INFECTIONS WITH MYCOBACTERIUM AVIUM SUBSP. PARATUBERCULOSIS
Infection of ruminant species with the facultative intracellular bacterium Mycobacterium avium subsp. paratuberculosis causes a fatal granulomatous enteritis known as Johne's disease or paratuberculosis. In cattle, Johne's disease is responsible for significant losses to the U.S. dairy industry (an estimated $220 million per year) (65). M. avium subsp. paratuberculosis can infect monogastric species (5, 13), and although this is still highly controversial, M. avium subsp. paratuberculosis has been implicated as a causal or exacerbating agent in human Crohn's disease (12, 46).
M. avium subsp. paratuberculosis preferentially resides in phagosomes or early endosomes of host macrophages, predominately those associated with ileal Peyer's patches (6, 19, 43). Entry of M. avium subsp. paratuberculosis is mediated by intestinal M cells (43) and is likely dependent upon expression of the bacterial major membrane protein, on binding of fibronectin, or both (3, 51, 52). In severe cases, M. avium subsp. paratuberculosis may be found in macrophages distributed throughout a number of tissues in infected animals (29, 33), although even in these cases, the majority of organisms are found associated with macrophages infiltrating intestinal tissues and adjacent draining lymph nodes (reviewed in reference 30).
Infections with M. avium subsp. paratuberculosis usually persist in a subclinical state for several years (32, 40, 41, 56). Intestinal lesions develop during the subclinical period of infection and are often of a diffuse granulomatous nature, largely restricted to the ileum and particularly to the ileocecal valve region of the small intestine (66). Pathology associated with M. avium subsp. paratuberculosis infection is largely due to chronic inflammation of infected tissues (11, 49, 57). Lymph nodes draining sites of M. avium subsp. paratuberculosis infection often exhibit hyperplasia with enhanced numbers of T cells, B cells, and infiltrating macrophages (11, 47). In addition, large numbers of acid-fast bacilli may be found in lymph nodes of infected cattle and sheep, predominantly associated with infiltrating macrophages (16, 19).
OVERVIEW OF IMMUNE RESPONSE TO M. AVIUM SUBSP. PARATUBERCULOSIS
Immune reactions to M. avium subsp. paratuberculosis have recently been reviewed (22, 48, 55) and thus will be presented here only in brief form. Following initial exposure to M. avium subsp. paratuberculosis, an appropriate T-cell response, characterized by release of proinflammatory cytokines such as gamma interferon (IFN-γ), interleukin-1α (IL-1α), and IL-6, as well as production of IL-2, develops (reviewed in reference 22). Because M. avium subsp. paratuberculosis is largely restricted to growth within macrophages, proinflammatory and cytotoxic responses are essential for control of infection. However, during the late subclinical phase of M. avium subsp. paratuberculosis infection, the proinflammatory type 1-like response is often muted or lost. Thus, in late-stage disease a type 2-like response, characterized by production of immunoglobulin G1 (IgG1) antibodies, predominates (reviewed in references 22 and 55). This shift in the predominant immune response is often associated with progression to clinical disease. A similar phenomenon has been linked to clinical disease associated with mycobacterial infections in other species, including mice and humans (2, 25). The mechanisms responsible for loss or reduction of type 1-like responses to mycobacteria, including M. avium subsp. paratuberculosis, are unknown but may be related to undefined host genetic factors, to constant exposure of immune cells to antigen released from infected macrophages, or to development of antigen-specific or general regulatory cell populations.
Recent evidence from transgenic knockout mice confirms that cytokine signaling plays a major role in the host defense against mycobacteria. Mice deficient in the immunoregulatory cytokine IL-10 are able to control mycobacterial infections better than their wild-type counterparts (45), presumably due to the immune-modulating effects of IL-10. In contrast, mice deficient in IL-4 (a classic type 2 cytokine) exhibit enhanced pathology associated with mycobacterial infections (59). Likewise, mice deficient in tumor necrosis factor alpha (TNF-α) production display enhanced dissemination of various mycobacteria following infection and exhibit diffuse granulomatous lesions at sites of active infection (31). TNF-α activates naive macrophages and is essential for formation of granulomas around sites of mycobacterial infection. Granuloma formation isolates the focus of infection, contains bacteria, and prevents dissemination to new sites (50). It is also well established that IFN-γ is required to fight infection with mycobacteria in a number of species (17, 26, 68), and thus, IFN-γ-regulating cytokines, such as IL-12 and IL-18, would seem to be important in limiting infection. Indeed, IL-12-deficient mice display enhanced susceptibility to mycobacterium-induced disease, although the p40 subunit of IL-12 appears to be more important in resistance than the smaller p35 subunit (18). The importance of the IL-12 p40 subunit may be related to formation of IL-23, a heterodimeric cytokine comprised of the IL-12 p40 subunit and another 19-kDa polypeptide (p19) (18). IL-23, like IL-12, stimulates production of IFN-γ, although IL-23 activity may be restricted to memory T cells (37). Similar to the case for IL-12 deficiency, IL-18-deficient mice are less able to restrict infections with various mycobacteria and display reduced levels of IFN-γ production at sites of infection (58).
Although the aforementioned studies have focused predominantly on mycobacteria other than M. avium subsp. paratuberculosis (primarily M. tuberculosis and M. bovis BCG), IFN-γ is also one of the major cytokine genes activated in response to M. avium subsp. paratuberculosis infection in cattle and other ruminants (10, 54). However, the role of other cytokines in M. avium subsp. paratuberculosis infections is less well delineated. Recent data, acquired through application of functional genomic tools and quantitative real-time reverse transcriptase PCR to studies of the immune response during natural and experimental infection of cattle with M. avium subsp. paratuberculosis, have begun to shed considerable light on the host response to this organism (1, 20, 21, 23, 24, 38, 63, 64). In this paper, a model for interactions between M. avium subsp. paratuberculosis and the bovine immune system is presented. The proposed model has been developed to be consistent with most lines of experimental evidence gathered to date and suggests several readily testable hypotheses that may help to focus future work. As with all models, it is expected that some proposed mechanisms will be substantiated by additional experimentation, some will be greatly expanded, and still other components of the model will be refuted. Early interactions of M. avium subsp. paratuberculosis with the host have recently been reviewed (22, 55), and thus, I begin describing the proposed model at the stage after initial infection is established and M. avium subsp. paratuberculosis has successfully colonized intestinal macrophages.
