Abstract
Gelatinase and serine protease were found to contribute in concert to pathogenesis in a rabbit model of endophthalmitis. However, a mutant defective in the fsr regulator was observed to be more attenuated than a mutant rendered defective in the expression of gelatinase and serine protease as the result of a polar transposon insertion into the former. This increased attenuation suggests that there are possible additional pleiotropic effects of the defect in fsr on expression of traits contributing to the pathogenesis of enterococcal infection.
Enterococci are gram-positive intestinal commensals of humans and other animals and are leading causes of nosocomial infections and subacute endocarditis (5, 13). The emergence of multidrug-resistant enterococci poses a formidable therapeutic challenge (5, 6, 10, 11). Efforts to identify enterococcal virulence factors with a view towards finding new therapeutic targets have led to the discovery of two quorum-regulated systems that contribute to enterococcal pathogenesis in several disease models (4, 15).
One of these quorum-sensing systems regulates cytolysin (4), a bipartite toxin produced by cytolytic strains of Enterococcus faecalis that contributes to virulence in all models tested (2, 3, 7, 9, 19), including endophthalmitis (9, 19). It was recently demonstrated that intraocular infection with the noncytolytic enterococcal strain OG1RF also follows a malignant course and that a significant portion of the virulence of OG1RF is regulated by a second quorum-sensing system termed fsr (14). The fsr quorum-sensing system has been shown to regulate two proteases, gelatinase (GelE) and serine protease (SprE), both of which contribute to virulence in an animal peritonitis model (15, 17, 18) and to killing in a Caenorhabditis elegans infection model (17). The serine protease gene sprE, which lies immediately downstream of and is cotranscribed with gelE, encodes a secreted 26-kDa serine protease that shares homology with Staphylococcus aureus V8 protease (15). Transcription of the gelE-sprE operon is positively regulated in a growth phase-dependent fashion by the fsr locus, the components of which have been termed fsrA, fsrB, and fsrC (16).
The rabbit endophthalmitis model provides a unique opportunity to study the role of quorum sensing in enterococcal infection, in that very low numbers of E. faecalis CFU can be used to establish infection (as few as 102 CFU or less) (9). Thus, in the rabbit endophthalmitis model, the quorum develops in vivo, as opposed to other models that require large inocula where quorum concentrations generated in vitro may be present upon injection. In addition, the endophthalmitis model provides an exquisitely sensitive infection system in which an impairment in organ function due to the infectious process can be directly assessed by electroretinography (ERG), which measures the electrical responses of the visual cells in the retina in reaction to light; many other parameters of infection can be monitored as the enterococcal quorum develops (9).
Because an fsrB mutant which was shown to be defective in the production of both gelatinase and serine protease was attenuated in virulence in the endophthalmitis model (14), it was of interest to determine whether this attenuation was caused by the loss of one protease or the other, or perhaps both. In this report we demonstrate that both gelatinase and serine protease contribute to virulence but that the combined loss of both protease activities does not fully account for the degree of attenuation observed for the fsr mutant; this finding suggests an additional contribution to virulence of one or more yet unidentified factors regulated by the fsr quorum-sensing system.
All strains were propagated in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, Mich.). For OG1RF and derivatives, the medium was supplemented with rifampin (25 μg/ml) and fusidic acid (10 μg/ml). For establishing endophthalmitis, bacteria were diluted to approximately 103 CFU/ml in phosphate-buffered saline and injected intravitreally as described below. Enumeration of organisms at the time of inoculation and after recovery from the vitreous was accomplished by plating duplicate serial dilutions on BHI agar (8) with selective antimicrobials as appropriate. The bacterial strains used are summarized in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this studya
| E. foecalis strain | Phenotype | Genotype and resistance phenotype | Reference(s) |
|---|---|---|---|
| OG1RF | WT | Wild-type strain; Rifr Fusr | 12 |
| TX 5266 | ΔfsrB | OG1RF fsrB in-frame deletion mutant with deletion of bp 79 to 684 from fsrB; Rifr Fusr | 16 |
| TX 5264 | GelE− SprE+ | OG1RF gelE in-frame deletion mutant; Rifr Fusr Emr | 17 |
| TX 5243 | GelE+ SprE− | OG1RF sprE insertional mutant; Rifr Fusr Emr | 15 |
| TX 5128 | GelE− SprE− | OG1RF polar insertional mutant in gelE with expression of sprE below detectable levels; Rifr Fusr Emr | 18, 15 |
Abbreviations: WT, wild type; Rif, rifampicin; Fus, fusidic acid; Em, erythromycin; GelE, gelatinase; SprE, serine protease.
