Significance
Entry of bacteria into host cells critically depends on their proper engulfment by the plasma membrane. So far, actin polymerization has been described as a major driving force in this process. However, our study reveals that the interaction of the bacterial surface lectin LecA with the host cell glycosphingolipid Gb3 is fully sufficient to promote engulfment of Pseudomonas aeruginosa, whereas actin polymerization is dispensable. Hence, the formation of a “lipid zipper” represents a previously unidentified mechanism of bacterial uptake and demonstrates that bacterial pathogens have evolved lipid-based invasion strategies that may function in addition to protein receptor-based ones. Furthermore, by identifying the LecA/Gb3 interaction as the major invasion-promoting factor, our study provides new targets for drugs that may prevent bacterial invasion and dissemination.
Keywords: membrane curvature, glycolipid, infection
Abstract
Glycosphingolipids are important structural constituents of cellular membranes. They are involved in the formation of nanodomains (“lipid rafts”), which serve as important signaling platforms. Invasive bacterial pathogens exploit these signaling domains to trigger actin polymerization for the bending of the plasma membrane and the engulfment of the bacterium—a key process in bacterial uptake. However, it is unknown whether glycosphingolipids directly take part in the membrane invagination process. Here, we demonstrate that a “lipid zipper,” which is formed by the interaction between the bacterial surface lectin LecA and its cellular receptor, the glycosphingolipid Gb3, triggers plasma membrane bending during host cell invasion of the bacterium Pseudomonas aeruginosa. In vitro experiments with Gb3-containing giant unilamellar vesicles revealed that LecA/Gb3-mediated lipid zippering was sufficient to achieve complete membrane engulfment of the bacterium. In addition, theoretical modeling elucidated that the adhesion energy of the LecA–Gb3 interaction is adequate to drive the engulfment process. In cellulo experiments demonstrated that inhibition of the LecA/Gb3 lipid zipper by either lecA knockout, Gb3 depletion, or application of soluble sugars that interfere with LecA binding to Gb3 significantly lowered P. aeruginosa uptake by host cells. Of note, membrane engulfment of P. aeruginosa occurred independently of actin polymerization, thus corroborating that lipid zippering alone is sufficient for this crucial first step of bacterial host-cell entry. Our study sheds new light on the impact of glycosphingolipids in the cellular invasion of bacterial pathogens and provides a mechanistic explication of the initial uptake processes.
Glycosphingolipids (GSLs) are essential components of biological membranes that have significant impact on the organization and the molecular architecture of the membrane (1, 2). Preferential association of GSLs with cholesterol, various other lipid species, and proteins induces formation of micro- and nanodomains. Such domains, which are also termed “lipid rafts,” are involved in the signal transduction across the membrane (3). Several invasive bacterial pathogens hijack these membrane domains as signaling platforms for actin polymerization, which leads to the engulfment of the bacterium by the host plasma membrane (PM) (4–6). For example, Listeria monocytogenes exploits lipid raft-dependent E-cadherin and HGF-R/Met signaling in the host cell to trigger actin polymerization for bacterial uptake (7). The opportunistic bacterium Pseudomonas aeruginosa can also invade and survive in diverse epithelial and endothelial cells (8–14), which significantly contributes to its pathogenicity (8, 10, 15). Numerous reports suggested various host cell factors, including cell surface receptors and signaling components, including α5β1 integrin, cystic fibrosis transmembrane conductance regulator, and Abelson tyrosine-protein kinase 1-dependent pathway (16–18), that are involved in the cellular uptake of P. aeruginosa. Host-cell GSLs have been identified as important molecules contributing to host specificity and adhesion of P. aeruginosa (19, 20). Recent observations suggest that GSLs might be of critical importance for the internalization of P. aeruginosa into nonphagocytic cells (9). The homotetrameric, galactophilic lectin LecA, which is localized to the outer bacterial membrane (21), belongs to the carbohydrate binding proteins expressed by P. aeruginosa that recognize GSLs (22, 23) and represents one of the virulence factors (24). The preferential binding of LecA to the GSL globotriaosylceramide (also known as Gb3/CD77 or Pk histo-blood group antigen) (22, 25) prompted us to investigate the role of LecA–Gb3 interaction in the cellular uptake of P. aeruginosa.
In our study, we demonstrated that GSLs have a major impact on the bending of the host plasma membrane and engulfment of the pathogen. Binding of GSL by P. aeruginosa induced negative membrane curvature on synthetic, giant unilamellar vesicles (GUVs), solely driven by LecA–Gb3 interaction. This interaction resulted in a bacterium tightly engulfed by the lipid bilayer of the GUV. Because the glycolipid component plays the major role in this process, we termed this mechanism “lipid zipper” as a novel zipper-type mode of bacterial entry (26). Further experiments revealed that the lipid zipper has a significant impact on P. aeruginosa uptake in several epithelial cell lines. Interestingly, actin polymerization, which was previously identified as a major driving force for membrane deformation and engulfment, was not required for the lipid-triggered process. Moreover, ectopic expression of LecA in Escherichia coli was sufficient to greatly increase its invasion efficiency in Gb3-positive cells.
Results
A LecA–Gb3 Lipid Zipper Is Sufficient for Membrane Engulfment of P. aeruginosa in a Synthetic Lipid Bilayer System.