MODEL FOR INTERACTIONS BETWEEN M. AVIUM SUBSP. PARATUBERCULOSIS AND THE HOST IMMUNE SYSTEM
Development of lesions typical of Johne's disease.
Infection with M. avium subsp. paratuberculosis leads, in many cases, to an early proinflammatory and cytotoxic response, which is appropriate for control of an intracellular pathogen (reviewed in reference 22). However, in later stages of infection, this response is replaced with a predominant humoral IgG1 response and, in many cases, an antigen-specific T-cell anergy is observed in peripheral immune cells (15, 34, 35). Recent studies with experimentally infected calves have challenged this dogma, demonstrating that humoral immune responses may occur very early after inoculation (62). However, the dosage, timing of exposure, and routes of entry in these studies were likely different than those in natural exposure, complicating interpretation. Other studies, using experimental infection through oral dosing and natural infection, suggest that the humoral response does not dominate until later in the infection cycle (42).
As infection with M. avium subsp. paratuberculosis progresses, macrophages are recruited to sites of active infection (9, 47). Genes encoding adhesion molecules and several factors likely responsible for macrophage recruitment are upregulated at sites of infection, including β1 integrin (CD29), NCAM (CD56), and monocyte chemotactic protein-2 (1). If properly activated (through interaction with IFN-γ), newly recruited macrophages may be successful in destroying M. avium subsp. paratuberculosis following phagocytosis (68). TNF-α signaling does not appear to be involved in macrophage activation at sites of M. avium subsp. paratuberculosis infection, and, in fact, expression of this cytokine may be specifically limited by M. avium subsp. paratuberculosis infection through as-yet-unknown mechanisms (1, 24, 38).
Activated intestinal macrophages produce increased amounts of IL-1α (1). In some cases, production of IL-1α may reach extremely high levels (>10-fold greater than normal), opening the possibility that some common outward signs of clinical Johne's disease are due to IL-1α toxicity (1). In the proposed model (Fig. 1), enhanced production of IL-1α at sites of infection is coincident with or leads to upregulation of TNF receptor-associated factor 1 (TRAF1) in infiltrating macrophages (1). TRAF1 is a strongly antiapoptotic protein that associates with TRAF2, limiting CD40-, TNF-α-, IL-1-, and Fas (CD95)-mediated signaling and programmed cell death (8, 27, 61). CD95 expression in monocytes and macrophages appears to be a proinflammatory event, and thus upregulation of TRAF1 may be one mechanism used to control this response. Enhanced expression of TRAF1 would also tend to limit signaling through CD40-CD40 ligand (CD154) (27). It is proposed that the combination of macrophage recruitment and prolonged macrophage survival is responsible for increases in numbers of macrophages surrounding sites of M. avium subsp. paratuberculosis infection. When combined with a failure to express sufficient TNF-α for efficient granuloma formation, macrophage recruitment and reduced apoptosis of resident macrophages could lead to the diffuse granulomatous appearance of many M. avium subsp. paratuberculosis infection sites. This model also opens the possibility that limiting macrophage apoptosis is one mechanism ensuring survival of intracellular M. avium subsp. paratuberculosis.
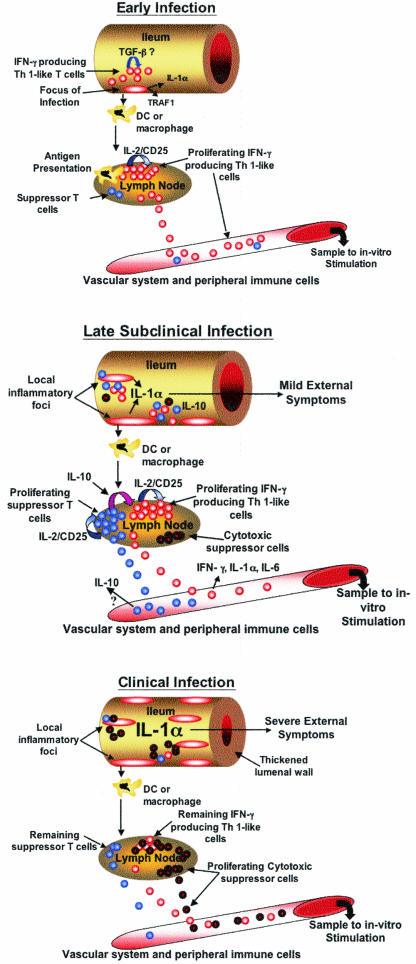
FIG. 1.
Proposed in vivo immune responses during progressive infection with M. avium subsp. paratuberculosis. (A) Early lesions associated with M. avium subsp. paratuberculosis infection likely develop in ileal Peyer's patches and consist of numerous infected macrophages. Infected macrophages express enhanced amounts of adhesion molecules, IL-1α,and TRAF1, leading to enhanced macrophage survival. Dendritic cells (DC) or macrophages successful in degrading M. avium subsp. paratuberculosis migrate to local lymph nodes, presenting antigen and stimulating reactive T cells to produce IFN-γ and proliferate via IL-2/CD25 signaling (red circles). IFN-γ-producing proinflamma-tory T cells released to the vascular system circulate, eventually migrating to sites of M. avium subsp. paratuberculosis infection, perhaps attracted by enhanced levels of IL-8 produced by infected macrophages. Both antigen-primed CD4+ T cells and infected macrophages produce IFN-γ, possibly activating newly recruited macrophages and helping to control or restrict the infection. A suppressor cell population capable of producing IL-10 is likely already present in lymph nodes, albeit in very small numbers (blue circles). At sites of infection, transforming growth factor β (TGF-β) may be expressed in a attempt to suppress or dampen the early proinflammatory response. (B) In late subclinical infection, continued expansion of the infection has occurred through migration of infected macrophages throughout the ileum. Failure to express TNF-α and to contain the infection in granulomas is one factor likely contributing to this disseminated infection. In late subclinical infection, the numerous macrophages within lesions produce significant amounts of IL-1α, perhaps causing mild IL-1α toxicity. Continued migration of macrophages and/or dendritic cells to local lymph nodes, combined with a prolonged proinflammatory response, has resulted in enhanced proliferation of an IL-10-producing suppressor cell population (blue circles). The balance of proinflammatory (red circles) and suppressor cells leads to loss of IFN-γ and IL-6 production at sites of infection and in local lymph nodes. Reduced production of IFN-γ may help propagate and expand the infection, as newly recruited macrophages would not be sufficiently activated to kill M. avium subsp. paratuberculosis. A population of cytotoxic suppressor cells has begun to proliferate within local lymph nodes (black-outlined red circles), although not in sufficient numbers to control the activated proinflammatory cells. (C) In clinically infected cattle, the ileum is heavily infected and is typically thickened, reducing transport of nutrients. It is proposed that large numbers of infected macrophages produce sufficient IL-1α to cause outward signs of toxicity. In local lymph nodes, continued expansion of a cytotoxic suppressor cell population (black-outlined red circles) has removed many of the M. avium subsp. paratuberculosis-reactive proinflammatory T cells (red circles) present during late subclinical infection. Loss of proinflammatory cells has reduced the need for IL-10-producing suppressor cells (blue circles). Indeed, if these cells are T regulatory cells, their continued proliferation may depend upon IL-2 released from the proinflammatory cells. Complete or nearly complete loss of an antigen-specific response allows the infection with M. avium subsp. paratuberculosis to progress essentially unchecked. Although not represented in this figure, antigen-reactive B-cell populations have continued to proliferate, and the remaining immune response is largely production of IgG1.