Experimental endophthalmitis was induced in female New Zealand White rabbits (weight, 1.75 to 2.25 kg), as previously described (9). The animals underwent general anesthesia by intramuscular administration of 35 mg of ketamine per kg of body weight (Ketaved; Phoenix Scientific Inc., St. Joseph, Mo.) and 5 mg of xylazine per kg (Rompun; Bayer Corp., Shawnee Mission, Kans.). The rabbits' eyes were dilated with topical 1% tropicamide and 2.5% phenylephrine hydrochloride. After paracentesis and the withdrawal of 0.1 ml of aqueous humor from the anterior chamber to reduce the intraocular pressure and prevent reflux of the inoculum, 100 CFU of the strain of interest suspended in 0.1 ml of sterile saline was injected into the midvitreous cavity with a sterile insulin syringe. Experimental groups consisted of at least eight animals each, and experiments were conducted at least in duplicate to demonstrate the reproducibility of results. Animals were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology regulations (http://www.arvo.org/AboutArvo/animalst.asp).
To detect potential differences in the intraocular growth rates of mutant and wild-type E. faecalis strains, the number of organisms in the vitreous at 12, 36, and 48 h after infection was determined. Eyes were enucleated at the indicated time points, and the anterior eye segment encompassing the cornea, iris, and ciliary body was separated from the posterior segment by a circumferential cut along the pars plana and the retina, with the attached vitreous entirely scraped out of the remaining scleral cup with a no. 10 scalpel blade. This material was homogenized by bead beating with 1.0-mm-diameter glass beads and a BeadBeater (Biospec Products, Bartlesville, Okla.) for 1 min at maximum speed, and duplicate serial 10-fold dilutions of homogenates were plated on BHI agar. After 24 h of incubation at 37°C, colonies were counted, and concentrations were expressed as CFU per milliliter of intraocular sample.
E. faecalis OG1RF and isogenic mutant strains with various levels of expression of gelatinase and serine protease were assessed by ERG for their abilities to affect retinal responsiveness to light stimulus. After general and topical anesthesia was administered as described above, and after pharmacologic dilation and dark adaptation, the B-wave amplitudes (trough of A wave to peak of B wave) in response to a bright flash (flash intensity of 700 cd/m2 in a Ganzfeld illumination sphere) were assessed simultaneously for the infected right eye and the saline-injected left eye (EPIC-2000 visual electrodiagnostic system; LKC Technologies, Gaithersburg, Md.). ERGs were performed at 12, 24, 36, and 48 h postinfection. Percent retinal function was defined as the ratio of the B-wave amplitude of the infected eye to the B-wave amplitude of the contralateral saline-injected eye.
All values represent the arithmetic means ± standard errors of the means. A two-tailed Student t test for unequal variances was used for statistical comparisons between groups. A P value of <0.05 was considered significant.
Eyes infected with wild-type and mutant E. faecalis strains were enucleated for histopathological analysis 48 h postinfection. The sclera anterior to the superior rectus muscle was marked with tattoo ink to ensure uniform orientation of the specimen during preparation of sections. Eyes were fixed in 4% paraformaldehyde for at least 24 h. Five serial sagittal sections were prepared and stained with hematoxylin and eosin. Since pathological findings were largely confined to the inferior portion of the retina, and inflammatory and structural changes elsewhere in the eye were subtle in comparison with changes resulting from infection with cytolytic E. faecalis or more virulent organisms such as S. aureus (1, 9), only this area was evaluated and assigned a score from 0 (normal) to 4 (most severe) as follows: 0, normal appearance; 1, cystoid changes and few infiltrates; 2, moderate infiltrate and photoreceptors recognizable; 3, retinal layers still discernible, marked inflammatory infiltrate, and no recognizable photoreceptors; 4, no discernible retinal layers and massive inflammatory infiltrate.