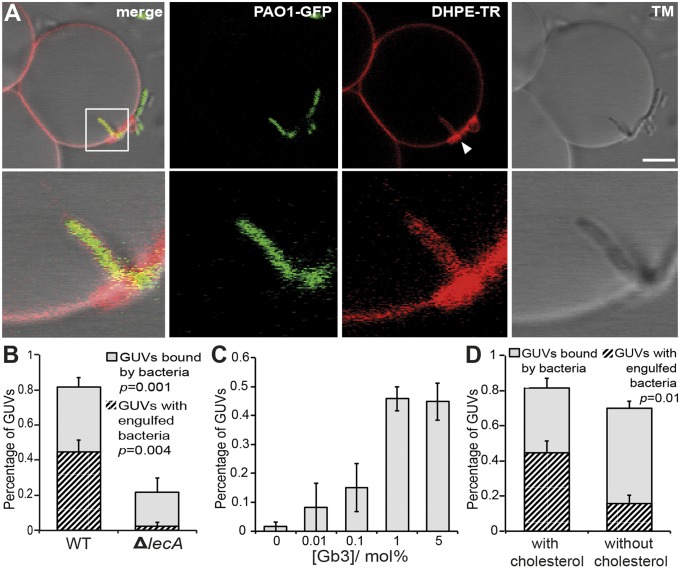
We used Gb3-containing GUVs as a membrane model system to directly address the impact of LecA–Gb3 interactions on the curvature of a lipid bilayer without any potential interference of cellular factors, particularly actin. We incubated GUVs with the invasive P. aeruginosa PAO1 WT strain and a lecA deletion mutant (ΔlecA) that did not express LecA (Fig. S1, Inset). Both strains were chromosomally GFP-tagged and did not differ in their growth rates (Fig. S1). PAO1 WT not only bound to Gb3-containing GUVs but also induced a highly curved GUV membrane at the contact point, which finally led to a complete engulfment of the WT bacteria by the GUV membrane (Fig. 1A and Fig. S2 A–C). Remarkably, we also observed membrane-engulfed bacteria that were connected via tether-like structures to the surrounding GUV membrane (Fig. S2 A, arrowhead and B). Interestingly, we observed clustering of lipid material at sites where bacteria attached to the GUVs and were engulfed (Fig. 1A). In total, 82 ± 6.5% of the analyzed GUVs were bound by PAO1 WT and of these 45 ± 6.4% showed bacteria, which were engulfed by the GUV membrane (Fig. 1B). In contrast, the ΔlecA mutant essentially did not induce membrane invaginations (Fig. S2 D–F). Although still 22 ± 7.7% of the GUVs were bound by the ΔlecA mutant (Fig. 1B), only 1 of 102 GUVs analyzed in total showed a membrane-engulfed bacterium. Lowering of the Gb3 concentration in GUVs from 5 to 0 mol% led to a dose-dependent decrease of GUVs containing membrane-engulfed bacteria (Fig. 1C), clearly indicating a crucial role of Gb3 in the process of bacterial membrane engulfment. Without Gb3, only 1 of the 106 GUVs analyzed showed a wrapped bacterium. In the next step we addressed the impact of the mechanical properties of the GUV membrane. When decreasing the surface tension by applying a hyperosmolar buffer solution from the outside (550 mOsm⋅L−1 outside vs. 290 mOsm⋅L−1 inside the GUVs), GUVs containing 0.1 mol% Gb3 showed a number of membrane-wrapped bacteria comparable to that obtained with 5 mol% Gb3 GUVs at iso-osmolar conditions (Fig. S3 and Fig. 1C).
Fig. 1.
P. aeruginosa induces a lipid zipper on giant unilamellar vesicles by binding of LecA to Gb3. (A) GUVs reconstituted with Texas Red-labeled 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (DHPE-TR) and Gb3 were inoculated with GFP-tagged P. aeruginosa PAO1 WT (PAO1-GFP) for 15 min at 37 °C. Lipid clustering at the bacterial attachment site is visible (arrowhead). (Scale bar, 5 µm.) (B) Relative number of GUVs containing bound or membrane-wrapped P. aeruginosa PAO1 WT or PAO1 ΔlecA. Bound GUVs were normalized to the total number of GUVs analyzed and invaginated GUVs were normalized to GUVs bound by bacteria. Bars represent mean values ± SEM of n = 4 independent experiments with ≥100 GUVs analyzed in total. (C) Relative number of GUVs with indicated Gb3 concentrations containing membrane-engulfed P. aeruginosa PAO1 WT. Bars represent mean values ± SEM of n ≥ 3 independent experiments. For 0 mol% Gb3, 106 GUVs were analyzed; for 0.01 mol% Gb3, 71 GUVs; for 0.1 mol% Gb3, 105 GUVs; for 1 mol%, 54 GUVs; and for 5 mol% Gb3, 100 GUVs. (D) Relative number of GUVs with bound or invaginated bacteria as normalized in B with lipid mixtures containing 5 mol% Gb3 with or without 30 mol% cholesterol. Bars represent mean values ± SEM of n ≥ 3 independent experiments with ≥100 GUVs analyzed in total.
Cholesterol plays a pivotal role in membrane physiology because it is implicated in the generation of lipid raft domains and can influence membrane rigidity, which might affect the formation of the lipid zipper. Therefore, we compared the efficiency of lipid zipper formation on GUVs in the presence or absence of cholesterol. The efficiency was reduced by 65% in the absence of cholesterol (45 ± 6.4% vs.15.7 ± 4.8%), which, apparently, was not due to a decrease in binding of bacteria to GUVs, which was just 10% less (70 ± 4.2%) compared with cholesterol-containing GUVs (81.7 ± 5.4%) (Fig. 1D). In summary, these data provide clear evidence that binding of LecA to Gb3 mediates a cholesterol-dependent, zipper-like engulfment of bacteria by the GUV membrane.
The LecA–Gb3 Interaction Provides Enough Energy for Complete Engulfment of P. aeruginosa by a Lipid Membrane.