Expression of IFN-γ and IL-6 transcripts is also enhanced at sites of active M. avium subsp. paratuberculosis infection in intestinal tissues (24), but expression of a proinflammatory phenotype may be partially tempered by enhanced production of transforming growth factor β in infected tissues (Fig. 1) (24, 38). IL-8 gene expression is enhanced in intestinal tissues from M. avium subsp. paratuberculosis-infected cattle relative to controls (24). Enhanced production of IL-8 may be a consequence of IL-1α overexpression and stimulation of macrophages (67). Curiously, although IL-8 is chemotactic for neutrophils and lymphocytes, lesions associated with M. avium subsp. paratuberculosis infection typically contain fewer neutrophils and lymphocytes than tissue from uninfected controls (34, 38, 44).
It is proposed that tissue damage at sites of M. avium subsp. paratuberculosis infection ensues due to the chronic inflammatory immune response, overproduction of IL-1α, and release of reactive oxygen species from activated macrophages and possibly from neutrophils (Fig. 1). At present, a role for eosinophils, recruited to sites of infection by enhanced expression of IL-5 (24), as a contributing factor in tissue damage early in infection and the continued proinflammatory reaction cannot be discounted, although histological evidence for eosinophils within M. avium subsp. paratuberculosis-induced lesions is lacking.
Development of a proinflammatory T-cell population and humoral response in lymph nodes draining sites of M. avium subsp. paratuberculosis infection.
As with any infection, macrophages and/or dendritic cells that have phagocytosed and processed M. avium subsp. paratuberculosis migrate to local lymph nodes and present antigen to resident T and B cells. A recent study however, indicates that macrophages persistently infected with M. avium subsp. paratuberculosis are deficient in stimulation of antigen-reactive T cells (69). Despite lower expression of major histocompatibility complex class II in some M. avium subsp. paratuberculosis-infected macrophages (63), lack of T-cell stimulation was not found to be due to differences in expression of major histocompatibility complex class II, ICAM-1, CD40, B7.1, or B7.2 (69). These results suggest that T-cell stimulation may be restricted to macrophages that successfully destroy M. avium subsp. paratuberculosis or to dendritic cells.
Pathogen antigens and activated helper T cells would stimulate B cells to differentiate and begin production of IgM and IgG1 antibodies against M. avium subsp. paratuberculosis antigens. The precise timing of antibody production likely depends upon the route of entry and dose, with higher initial doses of M. avium subsp. paratuberculosis leading to more rapid production of antibody (62). The potential role of tonsillar tissues in initial immune responses to M. avium subsp. paratuberculosis is an interesting recent proposal that may dramatically affect the timing of B-cell differentiation and antibody production (62).
Similar to the case for sites of infection, IL-8 mRNA expression is enhanced in local lymph nodes of infected cows relative to uninfected controls (24). Likely sources of IL-8 mRNA expression in these tissues are activated macrophages and/or dendritic cells, although a role of other cell types in production of IL-8 cannot be ruled out. Based on cytokine gene expression profiles (24), it is reasonable to postulate that an inflammatory or type 1-like response develops within lymph nodes draining sites of M. avium subsp. paratuberculosis infection following presentation of antigen to naive T cells (Fig. 1). This response, however does not appear to involve TNF-α (24, 38). Expansion of antigen-reactive T cells (proinflammatory cells) is likely to occur within lymph nodes, stimulated by enhanced IL-2 expression (24) and IL-2/CD25 signaling (Fig. 1).
Proinflammatory cells released into circulation during this period of infection would lead to delayed-type hypersensitivity responses, as well as positive responses in the in vitro IFN-γ stimulation test (Fig. 2). Results from gene expression profiling and real-time reverse transcriptase PCR suggest that the population of circulating proinflammatory T cells produces large amounts of IFN-γ mRNA, as well as enhanced levels of IL-6, IL-1α, and IL-8 mRNAs, without further antigen stimulation (21, 24). These data also support a previous report that peripheral blood mononuclear cells (PBMCs) from Johne's disease-positive cows expressed an IL-1-like comitogenic activity (36). In cases where M. avium subsp. paratuberculosis antigen stimulation of peripheral leukocytes has been carried out for extended periods in vitro, the predominant proliferating cell populations are CD4+ T cells and cytotoxic γδ T-cell receptor-positive (TCR+) T cells (15). It was postulated that the antigen-reactive CD4+ T cells were effector cells, while the γδ TCR+ T cells may serve a cytotoxic immunoregulatory role that could be dampened by the presence of a CD8+ T-cell population (14, 15). This interpretation is consistent with measures of relative amounts of IFN-γ produced by various T-cell subsets in response to M. avium subsp. paratuberculosis stimulation (4) and with the proposed eventual development of both suppressor and cytotoxic immunoregulatory T-cell populations in lymph nodes draining sites of M. avium subsp. paratuberculosis infection (Fig. 1).