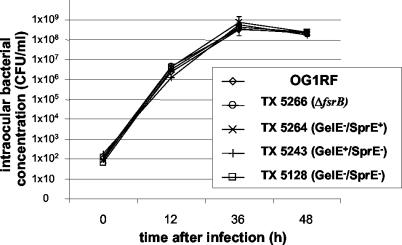
The intraocular growth kinetics of E. faecalis OG1RF, TX 5266 (ΔfsrB, phenotypically GelE− SprE−), TX 5264 (OG1RF GelE− SprE+), TX 5243 (OG1RF GelE+ SprE−), and TX 5128 (OG1RF which possesses a polar mini γδ insertion in gelE, which also reduces expression of sprE below detectable levels [15, 18], but has a functional fsr system) are depicted in Fig. 1. In vivo growth levels were similar for all strains studied. A steady increase in bacterial numbers was seen through 36 h postinoculation; the numbers of CFU reached a maximum at this time and declined slightly thereafter.
FIG. 1.
Intraocular growth of E. faecalis wild-type strains OG1RF and TX 5266 (OG1RF ΔfsrB), TX 5264 (GelE− SprE+), TX 5243 (GelE+ SprE−), and TX 5128 (GelE− SprE−) in vivo.
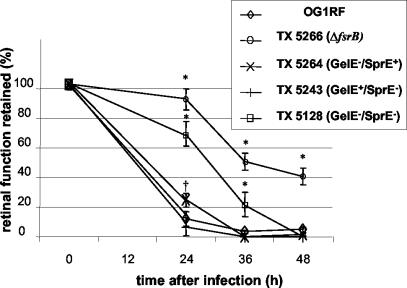
Figure 2 illustrates the effect of infection on retinal function as measured by ERG. As previously reported (14), infection with E. faecalis OG1RF resulted in a significantly greater reduction of B-wave amplitude than infection with the fsrB deletion mutant TX 5266 at the 24-h (P < 0.001), 36-h (P < 0.001), and 48-h (P < 0.005) time points. Infection with E. faecalis OG1RF resulted in a rapid loss of retinal responsiveness to light of about 85% at 24 h and a nearly complete loss by 36 h. In contrast, eyes infected with the fsrB deletion mutant TX 5266 retained over 90% of retinal function at 24 h, and retinal function did not decrease below 40% of the control value over the course of the entire experiment. Interestingly, infection with strains TX 5264 and TX 5243, which are deficient in production of gelatinase or serine protease individually, showed a course of disease that was nearly superimposable onto that caused by the parent strain. The TX 5264 (GelE− SprE+)-infected eyes retained better visual function (24%) at 24 h than the eyes infected with TX 5243 (GelE+ SprE−) or OG1RF (both below 10%), a difference that was statistically significant (P < 0.05) and reproducible in repeat experiments but transient.
FIG. 2.
Retinal function after intraocular infection with E. faecalis OG1RF and the mutants TX 5266 (OG1RF ΔfsrB), TX 5264 (GelE− SprE+), TX 5243 (GelE+ SprE−), and TX 5128 (GelE− SprE−). Rabbits were injected with 100 CFU, and retinal function was assessed at 24, 36, and 48 h after injection. Percent retinal function was defined as the ratio of the B-wave amplitude of the infected eye to the B-wave amplitude of the saline-injected contralateral eye. Error bars represent the standard errors of the mean. *, statistically significant result in comparison to results for the wild type, OG1RF (P < 0.05, two-tailed t test for unequal variances); †, statistically significant difference in results for TX 5264 (GelE− SprE+) and TX 5243 (GelE+ SprE−) at 24 h (P < 0.05).
