Significance
It has been reported that elevation of cGMP and activation of cGMP-dependent protein kinase I (cGKI) by inhibition of PDE5 activity with sildenafil prevents cardiac hypertrophy. We studied the roles of cGKI and PDE5 inhibition on angiotensin (AII)-induced hypertrophy and fibrosis using control (Ctr) mice and mice expressing cGKI only in cells where the smooth muscle SM22α promotor is active (βRM). In Ctr mice, sildenafil did not reduce the AII-induced increase of cardiomyocyte (CM) size or myocyte hypertrophic markers but did reduce fibrosis. In βRM mice, sildenafil had little or no effect on markers of fibrosis. These results show that the sildenafil/cGMP/cGKI cascade can have an inhibitory effect on cardiac fibrosis but not on CM hypertrophy.
Keywords: PKGI, PDE, cardiac failure, hypertension, NO/cyclic GMP system
Abstract
Conflicting results have been reported for the roles of cGMP and cGMP-dependent protein kinase I (cGKI) in various pathological conditions leading to cardiac hypertrophy and fibrosis. A cardioprotective effect of cGMP/cGKI has been reported in whole animals and isolated cardiomyocytes, but recent evidence from a mouse model expressing cGKIβ only in smooth muscle (βRM) but not in cardiomyocytes, endothelial cells, or fibroblasts has forced a reevaluation of the requirement for cGKI activity in the cardiomyocyte antihypertrophic effects of cGMP. In particular, βRM mice developed the same hypertrophy as WT controls when subjected to thoracic aortic constriction or isoproterenol infusion. Here, we challenged βRM and WT (Ctr) littermate control mice with angiotensin II (AII) infusion (7 d; 2 mg⋅kg−1⋅d−1) to induce hypertrophy. Both genotypes developed cardiac hypertrophy, which was more pronounced in Ctr animals. Cardiomyocyte size and interstitial fibrosis were increased equally in both genotypes. Addition of sildenafil, a phosphodiesterase 5 (PDE5) inhibitor, in the drinking water had a small effect in reducing myocyte hypertrophy in WT mice and no effect in βRM mice. However, sildenafil substantially blocked the increase in collagen I, fibronectin 1, TGFβ, and CTGF mRNA in Ctr but not in βRM hearts. These data indicate that, for the initial phase of AII-induced cardiac hypertrophy, lack of cardiomyocyte cGKI activity does not worsen hypertrophic growth. However, expression of cGKI in one or more cell types other than smooth muscle is necessary to allow the antifibrotic effect of sildenafil.
Cyclic GMP-dependent protein kinase I (cGKI) is expressed in a wide variety of cells including cardiomyocytes (CMs), cardiac myofibroblasts (CMFs), cardiac fibroblasts (CFs), endothelial cells (ECs), and smooth muscle cells (SMCs) (1). Increased cGKI activity has been reported to protect against cardiac hypertrophy induced by pressure overload (2, 3). For example, mice that carry a mutated form of cGKI unable to interact with downstream targets, develop increased pathologic hypertrophy, accelerated mortality, and congestive heart failure when subjected to thoracic aorta constriction (TAC) (4).
Hypertrophy induced by angiotensin II (AII) is thought to be attenuated by cGKI, because AII signaling through Gq/11 is abrogated by the regulator of G-protein signaling protein (RGS), a known substrate of cGKI (5). However, selective deletion of the AII receptor 1 (AT-R1) in the kidney ameliorated AII-induced cardiac hypertrophy, suggesting that the cardiac AT-R1 is dispensable for the induction of this response (6), whereas transgenic mice that overexpress the human AT-R1 specifically in CMs develop hypertrophy, fibrosis, dysfunctions, and early death (7). Furthermore, activation of TRPC channels followed by elevated [Ca2+]i may contribute to the induction of the cardiac hypertrophy gene response (8–11). Again, TRPC3 and TRPC6 channels are negatively regulated by cGKI (12–14).
It also has been reported that particulate guanylyl cyclase A, the major receptor for atrial natriuretic peptide (ANP) in the heart, couples to and directly activates the TRPC3/C6 channels in chronic cardiac hypertrophy, thus bypassing cGMP and cGKI (15). It was reported that cardiac cGMP can affects cardiac properties by modulating cAMP levels, bypassing again cGKI (16, 17). Thus, the site(s) of action of cGMP/cGKI are not entirely clear (18) and interpretation of cGMP/cGKI effects on cardiac hypertrophy may be quite complicated.
Sildenafil, a phosphodiesterase 5 (PDE5) inhibitor, elevates cardiac cGMP at high doses (100 mg⋅kg−1⋅d−1), increases cGKI activity, and has been reported to reverse TAC-induced cardiac hypertrophy (2). This effect is postulated to be caused by an increased activity of RGS2 (19), and in part by the direct regulation of TRPC3/6 conductance by cGKI (11, 14) mentioned above. However, two clinical studies did not report positive therapeutic results after prolonged treatment of diastolic dysfunction with sildenafil (20, 21).
