Significance
Epigenetic modifications, including various histone modifications, play important roles in regulating gene expression. The Trithorax group (TrxG) complex induces permissive histone modifications to activate transcription. We herein investigate the role for Menin, a component of the TrxG complex, in T helper (Th) cell differentiation, and find a critical role for Menin in differentiation and maintenance of Th17 cells. Menin is required for Th17 cell differentiation in vitro through the direct regulation of Il17a expression. Menin controls IL-17–mediated pathology in vivo. Menin is also required to maintain expression of Rorc, the gene encoding RORγt, a key transcription factor for Th17 cell function. Thus, Menin orchestrates Th17 cell differentiation and function by regulating both induction and maintenance of target gene expression.
Keywords: RNAPII, asthma, chromatin
Abstract
Epigenetic modifications, such as posttranslational modifications of histones, play an important role in gene expression and regulation. These modifications are in part mediated by the Trithorax group (TrxG) complex and the Polycomb group (PcG) complex, which activate and repress transcription, respectively. We herein investigate the role of Menin, a component of the TrxG complex in T helper (Th) cell differentiation and show a critical role for Menin in differentiation and maintenance of Th17 cells. Menin−/− T cells do not efficiently differentiate into Th17 cells, leaving Th1 and Th2 cell differentiation intact in in vitro cultures. Menin deficiency resulted in the attenuation of Th17-induced airway inflammation. In differentiating Th17 cells, Menin directly bound to the Il17a gene locus and was required for the deposition of permissive histone modifications and recruitment of the RNA polymerase II transcriptional complex. Interestingly, although Menin bound to the Rorc locus, Menin was dispensable for the induction of Rorc expression and permissive histone modifications in differentiating Th17 cells. In contrast, Menin was required to maintain expression of Rorc in differentiated Th17 cells, indicating that Menin is essential to stabilize expression of the Rorc gene. Thus, Menin orchestrates Th17 cell differentiation and function by regulating both the induction and maintenance of target gene expression.
Naive CD4 T cells adopt distinct cell fates including differentiation into T helper 1 (Th1), Th2, Th17, and regulatory T cells, and direct immune responses to facilitate the elimination of microorganisms (1, 2). Effector functions of these Th cells are defined by production of their signature cytokines and expression of lineage-specific transcription factors. Th1 cells express T-bet (encoded by the Tbx21 gene) and produce IFN-γ (3), and Th2 cells express GATA-3 and secrete interleukin 4 (IL-4), IL-5, and IL-13 (4–6). Th17 cells were identified by their ability to produce IL-17A and express high amounts of the RAR-related orphan receptor-γ, named RORγt, that is essential for Th17 differentiation (7–10). Although Th17 cells contribute to host defense against fungi and extracellular bacteria, the pathogenicity of IL-17–producing T cells has been recognized not only in autoimmune diseases but also in allergic diseases (11–13).
Although lineage-specific transcription factors are key regulators of helper T-cell differentiation, epigenetic modifications, such as the methylation of DNA and posttranslational modifications of histones, also play crucial roles (14, 15). Trithorax group (TrxG) and Polycomb group (PcG) genes were originally discovered in Drosophila melanogaster as activators and repressors of Homeobox genes, respectively (16). It has been recognized that epigenetic modification and chromatin accessibility mediated by the PcG or TrxG complexes is a critical factor for the commitment of helper T-cell lineages (17, 18). Mixed-lineage leukemia (MLL), which is a mammalian homolog of the Drosophila trithorax, controls the maintenance of Th2 cytokine gene expression by memory Th2 cells (19). MLL forms a multicomponent complex that includes Menin, and mediates its epigenetic transcriptional effector functions via SET domain-dependent histone methyltransferase activity (20). MLL specifically methylates lysine 4 in the N-terminal tail on histone H3, a modification typically associated with transcriptionally active regions of chromatin (16). Menin protein is encoded by multiple endocrine neoplasia 1 (Men1), and mutation of this gene is the cause of multiple endocrine neoplasia type 1 in humans (21). Menin is a highly specific partner for MLL proteins and is an essential component required for DNA binding of the TrxG/MLL complex (22). The binding of the Menin/TrxG complex is required for the maintenance of Gata3 expression and Th2 cytokine production in established Th2 cells (23), and the same mechanism was also recently found to function in human Th2 cells (24). However, it remains unclear whether the Menin/TrxG complex is involved in the differentiation and maintenance of other Th cell subsets. We herein show that Menin-deficient (Menin−/−) T cells displayed reduced ability to differentiate into Th17 cells in vitro, and that development of Th17 cell-mediated airway inflammation was attenuated in mice transferred with Menin−/− Th17 cells. We found that Menin recruitment to the Il17a locus was crucial for histone modification, RNA polymerase II (RNAPII) accumulation, and the subsequent expression of Il17a mRNA. The binding of Menin to the Rorc gene locus was required for the long-term maintenance of Rorc expression. Thus, these data point to a mechanism by which Menin regulates both the induction of Th17 differentiation and maintenance of Th17 cell function after differentiation.