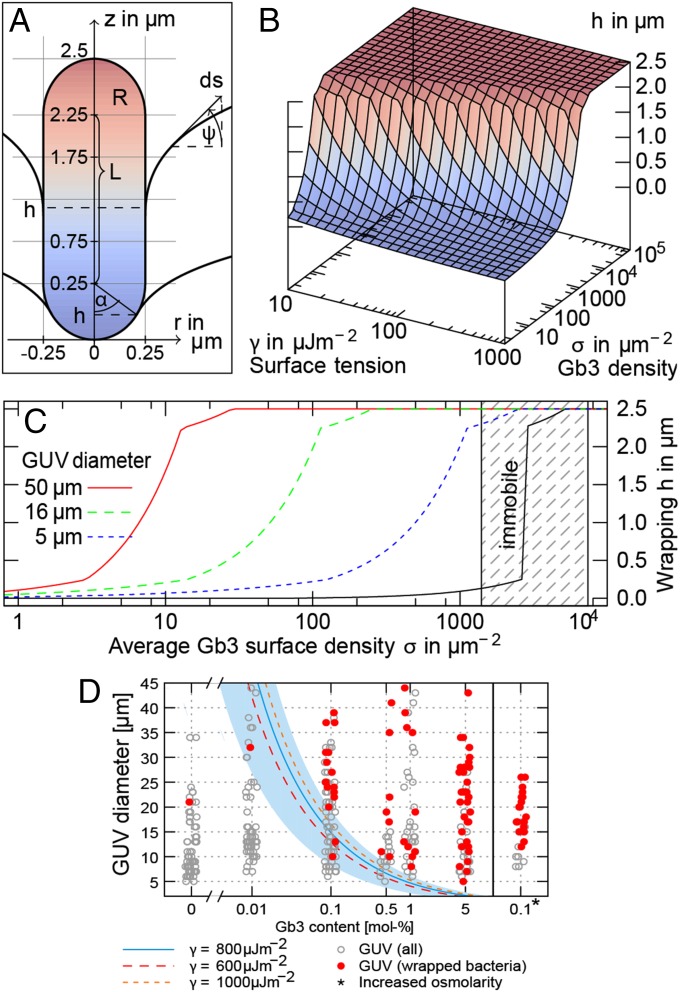
We developed a theoretical model to investigate whether the LecA–Gb3 interaction is sufficient to mediate membrane engulfment of bacteria. The GUV was described as an elastic surface consisting of mobile lipid constituents, and P. aeruginosa was modeled as a LecA-covered, rigid rod (Fig. 2A and SI Materials and Methods). The total energy of the system consists of three terms: (i) mechanical Helfrich energy (27), which favors flat membrane configurations; (ii) curvature-promoting adhesion energy owing to LecA–Gb3 interaction; and (iii) mixing entropy (28) of mobile lipids. The final configuration of the bacterium relative to the GUV membrane is the result of minimizing the total energy and depends solely on the physical properties of the system, that is, size of the bacterium, bending rigidity, and surface tension of the GUV membrane, as well as Gb3 density. In Fig. 2B we show the wrapping height h of a bacterium (L = 2.5 µm) in dependence of surface tension γ and Gb3 density σ for constant bending rigidity (SI Materials and Methods). For realistic values of GUV Gb3 densities (σ ≈ 105 µm−2), the model predicts a complete membrane engulfment of the bacterium. Similar effects are predicted for the host-cell PMs, for which Gb3 densities in the range of σ = 1,400−7,400 µm−2 (SI Materials and Methods) and surface tensions between 10 μJ⋅m−2 and 300 μJ⋅m−2 (29–31) are realistic. Furthermore, the model reveals that LecA-dependent clustering of mobile Gb3 lipids is crucial. When Gb3 is kept immobile, the global Gb3 concentration required to achieve full engulfment increased by two orders of magnitude (Fig. 2C; compare curves in color and black for mobile and immobile Gb3, respectively). Full engulfment occurred at local Gb3 densities well below critical values (i.e., close packing of Gb3) (compare Fig. 2C and Fig. S4C).
Fig. 2.
Gb3 binding and clustering predicts complete membrane engulfment of P. aeruginosa. (A) Rotationally symmetric geometry of a modeled rod-shaped bacterium engulfed by GUV membrane. (B) Wrapping height in dependence of surface tension and Gb3 surface density (GUV diameter d = 16 μm). (C) Invagination depth and local Gb3 density as function of the global Gb3 density and different GUV diameters for intermediate surface tension (γ = 10−4 J⋅m−2). Clustering of mobile Gb3 reduces the global Gb3 concentration necessary for full wrapping (compare colored curves for mobile vs. black curve for immobile Gb3). Shaded area corresponds to Gb3 densities in the PM of host cells. (D) Critical GUV sizes. Circles represent observed vesicles of different size and with different Gb3 content (they are scattered around x-values for improved visibility). Each wrapping event is denoted by red padding. Three lines represent the critical GUV size for full enclosing of bacteria at likely surface tensions γ. The shaded area shows the effect of uncertainty in effective Gb3 content in a single vesicle at γ = 800 μJ⋅m−2 (SI Materials and Methods). For the right column, the osmolarity of the outside buffer was increased from 290 to 550 mOsm⋅L−1, whereas the inside buffer was kept at 290 mOsm⋅L−1; the thereby reduced surface tension enhances wrapping at moderate GUV sizes. The theoretical curves show qualitative agreement with the observed statistics.
To further explore the dependence of the wrapping on the global Gb3 density and the vesicle size, we incubated GUVs prepared from lipid mixtures containing different Gb3 concentrations (Materials and Methods) with P. aeruginosa PAO1 WT. By microscopic analysis we measured the equatorial diameter of GUVs in a random field of view. On the basis of our model (see above), we calculated the minimal vesicle size for which the bacteria would be fully wrapped (h = 2.5 μm) for the global Gb3 density σ on the vesicle at the applied Gb3 concentrations. Fig. 2D compares the experimental results with the theoretical prediction for three different surface tensions (solid line, γ = 800 μJ⋅m−2). The shaded area in Fig. 2D denotes the uncertainty range for the minimal vesicle size assuming that the bacterium is at least wrapped up to the spherical cap (h = 2.25 μm) and a 40% variation in the Gb3 content (SI Materials and Methods). Additionally, we experimentally tested our model by variation of the surface tension of GUVs as a critical parameter for bacterial wrapping (Materials and Methods). According to the model the critical vesicle size should decrease with decreasing surface tension (compare in Fig. 2D the solid line for γ = 800 μJ⋅m−2 with the dashed line for γ = 600 μJ⋅m−2). This should lead to an increased fraction of bacteria-wrapped GUV in the low-diameter range. The experimental results for GUVs with a diameter of ≤20 μm show that the fraction of bacteria-wrapped GUVs is increased by 25% at higher osmolarity compared with iso-osmolar conditions (compare in Fig. 2E GUVs with 0.1 mol% Gb3 at 290 vs. 550 mOsm⋅L−1). Taken together, our theoretical model shows that for typical Gb3 densities and surface tensions of GUVs and cells the energy provided by LecA–Gb3 binding and clustering is sufficient to induce total membrane engulfment of bacteria-sized objects. For engulfment of smaller particles such as viruses additional mechanisms were required (SI Materials and Methods), which is in agreement with previous experimental and theoretical results (32, 33).
