AL, Heath WR, Carbone FR. Cutting edge: Prolonged antigen presentation after herpes simplex virus-1 skin infection. J Immunol. 2004;173(4):2241–2244. doi: 10.4049/jimmunol.173.4.2241. [DOI] [PubMed] [Google Scholar]
- 21.van Stipdonk MJ, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4(4):361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 22.Zehn D, King C, Bevan MJ, Palmer E. TCR signaling requirements for activating T cells and for generating memory. Cell Mol Life Sci. 2012;69(10):1565–1575. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldwin TA, Hogquist KA. Transcriptional analysis of clonal deletion in vivo. J Immunol. 2007;179(2):837–844. doi: 10.4049/jimmunol.179.2.837. [DOI] [PubMed] [Google Scholar]
- 24.Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109(Suppl):S57–S66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 25.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208(6):1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489(7414):160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Au-Yeung BB, et al. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11(12):1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin SE, Zhang C, Kadlecek TA, Shokat KM, Weiss A. Inhibition of ZAP-70 kinase activity via an analog-sensitive allele blocks T cell receptor and CD28 superagonist signaling. J Biol Chem. 2008;283(22):15419–15430. doi: 10.1074/jbc.M709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, et al. ZAP-70: An essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2(5):a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadlecek TA, et al. Differential requirements for ZAP-70 in TCR signaling and T cell development. J Immunol. 1998;161(9):4688–4694. [PubMed] [Google Scholar]
- 31.Corish P, Tyler-Smith C. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng. 1999;12(12):1035–1040. doi: 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- 32.Lourido S, et al. Optimizing small molecule inhibitors of calcium-dependent protein kinase 1 to prevent infection by Toxoplasma gondii. J Med Chem. 2013;56(7):3068–3077. doi: 10.1021/jm4001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Au-Yeung BB, et al. Quantitative and temporal requirements revealed for Zap70 catalytic activity during T cell development. Nat Immunol. 2014;15(7):687–694. doi: 10.1038/ni.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 35.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: Effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28(1):390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 36.Guy CS, et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol. 2013;14(3):262–270. doi: 10.1038/ni.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holst J, et al. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat Immunol. 2008;9(6):658–666. doi: 10.1038/ni.1611. [DOI] [PubMed] [Google Scholar]
- 38.Itoh Y, Germain RN. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J Exp Med. 1997;186(5):757–766. doi: 10.1084/jem.186.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458(7235):211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444(7120):724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 41.Siggs OM, et al. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27(6):912–926. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitmire JK, Ahmed R. Costimulation in antiviral immunity: Differential requirements for CD4(+) and CD8(+) T cell responses. Curr Opin Immunol. 2000;12(4):448–455. doi: 10.1016/s0952-7915(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 43.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 44.Butler TC, Kardar M, Chakraborty AK. Quorum sensing allows T cells to discriminate between self and nonself. Proc Natl Acad Sci USA. 2013;110(29):11833–11838. doi: 10.1073/pnas.1222467110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faroudi M, Zaru R, Paulet P, Müller S, Valitutti S. Cutting edge: T lymphocyte activation by repeated immunological synapse formation and intermittent signaling. J Immunol. 2003;171(3):1128–1132. doi: 10.4049/jimmunol.171.3.1128. [DOI] [PubMed] [Google Scholar]
- 46.Friedl P, Gunzer M. Interaction of T cells with APCs: The serial encounter model. Trends Immunol. 2001;22(4):187–191. doi: 10.1016/s1471-4906(01)01869-5. [DOI] [PubMed] [Google Scholar]
- 47.Rachmilewitz J, Lanzavecchia A. A temporal and spatial summation model for T-cell activation: Signal integration and antigen decoding. Trends Immunol. 2002;23(12):592–595. doi: 10.1016/s1471-4906(02)02342-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.