Abstract
Background
Most fragility fractures arise among the many women with osteopenia, not the smaller number with osteoporosis at high risk for fracture. Thus, most women at risk for fracture assessed only by measuring areal bone mineral density (aBMD) will remain untreated.
Methods
We measured cortical porosity and trabecular bone volume/total volume (BV/TV) of the ultradistal radius (UDR) using high-resolution peripheral quantitative computed tomography, aBMD using densitometry, and 10-year fracture probability using the country-specific FRAX tool in 68 postmenopausal women with forearm fractures and 70 age-matched community controls in Olmsted County, Minnesota.
Results
Women with forearm fractures had 0.4 standard deviations (SD) higher cortical porosity and 0.6 SD lower trabecular BV/TV. Compact-appearing cortical porosity predicted fracture independent of aBMD; odds ratio [OR] 1.92 (95%CI, 1.10–3.33). In women with osteoporosis at the UDR, cortical porosity did not distinguish those with, from those without, fractures because high porosity was present in 92% and 86% of each group respectively. By contrast, in women with osteopenia at the UDR, high porosity of the compact-appearing cortex conferred an OR for fracture of 4.00 (95%CI, 1.15–13.90).
Conclusion
In women with osteoporosis, porosity is captured by aBMD and so measuring UDR cortical porosity does not improve diagnostic sensitivity. However, in women with osteopenia, cortical porosity was associated with forearm fractures.
Keywords: bone mineral density, cortical porosity, forearm fractures, microarchitecture, trabecular bone
Introduction
The terms ‘osteoporosis’ and ‘osteopenia’ were originally coined to convey the notion that an individual is susceptible to sustaining a fracture following minimal trauma because there is ‘not enough bone’1–3. To formalize this imprecise notation, a working group of the World Health Organization (WHO) defined osteoporosis as a “systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture” 4. ‘Osteoporosis’ was quantified as a femoral neck (FN) aBMD T-score of −2.5 SD or more below the young normal mean, osteopenia as a T-score between −1 and −2.5 SD, and normal aBMD as a T-score above −1 SD 5.
Subsequent epidemiological research confirmed that fracture risk increases as aBMD decreases. However, these studies also showed that ~50% of all fractures, ~50% all recurrent fractures, and much of the accompanying morbidity, mortality and costs to the community arise from the large proportion of the population with osteopenia at modest risk for fracture, not the smaller fraction with osteoporosis at high risk for fracture 6–9. This observation identified a challenge to curbing the population burden of fractures.
Among the large group of women with osteopenia, there exists a substantial subgroup with bone fragility contributing to the burden of fractures. If an aBMD measurement alone is used in an osteoporosis screening program, women with osteopenia will be excluded from further investigation and so will not be offered treatment 10–12. One approach to case finding - identifying those at risk for fracture in need of treatment is the use of the fracture risk assessment tool (FRAX) 11,12. Another approach is to identify the structural basis of the bone fragility not captured by the aBMD measurement and thereby to quantify “microarchitectural deterioration of bone tissue”, the descriptive component of the definition of ‘osteoporosis’.
Trabecular bone loss and vertebral fractures are historical hallmarks of osteoporosis. However, ~80% of the skeleton is cortical; 80% of all fractures are non-vertebral and 30% of these are forearm fractures 13. Moreover, about 70% of all the appendicular bone lost during aging is cortical and results from intracortical remodelling which occurs throughout the cortex but is particularly vigorous in the cortico-trabecular junctional (transitional) zone where the cortical and trabecular compartments merge (figure 1) 14. Remodelling during advancing age becomes unbalanced and removes more bone than it deposits leaving residual cortical porosity, which increases bone fragility exponentially and is a quantifiable ‘footprint’ of bone loss 14–16.
