Significance
This study defines a cellular mechanism by which primary cilia mediate mechanosensation in intraocular pressure regulation. Changes in pressure are sensed by the interaction of the inositol phosphatase OCRL with transient receptor potential vanilloid 4 (TRPV4), a primary cilia-based calcium channel. Pediatric glaucoma (Lowe) syndrome patient cells with defective OCRL failed to respond to agonists of TRPV4, and targeting of TRPV4 lowered intraocular pressure in vivo. These findings significantly advance the current understanding of how intraocular pressure is regulated.
Abstract
Lowe syndrome is a rare X-linked congenital disease that presents with congenital cataracts and glaucoma, as well as renal and cerebral dysfunction. OCRL, an inositol polyphosphate 5-phosphatase, is mutated in Lowe syndrome. We previously showed that OCRL is involved in vesicular trafficking to the primary cilium. Primary cilia are sensory organelles on the surface of eukaryotic cells that mediate mechanotransduction in the kidney, brain, and bone. However, their potential role in the trabecular meshwork (TM) in the eye, which regulates intraocular pressure, is unknown. Here, we show that TM cells, which are defective in glaucoma, have primary cilia that are critical for response to pressure changes. Primary cilia in TM cells shorten in response to fluid flow and elevated hydrostatic pressure, and promote increased transcription of TNF-α, TGF-β, and GLI1 genes. Furthermore, OCRL is found to be required for primary cilia to respond to pressure stimulation. The interaction of OCRL with transient receptor potential vanilloid 4 (TRPV4), a ciliary mechanosensory channel, suggests that OCRL may act through regulation of this channel. A novel disease-causing OCRL allele prevents TRPV4-mediated calcium signaling. In addition, TRPV4 agonist GSK 1016790A treatment reduced intraocular pressure in mice; TRPV4 knockout animals exhibited elevated intraocular pressure and shortened cilia. Thus, mechanotransduction by primary cilia in TM cells is implicated in how the eye senses pressure changes and highlights OCRL and TRPV4 as attractive therapeutic targets for the treatment of glaucoma. Implications of OCRL and TRPV4 in primary cilia function may also shed light on mechanosensation in other organ systems.
Oculocerebrorenal syndrome of Lowe is a rare X-linked recessive disorder that presents in males with bilateral cataracts and glaucoma, as well as renal failure, muscular hypotonia, and mental retardation (1). The defective gene, OCRL, encodes an inositol polyphosphate 5-phosphatase; its substrates include phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] and phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3] (2). Decreased 5-phosphatase activity is demonstrated in fibroblasts from Lowe patients as well as a two- to threefold elevated ratio of PI(4,5)P2: PI(4)P (3). We and others have recently identified a novel link between OCRL and primary cilia (4–7).
The primary cilium is an evolutionarily conserved subcellular structure that protrudes from many postmitotic eukaryotic cells (8, 9). In response to changes in the extracellular environment, primary cilia coordinate signaling cascades that control cell differentiation, growth, and function (10). A highly specialized extension of the plasma membrane, the ciliary membrane is enriched with many signaling proteins, including transient receptor potential (TRP) channels (11). Upon extracellular stimulation, TRP channels initiate signal transduction cascades by inducing Ca2+ flow. Phosphoinositides within the ciliary membrane are essential second messengers for ciliary function, potentially through modulation of the activities of TRP channels (12, 13). Genetic defects in cilia formation or maintenance underlie a wide array of human diseases, including retinitis pigmentosa, renal cysts, polydactyly, and developmental delays, which are collectively called ciliopathies (14–17).
Although some forms of ciliopathies present with disease states of fluid dysregulation, including hydrocephalus (18, 19), it is unknown if elevated eye pressure may be caused by ciliary disease. Glaucoma is an optic neuropathy associated with elevated intraocular pressure (IOP) and is a leading cause of irreversible blindness in the world (20, 21). Consistent with the central role of increased pressure in the pathology of glaucoma, the only proven treatment for glaucoma is to lower eye pressure (22). Trabecular meshwork (TM) cells are responsible for the drainage of the majority of aqueous fluid (23), and dysfunction of the trabecular outflow leads to elevated IOP. In susceptible individuals, this results in the death of retinal ganglion cells, causing irreversible vision loss (24). However, the molecular events whereby elevated pressure results in aberrant mechanosensory signaling that leads to visual loss are poorly understood. Here we provide evidence that primary cilia are the pressure-sensing organelles in the TM; mutation of OCRL in TM cilia prevents proper functioning of the TRP vanilloid 4 (TRPV4) channel that in turn diminishes TM function and causes increased IOP.
Results
OCRL Mutation in a Patient with Congenital Glaucoma.