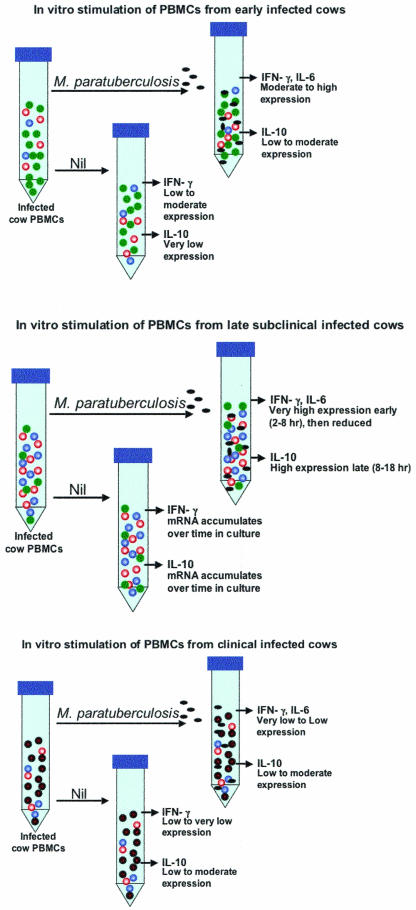
FIG. 2.
Proposed in vitro responses of PBMCs from progressively infected cows to stimulation with M. avium subsp. paratuberculosis. (A) Removal of peripheral immune cells from early-stage infected cows and stimulation in vitro with M. avium subsp. paratuberculosis results in a minor population of antigen-reactive proinflammatory T cells (red circles) producing significant amounts of IFN-γ. Expression of IL-6 and IL-1α is also upregulated in these proinflammatory cells. A small number of suppressor T cells (blue circles) may also be present in PBMCs from cattle in early stages of infection, but in numbers insufficient to control the proinflammatory response. Many peripheral immune cells are present in PBMC preparations that are not reactive to M. avium subsp. paratuberculosis antigens (green circles). Thus, the predominant response to in vitro stimulation of PBMCs from cattle at this stage of infection is production of IFN-γ, IL-6, and IL-1α.(B) PBMCs during the late subclinical stage of infection contain high numbers of M. avium subsp. paratuberculosis-reactive proinflammatory cells (red circles) that are already activated and produce significant amounts of IFN-γ and IL-6, even without further antigen stimulation in vitro (Nil). In vitro, production of IFN-γ, IL-6, and perhaps IL-1α is rapidly enhanced by introduction of M. avium subsp. paratuberculosis antigens, although this response is eventually quelled by IL-10 produced from large numbers of activated suppressor cells (blue circles). Overall, the percentage of non-M. avium subsp. paratuberculosis-reactive cells (green circles) has been reduced, although the balance of CD4+, CD8+, and γδ TCR+ T cells has not changed appreciably. (C) Due to the presence of large numbers of cytotoxic suppressor cells and reduced numbers of proinflammatory cells, PBMCs extracted during clinical stages of infection produce less IFN-γ and IL-6 in response to M. avium subsp. paratuberculosis antigens than cells from late subclinically infected cattle. Loss of IL-10-producing suppressor cells, which are present in large numbers during subclinical infection, also leads to lower production of this cytokine in PBMCs from clinically infected cattle. Prolonged in vitro stimulation (>16 h) of PBMCs from clinically infected cattle with M. avium subsp. paratuberculosis antigens results in apoptosis of many proinflammatory and cytotoxic suppressor cells. PBMCs from cattle in clinical stages of Johne's disease thus produce little IFN-γ, IL-6, and IL-1α in response to M. avium subsp. paratuberculosis antigens, although continued proliferation of the cytotoxic cell population has caused a profound change in the pattern of gene expression observed in PBMCs from these animals relative to those from uninfected cattle or cattle in earlier stages of infection. Although not shown in this figure, the predominant remaining response to M. avium subsp. paratuberculosis in clinically infected cattle is production of IgG1 from antigen-stimulated B cells. The proposed cytotoxic cell population may also kill non-M. avium subsp. paratuberculosis-reactive T cells in vitro (and perhaps in vivo), thus leading to an observed general immune cell anergy in PBMCs from clinically infected cattle.
Development of a suppressor T-cell population in lymph nodes draining sites of M. avium subsp. paratuberculosis infection.
It is proposed that, in an effort to stem tissue damage due to local inflammatory responses, a population of noncytotoxic suppressor T cells expressing IL-10, perhaps in an antigen-, cell-, or cytokine-restricted manner, eventually develops within lymph nodes draining sites of M. avium subsp. paratuberculosis infection (24) (Fig. 1). Development of such a cell population would be similar to those observed in antigen-induced T-cell tolerance (53) and may require the presence of specific CD5+ B cells (7). As with the proinflammatory cell population, expansion of suppressor T cells likely occurs through IL-2/CD25 autocrine or paracrine signaling but initially lags behind expansion of the proinflammatory T-cell population. In this model, as long as the proinflammatory/suppressor cell population ratio is skewed in favor of the proinflammatory population, infected cattle will show positive responses to the in vitro IFN-γ assay (Fig. 1 and 2).
Suppressor T cells may be of the γδ T-cell subtype and therefore may also be stimulated to proliferate by IL-15. However, it is more likely that the initial noncytotoxic suppressor cells are type 2-like CD4+ cells, producing IL-10, IL-4, and IL-5, or T regulatory cells, producing IL-10 and little, if any, IL-2, IL-4, and IL-5 (7). In either case, it is proposed that an unsteady equilibrium eventually is obtained, with suppression of the local proinflammatory response (Fig. 1) and perhaps accentuation of a B-cell response. This hypothesis is consistent with relatively low levels (compared to what is produced in PBMCs) of IFN-γ mRNA found within lesions and lymph nodes of infected cattle (24). Furthermore, low levels of IFN-γ at these sites is also consistent with continued production of IgG1 by antigen-stimulated B cells, because IFN-γ production is required for efficient class switching to IgG2. While helping to curb local inflammatory reactions, a reduction or loss of IFN-γ production may cause newly recruited macrophages to be susceptible to persistent infection with M. avium subsp. paratuberculosis (particularly without a significant TNF-α response), expanding foci of infection (Fig. 1).