In contrast to the gelE and sprE single mutants, a gelE sprE double mutant (TX 5128; GelE− SprE−) defective in production of both gelatinase and serine protease was significantly attenuated in the rabbit endophthalmitis model. TX 5128 resulted in infections characterized by a loss of retinal function of 31% at 24 h postinfection. Importantly, this loss of retinal function was significantly greater than the loss of 8% observed for infection caused by the fsrB deletion mutant TX 5266 (P < 0.05), which is also phenotypically defective in GelE and SprE. By 36 h, retinal function in eyes infected with the double knockout strain TX 5128 (GelE− SprE−) had decreased to 22% of preoperative levels, which was a significantly greater retention of function than that observed in eyes infected with mutants defective in only gelatinase (TX 5264 GelE− SprE+) or serine protease (TX 5243 GelE+ SprE−) production (P < 0.01) but a significantly greater loss than that observed in eyes infected with the fsrB deletion mutant TX 5266 (P < 0.005), which had retained 51% of retinal function. At 48 h, retinal function in the eyes infected with the mutant defective in both gelatinase and serine protease production (TX 5128) was undetectable and similar to that of eyes infected with the mutants defective in either gelatinase or serine protease production only, whereas the eyes infected with the fsrB deletion mutant TX 5266 still retained 41% of retinal function.
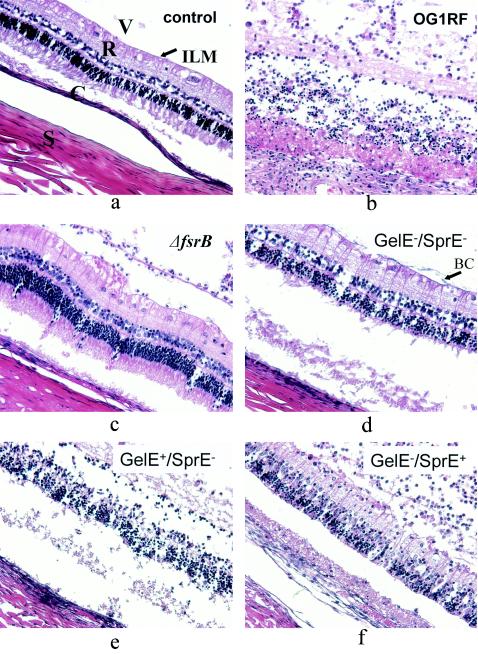
Histopathological slides were ranked according to severity, and the median severities of individual groups were compared. This approach was necessary because histopathological changes within the individual groups were variable, and changes overall were less extensive than those observed with cytolytic E. faecalis or more virulent organisms such as S. aureus (1, 9). In most eyes, the bacteria and the inflammatory infiltrate appeared to be concentrated in the inferior portion of the globe. The most dramatic histopathological changes were observed in the retina just below this accumulation of neutrophils and bacteria. As was observed previously (14), eyes infected with E. faecalis OG1RF showed marked vitreal polymorphonuclear infiltrate, cystoid changes in the ganglionic cell layer, decreased nuclear density of the inner and outer nuclear layers, and subretinal polymorphonuclear infiltrate; the majority of eyes showed an overall loss of structural integrity within 48 h. In contrast, most of the eyes injected with TX 5266 (OG1RF ΔfsrB) showed only mild vitreal polymorphonuclear infiltrate, preserved structure of all retinal layers, and no subretinal inflammatory infiltrate by 48 h, features which were similar to those of saline-injected control eyes (representative slides are shown in Fig. 3a to c).
FIG. 3.
Thin-section histopathology (representative slides, hematoxylin and eosin stain). (a) Saline-injected control eye. The vitreous (V) with some extracellular matrix and no inflammatory cells, the internal limiting membrane (arrow labeled ILM), the retina with intact nuclear and plexiform layers (R), the choroid (C), and the sclera (S) can be clearly discerned. (b) Infection with E. faecalis OG1RF after 48 h, showing marked vitreal polymorphonuclear infiltrate, cystoid changes in the ganglionic cell layer, decreased nuclear density of the inner and outer nuclear layers, mild subretinal polymorphonuclear infiltrate, and overall loss of structural integrity. (c) Infection with TX 5266 (OG1RF ΔfsrB) after 48 h, showing mild vitreal polymorphonuclear infiltrate, preserved structure of all retinal layers, and no subretinal inflammatory infiltrate. (d) Infection with TX 5128 (GelE− SprE−) after 48 h. The appearance is similar to that after infection with TX 5266 (OG1RF ΔfsrB). Note the bacterial clusters on the internal limiting membrane (arrow labeled BC). (e) Infection with TX 5243 (GelE+ SprE−) after 48 h. The appearance is similar to that after infection with the wild type, OG1RF. (f) Infection with TX 5264 (GelE− SprE+) after 48 h, with marked vitreal inflammation, mild to moderate retinal and subretinal infitration, and relatively preserved retinal structure.
