Abstract
Antibody (Ab) responses to polysaccharides (PS), such as Neisseria meningitidis group C PS (MCPS), are characterized as being thymus independent and are restricted with regard to clonotype and isotype expression. PS conjugated to proteins, e.g., MCPS coupled with tetanus toxoid or the diphtheria toxin derivative CRM197, elicit thymus-dependent responses. The present study developed a surface plasmon resonance approach to evaluate Ab responses to MCPS conjugate vaccines, including either O-acetylated (OAc+) or de-O-acetylated (OAc−) forms of the PS. The results were generally consistent with those obtained by enzyme-linked immunosorbent assay and showed that sera from mice immunized with conjugate vaccines contain Abs that bind more effectively to OAc+ and OAc− MCPS than sera from mice immunized with fixed bacteria. The data suggest a critical shared or overlapping epitope recognized by all the conjugate vaccine immune sera and strategies for assessing polyclonal Ab avidity.
A major virulence factor of many pathogenic bacteria that cause invasive diseases is the capsular polysaccharide (PS). Antibodies (Abs) against these PSs are protective (29, 30), but poor immune responses are observed in infants (19, 29, 49, 58). PSs are classified as thymus-independent type 2 (TI-2) antigens (Ags) because they do not require mature T cells to elicit a humoral response in vivo. The ability to elicit an Ab response to TI-2 Ags develops late in ontogeny (28, 49, 55) and in mice requires a subset of B cells that mature late and are defined by the expression of Lyb5 and other cell markers (46, 61). In general, TI-2 Ags elicit a poor memory response and fail to result in affinity maturation of the Ab response. In contrast, the ability to respond to a thymus-dependent (TD) Ag is present at birth and results in the formation of memory cells, and the Ab response undergoes subsequent affinity maturation upon reimmunization (59). For TI-2 Ag, immunoglobulin M (IgM) and IgG3 are the major isotypes expressed in mice, even after secondary immunization (50), whereas for TD Ag, the ratio of IgG to IgM increases after secondary immunization, with IgG1 being the major subclass (54, 60, 61).
The immunogenicity of TI-2 Ag has been shown to be enhanced by covalently binding TI-2 Ag to carrier proteins, thus converting the response to TD (7, 60, 61). Haemophilus influenzae type b (Hib) was once the most common cause of bacterial meningitis in children under 5 years of age in the United States, but immunization with TD conjugate vaccines has been remarkably successful in decreasing the incidence of Hib disease (1, 16, 47). The almost complete disappearance of Hib disease and the reduction in pharyngeal carriage of Hib (8) highlight the importance of these conjugate vaccines for public health (8, 16, 62).
Neisseria meningitidis remains one of the major causes of bacterial meningitis in children and young adults worldwide (33), with recent outbreaks of different serogroups (22, 24, 44). N. meningitidis PS vaccines have been widely available for >25 years (31); however, the PS is a TI-2 Ag that is poorly immunogenic in infants and has a short duration of protection in young children (15, 26, 27, 41). The meningococcal group C capsular PS (MCPS) is a linear homopolymer of α(2→9)-linked sialic acid residues that are O-acetylated (OAc+) at carbons 7 and/or 8 (10, 20). In nature, ∼85% of infections occur with OAc+ strains and the rest with de-O-acetylated (OAc−) strains (6), although in the United Kingdom the proportions of fatal cases caused by OAc+ and OAc− meningococcal group C strains were not significantly different (12).
Previous studies of mice showed that mainly IgG1 Abs to PS and carrier were produced in response to a single dose of meningococcal conjugate vaccine (9) and that IgG titers increased after a second dose (18, 54). Several oligosaccharide-protein conjugate vaccines that elicit a TD response to protect young children against invasive meningococcal disease (18, 35, 36) have been developed and evaluated in clinical trials around the world (4, 13, 14, 18, 21, 38, 40, 42, 43, 52, 53, 65). Recently, the meningococcal group C conjugate (MCC) vaccines have been approved for routine immunization in Europe (57). Licensure was based on immunogenicity and safety data alone, but recent estimates suggest the efficacy of the conjugate vaccine in teenagers and toddlers in England to be ∼90% (5, 51). Two of the three licensed MCC vaccines contain PSs that are OAc+ coupled to CRM197, a nontoxic mutant diphtheria toxin. In one, the MCPS is conjugated directly to the carrier; in the other, it is conjugated to the carrier using a bis-N-hydroxysuccinimide ester linker. The third vaccine consists of an OAc− MCPS conjugated to tetanus toxoid (MCC-TT). The presence or absence of OAc groups generates unique epitopes, and the specificity of Ab binding to MCPS can affect bactericidal activity against OAc+ and OAc− strains (6, 45, 56). It is possible that these differences in the compositions of the MCC vaccines affect their potential to offer protection against meningococcal strains expressing each form of the group C capsule (OAc+ or OAc−) (12). In addition to epitope specificity, Ab levels and affinity (63) play important roles in protection. Thus, assays that can measure Ab specificity, concentration, and affinity can contribute to the evaluation of sera from immunized individuals. In this study, we use BIAcore (BIAcore, Inc., Piscataway, N.J.) to evaluate sera from mice immunized with MCC vaccines.
BIAcore uses surface plasmon resonance (SPR) to measure the accumulation of soluble molecules, such as Abs, on a sensor chip matrix to which the ligand is covalently attached (37). The interaction between soluble molecules and the ligand molecules can be followed in real time, allowing the characterization of binding kinetics, as well as binding at equilibrium (3, 11, 39, 66). We have determined the binding and apparent dissociation half-times for monoclonal Abs (MAbs) specific for different MCPS epitopes (23) and sera from mice immunized with different conjugate vaccines using BIAcore with OAc−-bovine serum albumin (BSA) or OAc+-BSA immobilized on the sensor chip. The end point titer, as well as the relative amounts of IgM and IgG, was determined by fluorescence enzyme-linked immunosorbent assay (FELISA). We have also compared the two methods in assessing the binding of the polyclonal Abs and MAbs to MCPS epitopes.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female BALB/cAnN (BALB/c) mice were purchased from Charles River Laboratories and maintained under pathogen-free conditions in our animal rooms. All animal protocols were approved by the Center for Biologics Evaluation and Research (CBER) Animal Care and Use Committee or the Animal Ethics Committee at the National Institute for Biological Standards and Control.
PS.
The structures of the PSs used in these studies are shown in Table 1. Native MCPSs (OAc+) were prepared from N. meningitidis strain C11 and obtained from Merck, Inc., West Point, Pa. (lot 1815T). OAc− MCPS prepared from N. meningitidis strain MC19 was obtained from the Division of Bacterial, Parasitic and Allergenic Products, Office of Vaccines Research and Review, CBER. The Escherichia coli K92 and K1 PSs were obtained from Willie Vann, Division of Bacterial, Parasitic and Allergenic Products, Office of Vaccines Research and Review, CBER.
TABLE 1.
Structures of PSs used in this study
| PS | Structure |
|---|---|
| MCPS OAc+ | 9)-α-NeuNAC-(2→ |
| 7/8 | |
| ▴ | |
| O-acetyl | |
| MCPS OAc− | 9)-α-NeuNAC-(2→ |
| E. coli K92 | 8)-α-NeuNAC-(2→9)-α-NeuNAC-(2→ |
| E. coli K1 | 8)-α-NeuNAC-(2→ |
Conjugate vaccines.
Three conjugate vaccines were used in these studies; two, vaccine 1 (Vac1) and Vac2, consisted of oligosaccharides of OAc+ MCPS conjugated to CRM197. The third, Vac3, consisted of OAc− MCPS conjugated to TT. Three different manufacturers manufactured these conjugate vaccines.