Although it is proposed that IL-10 is a major early regulator of the proinflammatory response to M. avium subsp. paratuberculosis in subclinically infected cows (especially at sites of infection and adjacent lymph nodes), it is unlikely that IL-10 is the only mechanism used to control tissue injury. This is particularly evident in clinically infected cattle, where enhanced production of IL-10 mRNA in PBMCs exposed to M. avium subsp. paratuberculosis is not detected as it is in PBMCs from subclinically infected cows (24). One intriguing hypothesis is that a cell type capable of inducing apoptosis in proinflammatory cells (cytotoxic immunoregulatory cells) also develops within lymph nodes of infected cattle (Fig. 1). This suggestion is consistent with previous experimental evidence (14, 15) and with the proapoptotic gene expression profile observed in mixed PBMCs from Johne's disease-positive cows following overnight in vitro exposure to M. avium subsp. paratuberculosis (Fig. 1 and 2) (20, 23). Development of a cytotoxic regulatory cell would also tend to destroy proinflammatory cells in vivo, leading over time to antigen-specific anergy, as previously described (15, 35), and to a predominantly regulatory T-cell phenotype in clinically infected cattle (Fig. 1). Although it is possible that the cytotoxic regulatory cell population is CD8+, based on data from studies with humans (7), the TCR of these cells could be of either the αβ or the γδ type. Existing experimental evidence, although slight, suggests that in M. avium subsp. paratuberculosis-infected cattle, these cells are likely γδ TCR+ and CD8+ (14, 15).
In subclinical infections, it is proposed that B cells, as well as both proinflammatory and suppressor T cells, continue to proliferate in lymph nodes draining sites of M. avium subsp. paratuberculosis infection. Proliferation is likely fueled through continued presentation of antigen by incoming macrophages and/or dendritic cells and could explain the commonly observed hyperplasia of lymph nodes draining sites of active M. avium subsp. paratuberculosis infection (60). Interestingly, a role for IL-4 in M. avium subsp. paratuberculosis infections has not been established, as transcripts encoding this cytokine do not appear to be enhanced in lymph nodes of infected cattle and are not upregulated in PBMCs from infected cattle in response to M. avium subsp. paratuberculosis stimulation in vitro (24). The role of B cells in antigen presentation in lymph nodes of M. avium subsp. paratuberculosis-infected cattle has not been investigated. Given that B cells can be a significant source for antigen presentation, this would seem to be an area worthy of investigation. Furthermore, it is possible that presentation of soluble M. avium subsp. paratuberculosis-reactive TCR fragments by CD5+ or other B cells may play a role in development of suppressor T cells (cytotoxic and noncytotoxic).
Effect of foci of infection and draining lymph nodes on peripheral immune responses.
Cellular interactions within lesions and particularly within lymph nodes draining sites of M. avium subsp. paratuberculosis infection are likely to have a profound effect on peripheral immune responses, both in vivo and in vitro. Both types of T cells discussed above (proinflammatory and suppressor) would undoubtedly be able to enter the general circulation. It is proposed that, once released into circulation, activated proinflammatory cells are released from IL-10-mediated suppression and produce IFN-γ, IL-1α, and IL-6 (Fig. 2). Proinflammatory cells in peripheral circulation would then be responsible for observed delayed-type hypersensitivity activity (39) and may contribute to the common clinical signs of Johne's disease-positive cows (particularly by production of IL-1α).
Removing peripheral cells at this point and placing them in culture without antigen stimulation allows production of significant amounts of IFN-γ mRNA, as well as enhanced levels of IL-1α and IL-6 mRNAs (21, 24) (Fig. 2). Enhanced production of IFN-γ following in vitro stimulation of PBMCs from infected cows with M. avium subsp. paratuberculosis represents somewhat of a quandary, in view of the fact that IL-12 p35 appears to be downregulated in these same cells (24). It should also be noted that even in isolated macrophages, M. avium subsp. paratuberculosis only transiently enhances expression of IL-12 p35, despite continued presence of the pathogen in phagosomes (64). The answer to this quandary may come from the fact that, despite repressed expression of IL-12 p35, expression of mRNA encoding the IL-12 p40 subunit is enhanced in M. avium subsp. paratuberculosis-stimulated PBMCs (P. M. Coussens, N. Verman, A. McNulty, and C. Colvin, unpublished observations) and in M. avium subsp. paratuberculosis-infected macrophages (69). In association with p19, IL-12 p40 produces IL-23, a potent stimulator of IFN-γ expression in memory T cells (37). This hypothesis is entirely consistent with the differential regulation of genes encoding the IL-12 p35 and p40 subunits (28).
It is proposed that the noncytotoxic type 2-like suppressor cell population or T regulatory cell requires either contact with antigen, close contact with proinflammatory cells, or contact with products of these cells to produce significant amounts of IL-10. This hypothesis suggests that in circulation, suppressor cells may be relatively benign and do little to limit IFN-γ, IL-1, and IL-6 production by proinflammatory cells. However, if M. avium subsp. paratuberculosis antigens are added to total PBMCs in vitro (where close cell contact is much more likely than in vivo), proinflammatory T cells continue to produce IFN-γ, IL-1α, and IL-6 mRNAs until the suppressor population has been stimulated and produces enough IL-10 to quiet the proinflammatory response (Fig. 2). This hypothesis is supported by data demonstrating that PBMCs from Johne's disease-positive cows produce significant amounts of IFN-α mRNA in vitro without further antigen stimulation. These data also suggest that a significant population of activated T cells is circulating in vivo (21, 24). Furthermore, this hypothesis is consistent with data demonstrating that in vitro stimulation with M. avium subsp. paratuberculosis causes an increase in IL-10 mRNA production in PBMCs from subclinically infected cows (24) and that infection of macrophages results in significant increases in production of IL-10 mRNA (64). Finally, the temporal pattern of IFN-γ and IL-10 production in M. avium subsp. paratuberculosis-stimulated PBMCs from infected cows, where peak production of IL-10 mRNA (18 h of stimulation) lags behind peak production of IFN-γ mRNA (2 to 8 h of stimulation), is consistent with the proposed model (P. M. Coussens, S. K. Chiang, and B. C. Tooker, unpublished observations).
The presence of a potential cytotoxic regulatory cell type (possibly a type 2 CD8+ T cell or a γδ TCR+ T cell) in M. avium subsp. paratuberculosis-infected cows has been previously suggested (14, 15). If this is true, it is likely that this population develops or expands after proinflammatory and suppressor cell populations (Fig. 1). Postulation of such a cytotoxic cell population, whatever the subtype, is entirely consistent with the proposed model (Fig. 1 and 2). In fact, such a proposal offers one explanation for the general reduction in PBMC gene expression, antigen and perhaps general immune cell anergy, and lack of IL-10 response observed in M. avium subsp. paratuberculosis-stimulated PBMCs from clinically infected cows (24). As infections with M. avium subsp. paratuberculosis progress, a cytotoxic immunoregulatory cell would limit immunopathology associated with infection in a manner reminiscent of tolerance. By clearing antigen-reactive proinflammatory cells, such a mechanism would reduce the need for IL-10-producing suppressor T cells or limit the activation of these cells both in vivo and in vitro (Fig. 1 and 2). Thus, with expansion of a cytotoxic regulatory cell population, the major response of T cells to M. avium subsp. paratuberculosis stimulation in vitro would be apoptosis, a hypothesis consistent with experimental data (20, 23). In late-stage infection, following development of the proposed suppressor and cytotoxic immunoregulatory cells, the major observed immune response to M. avium subsp. paratuberculosis would be production of IgG1 by antigen-stimulated B cells.