Interestingly, infection with TX 5128 (GelE− SprE−) resulted in less severe histopathological changes after 48 h than those observed in the eyes infected with wild-type OG1RF, even though retinal function in the eyes infected with TX 5128 was undetectable at that time. In particular, inflammatory infiltration was less marked (representative slide shown in Fig. 3d), and complete loss of structural integrity could be observed only in 1 out of 10 specimens. These histopathological changes were never seen in the TX 5266 (OG1RF ΔfsrB)-infected eyes.
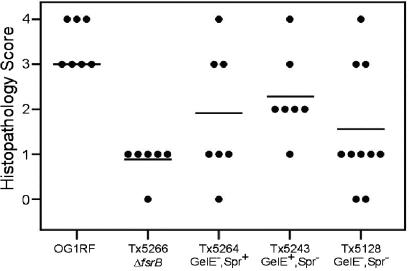
The median severity of disease as reflected in the stained sections of the eyes infected with mutants defective in the expression of gelatinase (TX 5264; GelE− SprE+) or serine protease (TX 5243; GelE+ SprE−) was greater than that observed for the TX 5266 (OG1RF ΔfsrB) or TX 5128 (GelE− SprE−) mutants. The majority of eyes infected with TX 5264 or TX 5243 demonstrated moderate to marked vitreal infiltration and various degrees of disintegration of retinal structure (Fig. 3e and f). A score of 0 to 4 was assigned to each histopathological slide according to the standardized grading system described above, and the results of this analysis are depicted in Fig. 4. At 48 h after infection, statistical significance could be demonstrated for the difference between wild-type OG1RF and every mutant studied (P < 0.02), corroborating the results obtained by ERG. However, the statistically significant differences for retinal function observed with ERG between the different mutants at 24 and 36 h did not translate into statistically significant differences in histopathological appearance at 48 h (Fig. 3 and 4) or 24 h (data not shown).
FIG. 4.
Histopathological results. Numerical scores ranging from 0 (normal) to 4 (most severe) were assigned to each slide according to a standardized grading system. The mean was significantly higher for the OG1RF-infected group than for the mutant-infected groups (P < 0.02). The apparent differences between the means for individual mutants are not statistically significant.
The E. faecalis fsr system is known to regulate two known proteases, a gelatinase and a serine protease (15). A structural relationship between the enterococcal fsr system and the global regulatory agr system in S. aureus has been observed based on sequence similarities between the two (15). The S. aureus agr system in concert with sar has been demonstrated to govern the expression of at least 19 exoproteins. This system has been shown to be involved in the pathogenesis of S. aureus infections, including endophthalmitis (1). It was previously demonstrated that in experimental enterococcal endophthalmitis, a mutation in the enterococcal fsr locus mitigated the course of retinal-function loss (14). In the present study, we sought to determine the individual contribution of the two proteases known to be regulated by fsr. We found that only when both proteases were knocked out simultaneously could a distinct reduction in severity be demonstrated. Infection with a mutant defective in the expression of sprE but which produces gelatinase showed a course of disease that was indistinguishable from that caused by the wild-type strain at all time points. Infection with a mutant defective in gelatinase (TX 5264; GelE− SprE+) led to a nominally but reproducibly attenuated course of disease as measured by ERG at 24 h but otherwise rapid and complete loss of this residual function 12 h later. In contrast to the results obtained with mutants defective singly in production of either protease, infection with a mutant defective in production of both GelE and SprE (TX 5128; GelE− SprE−) was significantly attenuated early; however, retinal-function loss was complete by 48 h. These data suggest that serine protease and gelatinase have redundant activities in the pathogenesis of endophthalmitis. Similar observations have been made in a C. elegans killing assay in which the strains with single mutations in either gelatinase or serine protease were observed to be only nominally less toxic than wild-type OG1RF (17).