We (22) and others (23) have tested the hypothesis that CM-cGKI attenuates cardiac hypertrophy by using the cGKIβ rescue mouse line (βRM). These animals express the cGKIβ isozyme under the SM22α promoter, which is active principally in SMCs and activated CMFs, but do not express cGKIβ in other cell types (24). Chronic infusion of isoproterenol or TAC induced in βRM mice a cardiac hypertrophy that was identical to that of WT littermate controls (22), a result that argues against the hypothesis that CM, EC, or CF cGKI is responsible for the antihypertrophic effects of cGMP in the heart. To examine the role(s) of sildenafil to inhibit cardiac hypertrophy and fibrosis, we now extend these experiments using AII-induced hypertrophy and high concentrations of sildenafil (0.6 mM) in the drinking water. Once again, the lack of cGKI in CM and CF did not cause an increased hypertrophic response as predicted (2). Moreover, little or no antihypertrophic effect of sildenafil was observed in the WT mice and no effect was seen in the βRM mice. However, a large effect of sildenafil was observed on fibrosis in WT but not the βRM mice, suggesting that cGKI present in cardiac SMCs or activated CMFs is an important regulator of cardiac fibrosis.
Results
The cGKIβ rescue mouse line (βRM) expresses the cGKIβ isozyme under the SM22α promoter, which is active principally in SMCs and activated CMFs, but does not express cGKIβ in other cell types (24). As previously reported (25), βRM mice are leaner than their control (Ctr) littermates (Fig. S1). However, the lack of cGKI in tissues other than smooth muscle does not affect the basic morphology and function of the heart of βRM mice (see below; Fig. S1 and refs. 22 and 24).
AII Induces Cardiac Hypertrophy in the Absence and Presence of Sildenafil.
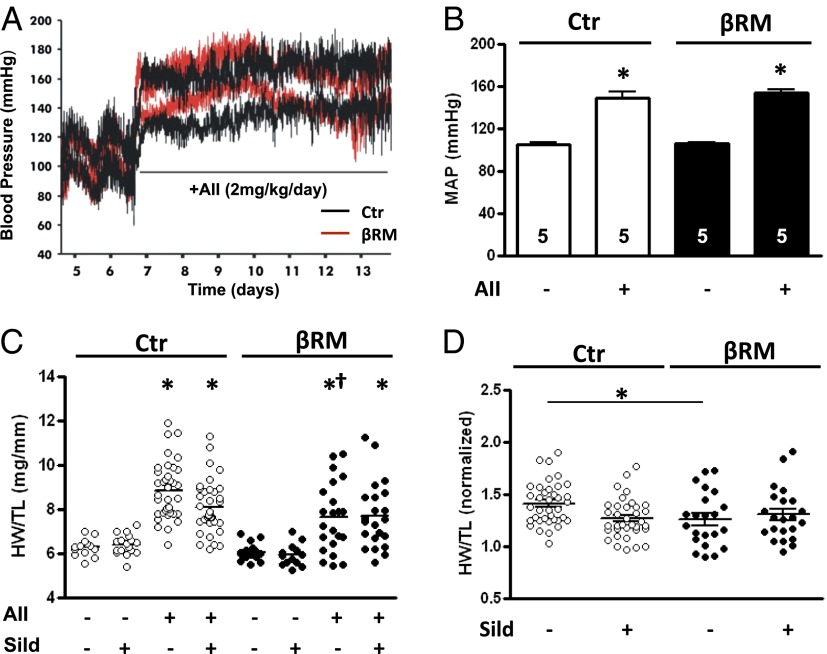
We exposed βRM and littermate Ctr mice to a 7-d continuous infusion of AII (2 mg⋅kg−1⋅d−1), a concentration known to establish chronic hypertension and cardiac hypertrophy (26). In addition, a subgroup of animals was treated with the PDE5 inhibitor sildenafil, administered through the drinking water at a concentration of 400 mg/L (0.6 mM). In some animals of both genotypes, systemic blood pressure was monitored before and during AII treatment using in vivo telemetry (Ctr: from 105 to 148 mmHg after AII, n = 5; βRM: from 105 to 153 mmHg after AII, n = 5; Fig. 1A). Median arterial blood pressure increased to the same extent within the first 24 h after implantation of the AII-delivering minipumps (Fig. 1B).
Fig. 1.