Immunization.
Sera were produced by immunizing groups of 10 female BALB/c mice with OAc+ or OAc− fixed bacteria. The mice used in the OAc+ or OAc− bacterial experiments were in a weight range of 18 to 20 g and were immunized intraperitoneally (i.p.) with 0.5 ml (108 CFU) of fixed bacteria on day 0 and bled by cardiac puncture on day 8. Groups of 10 mice weighing between 16 and 18 g were immunized with Vac1, Vac2, or Vac3 on day zero. They were immunized i.p. with one-fifth of a human dose (equivalent to 2 μg of MCPS) of vaccine on day 0, boosted i.p. with a further one-fifth dose of vaccine on day 28, and bled on day 42.
MAbs.
The specificities of the MAbs used for validation of binding in the SPR analyses and inhibition experiments were described previously (23, 56) and are summarized in Table 2. MAbs 2055.5 and 2016.3 were obtained by immunizing BALB/c mice with OAc+ fixed bacteria (56). MAbs C2/1076.10, C2/655.7, and C2/256.8 were obtained by immunizing BALB/c mice with MCPS-TT conjugate vaccine and boosting them with the same vaccine (23).
TABLE 2.
Fine specificities, avidities, and bactericidal activities of MAbsa
| MAb | Specificity by FELISA | Specificity by PPTb
|
Concn at 50% bindingc
|
Concn at 50% killingd
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| MCPS | OAc− | K92 | MCPS | OAc− | K92 | MCPS | OAc− | ||
| IgG3κ | |||||||||
| 2055.5 | MCPS | + | −e | − | 0.2 | −f | − | 0.18 | −g |
| 2016.3 | OAc− >> MCPS | + | + | − | >3.0 | 0.1 | − | 0.63 | 1.1 |
| IgG1κ | |||||||||
| C2/1076.10 | MCPS ≈ OAc− >>> K92 | + | + | −h | 0.005 | 0.002 | 1.5 | NDi | ND |
| C2/655.7 | OAc− >> MCPS | + | + | − | 0.3 | 0.003 | − | ND | ND |
| C2/256.8 | MCPS ≈ OAc− | + | + | − | 0.005 | 0.003 | − | ND | ND |
The fine specificity and precipitation with MCPS and OAc−, the concentration at 50% binding of MAb, and 50% killing were published previously (23, 56).
Determined by Ouchterlony precipitation (PPT) (48). Proteins were tested at 1 mg/ml. +, precipitation; −, no precipitation.
Concentration (micrograms per milliliter) at 50% binding were determined at the midpoint of the linear part of the titration curve on PS-coated plates. Proteins were tested by 12 threefold serial dilutions starting at 3 μg/ml.
The concentration (micrograms per milliliter) of antibody at which 50% of the bacteria were killed.
Not detectable at highest concentration tested (1 mg/ml).
Not detectable at highest concentration tested (3 μg/ml).
No detectable killing.
Negative using the purified protein, but undiluted ascites fluid was positive on all three Ags and negative on K1.
ND, not determined.
FELISA.
IgM and IgG isotypes in sera were determined by direct binding in a FELISA as described in detail elsewhere (55). End point titers are expressed as reciprocal dilutions and were determined by extrapolation to zero from the linear part of the titration curve using Unitcalc software (PhPlate AB, Stockholm, Sweden). The sera were tested by 8 to 12 threefold serial dilutions starting at a serum dilution of 1:30. The FELISA assay is similar to a conventional ELISA; however, it has three unique features. The MicroFLUOR “W” U plates (Dynatech Laboratories, Chantilly, Va.) are opaque, and PSs adhere well; the substrate, 4-methylumbelliferyl phosphate (Sigma Chemical Co., St. Louis, Mo.), is not hydrolyzed in water, resulting in a background that is stable over time; and the scale on the microfluor reader (Dynatech) is 0 to 4 rather than 0 to 2. The Ags with which the plates were coated for the assays used in these experiments were OAc+, OAc−, and K92 purified PSs. For isotype analysis of the immune sera, alkaline phosphatase-labeled goat anti-mouse IgM and IgG were used. These alkaline phosphatase anti-isotype reagents were purchased from Southern Biotechnology (Birmingham, Ala.).
SPR analyses.
Analyses were done using the BIAcore 3000 instrument, and data were analyzed using BIAevaluation version 3.0. The running buffer used for all experiments was HBS buffer, pH 7.4, containing 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20, filtered and degassed. Research grade CM5 sensor chips were activated by mixing equal amounts of N-ethyl-N′-(dimethylaminopropyl)carbodiide and N-hydroxysuccinimide. BSA-PS conjugates (OAc+-BSA and OAc−-BSA), kindly provided by Harold Jennings, National Research Council, Ottawa, Canada, were immobilized in 10 mM sodium acetate buffer, pH 4.0, followed by ethanolamine to block the unreacted dextran. The optimal pH to immobilize BSA and BSA conjugates (OAc+-BSA and OAc−-BSA) to the dextran matrix on the CM5 sensor chips was determined at 25°C. The pH range used was from 5.5 to 2.0 in gradations of 0.5. BSA or BSA conjugate (10 μg/ml) was passed through the chip (flow rate, 5 μl/min). Optimal immobilization occurred between pH 2.0 and 4.0, with some differences between the different ligands. To select the immobilization pH for experimental surfaces, we compared the binding of an anti-MCPS MAb to OAc+ conjugate surfaces immobilized at pH 2.0 and 4.0. There was no notable difference in the binding. However since de-O-acetylation can occur at low pH (34), we chose pH 4.0, as indicated above. Additional studies were done to evaluate the effects of the ligand concentration and dwell time on immobilization. Based on these studies, OAc+-BSA and OAc−-BSA conjugates were immobilized at a concentration of 50 μg/ml, with a contact time of 28 min and a flow rate of 5 μl/min. These conditions gave ∼200 to 350 resonance units (RU) of immobilized OAc+-BSA material. An RU corresponds to an immobilized protein concentration of ≈1 pg/mm2. Similar contact times and flow rates gave higher RU values for OAc−-BSA and BSA. To immobilize similar levels of OAc+-BSA and OAc−-BSA conjugates, a BIAcore sensor chip for the analysis of MAbs was prepared using a programmable feature for targeted immobilization. The chip was set up as follows: channel 1 was used as a blank, BSA (706.5 RU) was bound to channel 2, The OAc−-BSA conjugate (242.8 RU) was bound to channel 3, and channel 4 contained the OAc+-BSA conjugate (243.7 RU). Successful immobilization of Ags was verified by the use of well-defined MAbs (23, 56).
For the evaluation of binding, samples were diluted in HBS buffer and analyzed at several concentrations. Ab at the specified concentration was injected over the sensor chip surface for 4 min at a flow rate of 10 μl per min. The sensor chip was regenerated between cycles with two 1-min injections of 2.5 M NaCl at the same flow rate. The corrected response, in RU, was obtained by subtraction of the RU detected at the same MAb or serum concentration with unconjugated BSA coupled to the sensor chip. Thus, the resulting sensorgrams were corrected for background binding to the dextran matrix coupled to BSA, as indicated by Cooper et al. (17). For generation of concentration-dependent binding curves with the corrected sensorgrams, we calculated the concentration-dependent average RU values from an ∼20-s interval in the last 40 s of each 240-s injection. The binding was evaluated late in the injection to better reflect equilibrium values.