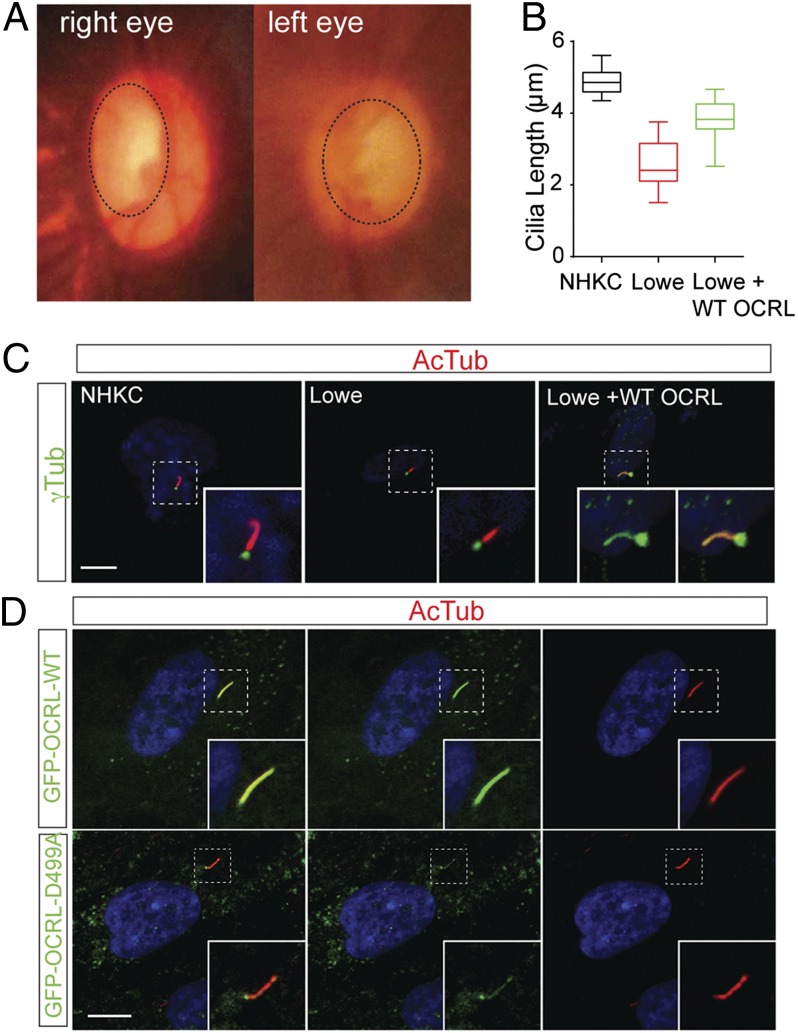
We have identified an 8-y-old male with Lowe syndrome who was born with bilateral congenital glaucoma and cataracts. Less than 12 h postpartum, the child was noted to have corneal edema and elevated pressures in both eyes and increased optic nerve cupping (Fig. 1A). The patient subsequently underwent multiple surgeries for glaucoma, including trabeculectomy and trabeculotomy, with poorly controlled disease (Fig. S1A). DNA sequencing revealed a novel missense mutation in the region encoding the 5-phosphatase domain of the OCRL gene (c.1661 A > C; p.D499A) (Fig. S1B). Based on its position in the OCRL structure, this aspartic acid residue is predicted to affect the 5-phosphatase activity of OCRL (Fig. S1C). Consistent with OCRL functioning in the cilia of retinal pigmented epithelial cells (4, 6, 7), the cilia in keratinocytes derived from this patient were defective. Following serum-starvation, these cells exhibited shortened cilia compared with keratinocytes from a healthy individual (Fig. 1 B and C). Upon reintroduction of wild-type OCRL, the shortened cilia phenotype was partially rescued (Fig. 1 B and C). To further assess the role of OCRL in cilia, the localization of OCRL was visualized in human trabecular meshwork (HTM) cells following serum-starvation by immunofluorescence. OCRL is concentrated in the axoneme or the membrane of the primary cilium (Fig. 1D). The OCRL mutant cDNA (OCRL D499A) was then expressed in HTM cells, which resulted in cilia from these cells forming significantly shorter structures than control cells upon serum-starvation (Fig. 1D and Fig. S1 D, E, and H). Additionally, OCRL knockdown HTM cells exhibited shortened cilia, which could be rescued by wild-type OCRL (Fig. S1 F and G). Taken together, these data support OCRL function in cilia formation in TM cells, which when defective resulted in glaucoma development in a human patient.
Fig. 1.
OCRL mutation in a Lowe syndrome patient with congenital glaucoma. (A) Optic nerve photos of the glaucoma patient. Photograph demonstrating glaucomatous optic nerve cupping in affected patient (cupping represented with dashed line). (B and C) Ciliary defect in a Lowe syndrome patient. After cilia induction by serum-starvation for 48 h, normal control (NHKC), Lowe syndrome patient (Lowe), and WT-GFP-OCRL transduced patient keratinocytes (Lowe + WT) were measured for ciliary length by staining with antiacetylated α-tubulin (red) and γ-tubulin (green) antibodies. Representative figure is shown in C. Average of three independent experiments, n = 50 cilia. Error bars represent SD. ANOVA, P < 0.001. (Scale bar, 10 µm.) (D) Primary cilia in HTM cells transfected with wild-type and mutant OCRL. HTM cells transfected with GFP-OCRL-WT or GFP-OCRL-D499A, followed by serum-starvation for 48 h, were then stained with acetylated α-tubulin. (Scale bar, 5 μm.)
Primary Cilia Are Present in the TM of the Eye.