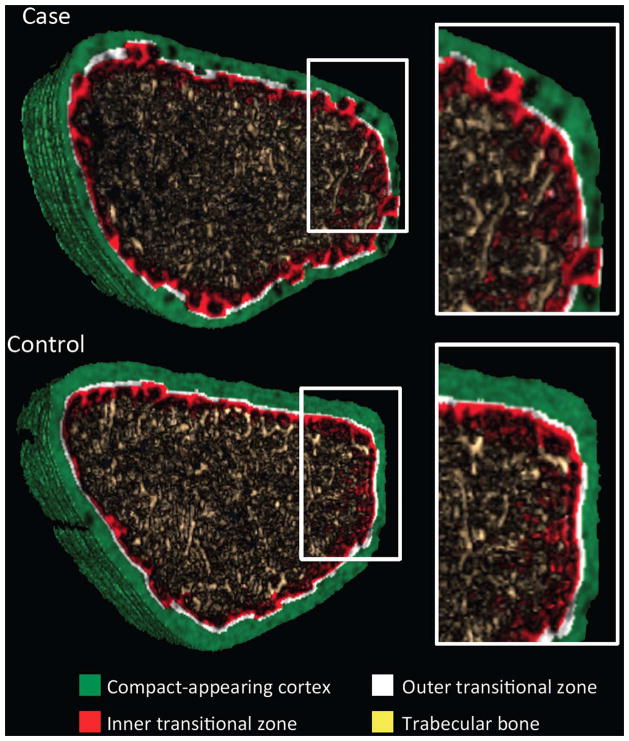
Figure 1.
Representative segmented image obtained at the ultradistal radius using non threshold-based image analysis in a postmenopausal women with (Case) and without (Control) forearm fracture. The full cross section and the magnified image show the presence of porosity within the compact appearing cortex (green) and the outer (white) and inner (red) transitional zones, and loss of trabecular bone (yellow) in the case, and less so in the control.
Quantifying porosity in vivo has become possible with the recent development of high-resolution peripheral quantitative computed tomography (HRpQCT), a noninvasive method of image acquisition, and StrAx1.0, a new method of image analysis that permits quantification of porosity, even porosity due to pores under 100 microns (the diameter of >80% of cortical pores), and quantifies porosity of the transitional zone 14,17. The aim of this study was to determine (i) whether bone microarchitecture, particularly cortical porosity, predicts fracture; (ii) whether porosity does so independent of aBMD and FRAX; and (iii) whether combining a measurement of forearm microarchitecture and aBMD (at the forearm or femoral neck) identifies more women with fractures than aBMD alone.
Methods
Participants
As previously reported, 100 postmenopausal women aged ≥50 years with a distal forearm fracture were matched with 105 controls from an age-stratified random sample of women from Olmsted County 18. The fracture occurred 7 (3–13) months (median (IQR)) before the investigation. Fragility fracture was defined on the basis of the original description of the fall that led to fracture using the classification of Palvanen et al. 19. This corresponds to the convention of a moderate trauma distal forearm fracture resulting from a fall from standing height or less. Controls had no history of a fracture after 35 years of age. Of the subjects analyzed here, 21/68 (31%) of cases and 21/70 (30%) of controls received bisphosphonate or estrogen therapy. Observations were reported in the whole group previously 18, and here were no different after excluding treated subjects. The cohort was >96% Caucasian. The study was approved by the Mayo Clinic Institutional Review Board and the present analysis was based on de-identified data.
Measurement of micro-architecture, aBMD and FRAX score
Microarchitecture was assessed at the non-fractured ultradistal radius (UDR) using HRpQCT (XtremeCT, Scanco Medical AG, Brüttisellen, Switzerland). Quality control was monitored by daily scans of phantoms (hydroxyapatite (HA) rods, QRM, Moehrendorf, Germany). Movement artefacts precluded use of scans in 32 cases and 35 controls leaving 68 cases (mean age ± SD, 63 ± 9 years) and 70 controls (mean age 66 ± 10 years; p = 0.09) 20. Age and aBMD did not differ between excluded and included subjects. The coefficient of variation calculated for the same patients at different times after repositioning was < 1.5% for volumetric density and 0.54 to 3.98% for cortical porosity 17,21.