Results
Menin Is Required for Th17 Cell Differentiation.
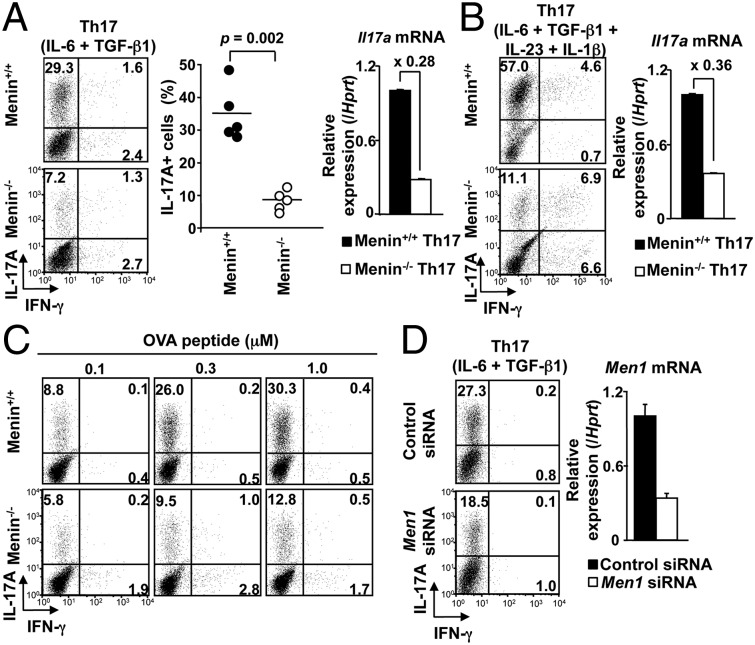
Menin is an essential component of the MLL/TrxG complex that is required for DNA binding (25). In the context of Th2 cells, we have reported that Menin is crucial for the maintenance of Gata3 expression and the function of Th2 cells after differentiation (23). However, it remains unclear whether the Menin/TrxG complex is involved in the differentiation or maintenance of function of Th17 cells. To address this question, we assessed the ability of Menin−/− naive CD4 T cells to differentiate into Th1, Th2, and Th17 cells. In in vitro Th1/Th2 cultures, Th1 and Th2 cell differentiation of Menin−/− T cells were not impaired as evidenced by IFN-γ and IL-4 production, respectively (Fig. S1 A and B) (23). In contrast, a dramatic reduction in the number of IL-17A–producing cells was observed in Menin−/− Th17 cell cultures (Fig. 1A, Left and Center). Likewise, a substantial decrease in Il17a mRNA expression was found in Menin−/− Th17 cells (Fig. 1A, Right). Moreover, at all concentrations of IL-6 tested, Menin−/− Th17 cells showed less IL-17A–positive cells compared with WT controls (Fig. S1C). As IL-1β, especially in synergy with IL-23, plays an essential role in the induction or expansion of IL-17A producers both in murine and human systems (26, 27), we examined whether IL-17A production by Menin−/− T cells was normalized by IL-1β and IL-23 under Th17 culture conditions. As shown in Fig. 1B, Menin−/− CD4 T cells showed decreased numbers of IL-17A–producing cells and reduced expression of Il17a even in the presence of IL-1β and IL-23. Menin−/− CD4 T cells showed a tendency for increased IFN-γ–producing cells in the culture, although anti–IFN-γ neutralizing antibody was added in this condition (Fig. 1B). The strength of T-cell receptor (TCR) signaling is also known to regulate IL-17 production (28, 29). We therefore investigated whether alteration of TCR stimulation could affect the reduced generation of IL-17–producing cells in Menin−/− T-cell cultures. OVA-specific DO11.10 TCR transgenic (Tg) CD4 T cells from WT or Menin-deficient mice were stimulated with various concentrations of antigenic OVA peptide together with antigen-presenting cells (APCs). Fig. 1C shows that, in Menin−/− CD4 T-cell cultures, the generation of IL-17A–producing cells was markedly reduced together with a slight increase in IFN-γ–producing cells at all concentrations of OVA peptide tested. Menin−/− CD4 T cells showed decreased generation of IL-17A–producing cells even at the early time points of the culture (day 2 and day 3; Fig. S1 D and E). Slightly accelerated cell division was detected in Menin−/− CD4 T cells compared with WT CD4 T cells (Fig. S1F). Knockdown experiments using Men1 siRNA in peripheral CD4 T cells confirmed that Menin is required for the differentiation of Th17 cells (Fig. 1D). Together, these results indicate that Menin is required for efficient differentiation of Th17 cells.
Fig. 1.