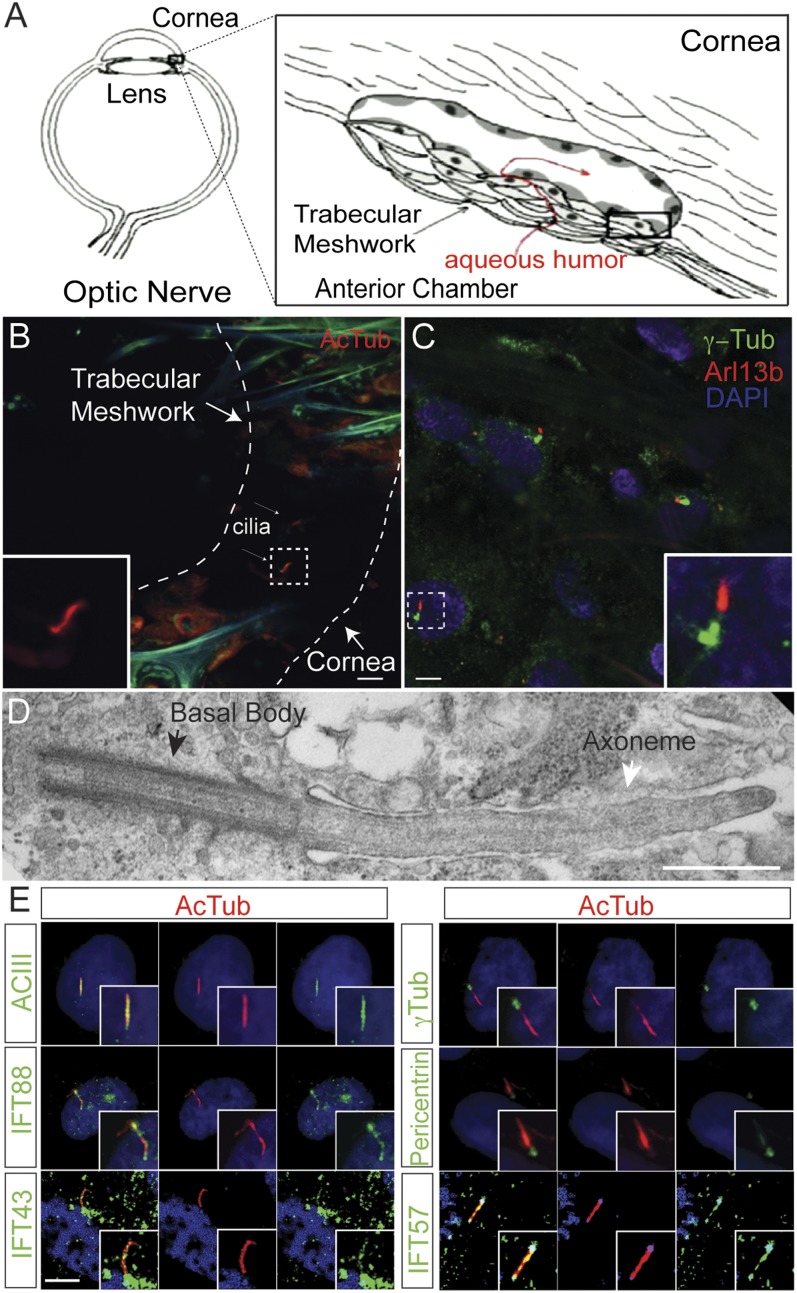
In aqueous humor regulation, the TM is generally accepted as the anatomical site of greatest resistance to outflow (23) (Fig. 2A). Although cilia-like structures have been observed in the pericanalicular region of the eye, primary cilia have not been characterized (25–27). Based on the results from the congenital glaucoma patient with Lowe syndrome, we sought to visualize cilia in isolated TM tissue from the anterior segment of human eyes by immunofluorescence and electron microscopy. The ciliary membrane covers a microtubule-based axoneme and is anchored by a basal body (10). Primary cilia were detected in TM tissues by immunostaining for acetylated α-tubulin or Arl13b, a small GTPase localized in the cilia, whereas the basal body was detected by staining for γ-tubulin. In cadaveric normal human eyes, immunofluorescence staining revealed numerous cilia-like structures in the uveoscleral region of the TM (Fig. 2B). Similar staining of cilia in TM tissues that were removed en face immediately following trabeculectomy from the eyes of glaucoma patients also detected primary cilia (Fig. 2C). Specificity for TM was verified by immunostaining of myocilin, a TM-enriched protein (Fig. S2A) (28). Similarly, primary cilia staining was noted in the TM of bovine, porcine, and murine eyes (Fig. S2 B–E). Electron microscopy revealed the characteristic “9+0” arrangement in the primary cilia of the TM cells (Fig. 2D and Fig. S2F). Thus, primary cilia are present in the TM cells of multiple mammalian species.
Fig. 2.
Primary cilia are conserved in mammalian TM. (A) Schematic of aqueous humor outflow in the eye. Aqueous humor is generated in the ciliary body, and drains out of the trabecular meshwork. (B and C) Primary cilia in normal and glaucomatous human TM. TM removed from human donor eyes (B) and surgical TM specimens during glaucoma surgery (C) were immunostained with anti-Arl13b (red), acetylated α-tubulin (AcTub, red), γ-tubulin (γ-Tub, green), and DAPI (blue). (Scale bar, 5 μm.) (D) Electron micrograph of a primary cilium in HTM in a glaucomatous eye. TM specimen removed during glaucoma surgery was imaged by transmission electron microscopy. (Scale bar, 500 nm.) (E) Characterization of primary cilia in cultured HTM cells. Serum-starved to induce ciliogenesis, HTM cells were immunostained with antiacetylated α-tubulin (AcTub, red), IFT88 (green), IFT43 (green), IFT57 (green), Adenylate cyclase III (green), pericentrin (green), and γ-tubulin (γ-Tub, green) antibodies. DAPI in blue. (Scale bar, 5 μm.)
Serum-starvation is a well-described method for the induction of primary cilia (29). The formation of cilia was therefore measured in cultured HTM cells at different times following serum-starvation, by detection of Arl13b, IFT88, IFT43, IFT57 (intraflagellar transport proteins), adenylate cyclase III (a cilia marker), and γ-tubulin by immunofluorescence (Fig. 2E). The average lengths of cilia increased following serum deprivation. At 24 h the cilia lengths were 2.2 ± 0.7 μm; at 48 h, 4.3 ± 0.6 μm; and 72 h, 5.6 ± 0.4 μm (Fig. S3). This finding confirms the presence of primary cilia in TM cells.
Pressure-Dependent Cilia Shortening and Gene Transcription.
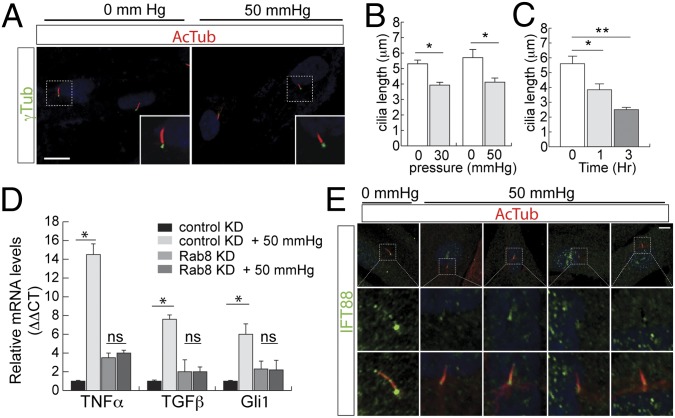
Because primary cilia mediate pressure sensation in the kidneys, brain, and bone (30–32), we examined the effect of pressure changes on cilia length in TM cells. In acute angle-closure glaucoma, intraocular pressure frequently can rise to 50 mmHg. HTM cells cultured in a pressure chamber were subjected to atmospheric (0 mmHg) or 50-mmHg hydrostatic pressure for 3 h. After fixation, cilia were imaged by immunostaining with acetylated α-tubulin (Fig. 3A). Average cilia lengths were 5.3 ± 0.2 μm in the normal pressure group and 3.9 ± 0.2 μm after 1 h of treatment (Fig. 3B). Overall, ciliary length in TM cells was reduced by pressure in a time-dependent fashion (Fig. 3C). Changes in ciliary length were both time- and dose-dependent processes.