StrAx1.0 is a new algorithm that segments cortical bone from background and from the trabecular compartment, into its compact appearing, outer and inner transitional zones and in so doing correctly assigns the trabecularized cortex (i.e., cortical fragments) to the transitional zone rather than to the medullary compartment which results in overestimation of ‘trabecular’ density 17. The outer transitional zone is defined as the trabecularized cortex that is adjacent to the compact-appearing cortex whereas the inner transitional zone is the trabecularized cortex adjacent to the medullary cavity. The latter also contains some true trabecular bone. The 40 most proximal slices are analyzed using ~3600 radial attenuation profile curves around each slice. All voxels within the periosteal envelope are analyzed and most are composite voxels as they contain both void volume and mineralized bone matrix volume. The proportions of each are quantified using an interpolation function derived from voxels with zero attenuation (porosity). Voxels with attenuation produced by fully mineralized bone (density of 1200 mgHA/cc or greater) are assigned 100%. The void volume of a voxel = 100 − mineralized bone volume fraction. Total porosity is the average of the summed void volume fractions of all voxels producing an attenuation ≤ 80% than produced by fully mineralized bone (≥1200 mgHA/cc). Attenuation between 80–100% of this maximum can be produced by either heterogeneity in bone tissue mineralization density or a small pore (≤ ~35 μm diameter) in a voxel containing fully mineralized bone matrix. Thus, porosity may be underestimated by erroneously discarding existing pores of ≤ 35μm diameter. UDR and femoral neck (FN) aBMD (g/cm2) were measured by DXA (Lunar Prodigy, GE Healthcare, USA).
The 10-year probability of a major fracture (hip, clinical spine, proximal humerus or forearm fracture) and of a hip fracture were assessed using the country-specific WHO risk assessment tool FRAX (version 3.8), including FN aBMD 22. Information on the FRAX input variables were available in all women. The forearm fracture was not included as the aim was to estimate the 10-year probability of the fracture before the event, not the probability of fracture after this event.
Statistical analysis
Fracture risk associated with aBMD and microstructure was estimated by odds ratios (OR) from logistic regression models adjusting for age, FRAX probabilities, UDR and FN aBMD. Group differences were summarized using the ΔSD, the mean value in cases standardized using the mean and SD in controls. Linear regression was used to study the relationship between microstructure and age and to test for differences in slopes and intercepts between cases and controls. The areas under a receiver operating curves (AUC) were compared using nonparametric methods 23,24. Cases and controls were pooled and partitioned into those with osteoporosis, osteopenia or normal aBMD at the UDR and FN. ORs for fracture were computed defining ‘high’ cortical porosity (>90th centile) and ‘low’ trabecular BV/TV (<10th centile) in 40 healthy premenopausal women in Melbourne (age 27 years, range 21–31). A p < 0.05 (two tailed) denoted statistical significance.
Results
Cortical porosity predicts forearm fractures independently of forearm aBMD
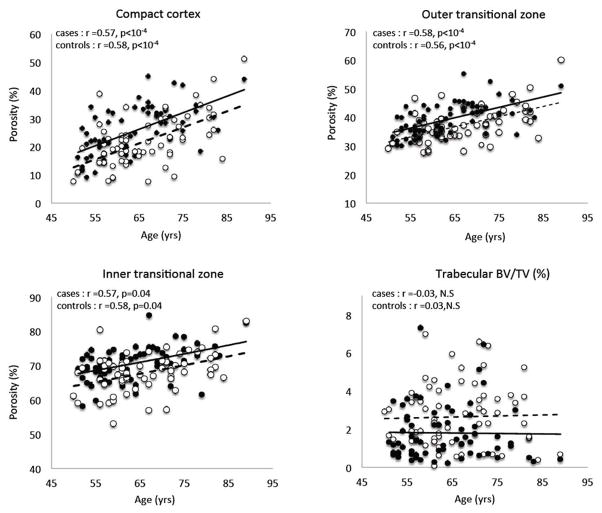
Women with forearm fractures had higher porosity of the compact-appearing cortex, outer and inner transitional zones, and lower trabecular BV/TV than controls (Table 1). Porosity increased linearly as age advanced with no difference in the slopes of the regression lines but the y-intercepts (porosity) were 0.35, 0.46 and 0.61 SD higher in the respective cortical compartments in women with fractures than controls (all p <0.001) (Figure 2).
Table 1.