LecA Promotes a Lipid Zipper on Epithelial Cells Independent of Actin Polymerization.
In further experiments, we directly assessed whether a lipid zipper mechanism is implicated in bacterial invasion of living host cells. We first verified that cell entry of P. aeruginosa into our cell model, the human lung epithelial cell line H1299, indeed depends on GSL expression, as previously reported (9). Quantification of the cellular invasion of P. aeruginosa showed an 80% reduction of invasion upon inhibition of GSL synthesis by d-threo-l-phenyl-2-palmitoylarmino-3-morpholino-l-propanol (PPMP) treatment, whereas bacterial attachment was not affected (Fig. S5A). Disappearance of Gb3 expression was verified by a loss of Shiga toxin B-subunit (StxB) binding to cells (Fig. S5B). In the following we studied the entry process of P. aeruginosa into H1299 cells by confocal fluorescence microscopy.
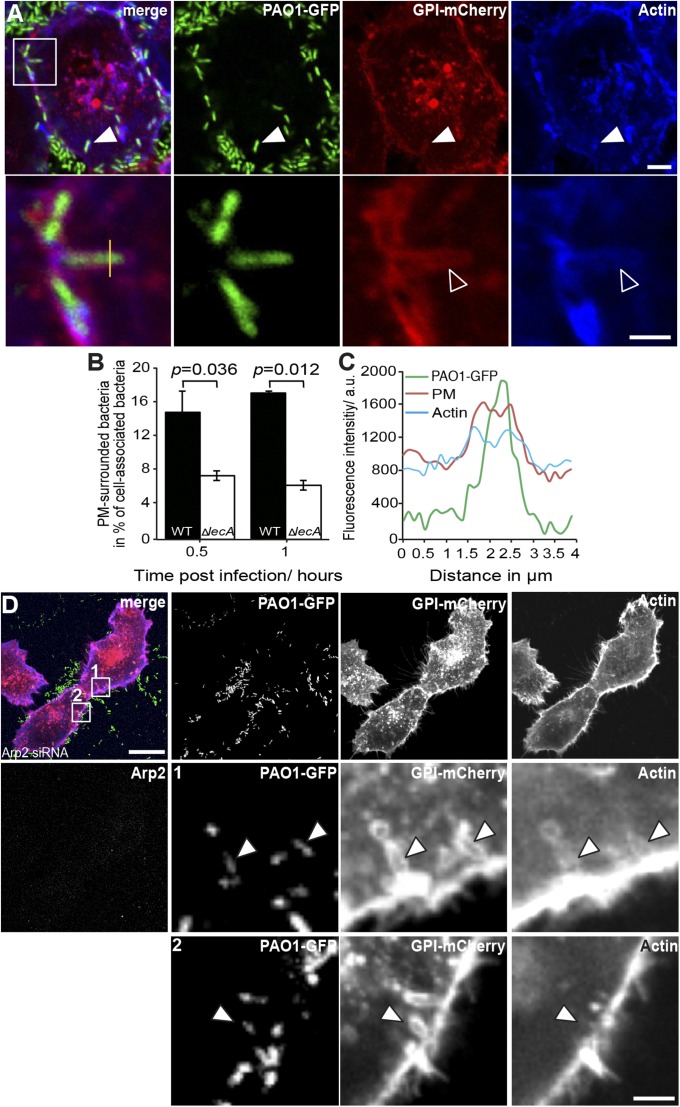
Shortly after binding to H1299 cells, bacteria induced negative PM curvature at the cellular entry site (Fig. S6A) that progressed into the engulfment of the bacterium by PM (Fig. 3A, arrowheads). For the ΔlecA mutant, the number of PM-surrounded bacteria was significantly lower (about 64.5%) at 1 h postinfection compared with the WT strain (Fig. 3B), suggesting that mainly LecA contributes to the zipper-like engulfment of bacteria by PM. In line with the GUV data, extraction of cholesterol by methyl-β-cyclodextrin treatment lowered the occurrence of zipper-like, PM-engulfed bacteria by about 50% (Fig. S7) and thereby to the same extent as observed for GUVs, with and without cholesterol (Fig. 1D). Interestingly, actin polymerization does not play a role for the PM engulfment of the bacteria, because actin-related protein 2/3 complex (Arp2) depletion (Fig. 3D and Fig. S6 B and D) (34) or latrunculin A treatment (Fig. S6E) (35) did not prevent initial envelopment of bacteria by PM. Nevertheless, accumulation of actin, as well as of the PM marker GPI-mCherry, was frequently seen close to invading bacteria even in Arp2-depleted cells (Fig. 3D). However, actin coverage seems not to be functionally linked to the formation of membrane invaginations, because we observed actin-covered, PM-enveloped bacteria under all perturbations (Fig. 3D and Fig. S6E, arrowheads). Most likely, actin coverage around invading bacteria represents persistent, cortical actin filaments. To further test whether actin polymerization is redundant for the zipper process, we studied the interactions of WT bacteria with plasma membrane spheres (PMS) induced in H1299 cells that stably expressed the actin marker LifeAct-mCherry. In PMS, no endocytic machinery is present and (cortical) actin is excluded (36). Intriguingly, we observed bacteria that were tightly engulfed by the membrane of PMS, which demonstrates membrane wrapping even in the absence of actin (Fig. S8). These PM-enveloped bacteria closely resembled those wrapped by GUV membranes (Fig. S2 B and C). Therefore, we propose a mechanistic model in which actin polymerization is dispensable for membrane engulfment but required for completion of the invasion process and closing of the invaginated membrane cup to form an intracellular bacteria-containing vesicle. This hypothesis is supported by the fact that P. aeruginosa requires actin polymerization for complete entry into lung cells, as indicated by the reduced invasion into Arp2-knockdown cells (Fig. S6C).