The bivalent nature of Abs can complicate the modeling of binding data for equilibrium and kinetic binding constants. In addition, since immune sera are polyclonal, the sensorgrams from these sera are the sums of a number of different Ab interactions, and the exact concentrations of the Abs are unknown. This makes modeling difficult, since the determination of equilibrium and kinetic association constants are concentration dependent. However in ideal Langmuir 1:1 binding, the dissociation can be modeled as a concentration-independent exponential decay. Thus, we attempted to model the dissociation of MAb and polyclonal-Ab binding to MCPS as an exponential decay. For evaluation of kinetics, we calculated the apparent half-life of the Ab bound to the Ag-coated surface. This was done by fitting the binding data from 40 to 120 s postinjection with an exponential decay model using BIAevaluation version 3.0 software. Comparison of the apparent half-life values was performed with the Student t test or with the Tukey-Kramer honestly significant difference (HSD) test for multiple comparisons. A P value of <0.05 for the Tukey-Kramer HSD test is significant, since it compensates for multiple comparisons, while a P value of <0.05 for the Student t test is suggestive.
Inhibition experiment.
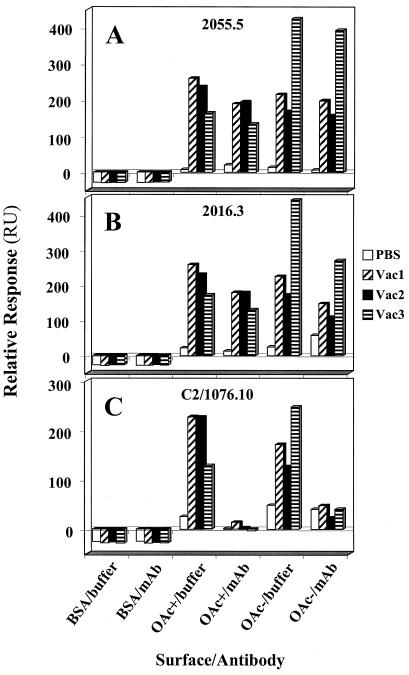
To determine if similar epitopes were bound by the Abs produced in response to vaccination with the different conjugate vaccines, we blocked OAc−-BSA- or OAc+-BSA-coated surfaces with MAbs of different specificities. MAbs 2055.5, 2016.3, C2/1076.10, and C2/256.8 were described previously (23, 56) (Table 2). Different concentrations of sera, starting at dilutions of 1:20, were injected across OAc+ or OAc− conjugate surfaces before and after the surface was blocked with a MAb. The amounts of binding pre- and postblockade were compared using the Student t test and the Tukey-Kramer HSD test for multiple comparisons. The significant differences at the 0.05 level for these tests and the percent inhibition [using the formula percent inhibition = (RU with MAb − RU with MAb control surface)/(RU − RU control surface)] were evaluated. The data shown for MAbs 2055.5, 2016.3, and C2/1076.10 (see Fig. 6) are the averages of duplicates for one dilution (1:20).
FIG. 6.
Inhibition of binding by MAbs with different specificities. The average of duplicates for a 1/20 dilution of PBS immune serum, Vac1 immune serum, Vac2 immune serum, and Vac3 immune serum are shown. The blocking MAbs were added at 100 μg/ml, or buffer was added as a control. (A) OAc+-specific MAb 2055.5. (B) OAc− ≫ OAc+-specific MAb 2016.3. (C) OAc+ ≈ OAc− >>> K92-specific MAb C2/1076.10.
RESULTS
FELISA.
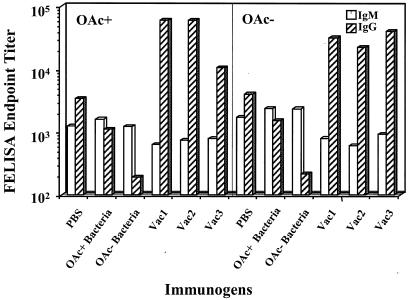
The levels of MCPS-specific IgM and IgG isotypes in sera were determined by FELISA and are shown in Fig. 1. Ab titers induced by immunization with bacteria were low compared with those induced by the conjugate vaccines. Figure 1 also indicates that immunization with fixed bacteria actually depleted the sera of specific Abs compared with the control sera. These phenomena have been observed previously (K. Stein, unpublished observations). Sera from mice immunized with OAc− bacteria contained more IgM than IgG Abs and bound the OAc+ and OAc− Ags with approximately equal avidities. Sera from mice immunized with OAc− bacteria showed slightly higher relative avidity for the OAc− Ag (Table 3). Sera from mice immunized with the conjugate vaccines had more MCPS-specific IgG than IgM. The two vaccines containing OAc+ PS (Vac1 and Vac2) elicited Ab responses of approximately equal reactivities to both the OAc+ and the OAc− Ags, whereas the OAc− vaccine (Vac3) induced higher-titer IgG Abs for OAc− than OAc+ Ag and a higher relative avidity for the OAc− Ag (Table 3). Sera from control mice immunized with phosphate-buffered saline (PBS) were approximately equally reactive with either OAc+ or OAc− MCPS and contained more IgG than IgM Abs. Table 3 shows that Vac2 and Vac3 stimulated somewhat higher-avidity Abs than Vac1 and that the bacterium-immune IgM has higher avidity than the IgG, something to be expected from nonmutated low-affinity Abs where the decavalent IgM shows higher relative avidity than the low-affinity bivalent IgG.
FIG. 1.
Total IgM and IgG end point titers, determined by FELISA, of anti-meningococcal serogroup C Abs in the pooled sera from groups of 10 mice immunized with different conjugate vaccines or fixed bacteria. PBS was used as a control. The titers are expressed as the log reciprocal dilution. The data represent the averages of three experiments.
TABLE 3.
Relative avidities of total IgM and IgG from murine sera by FELISA
| Immunization | Relative avidity for MCPS Ag on platea
|
|||
|---|---|---|---|---|
| OAc+
|
OAc−
|
|||
| IgM | IgG | IgM | IgG | |
| PBS | 0.05 | >1 | 0.03 | >1 |
| OAc+ bacteria | 0.005 | 0.4 | 0.004 | 0.35 |
| OAc− bacteria | 0.01 | 0.75 | 0.004 | 0.65 |
| Vac1 | 0.05 | 0.0002 | 0.04 | 0.001 |
| Vac2 | 0.03 | 0.0002 | 0.09 | 0.001 |
| Vac3 | 0.02 | 0.0006 | 0.03 | 0.0002 |
Values are reciprocal dilutions for 50% binding defined as an optical density of 2 on a scale of 0 to 4 for each serum from mice immunized with different bacterial strains or conjugate vaccines as indicated. The data are from similar results in two experiments. PBS was used as a control.
SPR analyses.