The course of disease observed in eyes infected with the mutant TX 5128, which is defective in both extracellular proteases as the result of a polar insertion in gelE (15), is significantly different from that observed for infection with TX 5266 (OG1RF ΔfsrB). This difference suggests that either the polar effect of the mini γδ insertion on sprE expression is not complete, even though sprE activity is reduced below levels that are detectable in vitro (15, 18), or that there are other pleiotropic effects of the deletion within fsrB which relate to the expression of other traits contributing to the pathogenesis of enterococcal infection.
Acknowledgments
This research was supported by NIH grants EY08289 and AI41108 (to M.S.G.), NIH grant EY12190 and an unrestricted grant from Research to Prevent Blindness for support of the Dean A. McGee Eye Institute (DMEI) Animal Facility, a grant from Aventis SA to Massachusetts General Hospital (to F.M.A. and S.B.C.), a Postdoctoral Research Fellowship for Physicians from the Howard Hughes Medical Institute (E.M.), and a project grant from the Georg und Hannelore Zimmermann Stiftung, Munich, Germany (to M.E.).
We thank Barbara E. Murray for providing us with mutant strains. The technical assistance of Mark Dittmar (DMEI animal facility) and Paula Pierce (DMEI Pathology) is greatly appreciated. We also thank Phillip S. Coburn and Christopher M. Pillar for helpful discussions.
Editor: J. N. Weiser
REFERENCES
- 1.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 65:1550-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow, J. W., L. A. Thal, M. B. Perri, J. A. Vazquez, S. M. Donabedian, D. B. Clewell, and M. J. Zervos. 1993. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 37:2474-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garsin, D. A., C. D. Sifri, E. Mylonakis, X. Qin, K. V. Singh, B. E. Murray, S. B. Calderwood, and F. M. Ausubel. 2001. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 98:10892-10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas, W., B. D. Shepard, and M. S. Gilmore. 2002. Two-component regulator of Enterococcus faecalis cytolysin responds to quorum-sensing autoinduction. Nature 415:84-87. [DOI] [PubMed] [Google Scholar]
- 5.Hancock, L. E., and M. S. Gilmore. 2002. The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall. Proc. Natl. Acad. Sci. USA 99:1574-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ike, Y., H. Hashimoto, and D. B. Clewell. 1984. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect. Immun. 45:528-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 9.Jett, B. D., H. G. Jensen, R. E. Nordquist, and M. S. Gilmore. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 60:2445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mundy, L. M., D. F. Sahm, and M. Gilmore. 2000. Relationships between enterococcal virulence and antimicrobial resistance. Clin. Microbiol. Rev. 13:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray, B. E. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710-721. [DOI] [PubMed] [Google Scholar]
- 12.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mylonakis, E., and S. B. Calderwood. 2001. Infective endocarditis in adults. N. Engl. J. Med. 345:1318-1330. [DOI] [PubMed] [Google Scholar]
- 14.Mylonakis, E., M. Engelbert, X. Qin, C. D. Sifri, B. E. Murray, F. M. Ausubel, M. S. Gilmore, and S. B. Calderwood. 2002. The Enterococcus faecalis fsrB gene, a key component of the fsr quorum-sensing system, is associated with virulence in the rabbit endophthalmitis model. Infect. Immun. 70:4678-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2000. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect. Immun. 68:2579-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2001. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J. Bacteriol. 183:3372-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sifri, C. D., E. Mylonakis, K. V. Singh, X. Qin, D. A. Garsin, B. E. Murray, F. M. Ausubel, and S. B. Calderwood. 2002. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect. Immun. 70:5647-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 178:1416-1420. [DOI] [PubMed] [Google Scholar]
- 19.Stevens, S. X., H. G. Jensen, B. D. Jett, and M. S. Gilmore. 1992. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental Enterococcus faecalis endophthalmitis. Investig. Ophthalmol. Vis. Sci. 33:1650-1656. [PubMed] [Google Scholar]