Comparison of areal bone mineral density (aBMD) at the ultradistal radius (UDR) and femoral neck (FN), FRAX score, cortical porosity and trabecular bone volume fraction (BV/TV) between postmenopausal women with (cases) or without forearm fracture (controls). Odds ratio (OR) and 95% confidence intervals (95% CI) are reported for 1 standard deviation (SD) increment. Area under the receiving operating curve (AUC) represents the ability of the variables to discriminate the fracture status.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Cases (n=68) | Controls (n=70) | Δ SD (95% CI) | OR for fracture (95% CI) | AUC (95% CI) | |||
| Mean (SD) | unajusted | adjusted for UDR aBMD | adjusted for FN aBMD | ||||
| Areal BMD | |||||||
| UDR (g/cm2) | 0.37 (0.08) | 0.42 (0.07) | − 0.51 (−0.82; −0.21) *** | 2.46 (1.57; 3.86) ** | - | - | 0.72 (0.63; 0.80) |
| FN (g/cm2) | 0.81 (0.10) | 0.88 (0.14) | − 0.53 (−0.84; −0.23) *** | 2.32 (1.48; 3.65) ** | - | - | 0.71 (0.62; 0.79) |
| FRAX score | |||||||
| Major fracture (%) | 12.51 (7.82) | 11.95 (8.03) | 0.07 (−0.17; 0.30) | - | - | - | 0.53 (0.43; 0.63) |
| Hip fracture (%) | 2.84 (4.93) | 2.70 (5.31) | 0.02 (−0.20; 0.25) | - | - | - | 0.57 (0.47; 0.46) |
| Porosity | |||||||
| compact-appearing cortex (%) | 30.07 (7.86) | 26.92 (7.62) | 0.40 (0.07; 0.73) ***,‡ | 2.59 (1.56; 4.33) ** | 1.92 (1.10; 3.33)* | 2.25 (1.33; 3.82) * | 0.72 (0.64; 0.81) |
| outer transitional zone (%) | 39.19 (5.53) | 36.98 (5.85) | 0.38 (0.05; 0.71) ***,‡ | 2.39 (1.47; 3.89) ** | 1.75 (1.02; 2.99)* | 2.01 (1.20; 3.34) * | 0.72 (0.63; 0.80) |
| Porosity inner transitional zone (%) | 81.37 (3.15) | 79.39 (3.41) | 0.54 (0.24; 0.84) *** | 2.11 (1.41; 3.14) ** | 1.38 (0.81; 2.28) | 1.75 (1.14; 2.68) * | 0.71 (0.63; 0.80) |
| Trabecular BV/TV (%) | 1.80 (1.46) | 2.64 (1.62) | − 0.58 (−0.88; −0.24) ** | 1.79 (1.21; 2.63) * | 1.04 (0.62; 1.74) | 1.43 (0.95; 2.17) | 0.67 (0.58; 0.76) |
p<0·05,
p<0·001,
p<0·0001
p<0·05 vs. controls adjusted for aBMD at UDR
Figure 2.
Cortical porosity in the compact-appearing cortex, outer transitional zone, inner transitional zone and trabecular bone volume fraction (BV/TV) versus age in women with distal forearm fractures (Cases, black dots, solid line) and without forearm fracture (controls, white open circles, dotted line).
Porosity of each cortical compartment and trabecular BV/TV predicted forearm fractures. Porosity of the compact appearing cortex and outer transitional zone, but not inner transitional zone or trabecular BV/TV, predicted fracture independently of UDR aBMD. Porosity discriminated cases from controls with AUCs ranging from 0.71 to 0.83 (all AUCs > 0.5 with p < 0.001) (Table 1).
Cortical porosity is captured by the diagnostic threshold of −2.5 SD for osteoporosis
Osteoporosis of the UDR was present in almost twice the number of cases than controls; 26 (38%) cases and 14 (20%) controls conferring an OR of 2.48 (95% CI, 1.15–5.31). High porosity in one or more cortical compartments was common in women with osteoporosis; 92% with a fracture and 86% without a fracture. Thus, porosity was captured by the diagnostic category of ‘osteoporosis’ at the UDR. Adding of a measure of porosity to aBMD did not identify more women with osteoporosis with fractures than aBMD alone.