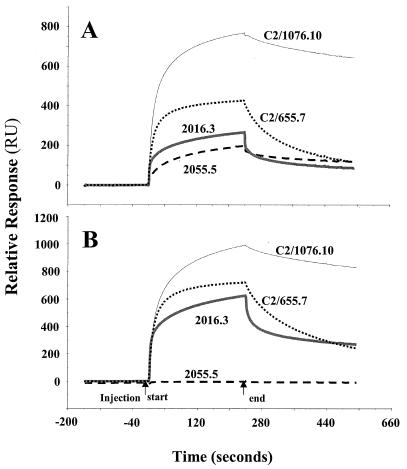
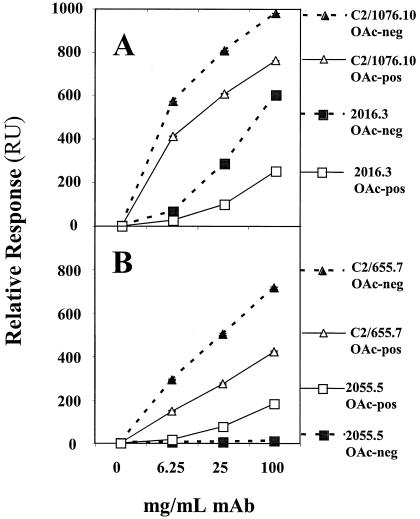
Studies with individual MAbs of known concentration established the ability of the BIAcore to discriminate between Abs with low and high affinity and specificity for either OAc+-BSA or OAc−-BSA Ag, as shown in Fig. 2. The sensorgrams represent real-time binding of the indicated MAbs at 100 μg/ml. The low-avidity MAb 2055.5 is reactive only with the OAc+ surface. MAb 2016.3 binds less well than the higher-avidity MAbs C2/655.7 and C2/1076.10 and has a preference for the OAc− surface. This binding pattern is similar to that seen by FELISA (Table 2). We also evaluated MAb binding at different concentrations. Figure 3 shows the near-equilibrium values of binding as a function of concentration. The low-avidity MAb 2055.5 has weak concentration-dependent binding to the OAc+ surface. The other MAbs bind the OAc+ surface; however, a greater preference for the OAc− surface is seen with MAbs 2016.3 and C2/655.7. Again, MAb C2/1076.10 has the best binding to both surfaces. This supports the FELISA data on these MAbs.
FIG. 2.
SPR sensorgrams of MCPS binding with four murine anti-MCPS MAbs, C2/1076.10, C2/655.7, 2016.3, and 2055.5, with different FELISA specificities. (A) Binding to OAc+-BSA conjugate (243.7 RU immobilized). (B) Binding to OAc−-BSA conjugate (242.8 RU immobilized).
FIG. 3.
Concentration-dependent MCPS binding of four murine anti-MCPS MAbs with different specificities. The markers represent the averages of near-equilibrium binding, and the error bars (within the symbols) represent the range of two determinations. (A) C2/1076.10 and 2016.3 binding to OAc− and OAc+ surfaces. (B) C2/655.7 and 2055.5 binding to OAc− and OAc+ surfaces. The OAc+ and OAc− MCPSs conjugated to BSA were used as the coating Ags.
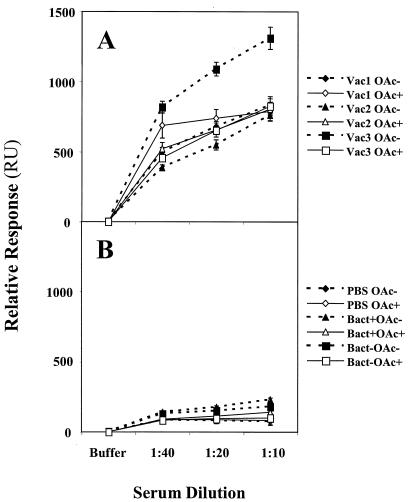
Sera from immunized mice were also studied by BIAcore for binding to OAc− and OAc+ surfaces. Dilutions of the sera were assessed for near-equilibrium binding (Fig. 4), and sera from conjugate vaccine-immunized mice had far greater binding than sera from fixed N. meningitidis- or PBS-immunized mice. All three MCCs induced Abs with similar specificities for the OAc+ Ag, while the OAc− conjugate vaccine (Vac3) induced Abs with a greater preference for the OAc− Ag. In contrast, fixed bacteria tended to induce Abs with a preferred specificity for OAc− Ag rather than for OAc+ Ag irrespective of the O-acetylation status of their PS capsules. These results are also similar to FELISA data (Fig. 1 and Table 3).
FIG. 4.
Concentration-dependent MCPS binding of murine serum Abs induced by immunization. The markers represent the averages of near-equilibrium binding, and the error bars represent the ranges of two determinations. (A) Sera from hosts immunized with MCC conjugate vaccines by Vac1, Vac2, and Vac3 binding to OAc− and OAc+ surfaces. (B) Sera from hosts immunized with PBS, OAc+ fixed bacteria, and OAc− fixed bacteria binding to OAc− and OAc+ surfaces.
One advantage of SPR analysis is the potential for calculating equilibrium and kinetic binding constants. However, the bivalent nature of Abs can complicate the modeling of binding data for equilibrium and kinetic binding constants. In addition, since some of the samples we have evaluated are polyclonal, the sensorgrams are the sum of a number of different Ab interactions, and the exact concentrations of the Abs are unknown. This makes modeling difficult, since the determinations of equilibrium and kinetic association constants are concentration dependent. However, in ideal Langmuir 1:1 binding, the dissociation can be modeled as a concentration-independent exponential decay. Thus, we attempted to model the dissociation of MAb and polyclonal-Ab binding to MCPS as an exponential decay. The apparent binding half-lives of anti-MCPS MAbs were calculated using this modeling (Fig. 5A). Overall, these values correlate with the equilibrium data from SPR and FELISA. The higher-avidity MAb C2/1076.10 has a longer binding half-life than the lower-avidity MAbs 2016.3 and 2055.5. This difference was statistically significant (P < 0.05; Tukey-Kramer HSD). The binding of C2/1076.10 had a long half-life for both OAc− and OAc+ surfaces, although the OAc+ half-life was significantly longer. This correlates with the slightly higher SPR equilibrium binding seen with MAb C2/1076.10 on an OAc+ surface. The results also suggest a longer half-life for binding to an OAc+ surface with the OAc+-specific MAb 2055.5 and a longer half-life for binding to an OAc− surface with the OAc−-preferring MAb C2/655.7. Although these results are similar to equilibrium results, some differences were noted. The C2/655.7 MAb had a high avidity for OAc− PS and a low avidity for OAc+ PS by FELISA. However, the calculated C2/655.7 half-lives for both OAc+ and OAc− surfaces are short. In addition, the 2016.3 MAb preference for OAc− PS is not observed in the half-lives of binding to OAc+ and OAc− surfaces. Possible explanations for the differences between the equilibrium and kinetic dissociation measurements may reflect bivalent versus monovalent Ab binding, differences in kinetic association constants, or technical aspects of the binding in different assays.
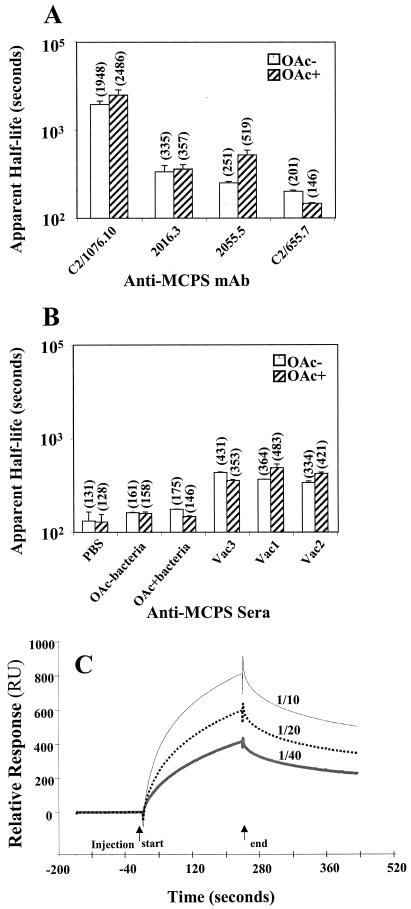
FIG. 5.