Effects of AII and sildenafil administration. (A) Example of systolic and diastolic blood pressure measurement by telemetry recording and (B) average median arterial pressure (MAP) before and after AII infusion (n = 5 in all groups; *P < 0.05 vs. Ctr group, one-way ANOVA). (C) Scatter plot diagram of measured heart mass as total heart weight/tibia length (HW/TL) (*P < 0.05 vs. corresponding Ctr group; †P < 0.05, βRM AII vs. Ctr AII; one-way ANOVA with Tukey’s multiple-comparison test; bars and whiskers represent median and SEM, respectively). For this ANOVA test, all eight groups were included. ANOVA test only for the four Ctr groups yielded a significance of P < 0.01 for Ctr plus AII vs. Ctr plus AII plus sildenafil. The same ANOVA test for βRM mice did not change the significance value. (D) The same values had been normalized on the average respective control group value (*P < 0.05, one-way ANOVA with Tukey’s multiple-comparison test).
Cardiac hypertrophy was evaluated at the end of the 7-d AII infusion. Fig. 1C (see also Table S1) shows the total heart weight/tibia length ratio in the different groups of Ctr and βRM mice. As expected, infusion of AII triggered an increase in the cardiac mass in both genotypes. In the βRM mice, which have no cGKI in their CMs, this increase was not greater but if anything slightly less prominent. This lack of an increase in the βRM mice also can be seen when the heart mass of the AII-treated animals is normalized on the average mass of the corresponding control group (Fig. 1D). This result suggests, in agreement with our previous findings (22), that the absence of cGKI in the CMs and ECs of the heart does not increase the extent of cardiac hypertrophy, at least under normal physiological conditions.
Coadministration of sildenafil (0.6 mM) in the drinking water during the AII infusion had no effect in βRM mice and only slightly reduced the hypertrophic response in WT mice, without reaching statistical significance (Fig. 1C). This concentration yields an approximate plasma level of 70 nM sufficient to greatly inhibit PDE5 (27). Application of the same dose mixed in the food yielded plasma levels of 10 nM and reversed TAC-induced cardiac hypertrophy (2). A parallel experiment was conducted on a separate cohort of WT mice that were infused with AII and treated with sildenafil by single daily administration via oral gavages (100 mg⋅kg−1⋅d−1). In this group, nearly identical results were seen (Fig. S2C).
βRM mice are more fragile and about 40% of the βRM mice died during the 7-d AII infusion, whereas almost all Ctr mice survived. Coadministration of sildenafil did not alter the survival trends (Fig. S2A). Control groups for both βRM and WT animals, which received infusion of vehicle solution, also did not show any difference in survival rate, which were always above 90% both in the presence or absence of sildenafil. Several different animal models were tested to unravel the cause of the death of the βRM mice during the AII infusion (see SI Text for details and Figs. S2–S4). The most likely cause was that hypertension activated platelets and thrombus formation (28, 29) and led to intravasal thrombi.
AII Infusion Induces Similar CM Size and Interstitial Fibrosis and Does Not Affect Cardiac Contractility in WT and βRM Mice.
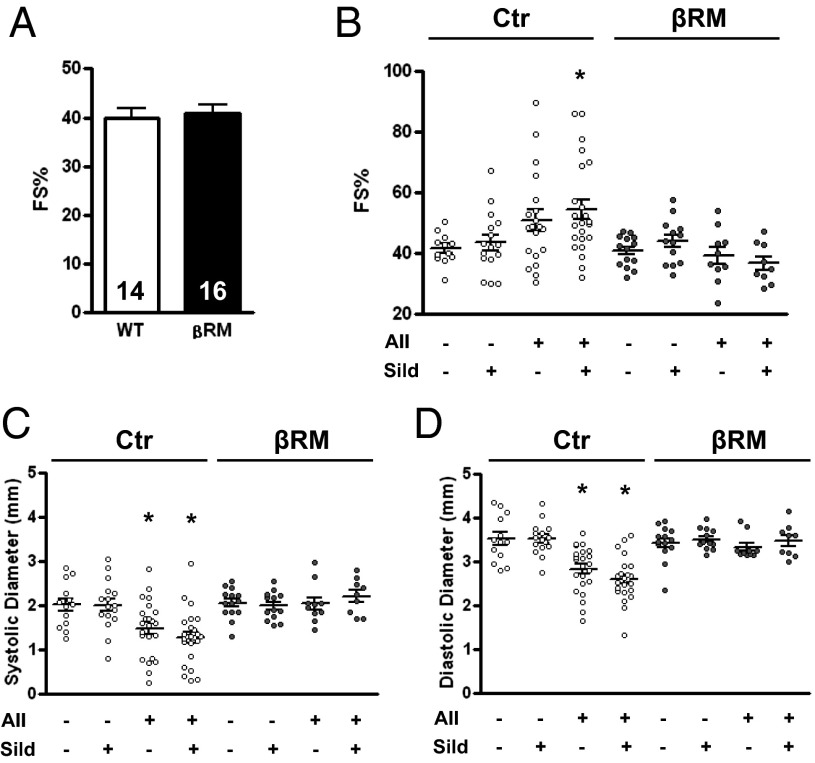
We studied the functional consequences of the experimental conditions on cardiac activity by monitoring heart contraction with echocardiography on the seventh day of AII treatment. Fractional shortening (%FS) did not vary significantly between genotypes either before AII treatment (FS in percentage: Ctr, 39.90 ± 1.87, vs. βRM, 40.97 ± 1.70; Fig. 2A) or after the 7-d AII infusion (Fig. 2B). The %FS showed a tendency toward an increase in Ctr animals infused with AII that did reach significance in the sildenafil-treated group (for Ctr AII-infused group ANOVA gave a P value of 0.053). A more clear effect of increased blood pressure was measured on systolic and diastolic left ventricle diameters, both of which were significantly reduced in Ctr AII-treated groups (Fig. 2 C and D). In βRM mice, ventricle diameters did not change upon AII treatment and consequently %FS did not vary. Sildenafil treatment showed no clear effect in any of the treated groups.