Menin is required for Th17 cell differentiation. (A) Naive CD4 T cells from WT or Menin-deficient (Menin−/−) mice were cultured under Th17 conditions for 5 d. The cultured cells were restimulated with phorbol 12-myristate 13-acetate plus ionomycin for 4 h, and IL-17A protein expression was analyzed by intracellular staining (Left). IL-17A protein expression data from five independent experiments are shown with mean values (Center). Expression Il17a mRNA was determined by quantitative RT-PCR (Right). The levels of transcripts normalized to Hprt signal in Menin−/− cells were depicted as the fold changes compared with those in WT cells. Mean values with SDs (n = 3) are shown. (B) Naive CD4 T cells were cultured under Th17 conditions in the presence of IL-23 (10 ng/mL) and IL-1β (10 ng/mL) for 5 d. The cultured cells were harvested and tested for intracellular staining (Left) and quantitative RT-PCR analysis (Right) as described in A. (C) Naive CD4 T cells from WT or Menin-deficient DO11.10 OVA-specific TCR Tg mice were cultured with splenic APCs under Th17 conditions in the presence of the indicated concentrations of OVA peptides (0.1–1.0 μM) for 6 d. IL-17A– and IFN-γ–secreting cells were assessed by intracellular staining. Three independent experiments were performed with similar results (B and C). (D) Control and Men1 siRNA were transfected into naive CD4 T cells from WT mice. These naive CD4 T cells were cultured under Th17 condition for 1 or 2 d before analysis. The IL-17A–producing cells (Left) and mRNA expression level of Men1 (Right) were assessed by intracellular staining on day 2 or quantitative RT-PCR on day 1, respectively. Two independent experiments were performed with similar results.
OVA-Induced Neutrophilic Airway Inflammation Is Attenuated in Mice Transferred with Menin−/− Th17 Cells and in Menin-Deficient Mice.
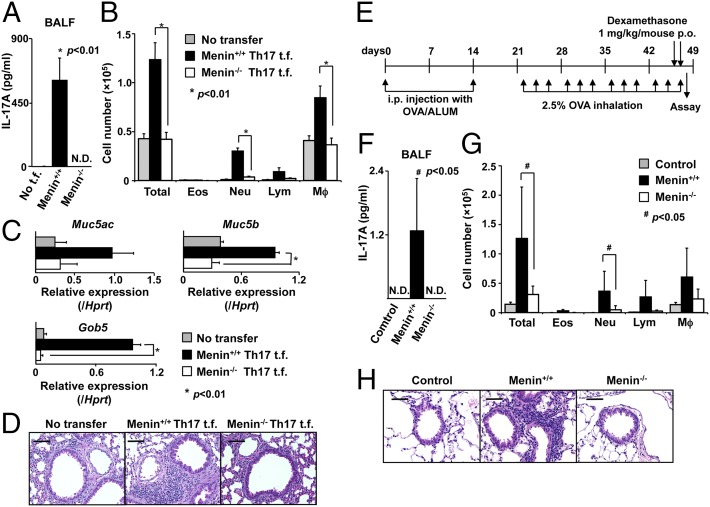
Based on our in vitro results, we reasoned that Menin could be an important factor regulating IL-17–dependent pathology in vivo. Some patients with severe asthma appear to have IL-17A–mediated airway inflammation with increased airway neutrophils, mucous cell metaplasia in airway epithelial cells, and increased airway hyperreactivity (30). Therefore, we next used a model of airway inflammation in which Th17 cells are key mediators of neutrophilic inflammation and pathology (31). We adoptively transferred Th17 cells from DO11.10 TCR Tg WT or Menin-deficient mice into syngeneic BALB/c recipient mice. First, we accessed the accumulation of transferred CD4 T cells before and after OVA challenge (Fig. S2A). Comparable numbers of Menin−/− T cells were engrafted in the lung, and 1 d after the last OVA challenge, a substantial increase was detected in the numbers of WT CD4 T cells but not Menin−/− CD4 T cells (Fig. S2B). There was little difference in the expression level of homing receptors between WT and Menin−/− Th17 cells (Fig. S2C). We assessed airway inflammation in BALB/c recipient mice, which were adoptively transferred with Th17 cells from DO11.10 TCR Tg WT or Menin-deficient mice followed by OVA inhalation (Fig. S2 D and E). The levels of IL-17A in bronchoalveolar lavage (BAL) fluid samples from mice that received Menin−/− Th17 cells were dramatically reduced (P < 0.01) in comparison with BAL fluid samples from mice that received WT Th17 cells (Fig. 2A). The total number of infiltrating leukocytes in the BAL fluid was significantly decreased (P < 0.01) in the group transferred with Menin−/− Th17 cells (Fig. 2B). Moreover, we detected a large increase in the number of neutrophils in the BAL fluid from mice transferred with WT Th17 cells that was absent from the mice transferred with Menin−/− Th17 cells (Fig. 2B). The mRNA expression levels of Muc5ac, Muc5b, and Gob5, molecular markers for goblet cell hyperplasia and mucus production, were decreased in the lungs of mice receiving Menin−/− Th17 cells (Fig. 2C). Consistent with these findings, deletion of Menin in Th17 cells resulted in diminished infiltration of mononuclear cells around the peribronchiolar and perivascular regions of the lungs (Fig. 2D). Next, to determine whether the impaired ability of transferred Menin−/− Th17 cells to induce airway inflammation is due to impaired expansion of Th17 cells or decreased IL-17A production in Th17 cells, we examined how many WT Th17 cells needed to be