Apparent dissociation half-lives of anti-MCPS Abs. The apparent dissociation half-lives were calculated by exponential decay modeling as described in Materials and Methods. (A) Averages of four determinations after injection of Abs C2/1076.10, 2016.3, and C2/655.7 at a concentration of 3.125 or 6.25 μg/ml are shown for OAc− and OAc+ surfaces. The error bars represent the standard deviations, and the numbers in parentheses are the numerical averages. For Ab 2055.5, the bars and averages reflect two determinations and the error bars reflect the range. (B) Averages of four determinations using polyclonal sera after injection of 1/20 or 1/40 dilutions; determinations are shown for OAc− and OAc+ surfaces. The error bars represent the standard deviations, and the numbers in parentheses are the numerical averages. (C) Examples of SPR sensorgrams of Vac2 immune sera binding to OAc−-BSA conjugate (242.8 RU immobilized) at concentrations of 1/10, 1/20, and 1/40.
The apparent half-life results with polyclonal anti-MCPS sera are shown in Fig. 5B. The apparent half-lives of all the conjugate vaccine sera are longer than those seen with either the PBS or the fixed N. meningitidis immune sera (P < 0.05; Tukey-Kramer HSD). Vac1 and Vac2 conjugate vaccine sera have longer half-lives (indicating higher avidity) for OAc+ Ag than for OAc− Ag (P < 0.05; Tukey-Kramer HSD), while the Vac3 conjugate vaccine serum has a longer apparent half-life for OAc− Ag than for OAc+ Ag (P < 0.05; Tukey-Kramer HSD). These results are comparable to SPR equilibrium data (Fig. 4) and to FELISA data (Table 4). One advantage of the apparent-half-life evaluation is that a comparison between the conjugate vaccines and MAbs can be made. Because the apparent dissociation half-life can be concentration independent, some comparison of polyclonal sera with MAbs can be made. Examples of SPR sensorgrams used for deriving apparent half-lives from polyclonal sera are shown in Fig. 5C. The conjugate vaccine polyclonal sera have similar or longer half-lives than the lower-avidity MAbs and shorter half-lives than the high-avidity MAb C2/1076.10. The polyclonal-serum half-lives do not vary greatly from dilutions of 1/10 to 1/40, and they are shorter than the C2/1076.10 half-life even when it is evaluated at high concentrations (data not shown).
TABLE 4.
Percent inhibition of conjugate vaccine immune-serum binding by anti-MCPS MAbs to OAc+ and OAc− MCPSsa
| MCPS Ag surface | Vaccine | % Inhibition with MAb
|
|||
|---|---|---|---|---|---|
| 2055.5 | 2016.3 | C2/1076.10 | C2/256.8 | ||
| OAc+ | Vac1 | 25b | 28c | 84b | 31 |
| Vac2 | 16c | 20 | 88b | 68b | |
| Vac3 | 17c | 21 | 84b | 25 | |
| OAc− | Vac1 | 8 | 31c | 62b | 102c |
| Vac2 | 7 | 32c | 68b | 118b | |
| Vac3 | 7 | 37b | 75b | 69b | |
Inhibition of conjugate vaccine immune sera binding to OAc+ and OAc− surfaces by anti-MCPS MAbs was calculated by the following equation: percent inhibition = (RU with MAb − RU with MAb control surface)/(RU − RU control surface). Duplicate experiments with 1/20 serum dilution were evaluated. Significance was assessed for RU with MAb versus RU without MAb using the Tukey-Kramer HSD and Student t tests.
Inhibition significant at a P value of < 0.05 by Tukey-Kramer HSD test for multiple comparisons in each Ab group.
Inhibition significant at a P value of <0.05 by paired Student t test.
Inhibition experiments.
To determine the principal epitopes recognized by each of the immune-serum samples, inhibition experiments were performed using three MAbs with different specificities (Table 2) to saturate the OAc−- or OAc+-BSA-conjugated surfaces. Different concentrations of conjugate immune sera were passed over the surfaces before and after they were blocked with the MAb, and the amounts of binding were compared. Significant inhibition of binding of Vac1 immune serum to the OAc+ surface was observed with the OAc+-specific MAb 2055.5 (P < 0.05; Tukey-Kramer HSD) (Fig. 6A). The percent inhibition and statistical significance are shown in Table 4 for all of the blocking experiments. The data suggest possible MAb 2055.5 blockade of Vac2 and Vac3 immune sera binding to the OAc+ surface (P < 0.05; Student t test). As expected, no significant inhibition by the OAc+-specific MAb 2055.5 was observed for sera binding to the OAc− surface. MAb 2016.3 (specificity, OAc− ≫ OAc+) significantly blocked Vac3 immune serum binding to the OAc− surface (P < 0.05; Tukey-Kramer HSD) (Fig. 6B and Table 4). This is expected, since both the Vac3 immune serum and MAb 2016.3 favor OAc− Ag. The data suggest possible MAb 2016.3 blockade of Vac1 and Vac2 immune sera binding to the OAc− surface (P < 0.05; Student t test). In addition, there was possible MAb 2016.3 blockade of Vac1 immune serum binding to the OAc+ surface (P < 0.05; Student t test). Although these low-avidity MAbs can significantly block some immune sera, the percent blockade is <40% (Table 4). With the high-avidity MAb C2/1076.10 (specificity, OAc+ ≈ OAc− >>> K92), the percent blockade is much higher with all immune sera on both OAc− and OAc+ surfaces (Table 4) and the blockade was significant in all cases (P < 0.05; Tukey-Kramer HSD). This high level of blockade suggests an epitope that overlaps with or is shared among the C2/1076.10 MAb and all the conjugate vaccine immune sera. The blockade seen with MAb C2/1076.10 may be due to high avidity for a broadly recognized epitope or an epitope unique to MAb C2/1076.10 (specificity, OAc+ ≈ OAc− >>> K92). To answer this question, we blocked the conjugate vaccine immune sera with MAb C2/256.8, which also has high avidity but differs in specificity from MAb C2/1076.10 (Table 2). Although MAb C2/256.8 also recognizes OAc+ and OAc− Ags, it lacks low-avidity interaction with K92. MAb C2/256.8 showed significant blockade of Vac2 and Vac3 immune sera (P < 0.05; Tukey-Kramer HSD) and suggested blockade of Vac1 immune serum (P < 0.05; Student t test) on an OAc− surface. A high percentage of the serum reactivity with the OAc− surface was blocked (Table 4). However, the blockade of serum reactivity with the OAc+ surface differed from that of MAb C2/1076.10. The percent blockade was lower, and only one immune serum was significantly inhibited. Thus, although the high avidity of MAb C2/1076.10 contributed to the high-percentage blockade observed, the epitope seen by the MAb was important for its broad blockade of conjugate vaccine immune sera.
DISCUSSION
The primary immune response to MCPS induced mostly Abs with IgM and IgG3 isotypes, and the secondary immunization with MCPS was similar to the primary immunization, typical of a TI type 2 response (54, 59). Previous data have demonstrated that the influence of the TD Ag during the primary immunization is to induce class switching and generate a memory B-cell population that can be boosted by either a TI-2 or a TD Ag (54). The TD response to MCPS-TT shifted the response to IgG1 Abs with bactericidal activity at least 10-fold higher than the response to MCPS. Furthermore, in studies with MAbs, it was demonstrated that IgG3 Abs were of lower avidity than were IgG1 Abs (23). Ab affinity is an important determinant of host defense and should be considered as important as the Ab concentration in evaluating Ab response to vaccination (63). Ahlstedt et al. (2) demonstrated that high-avidity Abs against E. coli O Ag were more protective against intraperitoneal infection in mice than Abs of low avidity. In the case of meningococcal disease, bactericidal Abs play an important role in protection against infection with group C bacteria (29). Evidence suggests that there is a close correspondence between the amount of high-avidity anti-group C Ab in serum and complement-mediated bactericidal activity (32).