Fig. 3.
P. aeruginosa induces plasma membrane invaginations depending on LecA. (A) PAO1 (green)-induced membrane invaginations (red) colocalize with actin (blue) in H1299 cells during cellular entry. The white squared area is shown enlarged in the lower panel. (Scale bars, 5 μm and 2 μm, respectively.) (B) Relative numbers of PM-engulfed PAO1 WT and ΔlecA during cell entry stage as shown in A, n = 3 independent experiments; mean ± SEM P value was calculated by two-tailed, paired t test. (C) Intensity profile across a membrane invagination in A (yellow line). (D) Actin polymerization is not required for PAO1-induced membrane invaginations. Bacteria still induce membrane invaginations (arrowheads) and are surrounded by host PM, either covered (zoom 1) or uncovered (zoom 2, recorded 0.5 μm from above the focal plane shown in the overview) by actin in Arp2 knockdown cells (see Fig. S6D for control siRNA-transfected cells). The white squared areas are shown enlarged in the lower panels. (Scale bars, 10 μm and 2.5 μm, respectively.)
LecA/Gb3-Mediated Lipid Zipper Contributes Measurable Impact on the Cellular Invasion of P. aeruginosa.
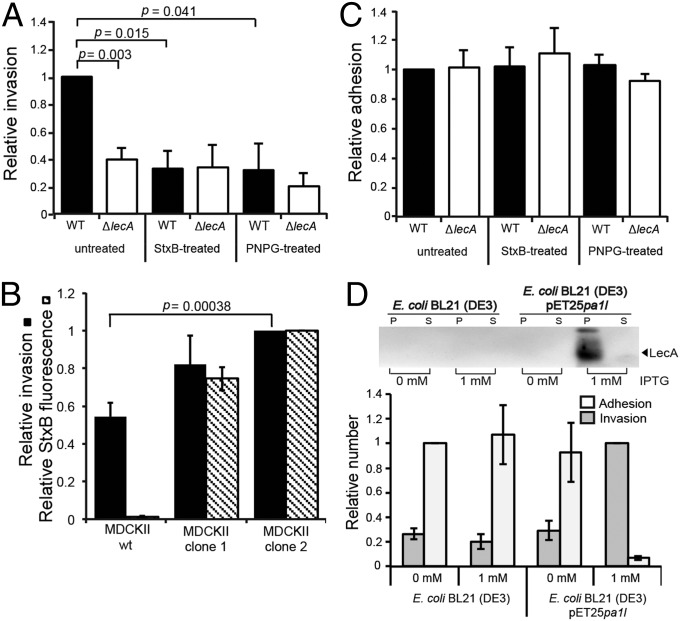
Because P. aeruginosa is capable of inducing a Gb3–LecA lipid zipper on GUVs (Fig. 1) and cells (Fig. 3) we quantified how the lipid zipper affects bacterial host-cell invasion efficiency. The P. aeruginosa PAO1 ΔlecA mutant strain showed a 61.3% reduced invasion into H1299 cells compared with PAO1 WT (Fig. 4A, “untreated”). Next, we depleted PM Gb3 levels by StxB-induced endocytosis of Gb3 (32) (Fig. S9 A and B). StxB-mediated depletion of Gb3 reduced the invasion of the WT strain into H1299 cells by 69.0% (Fig. 4A, “StxB-treated”) compared with untreated cells. No significant difference in invasion efficiency was observed with the ΔlecA strain on Gb3-depleted (“StxB-treated”) and Gb3-containing (“untreated”) H1299 cells. Additionally, we confirmed by ectopic expression of Gb3 in MDCKII cells that the invasion of PAO1 WT correlated with the amount of PM-localized Gb3 (Fig. 4B). Moreover, pretreatment of the bacteria with the LecA-binder para-nitrophenyl-α-d-galactopyranoside (PNPG) decreased the invasiveness of PAO1 WT by 68.6% (Fig. 4A, “PNPG-treated”). PNPG selectively prevented LecA, but not StxB, binding to cells (Fig. S9 C and D). In contrast, cellular invasion by the ΔlecA strain was not affected by PNPG, because the invasion efficiency was at a similar level compared with the untreated ΔlecA strain. None of the tested conditions significantly affected the adhesion of P. aeruginosa PAO1 WT or ΔlecA strains to host cells (Fig. 4C). These findings strongly suggest a major role of LecA and Gb3 as internalization factors rather than adhesion factors. Moreover, the cholesterol dependency of the lipid zipper formation in GUVs and cells correlated with the efficiency of cellular invasion by PAO1 WT. Invasion in cholesterol-depleted cells was significantly decreased by about 70% compared with control cells (Fig. S10). This suggests an overall critical role of cholesterol for the lipid zipper in vitro and in cellulo.
Fig. 4.
LecA in conjunction with Gb3 promotes efficient cellular invasion of P. aeruginosa. (A) Invasion of P. aeruginosa strains PAO1 WT and PAO1 ΔlecA into untreated or Gb3-depleted (StxB-treated) H1299 cells, and with LecA inhibition (PNPG-treated). Data represent mean values of n ≥ 3 independent experiments. The P value for WT vs. ΔlecA was calculated by a two-tailed, paired t test. (B) Ectopic expression of Gb3 in MDCKII cells enhances uptake of P. aeruginosa. Invasion of P. aeruginosa WT into untransfected MDCKII cells (wt) and Gb3-synthase-transfected MDCKII cells (clone 1 and clone 2) was evaluated. Both clones differ in their Gb3 expression, which was analyzed by FACS after incubation with StxB-Alexa488 (hatched bars). All data represent mean values ± SEM for n ≥ 3 independent experiments with normalized StxB fluorescence and invasion, to MDCKII clone 2. The P values were calculated by a two-tailed, paired t test. (C) Adhesion of PAO1 WT and ΔlecA to H1299 cells according to the treatments as in A. All data represent mean values ± SEM of n ≥ 4 independent experiments, normalized to the WT. (D, Upper) Induced expression of lecA by IPTG in E. coli BL21 (DE3) pET25pa1l detected by standard Western blot analysis. The parental, WT strain [BL21 (DE3)] serves as control (P, bacterial pellet; S, culture supernatant). (D, Lower) Invasion and adhesion of indicated E. coli strains to H1299 cells. Invasion upon IPTG-induced expression of lecA is strongly enhanced. Data represent mean values of normalized invasion ± SEM for n ≥ 4 independent experiments. For better visibility, adhesion was normalized to non-IPTG-treated E. coli BL21 (DE3).