CONCLUSION
In summary, a model is proposed by consolidating data from numerous laboratories, focusing on cytokine gene and protein expression and recent gene expression profiling work, to help explain interactions between M. avium subsp. paratuberculosis and the host immune system. The model begins after colonization of intestinal macrophages has been accomplished and infection has progressed to the early subclinical stage. Several key elements of the proposed model are early development of a proinflammatory T-cell population within lymph nodes draining sties of infection, followed by expansion of a noncytotoxic suppressor cell population capable of producing IL-10. At this time it is impossible to assign specific cell types to these populations, although previous data would suggest that the proinflammatory cell population is likely to be CD4+ (4) and that the noncytotoxic suppressor cell may be of the γδ TCR+ subtype, although experience with M. avium subsp. paratuberculosis and other pathogens would suggest that a CD4+ T cell is the more likely candidate (7, 60). In vivo, development of a suppressor T-cell population, although helpful in limiting tissue damage, severely restricts local proinflammatory and likely cytotoxic immune activity, thus allowing expansion of M. avium subsp. paratuberculosis infection. It is also proposed that IFN-γ-, IL-1α-, and IL-6-producing proinflammatory cells do not require antigen for cytokine expression, although the IL-10-producing suppressor population may require close contact with either antigen, proinflammatory cells, or products produced by these cells for activation. The proposed model also suggests that, depending upon the balance between proinflammatory and suppressor cell populations, PBMC responses to M. avium subsp. paratuberculosis antigens in vitro will be quite variable. In very-late-stage infections, it is proposed that a cytotoxic immunoregulatory cell develops and is in large part responsible for the T-cell anergy often observed in PBMCs of clinically infected cows.
Finally, in the proposed model, there is not actually an active switch from a proinflammatory to an predominately IgG1 response. The IgG1 response can develop at any time, depending upon the dose of microbe and route of entry. Rather, the IgG1 response is simply what is left after the proinflammatory and suppressor cells begin their uneasy balance. As infection progresses and the proinflammatory cells are depleted, the remaining predominant outward immune response would be production of IgG1. It is at this time that infection might be classified as both clinical and terminal.
Acknowledgments
I acknowledge Jeanne Burton, Abraham Aho, and Brian Tooker for many helpful discussions on bovine immunity. Much gratitude is extended to Carole Bolin and Bill Davis for critical review of the manuscript. The outstanding technical support of Chris Colvin, Marc A. Coussens, and Nitin Verman is also acknowledged.
This work was supported in part through the generous support of the Michigan Agriculture Experiment Station, The Center for Animal Functional Genomics at Michigan State University, and the MSU Foundation. Additional support from USDA-IFAFS grant 2001-52100-11211 and USDA-APHIS Veterinary Services number 03-9100-0794-GR is acknowledged.
Editor: J. B. Kaper
REFERENCES
- 1.Aho, A. D., A. M. McNulty, and P. M. Coussens. 2003. Enhanced expression of interleukin-1α and TRAF1 in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Infect. Immun. 71:6479-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg, R. 1994. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology 191:520-525. [DOI] [PubMed] [Google Scholar]
- 3.Bannantine, J. P., J. F. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061-2069. [DOI] [PubMed] [Google Scholar]
- 4.Bassey, E. O., and M. T. Collins. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect. Immun. 65:4869-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard, P. M., M. J. Daniels, D. Henderson, A. Pirie, K. Rudge, D. Buxton, S. Rhind, A. Greig, M. R. Hutchings, I. McKendrick, K. Stevenson, and J. M. Sharp. 2001. Paratuberculosis infection of nonruminant wildlife in Scotland. J. Clin. Microbiol. 39:1517-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendixen, P. H., B. Bloch, and J. B. Jorgensen. 1981. Lack of intracellular degradation of Mycobacterium paratuberculosis by bovine macrophages infected in vitro and in vivo: light microscopic and electron microscopic observations. Am. J. Vet. Res. 42:109-113. [PubMed] [Google Scholar]
- 7.Bloom, B. R., P. Salgame, and B. Diamond. 1992. Revisiting and revising suppressor T cells. Immunol. Today 13:131-136. [DOI] [PubMed] [Google Scholar]
- 8.Bradley, J. R., and J. S. Pober. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482-6491. [DOI] [PubMed] [Google Scholar]
- 9.Buergelt, C. D., C. Hall, K. McEntee, and J. R. Duncan. 1978. Pathological evaluation of paratuberculosis in naturally infected cattle. Vet. Pathol. 15:196-207. [DOI] [PubMed] [Google Scholar]
- 10.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1999. Interferon-gamma and interleukin-2 release by lymphocytes derived from the blood, mesenteric lymph nodes and intestines of normal sheep and those affected with paratuberculosis (Johne's disease). Vet. Immunol. Immunopathol. 68:139-148. [DOI] [PubMed] [Google Scholar]
- 11.Burrells, C., C. J. Clarke, A. Colston, J. M. Kay, J. Porter, D. Little, and J. M. Sharp. 1998. A study of immunological responses of sheep clinically-affected with paratuberculosis (Johne's disease). The relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet. Immunol. Immunopathol. 66:343-358. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlin, W., D. Y. Graham, K. Hulten, H. M. El-Zimaity, M. R. Schwartz, S. Naser, I. Shafran, and F. A. El-Zaatari. 2001. Mycobacterium avium subsp. paratuberculosis as one cause of Crohn's disease. Aliment. Pharmacol. Ther. 15:337-346. [DOI] [PubMed] [Google Scholar]