Fig. 2.
Physiological parameters of the animals before and after treatment. Cardiac contractility as measured by echocardiography and expressed as percentage fractional shortening (FS%) in basal conditions (A) and in the experimental groups (B). Left ventricle chamber diameter during systole (C) and diastole (D) (*P < 0.05 vs. corresponding Ctr group, one-way ANOVA with Tukey’s multiple-comparison test).
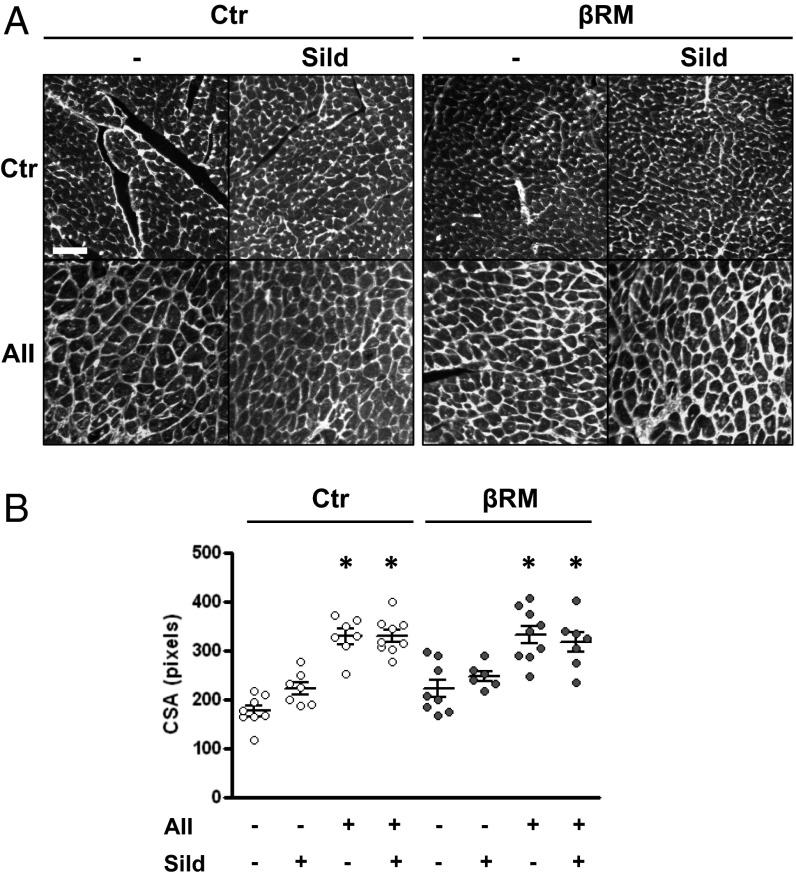
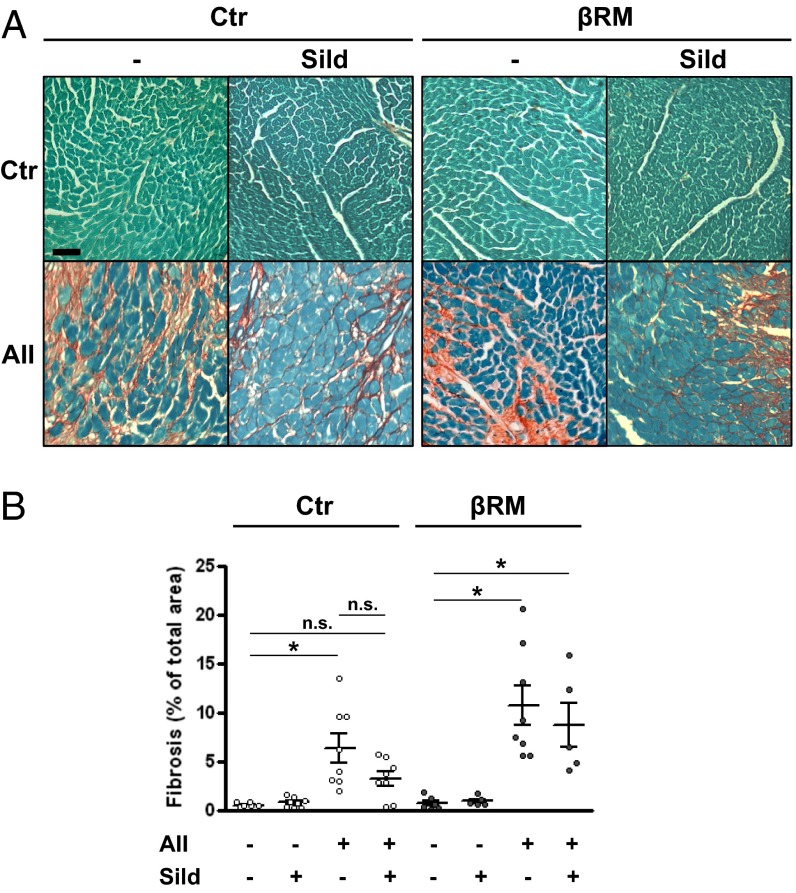
We then analyzed additional cardiac tissue samples. Ventricle CM cross-section area was measured after staining of plasma membranes with fluorophore-labeled wheat germ agglutinin. As shown in Fig. 3, AII infusions induced a marked increase in CM area, in a similar fashion for all treated groups. Concomitant sildenafil administration did not affect this increase, suggesting that the slight reduction in heart mass caused by sildenafil in Ctr mice is not due to a block of myocyte hypertrophy, and therefore may be caused by less intercellular matrix production. To test this hypothesis, another group of heart sections were stained with Sirius red to detect collagen deposition. Image analysis of the stained sections showed that AII infusion caused a marked but comparable deposition of interstitial collagen fibers in both Ctr and βRM mice (Fig. 4). Sildenafil