To test the capacity of LecA as an invasion factor, we explored the invasiveness of a noninvasive E. coli strain upon induced expression of lecA by isopropyl β-d-1-thiogalactopyranoside (IPTG) (25). Remarkably, ectopic expression of lecA in E. coli BL21 (DE3) transduced this strain into an invasive one, evidenced by a significant increase of invasion into H1299 cells of about 340% compared with non-lecA-expressing E. coli strains (Fig. 4D). Interestingly, adherence of lecA-expressing E. coli to H1299 cells was drastically reduced (approximately 20-fold) compared with the non-lecA-expressing E. coli strains (Fig. 4D). Probably LecA functionally interferes with endogenous adherence factors of E. coli, which consequently prevents efficient adhesion to H1299 cells. However, these findings highlight the dominant, invasin-like function of LecA. In summary, these data demonstrate that Gb3 and LecA represent key factors for the cellular entry of P. aeruginosa into nonphagocytic cells.
Discussion
By studying the interaction of P. aeruginosa with synthetic and cellular membranes we have identified a mechanism for bacterial entry that requires host GSLs and bacterial lectins but does not depend on actin polymerization for membrane engulfment. The lipid zipper highlights the particular importance of glycolipids in bacterial invasion processes. Numerous host-cell factors have been described to interact with P. aeruginosa to orchestrate cellular entry and infection of the bacterium. Therefore, it is intriguing that only two interacting factors, the host-cell GSL Gb3 and the bacterial, GSL-binding lectin LecA are sufficient to induce the initial processes of the cellular entry of P. aeruginosa.
GSLs are key components of lipid rafts and as such are involved in establishing signal transduction platforms in biological membranes (1, 37). Cholesterol has been described to cluster GSLs in the external leaflet of the PM and thereby to stabilize lipid raft domains. We show that the lipid zipper mechanism depends on lipid raft components such as cholesterol and the GSL Gb3, whereas a nonraft lipid, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), does not directly promote the induction of the lipid zipper. Our results suggest that cholesterol stabilizes LecA-induced domains with a sufficiently high Gb3 density to trigger the lipid zipper, and finally the efficient cellular invasion. Therefore, our findings expand the lipid raft concept because (i) they introduce an autonomous role for GSLs (i.e., Gb3) in triggering an endocytic event, uncoupled from cytoplasmic signaling processes, and (ii) consider raft domains to serve as membrane areas providing sufficiently high GSL densities for lipid zippering bacterial pathogens.
The effect of cholesterol on membrane stiffness is complex. Membrane stiffness is not necessarily decreased in cholesterol-depleted membranes. It could even be increased, at least in cholesterol-depleted cells (38). We found that cholesterol extraction on cells decreased the frequency of the lipid zipper, which could, in line with ref. 38, point to an increase in membrane stiffness, impairing the lipid zipper. Following a study on DOPC vesicles, cholesterol has no (significant) effect on bending rigidity (39). However, we found a significantly lower occurrence of the lipid zipper in cholesterol-free GUVs. Interpreting our results in the context of the above-mentioned report (39), cholesterol might affect the lipid zipper on GUVs by mechanisms other than membrane stiffness.
Studies of SV40 entry engaging the GSL GM1 for cellular entry (33) revealed that the chemical structure of the GSL (i.e., fatty acid chain length and saturation) is critical for the uptake. Therefore, it will be interesting to investigate in future studies whether and how different Gb3 species affect the uptake of P. aeruginosa.
It is well established that the cellular uptake of Gram-negative bacterial pathogens (e.g., Salmonella and Shigella species) critically depends on secreted bacterial effector proteins that induce a reorganization of the host cytoskeleton (40, 41). The expression of a type-III secretion system (T3SS) by P. aeruginosa and T3SS- and T6SS-secreted effector proteins have been reported to be required for the cellular invasion and are supposed to influence the growth-phase dependent cellular uptake of P. aeruginosa (11, 42–45). However, our data suggest that initial membrane curvature and wrapping of the bacterium by the host PM depends neither on a bacterial secretion system nor on secreted effector proteins. Furthermore, actin polymerization and an active endocytic machinery are not required for the formation of PM invaginations, as suggested by actin inhibition experiments. The GUV experiments and model calculations clearly demonstrate that the induction of membrane invaginations by P. aeruginosa through LecA–Gb3 interactions is a thermodynamically favored, ATP-independent process that does not depend on cytosolic factors or on bacterial effectors. However, other bacterial and cellular effector proteins might be necessary to accelerate and/or finalize the uptake. In analogy to a previous report indicating that actin dynamics drive membrane scission on StxB-induced membrane invaginations (46), actin could be actively involved in late stages of endocytic processing of PM invaginations. In addition, because P. aeruginosa induces membrane curvature, curvature-sensing Bin Amphiphysin Rvs. (BAR) domain-containing proteins (47) might be recruited to the entry site and activate signaling pathways to promote the cellular uptake of P. aeruginosa.
Moreover, our work demonstrates a previously unidentified pathophysiological relevance for LecA. So far, LecA has been related to bacterial adhesion and biofilm formation (20, 48) and represents one of the virulence factors contributing to lung injury during infection (24). Our data identify LecA as an invasion factor for P. aeruginosa, which might contribute to the dissemination of the pathogen within its host organism during infection. This is strongly supported by the finding that ectopic lecA expression induced an invasive phenotype of an originally noninvasive E. coli strain. Moreover, this finding shows that LecA does not need to interact with specific P. aeruginosa factors to fulfill its invasive function.