In addition to avidity, epitope specificity is another attribute of anti-MCPS that may have an impact on protection. The O-acetylation status of the PS moiety of conjugate vaccines determines the relative specificity of anti-PS Abs. Although the native MCPS has very few non-OAc+ sialic acid residues, with an average of 1.16 equivalents of O-acetyl per sialic acid (10), it still induced Abs that bind to both the OAc+ and OAc− Ags. The possibility that OAc− may be highly immunogenic is consistent with the observations of Glode et al. (25), who found a twofold-higher geometric mean anti-PS titer in sera from adult human volunteers immunized with OAc− PS than from those immunized with native MCPS (OAc+). In addition, immunization with native MCPS elicits Abs that are bactericidal for both C11 (native OAc+) and MC19 (OAc−) strains of N. meningitidis, and MC19 PS could adsorb a large part of the bactericidal activity for strain C11.
There are a number of conjugate vaccines for protection against N. meningitidis. The Vac1 and Vac2 conjugate vaccines utilize OAc+ oligosaccharides conjugated to CRM197, while Vac3 uses OAc− PS conjugated to TT. Because of the importance of avidity and specificity in the response to MCPS, we measured these parameters for the N. meningitidis conjugate vaccines using two methods, ELISA (FELISA) and SPR (BIAcore). ELISAs give important information about Ab binding; however, end point titers may not reflect true binding constants. SPR technology can assess real-time binding and allows kinetic as well as equilibrium measurements.
Preliminary experiments suggest that BIAcore data correlate with FELISA data, and SPR is a technology that can be used to measure both relative specificity and binding with serum samples. We first evaluated MAbs reactive with MCPS by using SPR. Concentration-dependent binding and OAc specificity for the MAbs correlated with FELISA data. Evaluation of conjugate vaccine immune sera for concentration-dependent binding to OAc− and OAc+ also correlated with FELISA data. For example, OAc− vaccine immune sera showed higher reactivity to OAc− Ags than the OAc+ vaccine immune sera with both FELISA and SPR assays. The SPR assays also verified the greater avidity of conjugate vaccine immune sera than fixed-bacteria immune sera. Although the two binding methodologies correlated in specificity and hierarchy, the FELISA detected some MAbs at nanogram-per-milliliter concentrations and may have greater sensitivity in this case. However, SPR offers the possibility of a more sophisticated analysis of binding.
We evaluated the global kinetics of MAb binding using a number of models, including those that compensate for bivalency or heterogeneous ligands (data not shown). The different models converged on equilibrium dissociation constants of ∼10−9 to 10−8 M for high-avidity Abs and ∼10−7 to 10−6 M for lower-avidity Abs. Due to uncertainty in the binding valency and lack of convincing fits, we decided to independently evaluate the apparent kinetic dissociation of the MAbs. Because ideal dissociation modeled as exponential decay is concentration independent, this strategy allowed us to also evaluate conjugate vaccine polyclonal immune sera. We verified that concentration was not a major factor in the apparent dissociation half-lives of the low-avidity MAbs; however, with high-avidity MAbs, such as C2/1076.10, we did observe a decrease in half-life as the concentration increased from 3.125 to 100 μg/ml (data not shown). A high-affinity Ab may bind in a monovalent manner at saturating concentrations and in a bivalent manner at lower concentrations and thus have a concentration-dependent change in half-life. Lower-affinity Abs may be detectable only with bivalent binding and thus have less half-life concentration dependence. We evaluated the MAb half-lives at concentrations of ≤6.25 μg/ml to facilitate half-life comparisons. These comparisons matched expectations from equilibrium binding with some exceptions. Possible explanations for differences between equilibrium and kinetic dissociation measurements may reflect restriction of a particular Ab to monovalent binding, differences in kinetic association constants, or other technical aspects of the binding or assay. The conjugate vaccine and fixed bacterial polyclonal immune sera were also evaluated for apparent dissociation half-lives. The conjugate vaccine immune sera have longer half-lives than fixed bacterial immune sera, half-lives similar to or longer than those of the lower-avidity MAbs, and shorter half-lives than the high-avidity MAb C2/1076.10. The polyclonal immune serum half-lives do not vary greatly from dilutions of 1/10 to 1/40, and they are shorter than the C2/1076.10 half-life even when it is evaluated at high concentrations (data not shown). Although there are difficulties with this type of evaluation, it strongly suggests that conjugate vaccine antiserum Abs have greater avidity than fixed bacterial antisera and some MAbs. Certain MAbs, such as C2/1076.10, may have higher avidity than the conjugate vaccine immune serum Abs. Such comparisons are impossible with equilibrium binding methods, since the serum Ab concentrations are unknown. Development of more sophisticated modeling for polyclonal-Ab binding will be of great value in evaluating immune sera. Advances in the use of SPR evaluations for polyclonal Abs will facilitate the evaluation of serum responses (64)
To assess whether the conjugate vaccine antisera shared epitopes with the anti-MCPS MAbs, we evaluated the binding of antisera pre- and post-Ab blockade of the SPR OAc+ and OAc− surfaces. The high-avidity MAb C2/1076.10 blocked all of the vaccine conjugate antisera on both surfaces. This suggests an epitope that is shared with or that overlaps all the conjugate vaccine immune sera and MAb C2/1076.10. This epitope does not include the O-acetyl moiety of the PS, so these observations are consistent with the suggestion that the principal role of the O-acetyl group may be to mask an immunodominant epitope (45). To distinguish between the C2/1076.10 specificity and avidity in the observed blockade, we evaluated the blockade of a second high-avidity MAb with a similar but not identical specificity, C2/256.8. This Ab strongly blocked all the conjugate vaccine immune sera on the OAc− surface but not on the OAc+ surface. This suggests that the specificity, as well as the avidity, contributes the pattern of blockade. Another MAb with a FELISA specificity similar to that of C2/1076.10 has not shown the same blockade pattern (data not shown). This suggests that there may be fine Ab specificity not revealed by FELISA studies.
Overall, our data suggest that FELISA and SPR near-equilibrium binding show similar results for the specificity and hierarchy of Ab responses to MCPS. Both methodologies demonstrate the high avidity of C2/1076.10, the higher avidity of conjugate vaccine antisera than fixed bacterial immune sera, and Ab preferences for OAc+ and OAc− PS Ags. Kinetic analysis using SPR allowed an initial comparison between polyclonal-antiserum and MAb avidities. SPR was a useful method to assess epitope specificity beyond O-acetylation using Ab epitope blockade. Monoclonal epitope blockade suggested a critical site recognized by MAb C2/1076.10 for MCPS reactivity. Although we conclude that all the conjugate vaccines used in this animal study generate relatively high-avidity Abs that can react with both OAc+ and OAc− PS Ags, there may be differences in the Ab specificities. Greater characterization of Ab specificity and correlation of the specificity with the clinical outcome may help to develop standard assays to assess successful immunizations.
Acknowledgments
We thank Carl E. Frasch, CBER/FDA, and David H. Margulies, LI/NIAID, for critical review of the manuscript.
The opinions expressed in this article are those of the authors and are not necessarily those of the Food and Drug Administration or the U.S. government.