appeared to reduce the average amount of collagen in Ctr hearts, although the difference did not reach statistical significance (P = 0.076) in unpaired T test or ANOVA. In βRM hearts, more collagen staining was seen in nearly all sections, but sildenafil had no effect on collagen deposition (Fig. 4B), suggesting that a nonsmooth muscle cell type (i.e., CMs, CFs, or ECs) are responsible for cGMP/cGKI-dependent regulation of AII-induced collagen deposition.
Fig. 3.
CM cross-sectional area (CSA). Representative images from heart sections (left ventricle at 20× magnification) stained with wheat germ agglutinin (WGA) (A), and scatter plot of CSA (B). Bar and whiskers represent median and SEM, respectively. *P < 0.05 vs. respective Ctr group; one-way ANOVA with Tukey’s multiple-comparison test. (Scale bar: 50 μm.) One pixel of the analyzed images is equivalent to 0.45 μm.
Fig. 4.
AII infusion-induced fibrosis in cardiac tissue. Representative images from heart sections (left ventricle at 20× magnification) stained with Fast Green and Sirius red to detect collagen deposition (A). The percentage fibrosis over the total section surface was determined by software-assisted image analysis (B). *P < 0.05, one-way ANOVA with Tukey’s multiple-comparison test against value minus AII. (Scale bars: 50 μm.)
Sildenafil Treatment Decreases Fibrotic Gene Markers.