Inhibition of the LecA/Gb3-dependent pathway reduced host-cell invasion by P. aeruginosa. Therefore, strategies to interfere with the binding of LecA to Gb3 could be the basis for new drugs that efficiently prevent the dissemination and persistence of P. aeruginosa. Moreover, many bacterial pathogens express lectins on their cellular surface that recognize host GSLs (49). In the context of our findings, it is possible that these pathogens use GSL-dependent mechanisms to trigger their uptake by host cells, alternatively or synergistically to protein receptor-based entry pathways, such as the invasin-induced entry of Yersinia (50) or the internalin-triggered pathway of Listeria spp. (51, 52).
Clearly, the lipid zipper mechanism that drives bacterial invasion shares some common features with the GSL-mediated endocytosis of toxins (32) and viruses (33, 53). Multivalent lectin–glycosphingolipid interactions trigger the formation of plasma membrane invaginations in the absence of cytosolic proteins (e.g., coat proteins and actin). However, for bacteria, in contrast to smaller particles such as toxins and viruses, the adhesion energy resulting from lectin-induced GSL clustering is already sufficient to overcome the energy penalty for membrane bending as an initial step for their cellular uptake.
Materials and Methods
Detailed materials, methods, and the theoretical model are described in SI Materials and Methods. Briefly, GUVs were prepared as described (54). H1299 and MDCKII cells stably expressing marker proteins or Gb3 synthase were cultured in RPMI or DMEM supplemented with Geneticin and 10% (vol/vol) (5% vol/vol) FBS. Recombinant LecA-, lecA-, and non-lecA-expressing E. coli BL21 (DE3) were produced as reported (25). GFP tags and LecA mutants of P. aeruginosa PAO1 were produced as described in SI Materials and Methods. Invasion assays were performed as essentially described (11). GSL synthesis was inhibited by incubation of cells for 3 d with 5 μM PPMP. For LecA inhibition, soluble LecA (2 μg⋅mL−1) or bacteria were incubated for 15 min at 37 °C with 10 mM PNPG. Gb3 and cholesterol was depleted using 5 μg⋅mL−1 Cy3-labled StxB and 10 mM methyl-β-cyclodextrin, respectively. Actin polymerization was inhibited by latrunculin A treatment (0.1 μM) or knockdown of Arp2 by siRNA. PMS were induced as described (36) and visualized by FM4-64 dye. Cells, GUVs, and P. aeruginosa infection were imaged on a confocal microscope (Nikon Eclipse Ti-E with A1R confocal laser scanner, 60× oil objective, N.A. 1.49). Statistical testing was performed with Excel with data of n ≥ 3 independent experiments using two-tailed, unpaired t test, unless stated otherwise.
Supplementary Material
Acknowledgments
W.R. acknowledges support by the Excellence Initiative of the German Research Foundation (EXC 294), the Ministry of Science, Research and the Arts of Baden-Württemberg (Az: 33-7532.20), a starting grant from the European Research Council (Programme “Ideas,” ERC-2011-StG 282105), the Excellence Initiative of the German Research Foundation (GSC-4, Spemann Graduate School). A.I. and A.A. acknowledge support from Neolect (ANR-11-BSV5-002), COST action BM1003, and Labex ARCANE (ANR-11-LABX-003). S.A. acknowledges support from the International Max Planck Research School for Molecular and Cellular Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402637111/-/DCSupplemental.
References
- 1.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.Lingwood CA. Glycosphingolipid functions. Cold Spring Harb Perspect Biol. 2011;3(7):a011874. doi: 10.1101/cshperspect.a004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 4.Riethmüller J, Riehle A, Grassmé H, Gulbins E. Membrane rafts in host-pathogen interactions. Biochim Biophys Acta. 2006;1758(12):2139–2147. doi: 10.1016/j.bbamem.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Lafont F, Abrami L, van der Goot FG. Bacterial subversion of lipid rafts. Curr Opin Microbiol. 2004;7(1):4–10. doi: 10.1016/j.mib.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124(4):715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Seveau S, Bierne H, Giroux S, Prévost MC, Cossart P. Role of lipid rafts in E-cadherin— and HGF-R/Met—mediated entry of Listeria monocytogenes into host cells. J Cell Biol. 2004;166(5):743–753. doi: 10.1083/jcb.200406078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi E, Mehl T, Nunn D, Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59(3):822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emam A, Carter WG, Lingwood C. Glycolipid-dependent, protease sensitive internalization of Pseudomonas aeruginosa into cultured human respiratory epithelial cells. Open Microbiol J. 2010;4:106–115. doi: 10.2174/1874285801004010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleiszig SM, Zaidi TS, Fletcher EL, Preston MJ, Pier GB. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62(8):3485–3493. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha U, Jin S. Growth phase-dependent invasion of Pseudomonas aeruginosa and its survival within HeLa cells. Infect Immun. 2001;69(7):4398–4406. doi: 10.1128/IAI.69.7.4398-4406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira SH, Cervante MP, Bentzmann S, Plotkowski MC. Pseudomonas aeruginosa entry into Caco-2 cells is enhanced in repairing wounded monolayers. Microb Pathog. 1997;23(4):249–255. doi: 10.1006/mpat.1997.0153. [DOI] [PubMed] [Google Scholar]
- 13.Plotkowski MC, Meirelles MN. Concomitant endosome-phagosome fusion and lysis of endosomal membranes account for Pseudomonas aeruginosa survival in human endothelial cells. J Submicrosc Cytol Pathol. 1997;29(2):229–237. [PubMed] [Google Scholar]
- 14.Plotkowski MC, Saliba AM, Pereira SH, Cervante MP, Bajolet-Laudinat O. Pseudomonas aeruginosa selective adherence to and entry into human endothelial cells. Infect Immun. 1994;62(12):5456–5463. doi: 10.1128/iai.62.12.5456-5463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleiszig SM, et al. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64(6):2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roger P, et al. Fibronectin and alpha5beta1 integrin mediate binding of Pseudomonas aeruginosa to repairing airway epithelium. Eur Respir J. 1999;13(6):1301–1309. [PubMed] [Google Scholar]