transferred to induce inflammation at the same level as transfer of 1 × 106 Menin−/− Th17 cells. We found that the number of neutrophils in the BAL fluid from mice transferred with 0.2 × 106 WT Th17 cells was comparable to that from the mice transferred with 1 × 106 Menin−/− Th17 cells (Fig. S2F). In the lungs of these recipient mice, the number of WT CD4 T cells was significantly lower (P < 0.05) than that of Menin−/− CD4 T cells (Fig. S2G). These results indicate that WT Th17 cells in the lung could induce inflammation with smaller number of cells than Menin−/− Th17 cells. Thus, we concluded that the impaired ability of transferred Menin−/− Th17 cells to induce airway inflammation was most likely due to decreased IL-17A production in Th17 cells rather than impaired expansion of Th17 cells in lung. Next, we examined neutrophilic airway inflammation directly in CD4-Cre+Meninfl/fl mice by using a previously reported steroid-resistant neutrophilic airway inflammation model (Fig. 2E) (32). The levels of IL-17A in BAL fluid samples from CD4-Cre+Meninfl/fl mice were dramatically reduced (P < 0.05) in comparison with BAL fluid samples from WT mice (Fig. 2F). In CD4-Cre+Meninfl/fl mice, the total number of infiltrating leukocytes in the BAL fluid was significantly decreased (P < 0.05) compared with that in WT mice (Fig. 2G). Moreover, CD4-Cre+Meninfl/fl mice showed a significant decrease in neutrophils in the BAL fluid compared with WT mice. Histological analysis also revealed that infiltration of mononuclear cells around the peribronchiolar and perivascular regions of the lungs was dependent on the ability of CD4 T cells to express Menin (Fig. 2H). Thus, Menin is required for the induction of Th17 cell-mediated neutrophilic airway inflammation in vivo.
Fig. 2.
Neutrophilic airway inflammation is attenuated by deficiency of Menin. (A) Airway inflammation was induced as described in Materials and Methods and Fig. S2D. The concentration of IL-17A in the BAL fluid was measured by ELISA. Mean values with SDs (n = 3) are shown (*P < 0.01). N.D., under the detection levels; t.f., transfer. (B) The cell number of eosinophils (Eos), neutrophils (Neu), lymphocytes (Lym), and macrophages (Mϕ) in the BAL fluid are shown. Mean values with SDs (n = 3) are shown (*P < 0.01). (C) The data represent the mean values of the indicated gene expression in the lungs of mice that received WT or Menin−/− Th17 cells (*P < 0.01). (D) The level of OVA-induced airway inflammation in recipient mice was examined by histological analysis (H&E staining). (Scale bars: 50 μm.) Data are representative of at least three independent experiments (A–D). (E) A schematic overview of the study protocol for the induction of steroid-resistant neutrophilic inflammation. p.o., per oral. (F) The concentration of IL-17A in the BAL fluid was measured by cytometric bead array (CBA). Mean values with SDs (n = 6) are shown (#P < 0.05). (G) The cell number of eosinophils (Eos), neutrophils (Neu), lymphocytes (Lym), and macrophages (Mϕ) in the BAL fluid are shown. Mean values with SDs (n = 3 for control group, n = 6 for WT, and n = 4 for Menin−/− group) are shown (#P < 0.05). (H) Antigen-induced leukocyte infiltration into the lungs was evaluated by H&E staining. (Scale bars: 50 μm.)
Menin Does Not Control the Expression of Other Key Transcription Factors That Can Regulate Th17 Cell Differentiation.
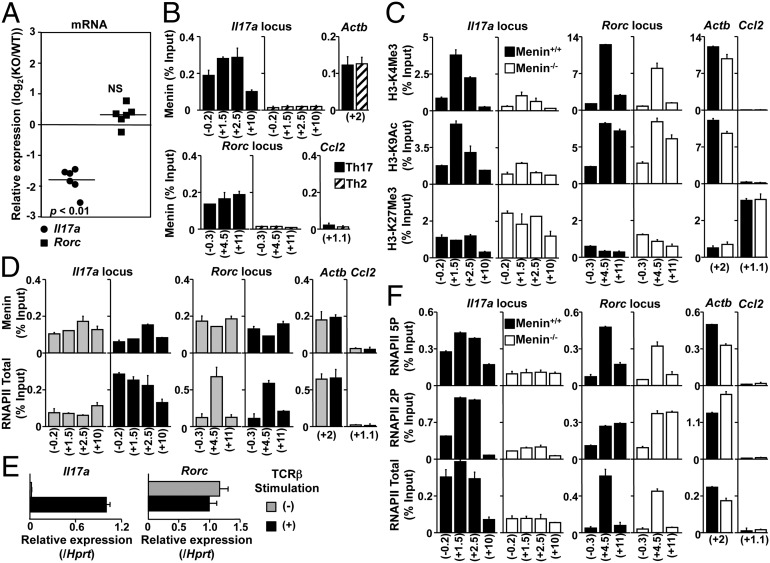
To further investigate the nature of the defect in IL-17A production in Menin−/− T cells, we next focused on the transcription factors involved in Th17 cell differentiation. Th17 cell differentiation is associated with the expression of several transcription factors (33). Despite the decrease of Il17a in Menin−/− Th17 cells, mRNA expression levels of all of these transcription factors including Rorc appeared to be normal in Menin−/− Th17 cells (Fig. 3A and Fig. S3A). The protein level of RORγt was also comparable (Fig. S3B, Top). IL-6–mediated phosphorylation of STAT3 in Menin−/− Th17 cells was equivalent to those in WT Th17 cells (Fig. S3B, Middle). Moreover IL-6–mediated phosphorylation of STAT3 in freshly isolated Menin−/− CD4 T cells was not altered (Fig. S3C). These results indicate that the expression of key transcription factors for Th17 cell differentiation, including the protein encoded by Rorc, was not affected by Menin deficiency.