Cortical porosity increases the diagnostic sensitivity of osteopenia, not normal aBMD
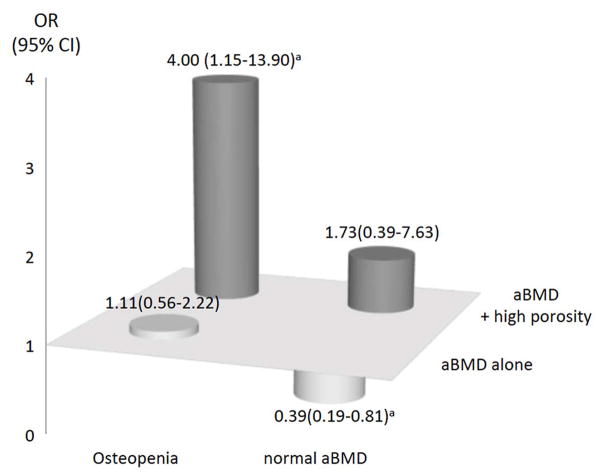
Osteopenia of the UDR was present in 26 (38%) cases and controls 25 (36%). Osteopenia alone was not associated with fracture as the OR was 1.11 (95% CI, 0.56–2.22) (Figure 3). However, over twice the number of women with osteopenia and a fracture had high porosity of the compact appearing cortex than did women with osteopenia without a fracture (50% versus 20% respectively), conferring an OR of 4.00 (95% CI, 1.15–13.90) and a specificity of 80%. Porosity of the outer and inner transitional zones conferred ORs of 3.17 (95% CI, 0.96–10.48) and 2.93 (95% CI, 0.84–10.25), respectively.
Figure 3.
Odds ratio (OR) and 95% confidence interval (95% CI) for distal fracture associated with areal bone mineral density (aBMD) alone and in combination with high cortical porosity assessed in the compact appearing cortex in women with osteopenia at the ultradistal radius. ap < 0.05.
Normal UDR aBMD was present in 16 (24%) cases and 31 (44%) controls and was protective, with an OR of 0.39 (95% CI, 0.19–0.81). High porosity was not more prevalent in those with, than without a fracture. Thus, adding a measure of porosity did not identify more women with normal aBMD with a fracture.
Cortical porosity, WHO diagnostic categories using FN aBMD, and forearm fracture risk
Compact-appearing and outer transitional zone porosity of the UDR predicted forearm fractures independently of FN aBMD (Table 1). Only 9% of women with forearm fractures had FN osteoporosis; 72% had FN osteopenia and 19% had normal FN aBMD. No significant associations with fracture were detected with FN osteoporosis alone [OR 2.16 (95% CI, 0.52–9.01)] or FN osteopenia alone [1.62 (95% CI, 0.79–3.31)]. Moreover, FN osteopenia plus high porosity of the UDR inner transitional zone, or FN osteopenia plus low UDR trabecular BV/TV were each associated with forearm fracture, conferring ORs of 2.92 (95% CI, 1.18–7.20) and 2.44 (95% CI, 1.01–5.92) respectively. Normal FN aBMD was protective with an OR 0.45 (95% CI, 0.21–0.99).
Cortical porosity predicts forearm fractures independently of FRAX
The ten-year probability of a major osteoporotic or hip fracture did not differ significantly between cases and controls (Table 1). By contrast, there were significant correlations between the probability of a major fracture and cortical porosity (r ranging from 0.26 to 0.40, all p<0.001). Compact-appearing cortex, outer and inner transitional zone porosities remained significantly associated with forearm fracture after adjustment for FRAX, with ORs of 2.11 (95% CI, 1.35–3.30), 2.06 (95% CI, 1.32–3.22) and 2.48 (95% CI, 1.65–3.73), respectively.
Discussion
We report the following: (i) women with forearm fractures had micro-architectural deterioration; cortical porosity was increased and trabecular bone volume fraction was reduced. (ii) Both predicted forearm fractures, but only cortical porosity did so independently of UDR or FN aBMD. (iii) The diagnostic threshold for ‘osteoporosis’ (T-score ≤ −2.5 SD), whether based on UDR or FN aBMD, captured high cortical porosity and low trabecular bone volume of the UDR whether a forearm fracture was present or not. Thus, after finding a BMD T-score ≤ −2.5 at the UDR or FN, measuring microstructure at the UDR did not identify more women with forearm fractures than measuring aBMD alone. These observations support the inclusion of “microarchitectural deterioration” in the WHO definition of osteoporosis.
While the diagnostic category of ‘osteoporosis’ captures microarchitectural deterioration in women at high risk for fracture, most fractures in the community arise from the larger segment of the population with an aBMD T-score less severely reduced than −2.5 SD 6–9,25. In this study, at the UDR, 38% of the women with a forearm fracture had osteopenia, while 24% had normal aBMD. At the FN, 72% had osteopenia and 19% had normal aBMD. As a group, these women were not at increased risk for fracture since neither UDR nor FN osteopenia alone was associated with fracture. Thus, physicians finding a UDR or FN aBMD T-score in the osteopenic range will be disinclined to initiate treatment even though most forearm fractures arise from this group.