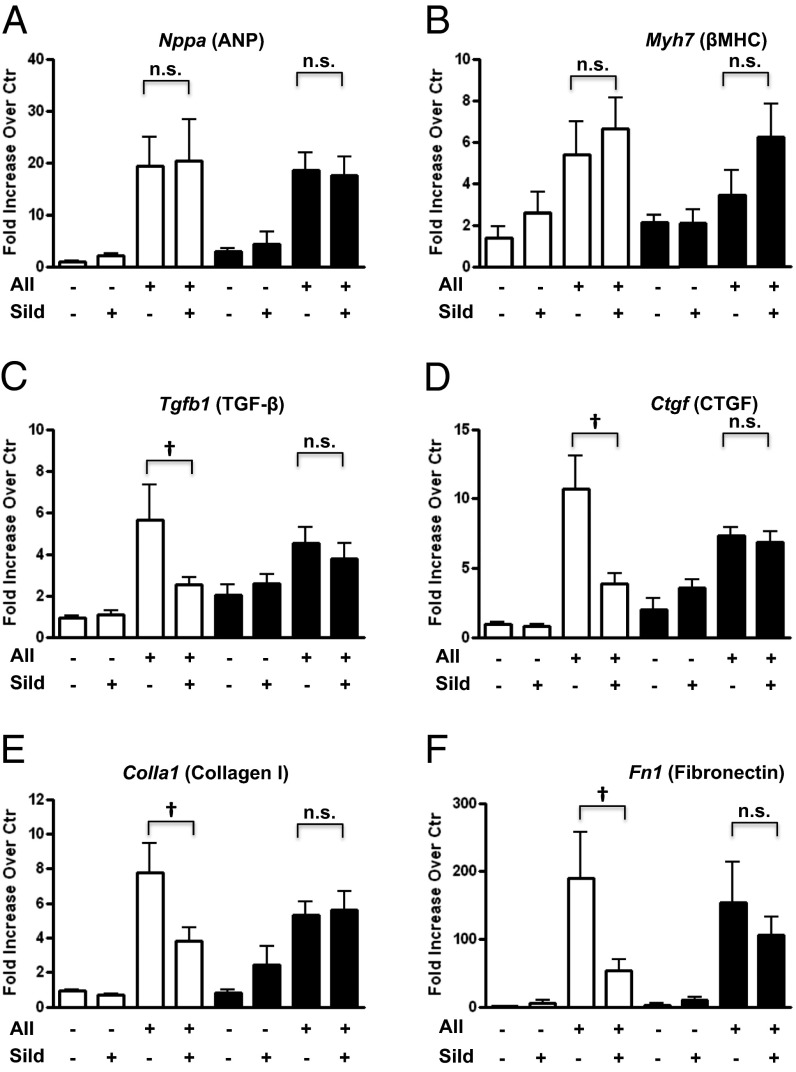
Next, quantitative real-time PCR was performed on total heart RNA samples to measure transcription of several genes associated with hypertrophy and fibrosis (primers are shown in Table S2) (Fig. 5). ANP mRNA levels, a classic marker of maladaptive cardiac hypertrophy, were equally increased in both genotypes by AII, and this increase was maintained in the presence of sildenafil (Fig. 5A). Again, the lack of cGKI in the CM did not increase this marker of hypertrophy. Similarly, the β-myosin heavy-chain gene transcript was elevated by AII in Ctr and βRM mice, although in βRM the increase was statistically significant only in the sildenafil-treated group (Fig. 5B). PDE5 and α-myosin transcript levels also were comparable in all of the groups (Fig. S5). These data strongly suggest that CM cGMP/cGKI does not directly regulate CM hypertrophy under these AII-induced conditions.
Fig. 5.
mRNA expression levels of Nppa (ANP) (A), Myh7 (ßMHC) (B), Tgfb1 (TGF-ß) (C), Ctgf (CTGF) (D), Colla1 (Collagen 1) (E), and Fn 1 (Fibronectin) (F). mRNA expression level was normalized to 18s rRNA and then to corresponding Ctr group average, assessed by real-time RT-PCR. The white bars refer to Ctr groups, and the black bars to βRM groups. Values for AII-treated animals are significantly different from Ctr animals at P < 0.05. †P < 0.05 vs. Ctr AII group; n.s., nonsignificant. Statistics was done with one-way ANOVA with Tukey’s multiple-comparison test. n = 5–7 for each group.
Analysis of the transcription of four genes involved in the fibrotic response [two profibrotic hormones, namely connective tissue growth factor (CTGF), and transforming growth factor β (TGF-β), and two extracellular matrix proteins, fibronectin1 (Fn1) and collagen 1] showed a significant increase both in Ctr and βRM hearts. Sildenafil treatment lowered this increase in Ctr, but not in βRM mice (Fig. 5 C–E). These findings indicate that cGKI in CMs or CFs, or ECs is necessary for the inhibitory effect of sildenafil on the transcription of these profibrotic genes.
Discussion
It is known that chronically elevated AII levels and hypertension induce cardiac hypertrophy and fibrosis (26). This study investigated the effects of activation of cGKI on the initial adaptation of heart muscle to pressure overload. We challenged mice that were genetically engineered to ablate cGKI expression in all cells except those expressing the smooth muscle SM22α promoter, because global KO of cGKI results in premature death of the animals in the first weeks after birth (30). Although it is very unlikely, we cannot rule out completely that some results are caused by undetected compensatory mechanisms.