Fig. 3.
Menin is required for the formation of permissive histone modifications and RNAPII accumulation at the Il17a locus. (A) Naive CD4 T cells from WT or Menin-deficient mice were cultured under Th17 conditions for 5 d. Expression of Il17a and Rorc mRNA were determined by quantitative RT-PCR as described in Fig. 1A. Data from six independent experiments with mean values are shown. (B and C) The binding levels of Menin and RNAPII, and modification of the histone H3-K4Me3, H3-K9Ac, and H3-K27Me3 levels at several regions around the Il17a, Rorc, Actb (active), and Ccl2 (silent) gene loci were determined by ChIP assays with quantitative PCR (qPCR) analysis. For ChIP with qPCR assay, percentages of input DNA ([specific antibody ChIP − control Ig ChIP]/input DNA; mean of three samples) is shown with SDs. (D and E) Menin and RNAPII binding or transcripts of the Il17a and Rorc genes were determined by qPCR in WT Th17 cells stimulated with (+) or without (−) immobilized anti-TCRβ mAb for 4 h. (F) RNAPII binding was measured after 4-h restimulation with TCRβ in WT or Menin−/− Th17 cells. Three independent experiments were performed with similar results (B–F).
Menin Is Required for the Formation of Permissive Histone Modifications at the Il17a Gene Locus.
To further understand the possible mechanism whereby Menin functions to regulate Il17a expression, we next assessed the binding of Menin and the histone modification states around the Il17a and Rorc gene loci together with the Actb and Ccl2 loci as heritably active and silent genes in Menin−/− Th17 cells by chromatin immunoprecipitation (ChIP) assays (Fig. S4 A and B). Five-day culture of WT cells under Th17-inducing conditions resulted in enhanced Menin binding at both the Il17a and Rorc gene loci compared with control Th2-inducing conditions (Fig. 3B). The accumulation of Menin at the Il17a gene locus was detected even after 48 h of stimulation (Fig. S4C). Levels of histone H3 trimethylated at Lys4 (H3-K4Me3) and histone H3 acetylated at Lys9 (H3-K9Ac), which frequently correlate with transcriptional activation, were decreased at the Il17a gene locus in Menin−/− Th17 cells compared with WT cells 5 d after TCR stimulation (Fig. 3C, Left). In addition, increased trimethylated histone H3 at Lys27 (H3-K27Me3), which correlates with genomic silencing was detected in Menin−/− Th17 cells (Fig. 3C, Left). In sharp contrast, loss of Menin had little effect on epigenetic histone modifications at the Rorc locus (Fig. 3C, Center).
Menin interacts specifically with RNAPII (34). In addition, eukaryotic gene expression is regulated by RNAPII through the phosphorylation of its carboxyl-terminal domain (CTD) (35). Ser-2 phosphorylation (2P) marks the elongation state, whereas Ser-5 is phosphorylated (5P) in the initiation phase (36). Therefore, we examined whether Menin is required for the recruitment of RNAPII to the Il17a gene locus in Th17 cells. The binding levels of Menin to the Il17a and Rorc gene loci in WT Th17 cells restimulated with an immobilized anti-TCRβ were similar to the binding levels in cells that had not been restimulated (Fig. 3D, Upper). Interestingly, however, the recruitment of RNAPII to the Il17a gene locus, but not to the Rorc gene locus, was markedly up-regulated after TCR restimulation (Fig. 3D, Lower, and Fig. S4D, Bottom). This is consistent with the observation that Il17a expression, but not Rorc, was up-regulated by TCR restimulation (Fig. 3E). The H3-K4Me3 and H3-K9Ac histone modifications at these gene loci were not altered by TCR restimulation (Fig. S4D, Top and Middle). Next, we assessed whether Menin was required for recruitment of RNAPII to the Il17a and Rorc gene loci. Compared with WT cells, the levels of RNAPII Ser-5 and Ser-2 phosphorylation together with a total RNAPll at the Il17a gene were much lower in Menin−/− Th17 cells restimulated with anti-TCRβ antibody (Fig. 3F). In contrast, the reduction of RNAPII at the Rorc gene locus in Menin−/− Th17 cells was much less dramatic. These results indicate that, in Th17 cells, Menin is primarily required for RNAPII accumulation and Ser-2/Ser-5 double phosphorylation at the Il17a gene locus.