Combining risk factors, as used in FRAX, assists in identifying individuals in need of treatment and avoids treating individuals at low risk for morbid events 4,10,11. Similarly, by adding a measurement of cortical porosity at the UDR, we identified a subset of women with UDR or FN osteopenia contributing to the burden of fractures. Thus, in women found to have osteopenia (the usual outcome in the community), the data suggest that it may be appropriate to also measure UDR porosity. Finding high porosity, which compromises bone strength out of proportion to the modest reduction in aBMD that characterizes osteopenia, identifies individuals in need of treatment who would not be identified otherwise.
Women with normal UDR or FN aBMD were relatively protected against fracture. Nevertheless, 24% of all women with forearm fractures had normal UDR aBMD. Measurement of porosity did not distinguish women with normal UDR aBMD with a fracture from those without a fracture because the prevalence of microarchitectural abnormalities was similar in those with and without fractures. In these women, the fracture may have been the result of more severe trauma than was estimated using the semiquantiative classification of trauma severity 19, or may be due to the result of abnormalities in bone material composition and structure yet to be identified.
Porosity throughout the cortex was associated with fracture, but only porosity of the compact-appearing cortex and outer transitional zone, not porosity of the inner transitional zone, predicted fracture independently of aBMD. This site-specific independence from aBMD may be due to the location of the porosity. While porosity of the inner transitional zone is larger than that of the compact appearing cortex and outer transitional zone, it is positioned nearer the neutral axis adjacent to the medullary canal. Deficits in mineralized bone matrix volume at this location may contribute less to loss of bending strength than deficits in mineralized bone matrix volume positioned further from the neutral axis 26.
Cortical porosity in adulthood is the net result of ‘peak’ porosity achieved during growth, constituted mainly by the Haversian and Volkmann canals, and the subsequent increase in porosity produced by age-related intracortical remodeling upon these canals which enlarges them focally and produces coalescent and giant pores in cross section as age advances 17,27. In this study, porosity increased across age, but the slope of porosity as a function of age was no greater in women with, than without, fractures, suggesting that women sustaining a fracture may assemble a bone with a higher peak porosity.
Porosity measured in this study was several-fold higher than the ≤1–16% porosity reported using HR-pQCT 28–32. While cortical bone is ‘compact’ relative to trabecular bone, the term ‘compact’ is a misnomer. Cortical bone is a three dimensional structure comprising mineralized bone matrix volume and a void volume formed at the nano-scale level by voids within and between collagen fibrils, and formed at the micro-scale level by the osteocyte lacunar-canalicular system and by the Haversian and Volkmann canals 33.
These voids are not ‘empty’; they are fluid filled and so the measurement of bone water content provides an accurate measurement of the component of cortical bone that is void volume. Direct measurements of cortical bone water content across species using deuterium oxide or dehydration experiments report a void volume ranging from 15 to 40% 34–37. Synchrotron radiation based μCT assessment of histomorphometric specimens suggests that the diameter of ~60% of pores is < 90 μm; ~20% is 90–180 μm and ~20% is > 180 μm 38,39. The values for porosity reported here are in agreement with the above and are compatible with the physiological role of the canals which house bone’s vasculature.
We suggest that the low porosities reported in previous studies are incompatible with the direct measurements cited above and are incompatible with the provision of an effective vascular supply since bone is a highly vascularized organ and receives 10 to 20% of total cardiac output essential for nutrient transport and waste removal 40. The discrepancies are likely to be the result of differences in image analysis techniques. While HR-pQCT has a voxel size of 82 μm, its in-plane resolution is about 130 μm. This precludes the quantification of pores lower than this value 41. As reported above, more than 60% of pores are under 100 microns. Excluding these, results in underestimation of porosity which depends upon the cortical compartment (compact-appearing or transitional zone) measured, age, sex, and the underlying disease present 17,38. Indeed, the larger pores may contribute disproportionately more to total porosity than smaller pore even though smaller pores are more numerous in samples from elderly in whom cortex is porous and fragmented. However, in samples from younger individuals, with a cortex mostly compact in appearance and not fragmented, the porosity created by smaller pores is likely to account for a larger proportion of the total porosity. The magnitude of the underestimation in each of these states will be the subject of future research.