Fig. 3.
Cilia shortening in response to pressure. (A and B) Elevated pressure resulted in ciliary shortening in HTM cells. HTM cells were serum-starved for 48 h, cultured in atmospheric (0 mmHg) or 50-mmHg pressure for 60 min, followed by immunostaining for acetylated α-tubulin. Cilia shown in Inset; cilia length was measured and shown in B. Error bars represent SD. n > 50 cilia, three independent experiments, paired t test. *P < 0.05. (Scale bar in A, 5 μm.) (C) HTM cells were serum-starved for 48 h, cultured in 50-mmHg pressure, for 0, 1, or 3 h, followed by immunostaining for acetylated α-tubulin. Cilia length was measured and shown. Error bars represent SD. n > 50 cilia, three independent experiments unpaired t test, *P < 0.01, **P < 0.001. (D) Pressure-mediated transcriptional activation requires primary cilia. Cilia formation in HTM cells (control and stable Rab8 knockdown) were induced by serum-starvation for 48 h followed by treatment with or without 50-mmHg pressure for 3 h. Levels of mRNA of TNF-α, GLI1, and TGF-β were measured. Three independent experiments with error bars represent SD. *P < 0.001; ns, not significant. (E) Altered distribution of IFT88 with elevated pressure. HTM cells were serum-starved to induce ciliogenesis, followed by treatment with or without elevated pressure (50 mmHg). Representative images of immunostaining with antibodies for IFT88 (green), acetylated α-tubulin (red), and DAPI (blue), showing the loss of IFT88 in the distal tip of cilia under elevated pressure conditions. (Scale bar, 10 μm.)
TNF-α and TGF-β are both highly abundant in the aqueous humor of patients with both acute and chronic forms of glaucoma (33–35). To determine whether the expression of TNF-α was up-regulated in response to pressure, its transcript levels were measured by quantitative RT-PCR (qRT-PCR) under different pressures. Following 1 h of application of 50-mmHg pressure, a robust elevation of TNF-α transcription was observed (Fig. 3D). The lack of changes in IL-33 transcript indicates that the effects on TNF-α are specific (Fig. S4A). Further, application of 50-mmHg pressure markedly elevated the transcript levels of TNF-α and TGF-β1, and had a modest effect on GLI1, a factor in the Sonic Hedgehog pathway (Fig. 3D). Cilia were ablated by shRNA knockdown of RAB8 (Fig. 3D and Fig. S4B), which inhibits ciliogenesis (36). Cells with reduced expression of Rab8 showed a reduction of gene transcription of TNF-α and TGF-β1 in response to pressure. In a complementary approach, the dependence of these effects on functional cilia was then determined by testing their sensitivity to chlorohydrate, which effectively removes cilia (30). The treatment of ciliated cells with chlorohydrate reduced the transcriptional activation in response to pressure (Fig. S4D). Taken together, these experiments strongly suggest that enhanced transcription of TNF-α and TGF-β1 by pressure occurs via a cilia-dependent process.
To further characterize the distribution of ciliary proteins under elevated pressure conditions, we examined intraflagellar transport (IFT) proteins. We examined the distribution of proteins involved in both anterograde (IFT57, IFT88) and retrograde (IFT20, IF43, IFT144) ciliary trafficking. Only IFT88 was found to have a markedly altered distribution under increased pressure conditions. In control HTM cells, IFT88 distributed to the base as well as to the tip of the axoneme; in HTM cells treated with pressure, IFT88 accumulated at the base of the cilia with a marked decrease at the ciliary tip (Fig. 3E and Fig. S4E). Thus, elevated pressure in cells may alter IFT distribution with a resultant change in ciliary protein trafficking.
Given the potential role of OCRL in pressure sensing, we assessed the requirement for OCRL for the activation of transcription in HTM cells in response to pressure. The expression of OCRL was silenced in HTM cells with lentiviral shRNA. Ciliogenesis was then induced by serum-starvation, followed by pressure stimulation (Fig. S4 C and F). Cells with reduced OCRL exhibited a significant loss of pressure-dependent TGF-β, TNF-α, and GLI1 transcriptional activity, which was restored by reexpression of wild-type OCRL (Fig. S4F).
In a separate approach, pressure-dependent transcription of TGF-β was assessed in primary fibroblasts obtained from wild-type control (NHF558 cells) versus primary fibroblasts from two previously described Lowe syndrome patients with nonfunctional OCRL protein (6) (Fig. S4G). The levels of TGF-β transcript modestly increased following elevated pressure in mutant cells, whereas cells expressing wild-type OCRL showed significant increased elevation of TGF-β in response to pressure. This result further supports that pressure activates the transcription of TNF-α and TGF-β in a manner that requires cilia, which in turn are dependent on functional OCRL.
TRPV4 and OCRL Interact and Localize to the Cilia.