STAT3 directly regulates not only gene expression but also epigenetic modifications of numerous genes involved in Th17 cell differentiation (37, 38). In the case of the Il17a and Rorc gene loci, STAT3 directly binds the promoter and also intergenic regions and induces alterations to the epigenetic signature of these genes. Consistent with these studies (37, 38), loss of STAT3 resulted in the disappearance of IL-17A–producing cells (Fig. S5A). The binding of STAT3 to Il17a and Rorc gene loci in Menin−/− Th17 cells was similar to that in WT cells (Fig. S5B). However, the binding of Menin to these loci in STAT3−/− Th17 cells was reduced (Fig. S5C). IL-6 stimulation alone could not induce Menin accumulation at the Il17a locus (Fig. S5D). These results indicate that STAT3 binding to the Il17a and Rorc gene loci was independent of Menin expression, whereas STAT3 in combination with TGF-β stimulation is required for the recruitment of Menin to these two gene loci.
Menin Is Crucial for Maintaining the Expression of Rorc and Permissive Histone Modifications at the Rorc Gene Locus in Differentiated Th17 Cells.
We have previously shown that, once Th2 cell differentiation takes place, Gata3 expression and Th2 function is maintained via recruitment of the Menin/TrxG complex to the Gata3 gene locus, even in the absence of IL-4–mediated STAT6 activation (23). We examined whether the expression of Rorc is maintained in a similar fashion via the binding of the Menin/TrxG complex in an IL-6/STAT3-independent manner. As TGF-β1 has been shown to be important, and IL-6 has been shown to be dispensable for the maintenance of IL-17A expression by Th17 cells (39), we cultured Th17 cells in the absence of IL-6 after the first cycle of Th17 differentiation. After initial differentiation, Th17 cells generated from WT T cells maintained the ability to produce IL-17A throughout two extra cycles of culture in the absence of IL-6 (Fig. 4A, Upper). Decreased numbers of IL-17A–producing cells and decreased expression of the Il17a gene were also observed throughout the culture period in Menin−/− Th17 cells compared with WT Th17 cells (Fig. 4 A, Lower, and B). The addition of IL-6 or IL-23 to the second culture did not rescue the number of IL-17A–producing Menin−/− cells (Fig. S6A; see 66.0% vs. 32.6% or 67.0% vs. 36.1%). The levels of histone H3-K4Me3 and H3-K9Ac at the Il17a gene locus were also lower in Menin−/− Th17 cells after the second cycle of culture without IL-6, and this defect appeared to be even more pronounced compared with that observed after initial differentiation (Figs. 3C, Left, and 4C, Left). The levels of H3-K27Me3 were also higher in Menin−/− Th17 cells after secondary culture (Fig. 4C, Left). Expression of Rorc in WT Th17 cells was efficiently maintained, whereas Rorc expression in Menin−/− Th17 cells was rapidly lost during extended culture in the absence of IL-6 (second and third cycles, Fig. 4B). In addition, the levels of H3-K4Me3 and H3-K9Ac at the Rorc gene locus in Menin−/− Th17 cells clearly decreased in the second cycle of culture (Fig. 4C, Middle). Furthermore, the expression of RORγt protein in Menin−/− Th17 cells was shown to be decreased after the second cycle of cultivation (Fig. 4D). Thus, these results indicate that Menin is essential for the maintenance of Rorc expression and permissive histone modifications at the Rorc gene locus.
Fig. 4.
Menin−/− Th17 cells fail to maintain the expression of Rorc. (A and B) Th17 cells (first, second, and third cycle) were generated as described in Materials and Methods. IL-17A– and IFN-γ–secreting cells were assessed by FACS (A), and Il17a and Rorc mRNA expression were determined by quantitative RT-PCR (B). Four independent experiments were performed with similar results (A and B). Mean values with SDs (n = 3) are shown (A). (C) The binding of Menin and levels of histone modifications after the second culture cycle at the indicated gene loci were determined by ChIP assays with qPCR as described in Fig. 3B. (D) The level of RORγt protein expression were determined by FACS. Data are representative of two independent experiments (C and D).
Discussion
Previous work has established that the MLL/Menin/TrxG complex plays a critical role in the maintenance of Th2 cell function in murine and human systems (17, 19, 23, 24, 40). We extended this research and herein report a crucial role for Menin in the regulation of Th17 cell differentiation and function. Our results show that Menin bound to the Il17a gene locus and induced subsequent histone modifications at the Il17a locus together with the expression of Il17a in the differentiation phase. Menin was not required for the induction of permissive histone modifications at the Rorc gene locus. In sharp contrast to the initial differentiation phase, Menin was required to preserve expression of Rorγt after differentiation. In vivo, Th17 cell-mediated neutrophilic airway inflammation was limited by the abrogation of Menin in Th17 cells, suggesting a physiological role of Menin in the regulation of IL-17–mediated pathology. Thus, these results point to an important role for Menin in the regulation of Th17 cell differentiation and function and also IL-17–dependent pathology.