This study has several limitations. The study was cross-sectional so determining the relative contributions of peak porosity and age-related bone loss to fracture risk was not possible. Moreover, the sample size may have been too small to detect an increased risk of fracture in some subgroups. About 33% of the patients enrolled in the study were excluded because of motion artifacts, a recognized problem being addressed by shorter scanning times and better forearm immobilization methods in newer imaging devices 20. Moreover, while the technique used to estimate porosity account for pores smaller than 100 μm, we acknowledge that it misses pore smaller than 35 μm.
In summary and conclusion, postmenopausal women with distal forearm fractures have microstructural deterioration characterized by high cortical porosity and reduced trabecular BV/TV. UDR aBMD in the osteoporosis range (T-score ≤ −2.5D) captured these abnormalities so measurement of microarchitecture did not identify a greater proportion of women with fracture than did areal BMD in this range. In women with osteopenia, the source of over 50% of all fractures, fracture risk was increased if high porosity was present so measuring porosity improved identification of women with osteopenia with forearm fractures. Thus, measuring porosity is likely to be clinically useful in identifying women at risk for fracture considered at low risk based on their aBMD measurement alone. Further research will be needed to determine whether assessment of microstructure at the spine and proximal femur improves the sensitivity and specificity in identification of women at risk for fracture at those locations.
Acknowledgments
This study was funded by NIH Grants R01 AR027065 and UL1 TR000135 (Mayo CTSA) and by NHMRC Grant 628701.
Footnotes
Author’s roles: Drafting manuscript: YB, ES, SK, JK, JM. Revising manuscript content: YB, ES, EA, RZ JP, JM, JK and SK. Approving final version of manuscript: YB, RZ, EA, AGZ, JP, AB, JM, HJ, JK, SK and ES. ES takes responsibility for the integrity of the data analysis.
Disclosures:
AGZ is one of the inventor of StrAx 1.0. ES & RZ are inventors of StrAx 1.0 algorithm and directors of Straxcorp. StrAx software is now being actively marketed. RZ has received Consulting fees / lecture fees / grant support from Amgen, MSD, Servier. JK has received Consulting fees / lecture fees / grant support from Amgen, D3A, GSK, Lilly, Medimaps, Merck. ES have received Consulting fees / lecture fees / grant support from Amgen, MSD, Novartis, Sanofi Aventis, Servier. RZ and ES are inventors of the Strax method and directors of StrAxcorp. Other Authors have no disclosures to declare.
References
- 1.Albright F. Osteoporosis. Ann Intern Med. 1947;27(6):861–82. doi: 10.7326/0003-4819-27-6-861. [DOI] [PubMed] [Google Scholar]
- 2.Cooper A, Cooper BB. A treatise on disloctations, and on fractures of the joints. London, United Kingdom: Churchill; 1822. [Google Scholar]
- 3.Schapira D, Schapira C. Osteoporosis: the evolution of a scientific term. Osteoporos Int. 1992;2(4):164–7. doi: 10.1007/BF01623921. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4(6):368–81. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Sanders KM, Nicholson GC, Watts JJ, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006;38(5):694–700. doi: 10.1016/j.bone.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164(10):1108–12. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 9.Langsetmo L, Goltzman D, Kovacs CS, et al. Repeat low-trauma fractures occur frequently among men and women who have osteopenic BMD. J Bone Miner Res. 2009;24(9):1515–22. doi: 10.1359/jbmr.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose G. Sick individuals and sick populations. International journal of epidemiology. 2001;30(3):427–32. doi: 10.1093/ije/30.3.427. discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriwaki K, Komaba H, Noto S, et al. Cost-effectiveness of alendronate for the treatment of osteopenic postmenopausal women in Japan. J Bone Miner Res. 2013;28(2):395–403. doi: 10.1002/jbmr.1755. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12(5):417–27. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 14.Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375(9727):1729–36. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 15.Carter DR, Hayes WC. The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am. 1977;59(7):954–62. [PubMed] [Google Scholar]
- 16.Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. J Biomech. 1988;21(1):13–6. doi: 10.1016/0021-9290(88)90186-8. [DOI] [PubMed] [Google Scholar]