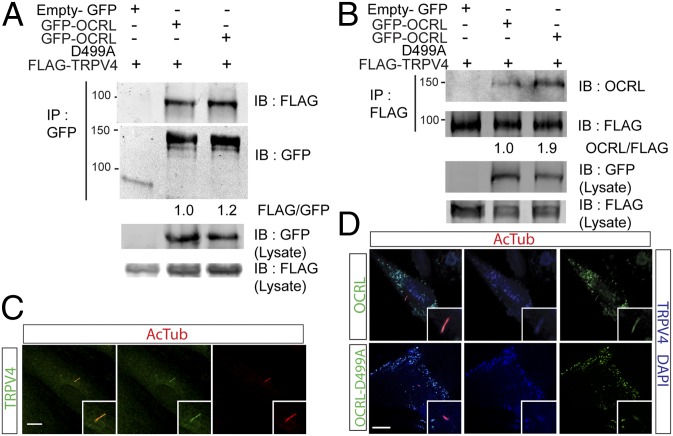
Because we found cilia of human TM cells to respond to pressure changes, and OCRL is known to transport membrane bound proteins, we hypothesized that it may interact with a mechanosensory channel within the cilia. TRPV4 is a mechanosensitive calcium-permeable cation channel that localizes to cilia, where it functions in osmotic regulation (32, 37–39). TRPV4 dysregulation has been implicated in diseases with fluid overload, such as hydrocephalus and heart failure (32, 40). Therefore, we investigated if OCRL and TRPV4 may play a role in TM cilia. First we showed that OCRL and TRPV4 interacted by coimmunoprecipitation assays. Coexpression of tagged GFP-OCRL and FLAG-TRPV4 followed by incubation with anti-GFP-Trap_A beads resulted in isolation of FLAG-tagged TRPV4 proteins (Fig. 4A and Fig. S5A). Conversely, reverse immunoprecipitation with anti-FLAG beads for TRPV4 was able to isolate OCRL-GFP (Fig. 4B). Second, we showed that in HTM cells TRPV4 is expressed and localized in the primary cilia, as indicated by staining for acetylated α-tubulin (Fig. 4C). To determine if the OCRL D499A mutant has an effect on the localization of TRPV4, the distribution of TRPV4 in the presence of wild-type OCRL or D499A mutant was determined. In HTM cells expressing both TRPV4 and OCRL, ciliary distribution of TRPV4 in OCRL D499A mutant cells was diminished (Fig. 4D). We assessed the function of the OCRL D499A mutant in vitro and found the enzymatic activity of PI(4,5)P2 5-phosphatase was decreased twofold compared with the wild-type enzyme (Fig. S5B). However, the stability of the protein was not significantly affected (Fig. S5C). Taking these data together, we show that OCRL interacts with TRPV4 calcium channel and both proteins localize within the primary cilia.
Fig. 4.
OCRL and TRPV4 interact and localize to the cilia. (A) OCRL coimmunoprecipitates TRPV4. HEK293 cells transfected with FLAG-TRPV4 and empty GFP vector, GFP-OCRL-WT, or GFP-OCRL-D499A were subjected to immunoprecipitation with GFP-Trap_A beads. Immunoblot for GFP and FLAG-TRPV4 was performed. Ratio of FLAG/GFP are shown as indicated. (B) TRPV4 coimmunoprecipitates OCRL. HEK293 cells transfected with FLAG-TRPV4 and empty GFP vector, GFP-OCRL-WT, or GFP-OCRL-D499A were subjected to immunoprecipitation with anti-FLAG beads. Immunoblot for GFP and FLAG-TRPV4 was performed. Ratio of OCRL/FLAG are shown as indicated. (C) TRPV4 localizes with HTM cilia. HTM cells serum-starved for 48 h were immunostained with anti-TRPV4 (green) and antiacetylated α-tubulin (red) antibodies. DAPI in blue. (Scale bar, 5 μm.) (D) OCRL-WT but not D499A mutant colocalize with TRPV4 in cilia. After serum-starvation for 48 h, HTM cells transfected with FLAG-TRPV4 and GFP-OCRL-WT or GFP-OCRL-D499A were immunostained with antiacetylated α-tubulin (red) and anti-FLAG antibodies. OCRL and OCRL-D499A in green. FLAG-TRPV4 and DAPI in blue. (Scale bar, 5 μm.)
OCRL and TRPV4 Regulate Calcium Flux in Human TM Cells and IOP in Mice.
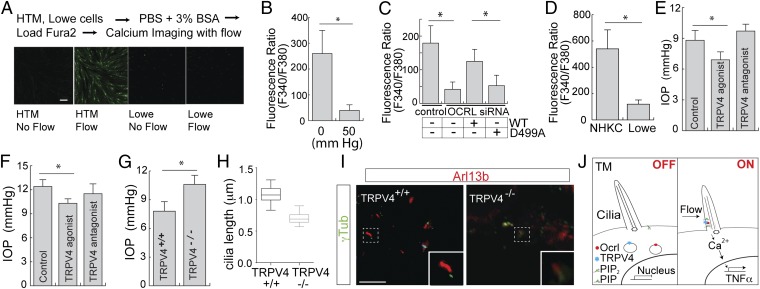
Based on the interaction and colocalization of OCRL and TRPV4, we assayed for the mechanosensory function of TRPV4 in HTM cells in response to flow. We showed that TRPV4 expression is similar in Lowe patients and normal HTM cells (Fig. S6 A and B). In Fura-2–loaded HTM or Lowe cells, Ca2+ flux was induced by laminar flow gradient (16 µL/s) over the cell surface (Fig. 5A) (32). Calcium flux was measured by F340/380 ratio in HTM cells poststimulation with the TRPV4 agonist GSK1016790A. Using this technique, we analyzed the stimulation of TRPV4 under various conditions. First we evaluated whether HTM cells treated with or without pressure responded differently to the TRPV4 agonist. In ciliated HTM cells treated with 50-mmHg hydrostatic pressure, we observed a significant decrease in TRPV4-induced calcium flux (Fig. 5B). Second, using OCRL siRNA or scrambled control siRNA-treated HTM cells, we showed a decrease in F340/F380 Fura-2 signal in the presence of TRPV4 agonist, which was rescued by wild-type OCRL but not by the D499A mutant (Fig. 5C). Furthermore, we isolated the Lowe syndrome patient’s keratinocytes and assayed for their response to TRPV4 treatment. The treatment with TRPV4 agonist failed to elicit calcium mobilization in Lowe patient keratinocytes compared with normal keratinocytes (Fig. 5D).