Editor: J. N. Weiser
REFERENCES
- 1.Adams, W. G., K. A. Deaver, S. L. Cochi, B. D. Plikaytis, E. R. Zell, C. V. Broome, and J. D. Wenger. 1993. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA 269:221-226. [PubMed] [Google Scholar]
- 2.Ahlstedt, S., J. Holmgren, and L. A. Hanson. 1974. Protective capacity of antibodies against E. coli O antigen with special reference to the avidity. Int. Arch. Allergy Appl. Immunol. 46:470-480. [DOI] [PubMed] [Google Scholar]
- 3.Altschuh, D., M. C. Dubs, E. Weiss, G. Zeder-Lutz, and M. H. Van Regenmortel. 1992. Determination of kinetic constants for the interaction between a monoclonal antibody and peptides using surface plasmon resonance. Biochemistry 31:6298-6304. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, E. L., T. Bowers, C. M. Mink, D. J. Kennedy, R. B. Belshe, H. Harakeh, L. Pais, P. Holder, and G. M. Carlone. 1994. Safety and immunogenicity of meningococcal A and C polysaccharide conjugate vaccine in adults. Infect. Immun. 62:3391-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arakere, G., and C. E. Frasch. 1991. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect. Immun. 59:4349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avery, O. T., and W. F. Goebel. 1931. Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J. Exp. Med. 54:437-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour, M. L. 1996. Conjugate vaccines and the carriage of Haemophilus influenzae type b. Emerg. Infect. Dis. 2:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuvery, E. C., R. W. van Delft, F. Miedema, V. Kanhai, and J. Nagel. 1983. Immunological evaluation of meningococcal group C polysaccharide-tetanus toxoid conjugate in mice. Infect. Immun. 41:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. P. Smith. 1975. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J. Biol. Chem. 250:1926-1932. [PubMed] [Google Scholar]
- 11.Borrebaeck, C. A., A. C. Malmborg, C. Furebring, A. Michaelsson, S. Ward, L. Danielsson, and M. Ohlin. 1992. Kinetic analysis of recombinant antibody-antigen interactions: relation between structural domains and antigen binding. Bio/Technology 10:697-698. [DOI] [PubMed] [Google Scholar]
- 12.Borrow, R., E. Longworth, S. J. Gray, and E. B. Kaczmarski. 2000. Prevalence of de-O-acetylated serogroup C meningococci before the introduction of meningococcal serogroup C conjugate vaccines in the United Kingdom. FEMS Immunol. Med. Microbiol. 28:189-191. [DOI] [PubMed] [Google Scholar]
- 13.Borrow, R., P. Richmond, E. B. Kaczmarski, A. Iverson, S. L. Martin, J. Findlow, M. Acuna, E. Longworth, R. O'Connor, J. Paul, and E. Miller. 2000. Meningococcal serogroup C-specific IgG antibody responses and serum bactericidal titres in children following vaccination with a meningococcal A/C polysaccharide vaccine. FEMS Immunol. Med. Microbiol. 28:79-85. [DOI] [PubMed] [Google Scholar]
- 14.Borrow, R., J. Southern, N. Andrews, N. Peake, R. Rahim, M. Acuna, S. Martin, E. Miller, and E. Kaczmarski. 2001. Comparison of antibody kinetics following meningococcal serogroup C conjugate vaccine between healthy adults previously vaccinated with meningococcal A/C polysaccharide vaccine and vaccine-naive controls. Vaccine 19:3043-3050. [DOI] [PubMed] [Google Scholar]
- 15.Ceesay, S. J., S. J. Allen, A. Menon, J. E. Todd, K. Cham, G. M. Carlone, S. H. Turner, L. L. Gheesling, W. DeWitt, B. D. Plikaytis, and B. Greenwood. 1993. Decline in meningococcal antibody levels in African children 5 years after vaccination and the lack of an effect of booster immunization. J. Infect. Dis. 167:1212-1216. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 1999. Impact of vaccines universally recommended for children—United States, 1990-1998. Morb. Mortal. Wkly. Rep. 48:243-248. [PubMed] [Google Scholar]
- 17.Cooper, L. J., D. Robertson, R. Granzow, and N. S. Greenspan. 1994. Variable domain-identical antibodies exhibit IgG subclass-related differences in affinity and kinetic constants as determined by surface plasmon resonance. Mol. Immunol. 31:577-584. [DOI] [PubMed] [Google Scholar]
- 18.Costantino, P., S. Viti, A. Podda, M. A. Velmonte, L. Nencioni, and R. Rappuoli. 1992. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine 10:691-698. [DOI] [PubMed] [Google Scholar]
- 19.Douglas, R. M., J. C. Paton, S. J. Duncan, and D. J. Hansman. 1983. Antibody response to pneumococcal vaccination in children younger than five years of age. J. Infect. Dis. 148:131-137. [DOI] [PubMed] [Google Scholar]
- 20.Egan, W. 1980. Structure of the capsular polysaccharide antigens from Haemophilus influenzae and Neisseria meningitidis by 13C NMR spectroscopy, p. 197. In J. S. Cohen (ed.), Magnetic resonance in biology, vol. 1. John Wiley & Sons, New York, N.Y.
- 21.Fairley, C. K., N. Begg, R. Borrow, A. J. Fox, D. M. Jones, and K. Cartwright. 1996. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in United Kingdom infants. J. Infect. Dis. 174:1360-1363. [DOI] [PubMed] [Google Scholar]
- 22.Ferson, M., L. Young, G. Hansen, J. Post, J. Tapsall, T. Shultz, A. Limnios, D. Lee, P. Reinbott, Y. Duffy, P. Robertson, P. Jones, G. Pontivivo, and K. Morgan. 1999. Unusual cluster of mild invasive serogroup C meningococcal infection in a university college. Commun. Dis. Intell. 23:261-264. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Ojeda, P. A., M. E. Monser, L. J. Rubinstein, H. J. Jennings, and K. E. Stein. 2000. Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 68:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmore, A., G. Jones, M. Barker, N. Soltanpoor, and J. M. Stuart. 1999. Meningococcal disease at the University of Southampton: outbreak investigation. Epidemiol. Infect. 123:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glode, M. P., E. B. Lewin, A. Sutton, C. T. Le, E. C. Gotschlich, and J. B. Robbins. 1979. Comparative immunogenicity of vaccines prepared from capsular polysaccharides of group C Neisseria meningitidis O-acetyl-positive and O-acetyl-negative variants and Escherichia coli K92 in adult volunteers. J. Infect. Dis. 139:52-59. [DOI] [PubMed] [Google Scholar]
- 26.Gold, R., M. L. Lepow, I. Goldschneider, T. F. Draper, and E. C. Gotshlich. 1979. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J. Infect. Dis. 140:690-697. [DOI] [PubMed] [Google Scholar]
- 27.Gold, R., M. L. Lepow, I. Goldschneider, T. L. Draper, and E. C. Gotschlich. 1975. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J. Clin. Investig. 56:1536-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold, R., M. L. Lepow, I. Goldschneider, and E. C. Gotschlich. 1977. Immune response of human infants to polysaccharide vaccines of group A and C Neisseria meningitidis. J. Infect. Dis. 136(Suppl.):S31-S35. [DOI] [PubMed] [Google Scholar]
- 29.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotschlich, E. C., I. Goldschneider, and M. S. Artenstein. 1969. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J. Exp. Med. 129:1367-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, and H. V. Raff. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison, L. H., D. M. Dwyer, C. T. Maples, and L. Billmann. 1999. Risk of meningococcal infection in college students. JAMA 281:1906-1910. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson, A. M. 1994. Characterization of glycoprotein oligosaccharides using surface plasmon resonance. Anal. Biochem. 220:303-307. [DOI] [PubMed] [Google Scholar]