The presented results can be summarized as follows: (i) AII infusion resulted in augmented cardiac mass in both Ctr and βRM mice. In contrast to expectation (2), the hypertrophy was not augmented in βRM mice (Fig. 1B). The βRM/AII infusion model allows us to conclude that lack of cGKI in CMs, ECs, and CFs does not prevent the basal AII-induced hypertrophic response. (ii) Sildenafil has a modest effect in reducing AII-induced cardiac hypertrophy in Ctr mice and little or no effect in βRM mice. This implies that some cell type other than the smooth muscle that still expresses significant amounts of cGKI is required for a sildenafil response. (iii) Sildenafil treatment suppressed the AII-dependent elevated transcript levels of two profibrotic hormones and two extracellular matrix proteins in Ctr but not in βRM mice. This result strongly suggests the major effects of sildenafil on these parameters are due to effects outside of the SMCs and CMFs. (iv) Similarly to what has been observed with isoproterenol infusion and aortic constriction (22), AII-induced hypertrophy was not augmented and, if anything, slightly reduced in βRM compared with WT mice despite an increase in fibrosis (Fig. 4). The mechanism leading to cardiac hypertrophy at this early stage was not elucidated (see SI Text and Figs. S5–S9).
In a previously published study (22), our group showed that βRM mice subjected to isoproterenol infusion (1 wk) or TAC (3 wk) develop the same degree of cardiac hypertrophy as their Ctr littermate controls. These observations and the results in this paper are in apparent contrast with the established hypothesis in the literature that activated cGKI serves as a general antihypertrophic brake in CMs. One likely possible explanation may be that the “basal” activity state of cGMP/cGKI has little effect as a break on hypertrophy unless there is also a strong tone of guanylyl cyclase. If so, it would imply that any therapy based on increased cGMP/cGKI will need to include a guanylyl cyclase activation component.
In this study, we treated mice with a high concentration of sildenafil, as used also by others (2, 3, 23). Although sildenafil had little effect on initial AII-induced cardiac hypertrophy, it showed a large effect on the profibrotic response as indicated by reductions of profibrotic and interstitial matrix gene expression in Ctr mice. It is well established that TGF-β is a key player in the profibrotic response of the heart to pressure overload and has an important role in the cross talk between CMs and CFs (31). cGKI activity has been shown to suppress TGF-β-induced CF proliferation and differentiation into myofibroblasts (32). CTGF, whose expression is induced by TGF-β, is another important intracellular messenger that is essential for TGF-β–induced collagen synthesis by CFs (33). The antifibrotic effects of sildenafil were only observed in Ctr mice and not in βRM mice, which lack cGKI in CFs.
Both CMs and CFs can be a source of TGF-β, although CFs likely represent the main source in heart (34). It is therefore possible that a suppressive effect of sildenafil on TGF-β production is occurring in either or both of these cell types. However, it seems most likely that the main effect is occurring in CFs, since they express high levels of PDE5 (22), and at this early time point of the hypertrophic growth response, an induction of PDE5 has not yet occurred (Fig. S6). Sildenafil had no obvious effect in the βRM mice that express cGKI in SMCs.
Finally, other cell types such as resident macrophages and ECs might also be important in this regard. ECs express PDE5 and cGKI in Ctr mice but no cGKI in βRM mice. They are a particularly interesting candidate for the source of the antifibrotic response because they respond to increased cGMP with production of C-type natriuretic peptide (CNP), which is a known cardiac protective agent (35, 36). For all of these reasons, the data do not allow us to determine which cell type is most involved in the sildenafil effect other than to say it is not SMCs.
In conclusion, this study suggests that ablation of cGKI in CMs, CFs, and/or ECs does not by itself potentiate the hypertrophic/fibrotic effect of AII under normal physiological conditions. The data also suggest that the major effect of sildenafil to reduce AII-induced hypertrophy/fibrosis is almost entirely on the fibrosis component of the response. Moreover, this antifibrotic effect is mediated by actions of sildenafil on one or more of the nonsmooth muscle cell types that express cGKI in the Ctr but not the βRM mice.
Materials and Methods
Experimental Animals.
The strategy to create the βRM mouse line was described earlier (24). For all of the experiments performed, 10- to 17-wk-old male βRM mice (genotype: SM22α+/Iβ; cGKIL−/L−) and littermate controls (Ctr; genotype SM22α+/Iβ; cGKI+/L− or SM22α+/+; cGKI+/L) were used. All experimental procedures were conducted according to the local government’s committee on animal care and welfare in Munich.
Statistical Analysis.
GraphPad (Prism) software, version 4, was used for statistical analysis. If not otherwise indicated, all values are presented as the mean ± SEM. To assess statistical significance, comparisons between groups, genotypes, and/or stimulation conditions were performed by using ANOVA or unpaired Student t test. In scatter plot diagrams, bar and whiskers represent mean value and SEM, respectively.
An extended and detailed materials and methods section is present in SI Text.
Supplementary Material
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (to F.H. and R.L.), the Sonderforschungsbereich 699 (to J.S.), the Fonds der Chemischen Industrie (to F.H.), the National Institutes of Health (AR056221 and GM083926) (to J.A.B.), and The Muscular Dystrophy Association of America (254895) (to J.A.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414364111/-/DCSupplemental.