Fig. 5.
OCRL and TRPV4 regulate calcium flux in HTM cells and IOP in mice. (A) Calcium flow experiment with HTM and Lowe cells. HTM or Lowe cells were loaded with Fura-2 AM dye for 1 h, and 16 μL/s flow was applied to the cells. Representative images are shown. (Scale bar, 10 μm.) (B) Defective TRPV4-mediated calcium signaling in HTM cells under elevated pressure. HTM cells were serum-starved (48 h) to induce ciliogenesis, followed by treatment with 0 or 50 mmHg for 3 h, Fura-2 AM dye was loaded for 1 h, followed with TRPV4 agonist GSK1016790A (0.1 µM). Ratio of F340/380 (value × 1000) that indicates calcium mobilization is shown, with average from three independent experiments. Error bars represent SD, paired t test, *P < 0.001. (C) OCRL is required for TRPV4-mediated calcium signaling in HTM cells. HTM cells were treated with OCRL siRNA or scrambled control siRNA, with or without wild-type OCRL or OCRL-D499A rescue. TRPV4 agonist GSK1016790A (0.1 µM) treatment was performed and calcium mobilization by F340/380 (value × 1000) was measured and shown. n > 50 cells, Representative of three independent experiments, error bars represent SD. paired t test, *P < 0.001. (D) Lowe syndrome patient cells exhibit defective TRPV4-mediated calcium flux. NHKC or Lowe keratinocytes were serum-starved, loaded with Fura-2 AM and then treated with TRPV4 agonist; F340/380 ratio (value × 1000) was determined. Average of three independent experiments, error bars represent SD, paired t test, *P < 0.001. (E) TRPV4 agonist but not antagonist lowers IOP in a rat model. Eight-d-old wpk−/− rats were treated with sham, TRPV4 agonist (GSK 1016790A, 20 ng⋅mL⋅d), or antagonist (HC 067047, 50 ng⋅mg⋅d) for 8 d. At day 17, IOP measurements (mmHg) were performed using a Tonolab tonometer. Error bars represent SD. n = 4, unpaired t test, *P < 0.05. (F) TRPV4 agonist but not an antagonist lowers IOP in mouse. Nine-week-old C57BL/6 WT mice were treated with sham, TRPV4 agonist GSK 1016790A or antagonist HC 067047 for 4 d. IOP was measured using a Tonolab tonometer 24 h after treatment. Error bars represent SD. n = 20, three independent experiments, unpaired t test, *P < 0.01. (G) TRPV4−/− mice exhibited higher IOP than TRPV4+/+ mice. IOP measurements of 7-mo-old TRPV4+/+ (n = 4) and TRPV4−/− (n = 6) were determined. Error bars represent SD, paired t test, *P < 0.05. (H and I) Shortened cilia in TM cells of TRPV4−/−. Primary cilia in paraffin-embedded sections from both TRPV4+/+ and TRPV4−/− mouse eyes were stained with acetylated α-tubulin (n = 5 per group, total of 50 cilia per group were measured). Quantification of cilia length (H) and representative images (I) are shown. Error bars represent SD, paired t test, *P < 0.05. (Scale bar, 5 μm.) (J) Model for OCRL and TRPV4 function in the cilia in TM cells.
The functional consequences of activating TRPV4 on eye pressure were therefore assessed in the mouse models. The Wistar polycystic kidney (Wpk) rat is a well-established model for studying ciliopathy, as these animals develop hydrocephalus (41). Previous work showed Wpk rats responded to TRPV4-targeted treatment in hydrocephalus; we thus used these animals to test the effect of TRPV4 agonist treatment on eye pressure. In 17-d-old Wpk−/− rats, intraocular pressure was significantly reduced by systemic GSK1016790A treatment but not by treatments with a TRPV4 antagonist (HC 067047) or sham control (Fig. 5E). We confirmed this finding in wild-type C57BL/6 mice; in animals treated with systemic TRPV4 agonist, antagonist, or sham control, daily over 4 d, we showed a decrease in IOP from 12.4 to 10.3 mmHg (Fig. 5F). Finally, we assessed IOP in TRPV4+/+ vs. TRPV4−/− mice. We showed that IOP in TRPV4−/− mice to be elevated compared with control TRPV4+/+ animals (Fig. 5G). The primary cilia in the TM of these animals were evaluated by immunofluorescence staining for ciliary markers with Arl13b and γ-tubulin; the cilia were found to be indeed shorter in the TRPV4−/− mice (Fig. 5 H and I). Thus, functional OCRL is indicated to be necessary for TRPV4 distribution, which in turn is required for calcium signaling and cilia growth in response to mechanical stimuli.
Discussion
Mechanosensation of pressure underlies a number of important human diseases, including the development of hypertension and glaucoma (22). Similar to the kidney, the eye is an organ with sensitive homeostatic regulation of fluid production and outflow (23). Defective sensation of pressure may result in imbalance of aqueous humor, resulting in elevated intraocular pressure (28). Low eye pressure results in structural changes of the retina and poor vision, whereas elevated eye pressure may damage the optic nerve (42). Here a role of primary cilia in the mechanosensation of pressure in HTM is presented. The presence of cilia that shortened in response to pressure in TM cells was identified by multiple complementary approaches. Furthermore, proper cilia function was found to be essential for pressure sensation in these cells. These effects were found to require both OCRL and TRPV4 in a manner where OCRL appears to be required for proper localization and function of TRPV4 in the cilia. This in turn is necessary for calcium flux that regulates the transcriptional programs that coordinate cilia function with pressure sensing.