- 35.Jennings, H. J., and C. Lugowski. 1981. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 127:1011-1018. [PubMed] [Google Scholar]
- 36.Jodar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 37.Jonsson, U., L. Fagerstam, B. Ivarsson, B. Johnsson, R. Karlsson, K. Lundh, S. Lofas, B. Persson, H. Roos, I. Ronnberg, et al. 1991. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. BioTechniques 11:620-627. [PubMed] [Google Scholar]
- 38.Joseph, H., E. Miller, M. Dawson, N. Andrews, I. Feavers, and R. Borrow. 2001. Meningococcal serogroup A avidity indices as a surrogate marker of priming for the induction of immunologic memory after vaccination with a meningococcal A/C conjugate vaccine in infants in the United Kingdom. J. Infect. Dis. 184:661-662. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson, R., A. Michaelsson, and L. Mattsson. 1991. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J. Immunol. Methods 145:229-240. [DOI] [PubMed] [Google Scholar]
- 40.Leach, A., P. A. Twumasi, S. Kumah, W. S. Banya, S. Jaffar, B. D. Forrest, D. M. Granoff, D. E. LiButti, G. M. Carlone, L. B. Pais, C. V. Broome, and B. M. Greenwood. 1997. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J. Infect. Dis. 175:200-204. [DOI] [PubMed] [Google Scholar]
- 41.Lepow, M. L., I. Goldschneider, R. Gold, M. Randolph, and E. C. Gotschlich. 1977. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics 60:673-680. [PubMed] [Google Scholar]
- 42.Lieberman, J. M., S. S. Chiu, V. K. Wong, S. Partidge, S. J. Chang, C. Y. Chiu, L. L. Gheesling, G. M. Carlone, and J. I. Ward. 1996. Safety and immunogenicity of a serogroup A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. JAMA 275:1499-1503. [PubMed] [Google Scholar]
- 43.MacDonald, N. E., S. A. Halperin, B. J. Law, B. Forrest, L. E. Danzig, and D. M. Granoff. 1998. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers: a randomized controlled trial. JAMA 280:1685-1689. [DOI] [PubMed] [Google Scholar]
- 44.Mayer, L. W., M. W. Reeves, N. Al-Hamdan, C. T. Sacchi, M. K. Taha, G. W. Ajello, S. E. Schmink, C. A. Noble, M. L. Tondella, A. M. Whitney, Y. Al-Mazrou, M. Al-Jefri, A. Mishkhis, S. Sabban, D. A. Caugant, J. Lingappa, N. E. Rosenstein, and T. Popovic. 2002. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electophoretic type-37 complex. J. Infect. Dis. 185:1596-1605. [DOI] [PubMed] [Google Scholar]
- 45.Michon, F., C. H. Huang, E. K. Farley, L. Hronowski, J. Di, and P. C. Fusco. 2000. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev. Biol. 103:151-160. [PubMed] [Google Scholar]
- 46.Mosier, D. E., I. M. Zitron, J. J. Mond, A. Ahmed, I. Scher, and W. E. Paul. 1977. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol. Rev. 37:89-104. [DOI] [PubMed] [Google Scholar]
- 47.Murphy, T. V., K. E. White, P. Pastor, L. Gabriel, F. Medley, D. M. Granoff, and M. T. Osterholm. 1993. Declining incidence of Haemophilus influenzae type b disease since introduction of vaccination. JAMA 269:246-248. [PubMed] [Google Scholar]
- 48.Ouchterlony, O. 1958. Diffusion-in-gel methods for immunological analysis. Prog. Allergy 51:1-78. [PubMed] [Google Scholar]
- 49.Peltola, H., H. Kayhty, A. Sivonen, and H. Makela. 1977. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics 60:730-737. [PubMed] [Google Scholar]
- 50.Perlmutter, R. M., D. Hansburg, D. E. Briles, R. A. Nicolotti, and J. M. Davie. 1978. Subclass restriction of murine anti-carbohydrate antibodies. J. Immunol. 121:566-572. [PubMed] [Google Scholar]
- 51.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 52.Richmond, P., R. Borrow, J. Findlow, S. Martin, C. Thornton, K. Cartwright, and E. Miller. 2001. Evaluation of de-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and de-O-acetylated serogroup C strains. Infect. Immun. 69:2378-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richmond, P., R. Borrow, E. Miller, S. Clark, F. Sadler, A. Fox, N. Begg, R. Morris, and K. Cartwright. 1999. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J. Infect. Dis. 179:1569-1572. [DOI] [PubMed] [Google Scholar]
- 54.Rubinstein, L. J., P. A. Garcia-Ojeda, F. Michon, H. J. Jennings, and K. E. Stein. 1998. Murine immune responses to Neisseria meningitidis group C capsular polysaccharide and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 66:5450-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubinstein, L. J., and K. E. Stein. 1988. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. I. Ontogeny. J. Immunol. 141:4352-4356. [PubMed] [Google Scholar]
- 56.Rubinstein, L. J., and K. E. Stein. 1988. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. II. Specificity. J. Immunol. 141:4357-4362. [PubMed] [Google Scholar]
- 57.Salisbury, D. 2001. Introduction of a conjugate meningococcal type C vaccine programme in the UK. J. Paediatr. Child Health 37:S34-S37. [DOI] [PubMed] [Google Scholar]
- 58.Sell, S. H., P. F. Wright, W. K. Vaughn, J. Thompson, and G. Schiffman. 1981. Clinical studies of pneumococcal vaccines in infants. I. Reactogenicity and immunogenicity of two polyvalent polysaccharide vaccines. Rev. Infect. Dis. 3(Suppl.):S97-S107. [DOI] [PubMed] [Google Scholar]
- 59.Stein, K. E. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 165(Suppl. 1):S49-S52. [DOI] [PubMed] [Google Scholar]
- 60.Stein, K. E., D. A. Zopf, B. M. Johnson, C. B. Miller, and W. E. Paul. 1982. The immune response to an isomaltohexosyl-protein conjugate, a thymus-dependent analogue of alpha(1 replaced by 6) dextran. J. Immunol. 128:1350-1354. [PubMed] [Google Scholar]
- 61.Stein, K. E., D. A. Zopf, C. B. Miller, B. M. Johnson, P. K. Mongini, A. Ahmed, and W. E. Paul. 1983. Immune response to a thymus-dependent form of B512 dextran requires the presence of Lyb-5+ lymphocytes. J. Exp. Med. 157:657-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinhoff, M. C. 1997. Haemophilus influenzae type b infections are preventable everywhere. Lancet 349:1186-1187. [DOI] [PubMed] [Google Scholar]
- 63.Steward, M. W., and A. M. Lew. 1985. The importance of antibody affinity in the performance of immunoassays for antibody. J. Immunol. Methods 78:173-190. [DOI] [PubMed] [Google Scholar]
- 64.Swanson, S. J., D. Mytych, and J. Ferbas. 2002. Use of biosensors to monitor the immune response. Dev. Biol. 109:71-78. [PubMed] [Google Scholar]
- 65.Twumasi, P. A., Jr., S. Kumah, A. Leach, T. J. O'Dempsey, S. J. Ceesay, J. Todd, C. V. Broome, G. M. Carlone, L. B. Pais, P. K. Holder, B. D. Plikaytis, and B. M. Greenwood. 1995. A trial of a group A plus group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J. Infect. Dis. 171:632-638. [DOI] [PubMed] [Google Scholar]
- 66.Zeder-Lutz, G., D. Altschuh, H. M. Geysen, E. Trifilieff, G. Sommermeyer, and M. H. Van Regenmortel. 1993. Monoclonal antipeptide antibodies: affinity and kinetic rate constants measured for the peptide and the cognate protein using a biosensor technology. Mol. Immunol. 30:145-155. [DOI] [PubMed] [Google Scholar]