References
- 1.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev. 2006;86(1):1–23. doi: 10.1152/physrev.00015.2005. [DOI] [PubMed] [Google Scholar]
- 2.Takimoto E, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, et al. Stress-dependent dilated cardiomyopathy in mice with cardiomyocyte-restricted inactivation of cyclic GMP-dependent protein kinase I. Eur Heart J. 2013;34(16):1233–1244. doi: 10.1093/eurheartj/ehr445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanton RM, et al. Protein kinase G Iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc. 2012;1(5):e003731. doi: 10.1161/JAHA.112.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang P, Mende U. Functional role, mechanisms of regulation, and therapeutic potential of regulator of G protein signaling 2 in the heart. Trends Cardiovasc Med. 2014;24(2):85–93. doi: 10.1016/j.tcm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowley SD, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103(47):17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci USA. 2000;97(2):931–936. doi: 10.1073/pnas.97.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bush EW, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281(44):33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 9.Kuwahara K, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116(12):3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onohara N, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25(22):5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res. 2011;108(2):265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, et al. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol. 2008;586(Pt 17):4209–4223. doi: 10.1113/jphysiol.2008.156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao X. TRPC, cGMP-dependent protein kinases and cytosolic Ca2+ Handb Exp Pharmacol. 2007;179(179):527–540. doi: 10.1007/978-3-540-34891-7_31. [DOI] [PubMed] [Google Scholar]
- 14.Koitabashi N, et al. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation: Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol. 2010;48(4):713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaiber M, et al. A cardiac pathway of cyclic GMP-independent signaling of guanylyl cyclase A, the receptor for atrial natriuretic peptide. Proc Natl Acad Sci USA. 2011;108(45):18500–18505. doi: 10.1073/pnas.1103300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142(2):136–143. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Götz KR, et al. Transgenic mice for real-time visualization of cGMP in intact adult cardiomyocytes. Circ Res. 2014;114(8):1235–1245. doi: 10.1161/CIRCRESAHA.114.302437. [DOI] [PubMed] [Google Scholar]
- 18.Lukowski R, Krieg T, Rybalkin S, Beavo J, Hofmann F. cGMP-dependent pathways as targets for 1 treating cardiac dysfunction: Boom, bust and beyond. Trends Pharmacol Sci. 2014;35(8):404–413. doi: 10.1016/j.tips.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Takimoto E, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119(2):408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redfield MM, et al. RELAX Trial Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309(12):1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen MJ, et al. Sildenafil and diastolic dysfunction after acute myocardial infarction in patients with preserved ejection fraction: The Sildenafil and Diastolic Dysfunction After Acute Myocardial Infarction (SIDAMI) trial. Circulation. 2013;127(11):1200–1208. doi: 10.1161/CIRCULATIONAHA.112.000056. [DOI] [PubMed] [Google Scholar]
- 22.Lukowski R, et al. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci USA. 2010;107(12):5646–5651. doi: 10.1073/pnas.1001360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaiber M, et al. Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: Role of cGMP-dependent protein kinase and RGS2. Basic Res Cardiol. 2010;105(5):583–595. doi: 10.1007/s00395-010-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber S, et al. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res. 2007;101(11):1096–1103. doi: 10.1161/CIRCRESAHA.107.154351. [DOI] [PubMed] [Google Scholar]
- 25.Leiss V, Friebe A, Welling A, Hofmann F, Lukowski R. Cyclic GMP kinase I modulates glucagon release from pancreatic α-cells. Diabetes. 2011;60(1):148–156. doi: 10.2337/db10-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devereux RB, Pickering TG, Cody RJ, Laragh JH. Relation of renin-angiotensin system activity to left ventricular hypertrophy and function in experimental and human hypertension. J Clin Hypertens. 1987;3(1):87–103. [PubMed] [Google Scholar]
- 27.Adamo CM, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107(44):19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massberg S, et al. Increased adhesion and aggregation of platelets lacking cyclic guanosine 3′,5′-monophosphate kinase I. J Exp Med. 1999;189(8):1255–1264. doi: 10.1084/jem.189.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez AM, et al. Inhibition of platelet activation in stroke-prone spontaneously hypertensive rats: Comparison of losartan, candesartan, and valsartan. J Cardiovasc Pharmacol. 2001;37(4):406–412. doi: 10.1097/00005344-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer A, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17(11):3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koitabashi N, et al. Pivotal role of cardiomyocyte TGF-β signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121(6):2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, et al. Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102(2):185–192. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 33.Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89(2):265–272. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 34.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74(2):184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horio T, et al. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144(6):2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- 36.Suga S, et al. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system.”. J Clin Invest. 1992;90(3):1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.