Once thought as a vestigial organelle, the primary cilium has increasingly become recognized as a critical regulator of mechanotransduction. In the kidney epithelium, ciliary proteins polycystins (PC1/2) are important for flow-dependent calcium flux (43). In the lining of the ventricles, cerebrospinal fluid is also regulated by cilia (18, 44). This finding is highlighted by the ciliopathy and hydrocephalus that present in Bardet–Biedl syndrome 1 (BBS1) and BBS3 mutant mice (18, 44). Our study shows that cilia in the TM cells of the eye are similarly required for the regulation of pressure, which when dysregulated is strongly implicated in the pathogenesis of glaucoma. We propose that the mechanosensing properties of primary cilia that we have identified in TM cells are likely also in the cells within the kidneys and the brain (Fig. 5J).
OCRL is an inositol polyphosphate 5-phosphatase that is implicated in Lowe syndrome and Dent disease (1, 45, 46). The recent discovery of its localization to cilia suggested its potential role in the pathophysiology of glaucoma in the TM of eyes (4, 6, 7). TRPV4 has been well recognized to be a mechanosensor in kidney epithelial cells, as well as in a number of other fluid-regulating cell types (32, 37, 38, 40). Here we show that OCRL interacts with TRPV4. Furthermore, we have identified a patient with Lowe syndrome that developed congenital glaucoma at birth, requiring filtration surgery to lower intraocular pressure. Although TRPV4 is present in the keratinocytes isolated from this patient, its inability to be stimulated by its agonist is strongly suggested by our work to be a result of an enzymatic-deficient OCRL. Based on our results, TRPV4 trafficking to the cilia is an essential step for the release of intracellular calcium, which may lead to subsequent enhanced Ca2+/calmodulin-dependent protein kinase II activity and up-regulate endothelial nitric oxide synthase (47). Nitric oxide has been shown as an effector pathway for lowering intraocular pressure (48); we propose that the primary cilia within TM cells may serve as an afferent pathway for signal transduction.
Interestingly, TRPV6, another TRP channel, was reported to be suppressed by OCRL in a Xenopus model (49), suggesting that there can be differential regulation of TRP channels by OCRL and the PI(4,5)P2 5-phosphatase activity. Indeed, depletion of PI(4,5)P2 inactivates TRPV6 channel-mediated Ca2+ activity. However, the role of phosphoinositides in TRP channel regulation is far from clear as TRPV1 is negatively regulated by PI(4,5)P2, whereas hydrolysis of PIP2 results in activation of TRPV1 (50). Thus, future studies are needed to distinguish the effects of OCRL on different TRP channels in the eye as well as in other tissues.
Recently, bbs-3, which is an ADP ribosylation factor-like small GTPase but not a component of the BBsome, was noted to play a role in blood pressure regulation (18). Inactivation of bbs-3 in mice resulted in elevated blood pressure as well as hydrocephalus. Furthermore, a sensory role for primary cilia was found when the PC1/2 complex stimulates the recruitment of STAT6 to promote STAT6 signaling (51). Because the loss of PC1/2 reduces the activation of STAT6 in response to flow, the primary cilium is likely a conserved mechanism for pressure mechanosensation across different organ systems, including the kidney, brain, and eyes. Consistently, we show that in response to elevated pressure, the primary cilia of TM cells are shortened in both in vitro culture and mammalian eyes. The identification of primary cilia in the TM, and the central role of OCRL and TRPV4 in their function, begins to define our understanding of aqueous regulation and therefore points to novel targets for glaucoma therapy.
Materials and Methods
TRPV4 agonist (GSK 1016790A) and antagonist (HC 067047) were obtained from Sigma and TOCRIS, respectively. Cycloheximide was obtained from Sigma (C7698). Antiacetylated α-tubulin, anti–β-actin, and anti–γ-tubulin monoclonal antibodies were purchased from Sigma. For more details, see SI Materials and Methods and Table S1.
Supplementary Material
Acknowledgments
We thank Dr. Wolfgang Liedtke (Duke University) and Dr. David Robertson [Vanderbilt University; National Institutes of Health (NIH)/NHL P01-056693] for the gift of transient receptor potential vanilloid 4 (TRPV4) knockout mice; Charlene Finney and Ratna Prasad for TRPV4-related mouse work; Akhilesh Kumar for assistance with TRPV4 imaging; Vincent Gattone II for assistance with wpk rats; and Drs. Jeffrey Travers and Michael Boulton for thoughtful comments. This work was funded by NIH/NEI022058, Knights Templar Eye Foundation, a Clinician-Scientist award (American Glaucoma Society), a Lowe Syndrome Association grant, and an unrestricted grant from Research to Prevent Blindness-Glick Eye Institute (all to Y.S.); NIH/NCI151765 (to C.D.W.); NIH/NHL115140 (to A.G.O.); NIH/NEI22639 and Research to Prevent Blindness Dolly Green Special Scholar Award (to C.I.); NIH/KL2TR001106 (to T.W.C.); and National Eye Institute Core Grant 5P30EY08126, Joseph Ellis Family and William Black Research funds, and Research to Prevent Blindness Unrestricted Departmental Grant-Vanderbilt (all to K.J.).
Footnotes
Conflict of interest statement: Y.S. is an inventor on a patent related to the work described, which is managed by Indiana University School of Medicine.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323292111/-/DCSupplemental.