- 17.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94(22):12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pielage JF, Powell KR, Kalman D, Engel JN. RNAi screen reveals an Abl kinase-dependent host cell pathway involved in Pseudomonas aeruginosa internalization. PLoS Pathog. 2008;4(3):e1000031. doi: 10.1371/journal.ppat.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Bentzmann S, et al. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect Immun. 1996;64(5):1582–1588. doi: 10.1128/iai.64.5.1582-1588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilboa-Garber N, Sudakevitz D, Sheffi M, Sela R, Levene C. PA-I and PA-II lectin interactions with the ABO(H) and P blood group glycosphingolipid antigens may contribute to the broad spectrum adherence of Pseudomonas aeruginosa to human tissues in secondary infections. Glycoconj J. 1994;11(5):414–417. doi: 10.1007/BF00731276. [DOI] [PubMed] [Google Scholar]
- 21.Glick J, Garber N. The intracellular localization of Pseudumonas aeruginosa lectins. J Gen Microbiol. 1983;129:3085–3090. doi: 10.1099/00221287-129-10-3085. [DOI] [PubMed] [Google Scholar]
- 22.Lanne B, Cîopraga J, Bergström J, Motas C, Karlsson KA. Binding of the galactose-specific Pseudomonas aeruginosa lectin, PA-I, to glycosphingolipids and other glycoconjugates. Glycoconj J. 1994;11(4):292–298. doi: 10.1007/BF00731201. [DOI] [PubMed] [Google Scholar]
- 23.Gilboa-Garber N. Inhibition of broad spectrum hemagglutinin from Pseudomonas aeruginosa by D-galactose and its derivatives. FEBS Lett. 1972;20(2):242–244. doi: 10.1016/0014-5793(72)80805-6. [DOI] [PubMed] [Google Scholar]
- 24.Chemani C, et al. Role of LecA and LecB lectins in Pseudomonas aeruginosa-induced lung injury and effect of carbohydrate ligands. Infect Immun. 2009;77(5):2065–2075. doi: 10.1128/IAI.01204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanchard B, et al. Structural basis of the preferential binding for globo-series glycosphingolipids displayed by Pseudomonas aeruginosa lectin I. J Mol Biol. 2008;383(4):837–853. doi: 10.1016/j.jmb.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Cossart P, Sansonetti PJ. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science. 2004;304(5668):242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 27.Helfrich W. Elastic properties of lipid bilayers: Theory and possible experiments. Z Naturforsch C. 1973;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 28.Reichl L. A Modern Course in Statistical Physics. Weinheim, Germany: Wiley-VCH; 2009. [Google Scholar]
- 29.Lieber AD, Yehudai-Resheff S, Barnhart EL, Theriot JA, Keren K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr Biol. 2013;23(15):1409–1417. doi: 10.1016/j.cub.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 30.Pietuch A, Brückner BR, Janshoff A. Membrane tension homeostasis of epithelial cells through surface area regulation in response to osmotic stress. Biochim Biophys Acta. 2013;1833(3):712–722. doi: 10.1016/j.bbamcr.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77(6):3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Römer W, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450(7170):670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 33.Ewers H, et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat Cell Biol. 2010;12(1):11–18; sup pp 11-12. doi: 10.1038/ncb1999. [DOI] [PubMed] [Google Scholar]
- 34.Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- 35.Coué M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213(2):316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 36.Lingwood D, Ries J, Schwille P, Simons K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci USA. 2008;105(29):10005–10010. doi: 10.1073/pnas.0804374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 38.Byfield FJ, Aranda-Espinoza H, Romanenko VG, Rothblat GH, Levitan I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys J. 2004;87(5):3336–3343. doi: 10.1529/biophysj.104.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gracia RS, Bezlyepkina N, Knorr RL, Lipowsky R, Dimova R. Effect of cholesterol on the rigidity of saturated and unsaturated membranes: Fluctuation and electrodeformation analysis of giant vesicles. Soft Matter. 2010;6(7):1472–1482. [Google Scholar]
- 40.McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V. Salmonella takes control: Effector-driven manipulation of the host. Curr Opin Microbiol. 2009;12(1):117–124. doi: 10.1016/j.mib.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa M, Handa Y, Ashida H, Suzuki M, Sasakawa C. The versatility of Shigella effectors. Nat Rev Microbiol. 2008;6(1):11–16. doi: 10.1038/nrmicro1814. [DOI] [PubMed] [Google Scholar]
- 42.Galán JE, Collmer A. Type III secretion machines: Bacterial devices for protein delivery into host cells. Science. 1999;284(5418):1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 43.Jendrossek V, et al. Apoptotic response of Chang cells to infection with Pseudomonas aeruginosa strains PAK and PAO-I: Molecular ordering of the apoptosis signaling cascade and role of type IV pili. Infect Immun. 2003;71(5):2665–2673. doi: 10.1128/IAI.71.5.2665-2673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbieri JT, Sun J. Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol. 2004;152:79–92. doi: 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 45.Sana TG, et al. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J Biol Chem. 2012;287(32):27095–27105. doi: 10.1074/jbc.M112.376368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Römer W, et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell. 2010;140(4):540–553. doi: 10.1016/j.cell.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Peter BJ, et al. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science. 2004;303(5657):495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 48.Diggle SP, et al. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ Microbiol. 2006;8(6):1095–1104. doi: 10.1111/j.1462-2920.2006.001001.x. [DOI] [PubMed] [Google Scholar]
- 49.Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta. 2006;1760(4):527–537. doi: 10.1016/j.bbagen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 51.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103(3):501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 52.Mengaud J, Ohayon H, Gounon P, Cossart P, Cossart P. Mege R-M E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84(6):923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 53.Rydell GE, Svensson L, Larson G, Johannes L, Römer W. Human GII.4 norovirus VLP induces membrane invaginations on giant unilamellar vesicles containing secretor gene dependent α1,2-fucosylated glycosphingolipids. Biochim Biophys Acta. 2013;1828(8):1840–1845. doi: 10.1016/j.bbamem.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Mathivet L, Cribier S, Devaux PF. Shape change and physical properties of giant phospholipid vesicles prepared in the presence of an AC electric field. Biophys J. 1996;70(3):1112–1121. doi: 10.1016/S0006-3495(96)79693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.