- 13.Chiodini, R. J., and C. D. Buergelt. 1993. Susceptibility of Balb/c, C57/B6 and C57/B10 mice to infection with Mycobacterium paratuberculosis. J. Comp. Pathol. 109:309-319. [DOI] [PubMed] [Google Scholar]
- 14.Chiodini, R. J., and W. C. Davis. 1993. The cellular immunology of bovine paratuberculosis: immunity may be regulated by CD4+ helper and CD8+ immunoregulatory T lymphocytes which down-regulate gamma/delta+ T-cell cytotoxicity. Microb. Pathog. 14:355-367. [DOI] [PubMed] [Google Scholar]
- 15.Chiodini, R. J., and W. C. Davis. 1992. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb. Pathog. 13:447-463. [DOI] [PubMed] [Google Scholar]
- 16.Coetsier, C., X. Havaux, F. Mattelard, S. Sadatte, F. Cormont, K. Buergelt, B. Limbourg, D. Latinne, H. Bazin, J. F. Denef, and C. Cocito. 1998. Detection of Mycobacterium avium subsp. paratuberculosis in infected tissues by new species-specific immunohistological procedures. Clin. Diagn. Lab. Immunol. 5:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper, A. M., A. Kipnis, J. Turner, J. Magram, J. Ferrante, and I. M. Orme. 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 168:1322-1327. [DOI] [PubMed] [Google Scholar]
- 19.Corpa, J. M., J. Garrido, J. F. Garcia Marin, and V. Perez. 2000. Classification of lesions observed in natural cases of paratuberculosis in goats. J. Comp. Pathol. 122:255-265. [DOI] [PubMed] [Google Scholar]
- 20.Coussens, P., C. Colvin, A. Abouzied, K. Wiersma, and S. Sipkovsky. 2002. Gene expression profiling of peripheral blood mononuclear cells from cattle infected with Mycobacterium paratuberculosis. Infect. Immun. 70:5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coussens, P., C. Colvin, J. M. G. Rosa, J. Perez-Laspuir, and M. Elftman. 2003. Evidence for a novel gene expression program in peripheral blood mononuclear cells from Mycobacterium paratuberculosis-infected cattle. Infect. Immun. 71:6487-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coussens, P. M. 2001. Interactions between Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2:141-161. [PubMed] [Google Scholar]
- 23.Coussens, P. M., A. Jeffers, and C. Colvin. 2003. Rapid and transient activation of gene expression in peripheral blood mononuclear cells from Johne's disease positive cows exposed to Mycobacterium paratuberculosis in vitro. Microb. Pathog. 36:93-108. [DOI] [PubMed] [Google Scholar]
- 24.Coussens, P. M., N. Verman, M. A. Coussens, and A. M. McNulty. 2004. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subspecies paratuberculosis: evidence for an inherent proinflammatory gene expression pattern. Infect. Immun. 72:1409-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dlugovitzky, D., M. L. Bay, L. Rateni, L. Urizar, C. F. Rondelli, C. Largacha, M. A. Farroni, O. Molteni, and O. A. Bottasso. 1999. In vitro synthesis of interferon-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand. J. Immunol. 49:210-217. [DOI] [PubMed] [Google Scholar]
- 26.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fotin-Mleczek, M., F. Henkler, A. Hausser, H. Glauner, D. Samel, A. Granes, P. Scheurich, D. Mauri, and H. Wajant. 2004. TNF receptor associated factor (TRAF) 1 regulates CD40-induced TRAF2-mediated NF-kappa B activation. J. Biol. Chem. 279:677-685. [DOI] [PubMed] [Google Scholar]
- 28.Goodridge, H. S., W. Harnett, F. Y. Liew, and M. M. Harnett. 2003. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology 109:415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwozdz, J. M., M. P. Reichel, A. Murray, W. Manktelow, D. M. West, and K. G. Thompson. 1997. Detection of Mycobacterium avium subsp. paratuberculosis in ovine tissues and blood by the polymerase chain reaction. Vet. Microbiol. 57:233-244. [DOI] [PubMed] [Google Scholar]
- 30.Hines, M. E., J. M. Kreeger, and A. J. Herron. 1995. Mycobacterial infections of animals: pathology and pathogenesis. Lab. Anim. Sci. 45:334-351. [PubMed] [Google Scholar]
- 31.Kaneko, H., H. Yamada, S. Mizuno, T. Udagawa, Y. Kazumi, K. Sekikawa, and I. Sugawara. 1999. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab. Invest. 79:379-386. [PubMed] [Google Scholar]
- 32.Kennedy, D. J., and G. Benedictus. 2001. Control of Mycobacterium avium subsp. paratuberculosis infection in agricultural species. Rev. Sci. Technol. 20:151-179. [DOI] [PubMed] [Google Scholar]
- 33.Koenig, G. J., G. F. Hoffsis, W. P. Shulaw, S. Bech-Nielsen, D. M. Rings, and G. St-Jean. 1993. Isolation of Mycobacterium paratuberculosis from mononuclear cells in tissues, blood, and mammary glands of cows with advanced paratuberculosis. Am. J. Vet. Res. 54:1441-1445. [PubMed] [Google Scholar]
- 34.Koets, A., V. Rutten, A. Hoek, F. van Mil, K. Muller, R. D. Bakke, E. Gruys, and W. van Eden. 2002. Progressive bovine paratuberculosis is associated with local loss of CD4+ T cells, increased frequency of gamma delta T cells, and related changes in T-cell function. Infect. Immun. 70:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koets, A. P., V. P. Rutten, A. Hoek, D. Bakker, F. van Zijderveld, K. E. Muller, and W. van Eden. 1999. Heat-shock protein-specific T-cell responses in various stages of bovine paratuberculosis. Vet. Immunol. Immunopathol. 70:105-115. [DOI] [PubMed] [Google Scholar]
- 36.Kreeger, J. M., T. G. D. Snider, and B. M. Olcott. 1991. Spontaneous murine thymocyte comitogenic activity consistent with interleukin-1 in cattle naturally infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 28:317-326. [DOI] [PubMed] [Google Scholar]
- 37.Lankford, C. S., and D. M. Frucht. 2003. A unique role for IL-23 in promoting cellular immunity. J. Leukoc. Biol. 73:49-56. [DOI] [PubMed] [Google Scholar]
- 38.Lee, H., J. R. Stabel, and M. E. Kehrli, Jr. 2001. Cytokine gene expression in ileal tissues of cattle infected with Mycobacterium paratuberculosis. Vet. Immunol. Immunopathol. 82:73-85. [DOI] [PubMed] [Google Scholar]