References
- 1.Loi M. Lowe syndrome. Orphanet J Rare Dis. 2006;1:16. doi: 10.1186/1750-1172-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, Jefferson AB, Auethavekiat V, Majerus PW. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1995;92(11):4853–4856. doi: 10.1073/pnas.92.11.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ungewickell AJ, Majerus PW. Increased levels of plasma lysosomal enzymes in patients with Lowe syndrome. Proc Natl Acad Sci USA. 1999;96(23):13342–13344. doi: 10.1073/pnas.96.23.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rbaibi Y, et al. OCRL1 modulates cilia length in renal epithelial cells. Traffic. 2012;13(9):1295–1305. doi: 10.1111/j.1600-0854.2012.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo N, et al. Compensatory role of inositol 5-phosphatase INPP5B to OCRL in primary cilia formation in oculocerebrorenal syndrome of Lowe. PLoS ONE. 2013;8(6):e66727. doi: 10.1371/journal.pone.0066727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo N, et al. OCRL localizes to the primary cilium: A new role for cilia in Lowe syndrome. Hum Mol Genet. 2012;21(15):3333–3344. doi: 10.1093/hmg/dds163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coon BG, et al. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum Mol Genet. 2012;21(8):1835–1847. doi: 10.1093/hmg/ddr615. [DOI] [PubMed] [Google Scholar]
- 8.Yuan S, Sun Z. Expanding horizons: Ciliary proteins reach beyond cilia. Annu Rev Genet. 2013;47:353–376. doi: 10.1146/annurev-genet-111212-133243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Dynlacht BD. Assembling a primary cilium. Curr Opin Cell Biol. 2013;25(4):506–511. doi: 10.1016/j.ceb.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa H, Marshall WF. Ciliogenesis: Building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12(4):222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 11.Clapham DE. SnapShot: Mammalian TRP channels. Cell. 2007;129(1):220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Sarmah B, Wente SR. Zebrafish inositol polyphosphate kinases: New effectors of cilia and developmental signaling. Adv Enzyme Regul. 2010;50(1):309–323. doi: 10.1016/j.advenzreg.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond GR, et al. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337(6095):727–730. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Reeuwijk J, Arts HH, Roepman R. Scrutinizing ciliopathies by unraveling ciliary interaction networks. Hum Mol Genet. 2011;20(R2):R149–R157. doi: 10.1093/hmg/ddr354. [DOI] [PubMed] [Google Scholar]
- 15.Lee JE, Gleeson JG. A systems-biology approach to understanding the ciliopathy disorders. Genome Med. 2011;3(9):59. doi: 10.1186/gm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: Focus on CEP290, RPGR and their interacting proteins. Cilia. 2012;1(1):22. doi: 10.1186/2046-2530-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beales P, Jackson PK. Cilia–The prodigal organelle. Cilia. 2012;1(1):1. doi: 10.1186/2046-2530-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, et al. Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc Natl Acad Sci USA. 2011;108(51):20678–20683. doi: 10.1073/pnas.1113220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotak BN, Gleeson JG. Can’t get there from here: Cilia and hydrocephalus. Nat Med. 2012;18(12):1742–1743. doi: 10.1038/nm.3011. [DOI] [PubMed] [Google Scholar]
- 20.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 21.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: Novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev Drug Discov. 2012;11(7):541–559. doi: 10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman PL, Rasmussen CA. Advances in glaucoma treatment and management: Outflow drugs. Invest Ophthalmol Vis Sci. 2012;53(5):2495–2500. doi: 10.1167/iovs.12-9483m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickells RW, Semaan SJ, Schlamp CL. Involvement of the Bcl2 gene family in the signaling and control of retinal ganglion cell death. Prog Brain Res. 2008;173:423–435. doi: 10.1016/S0079-6123(08)01129-1. [DOI] [PubMed] [Google Scholar]
- 25.Hogan MJ, Alvarado JA, Weddell JR. Histology of the Human Eye. Philadelphia: W. B. Saunders; 1971. [Google Scholar]
- 26.Wickham MG, Worthen DM. Centrioles and cilia in the mesothelial cells of the pericanalicular region. Invest Ophthalmol. 1976;15(6):499–502. [PubMed] [Google Scholar]
- 27.Samuelson DA, Gelatt KN. Aqueous outflow in the beagle. I. Postnatal morphologic development of the iridocorneal angle: Pectinate ligament and uveal trabecular meshwork. Curr Eye Res. 1984;3(6):783–794. doi: 10.3109/02713688409000790. [DOI] [PubMed] [Google Scholar]
- 28.Alward WL, et al. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) N Engl J Med. 1998;338(15):1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- 29.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Malone AM, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci USA. 2007;104(33):13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshiba S, et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science. 2012;338(6104):226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Köttgen M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182(3):437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roh M, et al. Etanercept, a widely used inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal ganglion cell loss in a rat model of glaucoma. PLoS ONE. 2012;7(7):e40065. doi: 10.1371/journal.pone.0040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiggs JL, et al. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012;8(4):e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang MH, Oh DJ, Kang JH, Rhee DJ. Regulation of SPARC by transforming growth factor β2 in human trabecular meshwork. Invest Ophthalmol Vis Sci. 2013;54(4):2523–2532. doi: 10.1167/iovs.12-11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, Khanna H. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: Implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet. 2010;19(18):3591–3598. doi: 10.1093/hmg/ddq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye L, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151(1):96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonkusare SK, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336(6081):597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorneloe KS, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4(159):ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- 41.Smith UM, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38(2):191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 42.Springer AD, Hendrickson AE. Development of the primate area of high acuity, 3: Temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci. 2005;22(2):171–185. doi: 10.1017/S095252380522206X. [DOI] [PubMed] [Google Scholar]
- 43.Nauli SM, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 44.Carter CS, et al. Abnormal development of NG2+PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med. 2012;18(12):1797–1804. doi: 10.1038/nm.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta ZB, Pietka G, Lowe M. The cellular and physiological functions of the Lowe syndrome protein OCRL1. Traffic. 2014;15(5):471–487. doi: 10.1111/tra.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pirruccello M, De Camilli P. Inositol 5-phosphatases: Insights from the Lowe syndrome protein OCRL. Trends Biochem Sci. 2012;37(4):134–143. doi: 10.1016/j.tibs.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YS, Lu MJ, Huang HS, Ma MC. Mechanosensitive transient receptor potential vanilloid type 1 channels contribute to vascular remodeling of rat fistula veins. J Vasc Surg. 2010;52(5):1310–1320. doi: 10.1016/j.jvs.2010.05.095. [DOI] [PubMed] [Google Scholar]
- 48.Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52(13):9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu G, et al. Suppression of intestinal calcium entry channel TRPV6 by OCRL, a lipid phosphatase associated with Lowe syndrome and Dent disease. Am J Physiol Cell Physiol. 2012;302(10):C1479–C1491. doi: 10.1152/ajpcell.00277.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77(4):667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Low SH, et al. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10(1):57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.