An interesting finding of the present study is that Menin appeared to function differently at the Il17a locus and the Rorc locus during the initial Th17 cell differentiation phase. Menin was not required for the induction of permissive histone modifications and transcriptional expression of the Rorc locus during Th17 cell differentiation, even though Menin bound strongly to the Rorc loci in WT Th17 cells. In the case of Th2 cells, the Menin/TrxG complex did not affect the expression of either the Gata3 locus or Th2 cytokine loci during the naive to effector Th2 differentiation phase (23). The polycomb protein Ezh2, which can antagonize TrxG function and specifically trimethylate H3K27 to induce repressive histone modifications, appears to regulate effector Th1/Th2 cell differentiation primarily via control of the expression of lineage-specific transcription factor genes rather than the cytokine genes (18). Although the underlying mechanisms that determine which genes the TrxG and PcG complexes functionally target remain unclear, the binding of these chromatin-modifying complexes alone does not always correlate with the expected modification of histones or transcription. Our preliminary results indicate that the Il17f locus behaves in a similar fashion to the Rorc locus in terms of the binding of Menin and the state of histone modifications, i.e., Menin was not required for the induction of Il17f expression and permissive histone modifications in differentiating Th17 cells.
In contrast to the initial induction phase of Th17 cell differentiation, the Menin/TrxG complex plays a crucial role in the maintenance of Rorc expression and permissive histone modifications that are required to support appropriate function of Th17 cells. Indeed, IL-6 is required for the induction of Th17 cell differentiation but not for the maintenance of Th17 function (39), indicating that the underlying mechanisms governing the expression of Rorc and Il17a are likely to be different between the differentiation and maintenance phases. Our previous report showed that the Menin/TrxG complex is essential to retain Gata3 expression after differentiation, whereas IL-4–mediated STAT6 activation is dispensable (23). GATA3 expression is required for the maintenance of Th2 cell function during long-term culture (23) and also during the memory phase in both mouse and human systems (24, 41). Our finding that STAT3 deficiency in Th17 cells results in impaired Menin binding to the Il17a and Rorc loci, indicates that STAT3 and TGF-β–mediated recruitment of Menin is likely an important mechanism in the induction of permissive histone modifications at these genes in differentiating Th17 cells.
It has been reported that the Menin/TrxG complex binds to DNA through RNAPII (22, 40). We demonstrate that Menin directly bound to the Il17a gene locus and induced permissive histone modifications in differentiating Th17 cells (Fig. 3 and Fig. S4). Menin may bind to DNA through low levels of RNAPII bound to the Il17a gene locus in differentiating Th17 cells. However, we also found that Menin is required for TCR restimulation-induced RNAPII recruitment and Ser-2/Ser-5 double phosphorylation of RNAPII at the Il17a gene locus that accompanies the dramatic induction of Il17a expression after TCR restimulation (Fig. 3E). This reveals a previously unidentified unexpected mechanism for the Menin/TrxG complex in the regulation of gene expression, i.e., Menin is essential for rapid recruitment of the RNAPII transcription complex and high-level transcription of target genes such as Il17a. The sequential recruitment of the Menin/TrxG complex and the RNAPII transcription complex appears to be important to establish a fully active transcriptional state capable of rapidly inducing target gene expression after exposure to an external stimuli such as TCR stimulation. It will thus be important to determine to what extent Menin retains this function as a facilitator of RNAPII recruitment at other genes and in other cell types.
We found that Th17 cell-mediated neutrophilic airway inflammation is markedly attenuated in the mice transferred with Menin−/− Th17 cells and CD4-Cre+Meninfl/fl mice (Fig. 2). The dramatic effect observed in these experimental settings may reflect the decreased numbers of IL-17A–producing cells and also the impaired maintenance of Th17 cell function of the Menin−/− Th17 cells. Noneosinophilic asthma associated with neutrophilic inflammation is generally refractive to treatment with steroids (42, 43). Multiple mechanisms have been identified by which Th17 cells can cause steroid-resistant asthma (44). It is recognized that IL-17 family members induce granulopoiesis, neutrophil chemotaxis, and the antiapoptotic properties of G-CSF (45, 46). Our study revealed that neutrophilic airway inflammation induced by Th17 cells was attenuated in the mice transferred with Menin−/− Th17 cells and CD4-Cre+Meninfl/fl mice. Menin may also play an important role in some types of neutrophilic airway inflammation in humans.
In summary, our study highlights that Menin regulates both the induction and maintenance of Th17 differentiation and function in vitro, and contributes IL-17–mediated pathogenicity in vivo. Thus, the components of Menin/TrxG complex could represent unique therapeutic targets for the treatment of Th17 cell–mediated steroid-resistant asthma in humans.