- 39.Lepper, A. W., C. R. Wilks, M. Kotiw, J. T. Whitehead, and K. S. Swart. 1989. Sequential bacteriological observations in relation to cell-mediated and humoral antibody responses of cattle infected with Mycobacterium paratuberculosis and maintained on normal or high iron intake. Aust. Vet. J. 66:50-55. [DOI] [PubMed] [Google Scholar]
- 40.Manning, E. J., and M. T. Collins. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev. Sci. Technol. 20:133-150. [DOI] [PubMed] [Google Scholar]
- 41.Merkal, R. S., A. B. Larsen, and G. D. Booth. 1975. Analysis of the effect of inapparent bovine paratuberculosis. Am. J. Vet. Res. 36:837-838. [PubMed] [Google Scholar]
- 42.Molina, J. M., A. Anguiano, and O. Ferrer. 1996. Study on immune response of goats vaccinated with a live strain of Mycobacterium paratuberculosis. Comp. Immunol. Microbiol. Infect. Dis. 19:9-15. [DOI] [PubMed] [Google Scholar]
- 43.Momotani, E., D. L. Whipple, A. B. Thiermann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet. Pathol. 25:131-137. [DOI] [PubMed] [Google Scholar]
- 44.Mukaida, N., A. Harada, and K. Matsushima. 1998. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 9:9-23. [DOI] [PubMed] [Google Scholar]
- 45.Murray, P. J., and R. A. Young. 1999. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 67:3087-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naser, S. A., I. Shafran, D. Schwartz, F. El-Zaatari, and J. Biggerstaff. 2002. In situ identification of mycobacteria in Crohn's disease patient tissue using confocal scanning laser microscopy. Mol. Cell Probes 16:41-48. [DOI] [PubMed] [Google Scholar]
- 47.Navarro, J. A., G. Ramis, J. Seva, F. J. Pallares, and J. Sanchez. 1998. Changes in lymphocyte subsets in the intestine and mesenteric lymph nodes in caprine paratuberculosis. J. Comp. Pathol. 118:109-121. [DOI] [PubMed] [Google Scholar]
- 48.Rideout, B. A., S. T. Brown, W. C. Davis, J. M. Gay, R. A. Giannella, M. E. Hines, W. D. Hueston, and L. J. Huctchinson. 2003. Diagnosis and control of Johne's disease. The National Academies Press, Washington, D.C. [PubMed]
- 49.Rook, G. A., R. A. Attiyah, and N. Foley. 1989. The role of cytokines in the immunopathology of tuberculosis, and the regulation of agalactosyl IgG. Lymphokine Res. 8:323-328. [PubMed] [Google Scholar]
- 50.Saunders, B. M., and A. M. Cooper. 2000. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol. Cell Biol. 78:334-341. [DOI] [PubMed] [Google Scholar]
- 51.Secott, T. E., T. L. Lin, and C. C. Wu. 2001. Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 69:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Secott, T. E., T. L. Lin, and C. C. Wu. 2002. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 70:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seewaldt, S., J. Alferink, and I. Forster. 2002. Interleukin-10 is crucial for maintenance but not for developmental induction of peripheral T cell tolerance. Eur. J. Immunol. 32:3607-3616. [DOI] [PubMed] [Google Scholar]
- 54.Stabel, J. R. 1996. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet. Diagn. Invest. 8:345-350. [DOI] [PubMed] [Google Scholar]
- 55.Stabel, J. R. 2000. Transitions in immune responses to Mycobacterium paratuberculosis. Vet. Microbiol. 77:465-473. [DOI] [PubMed] [Google Scholar]
- 56.Storset, A. K., H. J. Hasvold, M. Valheim, H. Brun-Hansen, G. Berntsen, S. K. Whist, B. Djonne, C. M. Press, G. Holstad, and H. J. Larsen. 2001. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study. Vet. Immunol. Immunopathol. 80:271-287. [DOI] [PubMed] [Google Scholar]
- 57.Strohmeier, G. R., and M. J. Fenton. 1999. Roles of lipoarabinomannan in the pathogenesis of tuberculosis. Microbes Infect. 1:709-717. [DOI] [PubMed] [Google Scholar]
- 58.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugawara, I., H. Yamada, S. Mizuno, and Y. Iwakura. 2000. IL-4 is required for defense against mycobacterial infection. Microbiol. Immunol. 44:971-979. [DOI] [PubMed] [Google Scholar]
- 60.Valheim, M., H. J. Hasvold, A. K. Storset, H. J. Larsen, and C. M. Press. 2002. Localisation of CD25+ cells and MHCII+ cells in lymph nodes draining Mycobacterium avium subsp. paratuberculosis vaccination granuloma and the presence of a systemic immune response. Res. Vet. Sci. 73:77-85. [DOI] [PubMed] [Google Scholar]
- 61.Wang, C.-Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-kappaB antiapoptosis: induction of TRAF1 and cIAP1 and cIAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 62.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss, D. J., O. A. Evanson, D. J. McClenahan, M. S. Abrahamsen, and B. K. Walcheck. 2001. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect. Immun. 69:1002-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss, D. J., O. A. Evanson, A. Moritz, M. Q. Deng, and M. S. Abrahamsen. 2002. Differential responses of bovine macrophages to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Infect. Immun. 70:5556-5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wells, S. J., S. L. Ott, and A. H. Seitzinger. 1998. Key health issues for dairy cattle—new and old. J. Dairy Sci. 81:3029-3035. [DOI] [PubMed] [Google Scholar]
- 66.Whitlock, R. H., and C. Buergelt. 1996. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. N. Am Food Anim. Pract. 12:345-356. [DOI] [PubMed] [Google Scholar]
- 67.Wolf, J. S., Z. Chen, G. Dong, J. B. Sunwoo, C. C. Bancroft, D. E. Capo, N. T. Yeh, N. Mukaida, and C. Van Waes. 2001. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin. Cancer Res. 7:1812-1820. [PubMed] [Google Scholar]
- 68.Zurbrick, B. G., D. M. Follett, and C. J. Czuprynski. 1988. Cytokine regulation of the intracellular growth of Mycobacterium paratuberculosis in bovine monocytes. Infect. Immun. 56:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zur Lage, S., R. Goethe, A. Darji, P. Valentin-Weigand, and S. Weiss. 2003. Activation of macrophages and interference with CD4+ T-cell stimulation by Mycobacterium avium subspecies paratuberculosis and Mycobacterium avium subspecies avium. Immunology 108:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]