- 17.Zebaze R, Ghasem-Zadeh A, M’bala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantificatino of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013;54:8–20. doi: 10.1016/j.bone.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ, 3rd, Christen D, Riggs BL, et al. Assessing forearm fracture risk in postmenopausal women. Osteoporos Int. 2010;21(7):1161–9. doi: 10.1007/s00198-009-1047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palvanen M, Kannus P, Parkkari J, et al. The injury mechanisms of osteoporotic upper extremity fractures among older adults: a controlled study of 287 consecutive patients and their 108 controls. Osteoporos Int. 2000;11(10):822–31. doi: 10.1007/s001980070040. [DOI] [PubMed] [Google Scholar]
- 20.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50(1):111–8. doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Bjornerem A, Bui QM, Ghasem-Zadeh A, Hopper JL, Zebaze R, Seeman E. Fracture risk and height: An association partly accounted for by cortical porosity of relatively thinner cortices. J Bone Miner Res. 2013;28(9):2017–26. doi: 10.1002/jbmr.1934. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA on the behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at the primary health-care level. University of Sheffield; 2007. [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 24.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. American journal of epidemiology. 2004;159(9):882–90. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 25.Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17(9):1404–9. doi: 10.1007/s00198-006-0135-9. [DOI] [PubMed] [Google Scholar]
- 26.Burr DB. Cortical bone: a target for fracture prevention? Lancet. 2010;375(9727):1672–3. doi: 10.1016/S0140-6736(10)60444-8. [DOI] [PubMed] [Google Scholar]
- 27.Keshawarz NM, Recker RR. Expansion of the medullary cavity at the expense of cortex in postmenopausal osteoporosis. Metabolic bone disease & related research. 1984;5(5):223–8. doi: 10.1016/0221-8747(84)90063-8. [DOI] [PubMed] [Google Scholar]
- 28.Boutroy S, Walker MD, Liu XS, et al. Lower cortical porosity and higher tissue mineral density in Chinese-American versus white women. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2057. [DOI] [PubMed] [Google Scholar]
- 29.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25(5):983–93. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25(4):882–90. doi: 10.1359/jbmr.091020. [DOI] [PubMed] [Google Scholar]
- 32.Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. 2012;27(2):273–82. doi: 10.1002/jbmr.552. [DOI] [PubMed] [Google Scholar]
- 33.Cowin SC, Gailani G, Benalla M. Hierarchical poroelasticity: movement of interstitial fluid between porosity levels in bones. Philosophical transactions Series A, Mathematical, physical, and engineering sciences. 2009;367(1902):3401–44. doi: 10.1098/rsta.2009.0099. [DOI] [PubMed] [Google Scholar]
- 34.Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology. 2008;248(3):824–33. doi: 10.1148/radiol.2482071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JW. Observations on the Water Content of Bone. The Journal of bone and joint surgery. 1964;46:553–62. British volume. [PubMed] [Google Scholar]
- 36.Mueller KH, Trias A, Ray RD. Bone density and compostiton. Age-related and pathological changes in water and mineral content. J Bone Joint Surg Am. 1966;48(1):140–8. [PubMed] [Google Scholar]
- 37.Biltz RM, Pellegrino ED. The chemical anatomy of bone. I. A comparative study of bone composition in sixteen vertebrates. J Bone Joint Surg Am. 1969;51(3):456–66. [PubMed] [Google Scholar]
- 38.Chappard C, Bensalah S, Olivier C, et al. 3D characterization of pores in the cortical bone of human femur in the elderly at different locations as determined by synchrotron micro-computed tomography images. Osteoporos Int. 2013;24(3):1023–33. doi: 10.1007/s00198-012-2044-4. [DOI] [PubMed] [Google Scholar]
- 39.Qiu S, Fyhrie DP, Palnitkar S, Rao DS. Histomorphometric assessment of Haversian canal and osteocyte lacunae in different-sized osteons in human rib. Anat Rec A Discov Mol Cell Evol Biol. 2003;272(2):520–5. doi: 10.1002/ar.a.10058. [DOI] [PubMed] [Google Scholar]
- 40.Brookes M, Revell JW. The blood supply of bone: scientific aspects. New York: Springer; 1998. [Google Scholar]
- 41.Tjong W, Kazakia GJ, Burghardt AJ, Majumdar S. The effect of voxel size on high-resolution peripheral computed tomography measurements of trabecular and cortical bone microstructure. Med Phys. 2012;39(4):1893–903. doi: 10.1118/1.3689813. [DOI] [PMC free article] [PubMed] [Google Scholar]