Materials and Methods
C57BL/6 and BALB/c mice were purchased from CLEA. Meninfl/fl mice (47) were purchased from The Jackson Laboratory and backcrossed at Chiba University to C57BL/6 or BALB/c background more than 10 times. CD4-Cre Transgenic mice were purchased from Taconic Farms. STAT3fl/fl mice were provided by T. Hirano (Osaka University, Osaka) (48). All mice used in this study were maintained under specific pathogen-free conditions and ranged from 6 to 8 wk of age. All animal care was performed in accordance with the guidelines of Committee on the Ethics of Animal Experiments of Chiba University.
Detailed descriptions of all materials and methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Kaoru Sugaya, Hikari Kato, Miki Kato, and Toshihiro Ito for their excellent technical assistance. This work was supported by the Global Centers of Excellence Program (Global Center for Education and Research in Immune System Regulation and Treatment), and by grants from the Ministry of Education, Culture, Sports, Science and Technology [Grants-in-Aid for Scientific Research (S) 26221305; (C) 24592083; Young Scientists (B) 22790452, 25860351, and 25860352; Research Activity Start-up 25893032 and 25893033], the Ministry of Health, Labour and Welfare, the Astellas Foundation for Research on Metabolic Disorders, the Uehara Memorial Foundation, Osaka Foundation for Promotion of Fundamental Medical Research, Princes Takamatsu Cancer Research Fund, and Takeda Science Foundation. D.J.T. was supported by Japanese Society for the Promotion of Science Postdoctoral Fellowship 2109747.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321245111/-/DCSupplemental.
References
- 1.Reiner SL. Development in motion: Helper T cells at work. Cell. 2007;129(1):33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 2.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 4.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 5.Löhning M, Richter A, Radbruch A. Cytokine memory of T helper lymphocytes. Adv Immunol. 2002;80:115–181. doi: 10.1016/s0065-2776(02)80014-1. [DOI] [PubMed] [Google Scholar]
- 6.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu Rev Immunol. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 7.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Stockinger B, Veldhoen M, Martin B. Th17 T cells: Linking innate and adaptive immunity. Semin Immunol. 2007;19(6):353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 13.Molet S, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 14.Allan RS, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487(7406):249–253. doi: 10.1038/nature11173. [DOI] [PubMed] [Google Scholar]
- 15.Kanno Y, Vahedi G, Hirahara K, Singleton K, O’Shea JJ. Transcriptional and epigenetic control of T helper cell specification: Molecular mechanisms underlying commitment and plasticity. Annu Rev Immunol. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128(4):735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama T, Yamashita M. Critical role of the Polycomb and Trithorax complexes in the maintenance of CD4 T cell memory. Semin Immunol. 2009;21(2):78–83. doi: 10.1016/j.smim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Tumes DJ, et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4+ T helper type 1 and type 2 cells. Immunity. 2013;39(5):819–832. doi: 10.1016/j.immuni.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita M, et al. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24(5):611–622. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Milne TA, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10(5):1107–1117. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekharappa SC, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama A, et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123(2):207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Onodera A, et al. STAT6-mediated displacement of polycomb by trithorax complex establishes long-term maintenance of GATA3 expression in T helper type 2 cells. J Exp Med. 2010;207(11):2493–2506. doi: 10.1084/jem.20100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakata Y, et al. c-Myb, Menin, GATA-3, and MLL form a dynamic transcription complex that plays a pivotal role in human T helper type 2 cell development. Blood. 2010;116(8):1280–1290. doi: 10.1182/blood-2009-05-223255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guru SC, et al. Identification and characterization of the multiple endocrine neoplasia type 1 (MEN1) gene. J Intern Med. 1998;243(6):433–439. doi: 10.1046/j.1365-2796.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- 26.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 27.Ghoreschi K, et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iezzi G, et al. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci USA. 2009;106(3):876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Rodriguez J, et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31(4):587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Ramli W, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 31.McKinley L, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181(6):4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito K, et al. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am J Respir Cell Mol Biol. 2008;39(5):543–550. doi: 10.1165/rcmb.2008-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirahara K, et al. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21(6):425–434. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes CM, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13(4):587–597. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 35.Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108(4):439–451. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 36.Czudnochowski N, Bösken CA, Geyer M. Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition. Nat Commun. 2012;3:842. doi: 10.1038/ncomms1846. [DOI] [PubMed] [Google Scholar]
- 37.Durant L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32(5):605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama T, Yamashita M. Initiation and maintenance of Th2 cell identity. Curr Opin Immunol. 2008;20(3):265–271. doi: 10.1016/j.coi.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Endo Y, et al. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity. 2011;35(5):733–745. doi: 10.1016/j.immuni.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353(9171):2213–2214. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- 43.Pavord ID. Non-eosinophilic asthma and the innate immune response. Thorax. 2007;62(3):193–194. doi: 10.1136/thx.2006.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 45.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crabtree JS, et al. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23(17):6075–6085. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda K, et al. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: Generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161(9):4652–4660. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.